Abstract
Lignin, the most abundant aromatic biopolymer on Earth, is extremely recalcitrant to degradation. By linking to both hemicellulose and cellulose, it creates a barrier to any solutions or enzymes and prevents the penetration of lignocellulolytic enzymes into the interior lignocellulosic structure. Some basidiomycetes white-rot fungi are able to degrade lignin efficiently using a combination of extracellular ligninolytic enzymes, organic acids, mediators and accessory enzymes. This review describes ligninolytic enzyme families produced by these fungi that are involved in wood decay processes, their molecular structures, biochemical properties and the mechanisms of action which render them attractive candidates in biotechnological applications. These enzymes include phenol oxidase (laccase) and heme peroxidases [lignin peroxidase (LiP), manganese peroxidase (MnP) and versatile peroxidase (VP)]. Accessory enzymes such as H2O2-generating oxidases and degradation mechanisms of plant cell-wall components in a non-enzymatic manner by production of free hydroxyl radicals (·OH) are also discussed.
Keywords: Lignocellulose, bioconversion, fungi, lignin, ligninases
1. Lignocellulosic materials
Lignocellulose is a renewable organic material and is the major structural component of all plants. Lignocellulosic wastes are produced in large amounts by many industries including those of forestry, pulp and paper, agriculture, and food. Such wastes are also present in municipal solid waste (MSW), and animal wastes [1-6]. These potentially valuable materials were treated as waste in many countries in the past, and still are today in some developing counties, which raises many environmental concerns [7,8].
Lignocellulose consists of three major components: cellulose, hemicellulose and lignin. In addition, small amounts of other materials such as ash, proteins and pectin can be found in lignocellulosic residues in varying degrees based on the source [9]. Cellulose, the major constituent of all plant material and the most abundant organic molecule on Earth, is a linear biopolymer consisting of a nhydroglucopyranose-molecules (glucose) connected by β-1,4-glycosidic bonds. Coupling of adjacent cellulose chains via hydrogen bonds, hydrophobic interactions and Van der Waal's forces results in the parallel alignment of crystalline structures known as microfibrils [10]. Unlike cellulose, hemicelluloses are heterogeneous polymers of pentoses (including xylose and arabinose), hexoses (mainly mannose, less glucose and galactose) and sugar acids. The highly variable composition of hemicelluloses is dependent on its plant source [11,12]. Lignin, the second-most abundant biopolymer on Earth and a heterogeneous polymer in lignocellulosic residues, is the only naturally synthesised polymer with an aromatic backbone. It generally contains three precursor aromatic alcohols including coniferyl alcohol, sinapyl and p-coumaryl [13]. These precursors form the guaiacyl- (G), syringyl- (S) and p-hydroxyphenyl (H) subunits in the lignin molecule, respectively [14]. The subunits ratio, and consequently, the lignin composition, varies between different plant groups. Oxidative coupling of these lignin aromatic alcohol monomers creates a complex structure in lignin which is highly recalcitrant to degradation [15]. By linking to both hemicelluloses and cellulose, lignin acts as a barrier to any solutions or enzymes and prevents penetration of lignocellulolytic enzymes to the interior lignocellulosic structure. Not surprisingly, of the components of lignocellulosic material, lignin is the most resistant to degradation [9,16]. Although lignin resists attack by most microorganisms, basidiomycetes white-rot fungi, are able to degrade lignin efficiently [15,17].
2. Lignocellulolytic enzyme-producing fungi
Lignocellulolytic enzymes-producing fungi are widespread, and include species from the ascomycetes (e.g. Trichoderma reesei) and basidiomycetes phyla such as white-rot (e.g. Phanerochaete chrysosporium) and brown-rot fungi (e.g. Fomitopsis palustris). In addition, a few anaerobic species (e.g. Orpinomyces sp.) are found to be able to degrade cellulose in the gastrointestinal tracts of ruminant animals [18,19]. Biomass degradation by these fungi is performed by complex mixtures of cellulases [20], hemicellulases [18] and ligninases [9,21], reflecting the complexity of the materials. In nature, degradation of cellulosic biomass is performed by mixtures of hydrolytic enzymes collectively known as cellulases. Cellulases include endo-acting (endoglucanases) and exo-acting (cellobio-hydrolases) enzymes, which behave in a synergistic manner in biomass-degrading microbes. Many microorganisms, including fungi and bacteria, have been found to be capable of degrading cellulose and other plant cell wall fibres. By 1976, over 14,000 fungal species expressing this ability had been isolated, but only a few of them were subjected to in-depth studies [22]. Most fungal strains produce and secrete various lignocellulolytic synergistically-acting enzymes into the environment, thus contributing significantly to the decay of lignocellulosic residues in nature. The breakdown of lignocellulosic biomass involves the formation of long-chain polysaccharides, mainly cellulose and hemicellulose, and the subsequent hydrolysis of these polysaccharides into their component 5- and 6-carbon chain sugars. In biofuel production, these sugars can be further converted to bioethanol through fermentation processes [23,24].
In nature, efficient lignin degradation during the process of wood decay became possible mainly by basidiomycetes white-rot fungi. Many white-rot fungi simultaneously attack lignin, hemicellulose and cellulose whereas some other white-rot fungi preferentially work on lignin in a selective manner. For example, while Ceriporiopsis subvermispora [25], Phlebia spp. [26,27], Physisporinus rivulosus [28] and Dichomitus squalens [27] selectively attack lignin, Trametes versicolor [29], Heterobasidium annosum [30], P. chrysosporium [9] and Irpex lacteus [31] simultaneously degrade all cell wall components. Selective lignin degraders may have significant potential biotechnological applications when the removal of lignin is required to obtain intact cellulose such as in biopulping processes and also in procedures where the main objective is to provide an unprotected carbohydrate for subsequent use (e.g. animal feed and/or biofuel substrate) [32,33]. For example C. subvermispora, which lacks cellulase, has been selected for biopulping processes as a selective lignin degrader [32,34].
In contrast to white-rot fungi, brown-rot fungi, such as Postia placenta, Laetiporus portentosus, Piptoporus betulinus and Gloeophyllum trabeum, can degrade wood carbohydrates, but not oxidized lignin. As a result, brown-colored rot ensues [14,15]. Ascomycetes are mostly able to degrade cellulose and hemicellulose, while their ability to convert lignin is limited [14]. Plant pathogenic fungi such as Fusarium solani f. sp. glycines are able to degrade lignin by production of laccase and lignin peroxidase. Lignin degradation by the fungi is suggested to play a role in sudden death syndrome (SDS) in soybean [35].
2.1. Fungal extracellular ligninases
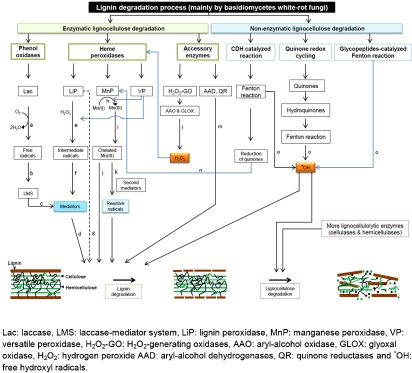
Fungi degrade lignin by secreting enzymes collectively termed “ligninases”. Ligninases can be classified as either phenol oxidases (laccase) or heme peroxidases [lignin peroxidase (LiP), manganese peroxidase (MnP) and versatile peroxidase (VP)] (Table 1) [14]. In general, laccases use molecular oxygen as electron acceptors while peroxidases use hydrogen peroxide as a co-substrate [33]. White-rot fungi variously secrete one or more of the lignin-modifying enzymes (LMEs) in addition to other compounds necessary for effective lignin degradation (discussed in section 2.2.) [36]. It has been shown that P. chrysosporium produces several UP and MnP isoenzymes but no laccase [37]. Correspondingly, the genome of P. chrysosporium contains ten UP and five MnP genes [38]. In addition, H2O2-generating enzyme, glyoxal oxidase (GLOX) has been found in P. chrysosporium cultures [39]. White-rot basidiomycetes, such as Coriolus versicolor [40], P. chrysosporium and T. versicolor [41], have been found to be the most efficient lignin-degrading microorganisms studied. Although LiP is able to oxidize the non-phenolic part of lignin (which forms 80-90% of lignin composition), it is absent from many lignin degrading fungi [40]. In addition, electron microscopy studies of the early stages of the fungal degradation of wood have shown that oxidative ligninolytic enzymes are too large to penetrate into the wood cell wall micropores [42]. Thus, it has been suggested that prior to the enzymatic attack, low-molecular weight diffusible reactive oxidative compounds must initiate changes to the lignin structure (as discussed below) [42,43]. Figure 1 summarized the major steps and enzymes involved in lignin degradation by basidiomycetes white-rot fungi.
Table 1.
The features of the main two groups of fungal ligninolytic enzymes
| Type of enzymes | Reaction1 | Cofactor | Metals or ions2 | Mediators | Subunits & molecular mass (kDa) | Range of optimum temperature (°C) | Range of pH optimum | Localization | Glycosylation | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | Phenols, aniline, | Monomeric, (43-100) dimeric, trimeric & oligomeric | ||||||||
| Cd2+ | 3-HAA, NHA, | |||||||||
| Cu2+ | syringaldehyde, | |||||||||
| Phenol oxidase (laccase) | 4 benzenediol + O2 = 4 | H2O2 | hydroxybenzotri | Mostly extracellular3 | Yes (N-glycosylated)4 | [36,44,45,68,84,131] | ||||
| benzosemiquinone + 2 H2O | N/A | Imidazole | azole and ABTS | 20-80 | 2-10 | |||||
| K+ | ||||||||||
| K2SO4 | (e.g. tetramers with ~ 390 KDa | |||||||||
| Mn2+ | ||||||||||
| Na2SO4 | ||||||||||
| (NH4)2SO4 | ||||||||||
| Peroxidases: | 1,2-bis(3,4-dimethoxyphenyl)propane-1,3-diol + H2O2 = 3,4-dimethoxybenzaldehyde + 1-(3,4-dimethoxyphenyl)ethane-1,2-diol + H2O | |||||||||
| a) Lignin peroxidase (LiP) | Heme | Iron | Veratryl alcohol | Monomeric (37-50) | 35-55 | 1-5 | extracellular | Yes (N-glycosylated)4 | [36,68,84,132] | |
| b) Manganese peroxidase (MnP) | Ca2+ | Organic acid as chelators, thiols, | ||||||||
| 2Mn(II) + 2H+ + H2O2 = | Heme | Cd2+ | unsaturated | Monomeric | 30-60 | 2.5-6.8 | extracellular | Yes (N-glycosylated)4 | [36,68,84,133,134] | |
| 2Mn(III) + 2H2O | Mn2+ | fatty acids | (32-62.5) | |||||||
| Sm3+ | ||||||||||
| c) Versatile peroxidase | donor + H2O2 = oxidized | Veratryl alcohol, compounds | ||||||||
| donor + 2H2O | ||||||||||
| Mn2+ | similar to LiP and MnP mediators | |||||||||
| (e.g. reactive black 5 + H2O2 and = oxidized reactive black 5 + 2H2O) | Heme | Ca2+ | Monomeric | Not known | 3-5 | extracellular | Yes | [68,101,135] | ||
| Cu2+ |
General reactions.
Differnet enzymes from different species need different metal(s) or ion(s).
Fungal laccases are mostly extracellular enzymes but cytoplacmic or intracellular laccases were also found especially in plants and bacteria [45].
Glycosylation degree varies between different fungal ligninolytic enzymes [68]. N/A: not applicable; 3-HAA: 3-hydroxyanthranilic acid; NHA: N-hydroxyacetanilide; ABTS: 2,2'-azinobis(3-ethylbenzthiazoline-6-sulphonate).
Figure 1.
Schematic diagram of lignin degradation by basidiomycetes white-rot fungi: the major steps and enzymes involved (refer to text).
2.1.1. Phenol oxidases (laccases) (benzenediol:oxygen oxidoreductases, EC 1.10.3.2)
Laccases are glycosylated blue multi-copper oxidoreductases (BMCO) that use molecular oxygen to oxidize various aromatic and non-aromatic compounds through a radical-catalyzed reaction mechanism (Table 1) [44,45]. Laccases couple the electron reduction of dioxygen into two molecules of water with the oxidation of a vast variety of substrates, such as phenols, arylamines, anilines, thiols and lignins (Figure 1, a) [46]. Four copper ions in their catalytic center mediate the redox process. These are classified as being type-1 (T1), type-2 (T2) and two type-3 (T3 and T3'), based on the copper's coordination and spectroscopic properties [47]. The oxidation reactions catalyzed by laccases lead to the formation of free radicals which act as intermediate substrates for the enzymes (Figure 1, b) [48]. These mediators can leave the enzyme site and react with a broad range of high-redox potential substrates and thus create non enzymatic routes of oxidative polymerizing or depolymerizing reactions (Figure 1, c). Ultimately, laccase-mediator system (LMS) becomes involved in a range of physiological functions such as lignolysis (Figure 1, d), lignin synthesis, morphogenesis, pathogenesis and detoxification [49].
Initially discovered in the Japanese lacquer tree Rhus vernicifera [50], laccases have since been found in many other plants and insects [44]. However, for the most part, laccases have been found and studied in white-rot fungi, such as Lentinus tigrinus [48], Pleurotus ostreatus D1 [51], Cerrena unicolor strain 137 [52], T. versicolor [53], Trametes sp. strain AH28-2 [54], Trametes pubescens [55] and Cyathus bulleri [56]. Laccase production using a liquid culture has also been reported in brown-rot fungi, including Coniophora puteana [57]. Also, ascomycetes such as Melanocarpus albomyces [58], Chaetomium thermophile [59], Magnaporthe grisea [60], Myrothecium verrucaria 24G-4 [61] and Neurospora crassa [62] are be able to produce laccases. In addition by application of gene-specific PCR primers, laccase genes were detected in a few different fungal species including Pycnoporus cinnabarinus, Pycnoporus coccineus, Pycnoporus sanguineus, Cyathus sp. and also in xylariaceous ascomycetes Xylaria sp. and Hypoxylon sp. [63]. Interestingly, laccases were also detected in some bacteria such as Bacillus subtilis [64], while other bacteria, like Streptomyces griseus [65], produce a laccase-like phenol oxidase. Also, laccase genes were detected in bacteria such as Bacillus licheniformis [66] and B. subtilis [67]. For extensive information please refer to the following website http://www.brenda-enzymes.org [68].
As laccases work efficiently on a broad range of substrates without cofactors, they may have significant value in many biotechnological applications, such as pulp bio-bleaching [69], biosensors [70], food industries [71], textile industries [72], soil bioremediation [73] and in the production of complex polymers in synthetic chemistry [74]. However, commercial application of laccases face major obstacles, such as the lack of sufficient enzyme stocks and the cost of redox mediators [75]. Heterologous expression of the enzymes with protein engineering allows for the cost-effective creation of more robust and active enzymes. In addition, an improvement in immobilization methods would result in greater stability of laccases with long life times [76].
2.1.2. Heme Peroxidases
2.1.2.1. Lignin peroxidases (LiP)(1,2-bis(3,4-dimethoxyphenyl)propane-l, 3-diol:hydrogen-peroxide oxidoreductases, EC 1.11.1.14)
LiPs are heme-containing glycoproteins and play a central role in the biodegradation of the cell wall constituent, lignin [77]. LiPs catalyze the H2O2dependent oxidative depolymerization of a variety of non-phenolic lignin compounds (diarylpropane), β-O-4 non-phenolic lignin model compounds and a wide range of phenolic compounds (e.g. guaiacol, vanillyl alcohol, catechol, syringic acid, acteosyringone) with redox potentials up to 1.4 V (Table 1) [15]. LiPs oxidize the substrates in multi-step electron transfers and form intermediate radicals, such as phenoxy radicals and veratryl alcohol radical cations (Figure 1, e). These intermediate radicals undergo non-enzymatic reactions such as radical coupling and polymerization, side-chain cleavage, demethylation and intramolecular addition and rearrangement (Figure 1, f) [15]. Unlike the other peroxidises, like MnP, LiP is able to oxidize non-phenolic aromatic substrates and does not require the participation of mediators due to its unusually high redox potential (Figure 1, g) [15,40]. The crystal structure of the first LiP has shown that the heme group is buried in the interior of the protein and has access to the outer medium through a channel. Although the size of the channel is not sufficient to allow the large polymer lignin to access the heme group, small molecule substrates can find a suitable binding site [77].
Since the discovery of LiP in P. chrysosporium [78] in 1983, more LiPs have been found in different P. chrysosporium strains [79,80] and other white-rot fungi, such as T. versicolor [81]. In addition, LiP genes were detected in a few different fungal species including Panus sp., P. coccineus, P. sanguineus and Perenniporia medulla-panis [63]. LiP was also detected in some bacteria, such as Acinetobacter calcoaceticus NCIM 2890 [82] and Streptomyces viridosporus T7A[83].
2.1.2.2. Manganese peroxidases (MnP)(Mn (ll):hydrogen-peroxide oxidoreductases, EC 1.11.1.13)
MnPs are extracellular glycoproteins and are secreted in multiple isoforms which contain one molecule of heme as iron protoporphyrin IX [84]. MnP catalyzes the peroxide dependent oxidation of Mn(ll) (as the reducing substrate) to Mn(lll) (Figure 1, h), which is then released from the enzyme surface in complex with oxalate or with other chelators (Figure 1, i). Chelated Mn (III) complex acts as a reactive low molecular weight, diffusible redox-mediator (Figure 1, j) of phenolic substrates including simple phenols, amines, dyes, phenolic lignin substructures and dimers (Table 1) [15,36,84]. The oxidation potential of Mn(lll) chelator is only limited to phenolic lignin structures. However, for the oxidation of non-phenolic substrates by Mn(lll), reactive radicals must be formed in the presence of a second mediator (Figure 1, k). Organic acids, such as oxalate and malonate, are the primary compounds that act as second mediators in the production of reactive radicals like carbon-centered radicals (acetic acid radicals, COOH-C·H2), peroxyl radicals (COOH-CH2OO·), superox-ide (O2·-) and formate radicals (CO2·-) [15,36,84]. In the absence of H2O2(e.g in fungi lacking H2O2-generating oxidases), these radicals can be used by MnP as a source of peroxides and increase the lignin-degrading efficiency of the fungi [15,36,85].
Since the discovery of MnP in P. chrysosporium in 1985 [86], more MnPs have been found in other basidiomycetes, such as Panus tigrinus [87], Lenzites betulinus [88], Phanerochaete flavido-alba [89], Agaricus bisporus [90], Bjer-kandera sp. [91] and Nematoloma frowardii b19 [92].
MnPs may be capable of rivalling the potential applications of laccases in biotechnology. This is evident in studies which illustrate that the presence of MnP can increase the degree of dye decolorization. One study in particular found that MnP was the main enzyme involved in dye decolorization by P. chrysosporium [93]. Also, in another study, MnP produced by P. chrysosporium was used for the decolonization of sulfonphthalein (SP) dyes [94]. Complete decolorization took place at pH 4.0. In addition, MnP from white-rot fungi is considered the primary enzyme responsible for biobleaching of kraft pulps. The main drawback in commercial applications of MnP is the unavailability of the enzyme in large quantities; this can be resolved with the use of DNA recombinant technology [95]. For example, wild-type MnP from white-rot fungi [96] and recombinant MnP (rMnP) expressed in Pichia pastoris [97] have been used to remove lignin from cellulose fibers in pulp bleaching experiments [95]. The rMnP used in the study was found to be effective in lignin degradation and removal in both hardwood and softwood unbleached kraft pulps [95].
2.1.2.3. Versatile peroxidases (VP) (EC 1.11.1.16)
VPs are glycoproteins with hybrid properties capable of oxidizing typical substrates of other basidiomycetes peroxidases including Mn(ll) and also veratryl alcohol (VA), MnP and the typical LiP substrate, respectively (Figure 1) [36,84,98]. VPs form an attractive ligninolytic enzyme group due to their dual oxidative ability to oxidize Mn(ll) and also phenolic and nonphenolic aromatic compounds (Table 1) [36]. It has been found that VPs can also efficiently oxidize high redox-potential compounds such as dye Reactive Black 5 (RB5) as well as a wide variety of phenols, including hydroquinones [99,100]. It has been suggested that VPs can oxidize substrates spanning a wide range of potentials, including low- and high-redox potentials. This is a result of their hybrid molecular structures which provide multiple binding sites for the substrates [101]. This makes VPs superior to both LiPs and MnPs, which are not able to efficiently oxidize phenolic compounds in the absence of VA or oxidize phenols in the absence of Mn(ll), respectively [98]. Similar to the MnP mechanism, Mn(lll) is released from VPs and acts as a diffusible oxidizer of phenolic lignin and free phenol substrates (Figure 1, h, i and j). Like other members of heme peroxidases, heme is buried in the interior of the protein and has access to the outer medium through two channels [100,101]. The function of the first channel is similar to that described for LiP and is conserved among all heme peroxidases. Conversely, the second channel is found to be specific to VP and MnP and is where the oxidation of Mn(ll) to Mn(lll) takes place [98].
Since the discovery of VP in 1999 in members of the genus Pleurotus, such as P. eryngii [101,102] and P. ostreatus [103], more VPs have been found in other basidiomycetes such as Bjerkandera adusta [100,104], Bjerkandera sp. strain B0S55 [105], Bjerkandera sp. (B33/3) [106], Bjerkandera fumosa [107] and Pleurotus pulmonarius [108]. Although P. chrysosporium did not show any VP activity, a putative extracellular peroxidase related to Pleurotus VP has been identified in its genome [109]. In addition, VP was detected in Polyporales basidiomycetes, including species from the genera Panus (e.g. P. tigrinus 8/18) [110].
Among basidiomycetes peroxidases, VPs have attracted the greatest biotechnological attention due to their catalytic versatility, which includes the degradation of compounds that other peroxidases are not able to oxidize directly. This unique feature allows VP to oxidize not only Mn (II) but also VA, phenolic, non-phenolic and high molecular weight compounds, including dyes in Mn-independent reactions [15,84]. Like MnP, VP's primary disadvantage in commercial applications is its limited availability in large quantities, which can be resolved with the use of DNA recombinant technology [95,98]. Efforts have been made to produce VP using heterologous expression systems. For example, in an experiment in which VP from Pleurotus eryngii was expressed under control of the alcohol dehydrogenase (alcA) promoter of Aspergillus nidulans, and lowering the growing temperature further improved the expression level [111]. Alternatively, other basidiomycete peroxidases such as LiP or MnP can be engineered to create peroxidases with new functions that emulate those of natural occurring VPs. For example, the Mn(ll) binding site was introduced into a P. chrysosporium LiP by site-directed mutagenesis and the engineered enzyme also showed MnP activity while retaining its ability to oxidize VA (LiP activity) [112]. In another experiment, when a tryptophan residue analogous to the essential one in LiP was introduced to P. chrysosporium MnP by site-directed mutagenesis (single mutation, S168W), MnP with LiP activity was created while full MnP activity was maintained [113].
2.1.3. Other lignin degrading enzymes and accessory enzymes
In addition to ligninases, other fungal extracellular enzymes which act as accessory enzymes have been found to be involved in lignin degradation. These include oxidases generating H2O2, which provide the hydrogen peroxide required by peroxidases, and mycelium-associated dehydrogenases, which reduce lignin-derived compounds (Figure 1, I) [14]. Oxidases generating H2O2 include aryl-alcohol oxidase (AAO) (EC 1.1.3.7) found in various fungi, such as P. eryngii, and glyoxal oxidase (GLOX, a copperradical protein) found in P. chrysosporium [39,114]. In addition, aryl-alcohol dehydrogenases (AAD) (a flavoprotein) and quinone reductases (QR) are also involved in lignin degradation by fungi (Figure 1, m) [115]. Moreover, it has been shown that cellobiose dehydrogenase (CDH), which is produced by many different fungi under cellulolytic conditions, is also involved in lignin degradation in the presence of H2O2 and chelated Fe ions [116]. It is proposed that the effect of CDH on lignin degradation is through the reduction of quinones, which can be used by ligninolytic enzymes or the support of a Mn-peroxidase reaction (Figure 1, n) (for detailed information please refer to the review by Henriksson et al. 2000 [117]).
2.2. Oxidative (non-lignocellulolytic) lignocellulose-degradation mechanisms in higher fungi
Over the last few decades, there has been emerging evidence in support of the involvement of non-enzymatic mechanisms in plant cell -wall polysaccharide degradation. These mechanisms are mostly assisted by oxidation through the production of free hydroxyl radicals (·OH). Many white and brown-rot fungi have been shown to produce hydrogen peroxide (H2O2) which enters the Fenton reaction and results in release of ·OH (Figure 1, o) [114,118]. By attacking polysaccharides and lignin in plant cell walls in a non-specific manner, these radicals create a number of cleavages which facilitate the penetration of the cell wall by lignocellulolytic enzymes [119,120]. The pathways by which fungi generate free ·OH radicals are: cel-lobiose dehydrogenase (CDH) catalyzed reactions, low molecular weight peptides/quinone redox cycling and glycopeptide-catalyzed Fenton reactions (Figure 1 and Table 2) [121].
Table 2.
Mechanisms and enzymes involved in the production of •OH in different fungi
| Fungi | Mechanisms | Other enzymes involved/their function | References |
|---|---|---|---|
| White-rot fungi (e.g. D. squalens) | CDH catalyzed reaction | Oxalate decarboxylase/regulation of oxalate concentration | [136,137] |
| Brown and white-rot fungi (e.g. C. puteana, P. chrysosporium) | Quinone redox cycling | Benzoquinone reductases, CDH, sugar dehydrogenases/convert quinones to hydroquinones | [117,138] |
| Brown and white-rot fungi (e.g. F. palustris, P. chrysosporium) | Glycopeptides-catalyzed Fenton reaction | Cell wall-associated reductase/reduction of glycopeptides | [139] |
CDH, an extracellular monomeric protein with some glycosylation, has been identified in a number of wood- and cellulose-degrading fungi, including basidiomycetes (mostly white-rot fungi) and ascomycetes, growing on cellulosic medium. This enzyme is able to oxidize cellobiose, higher cellodextrins and other disaccharides or oligosaccharides with β-1,4 linkages. In addition, CDH has been found with cellulose binding module (CBM) (in ascomycetes) and without CBM (in basidiomycetes). In the absence of CBM, CDH is able to bind to cellulose through hydrophobic interactions [122]. In some fungi, under cellulolytic conditions, CDH production increases, which in turn helps cellulases and hemicellulases [117,123]. It is now widely accepted that CDH is able to degrade and modify all three major components of the lignocellulosic residues (cellulose, hemicellu-loses and lignin) by producing free hydroxyl radicals in a Fenton-type reaction (Figure 1) (for detailed information please refers to the review by Baldrin and Valaskova, 2008 [121]).
It has been shown that white and brown-rot fungi produce low molecular weight chelators which are able to penetrate into the cell wall. For example, G. trabeum produces a low molecular weight peptide (known as short fiber generating factor, SFGF) which can degrade cellulose into short fibers by an oxidative reaction [120,124]. It has also been reported that some of these low molecular weight compounds are quinones, which must first be converted to hydroquinones by particular fungal enzymes (Table 2) before free hydroxyl radicals can be produced through the Fenton reaction (Figure 1, o) [40].
Glycopeptides of varying molecular weights (ranging from 1.5 to 12 kDa) have been found in many brown-rot fungi, such as G. trabeum [125] and white-rot fungi, such as P. chrysosporium [43,126]. Similar to other mechanisms, glycopeptides are able to catalyze redox reactions and thus produce free hydroxyl radicals (Figure 1, o).
3. Concluding remarks
Lignocellulolytic microorganisms, especially fungi, have attracted a great deal of interest as potential biomass degraders for large-scale applications due to their ability to produce vast amounts of extracellular lignocellulolytic enzymes. Lignin, the most recalcitrant component of lignocellulosic material, acts as a barrier for any solutions or enzymes by linking to both hemicelluloses and celluloses and prevents penetration of lignocellulolytic enzymes to the interior lignocellulosic structure. It is primarily basidiomycetes white-rot fungi that are responsible for efficient lignin degradation in wood decay processes. Their production of extracellular enzymes known as lignin-modifying enzymes (LMEs) facilitate the degradation process. Based on their activity on lignin and other lignocellulosic materials, white-rot fungi are categorized into two groups, simultaneous and selective degraders. Selective lignin degraders white-rot fungi are most attractive for their potential biotechnological applications in removing lignin, as in biopulping processes, and for providing an unprotected carbohydrate for subsequent use, as in animal feed and/or biofuel substrate. LMEs include mainly two ligninolytic enzyme families; i) phenol oxidase (laccase) and ii) heme peroxidases (LiP, MnP and VP). LMEs, especially VPs require more research to understand the efficiency of the enzymes on lignin oligomers and the mechanisms of their action. In addition, accessory enzymes, such as oxidases that generate the H2O2 required by peroxidases, have been found to be involved in lignin degradation. Hydrogen peroxide is used by many white and brown-rot fungi to produce ·OH in the Fenton reaction. Ultimately, these free radicals attack polysaccharides as well as lignin in plant cell walls in a non-specific manner creating some cleavage sites which allow for easier penetration by lignocellulolytic enzymes.
Genome sequencing projects of 62 fungal species, including six basidiomycetes and 27 ascomycetes, have been completed thus far [127]. Availability of the full genome sequence of white -rot fungi, such as that of P. chrysosporium, now allows for the creation of proteomic methods to identify all enzymes involved in lignin degradation. In turn, such methods may lead to the discovery of new enzymes involved in the degradation [17]. In order to achieve this, small sample sizes were used to identify the proteins released by P. chrysosporium grown in a solid-substrate culture (red oak wood chips). Traditional two-dimensional (2-D) gel electrophoresis was coupled to advanced instrumentation, such as matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF/MS) or capillary liquid chromatography-nanoelectrospray ionization-tandem MS (CapLC-ESI-MS/MS). Using this combination, 16 extracellular proteins were identified from over 40 proteins spotted on 2-D gel [17]. However, it was predicted that P. chrysosporium, which was found to have a 30-Mb genome with more than 10,000 gene models (based on computational analysis), could potentially release about 790 proteins into the culture medium [128]. The goal of this and other on-going projects is to identify the extracellular proteome (proteomic secretome) of P. chrysosporium or other basidiomycetes white-rot fungi when grown on a solid substrate. More recently, the first genomelevel transcriptome study of P. chrysosporium was performed to determine all the gene products involved in wood degradation using red oak as a carbon source. The results have shown that in addition to other lignocellulolytic enzymes, lignin peroxidase and alcohol oxidase (H2O2-generating enzyme) are highly expressed during lignin degradation [129]. Further investigation is needed to identify the novel proteins involved in fungal lignin degradation and their mechanisms of action. In addition, the suppression of lignin biosynthesis enzymes via plant genetic engineering may be of potential use in overcoming some of the problems related to the recalcitrance of lignin [130].
Acknowledgements
This work was supported by a grant from OGS (Ontario Graduate Scholarship) to M.D. and NSERC-RCD and Ontario Research Chair funding to W.Q.
References
- [1].Kim S, Dale BE. Global potential bioethanol production from wasted crops and crop residues. Biomass and Bioenergy. 2004;26:361–375. [Google Scholar]
- [2].Kalogo Y, Habibi S, MacLean HL, Joshi SV. Environmental implications of municipal solid waste-derived ethanol. Environ Sci Technol. 2007;41:35–41. doi: 10.1021/es061117b. [DOI] [PubMed] [Google Scholar]
- [3].Pokhrel D, Viraraghavan T. Municipal solid waste management in Nepal: practices and challenges. Waste Manag. 2005;25:555–562. doi: 10.1016/j.wasman.2005.01.020. [DOI] [PubMed] [Google Scholar]
- [4].Statistics Canada Human Activity and the Environment, Annual Statistics 2005, Solid Waste in Canada. Catalogue no. 16-201-XIE November. 2005. p. pp 8.
- [5].Champagne P. Feasibility of producing bioethanol from waste residues: A Canadian perspective Feasibility of producing bio-ethanol from waste residues in Canada. Resources, Conservation and Recycling. 2007;50:211–230. [Google Scholar]
- [6].Wen Z, Liao W, Chen S. Hydrolysis of animal manure lignocellulosics for reducing sugar production. Bioresour Technol. 2004;91:31–39. doi: 10.1016/s0960-8524(03)00166-4. [DOI] [PubMed] [Google Scholar]
- [7].Palacios-Orueta A, Chuvieco E, Parra A, Carmona-Moreno C. Biomass burning emissions: a review of models using remote-sensing data. Environ Monit Assess. 2005;104:189–209. doi: 10.1007/s10661-005-1611-y. [DOI] [PubMed] [Google Scholar]
- [8].Levine JS. The MIT Press; Cambridge, Massachusetts, USA; 1996. Biomass burning and global change. In: Levine JS (eds) (vol. 1) Remote sensing and inventory development and biomass burning in Africa; p. 35. [Google Scholar]
- [9].Sanchez C. Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv. 2009;27:185–194. doi: 10.1016/j.biotechadv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- [10].Percival Zhang YH, Himmel ME, Mielenz JR. Outlook for cellulase improvement: screening and selection strategies. Biotechnol Adv. 2006;24:452–481. doi: 10.1016/j.biotechadv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- [11].Saha BC. Alpha-L-arabinofuranosidases: biochemistry, molecular biology and application in biotechnology. Biotechnol Adv. 2000;18:403–423. doi: 10.1016/s0734-9750(00)00044-6. [DOI] [PubMed] [Google Scholar]
- [12].Saha BC. Hemicellulose bioconversion. J Ind Microbiol Biotechnol. 2003;30:279–291. doi: 10.1007/s10295-003-0049-x. [DOI] [PubMed] [Google Scholar]
- [13].Wei H, Xu Q, Taylor LE, 2nd, Baker JO, Tucker MP, Ding SY. Natural paradigms of plant cell wall degradation. Curr Opin Biotechnol. 2009;20:330–338. doi: 10.1016/j.copbio.2009.05.008. [DOI] [PubMed] [Google Scholar]
- [14].Martinez AT, Speranza M, Ruiz-Duenas FJ, Ferreira P, Camarero S, Guillen F, Martinez MJ, Gutierrez A, del Rio JC. Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol. 2005;8:195–204. [PubMed] [Google Scholar]
- [15].Wong DW. Structure and action mechanism of ligninolytic enzymes. Appl Biochem Biotechnol. 2009;157:174–209. doi: 10.1007/s12010-008-8279-z. [DOI] [PubMed] [Google Scholar]
- [16].Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science. 2007;315:804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- [17].Abbas A, Koc H, Liu F, Tien M. Fungal degradation of wood: initial proteomic analysis of extracellular proteins of Phanerochaete chrysosporium grown on oak substrate. Curr Genet. 2005;47:49–56. doi: 10.1007/s00294-004-0550-4. [DOI] [PubMed] [Google Scholar]
- [18].Ljungdahl LG. The cellulase/hemicellulase system of the anaerobic fungus Orpinomyces PC-2 and aspects of its applied use. Ann N Y Acad Sci. 2008;1125:308–321. doi: 10.1196/annals.1419.030. [DOI] [PubMed] [Google Scholar]
- [19].Yoon JJ, Cha CJ, Kim YS, Son DW, Kim YK. The brown-rot basidiomycete Fomitopsis palustris has the endo-glucanases capable of degrading microcrystalline cellulose. J Microbiol Biotechnol. 2007;17:800–805. [PubMed] [Google Scholar]
- [20].Bayer EA, Chanzy H, Lamed R, Shoham Y. Cellulose, cellulases and cellulosomes. Curr Opin Struct Biol. 1998;8:548–557. doi: 10.1016/s0959-440x(98)80143-7. [DOI] [PubMed] [Google Scholar]
- [21].Weng JK, Li X, Bonawitz ND, Chapple C. Emerging strategies of lignin engineering and degradation for cellulosic biofuel production. Curr Opin Biotechnol. 2008;19:166–172. doi: 10.1016/j.copbio.2008.02.014. [DOI] [PubMed] [Google Scholar]
- [22].Mandels M, Sternberg D. Recent advances in cellulase technology. Ferment. Technol. 1976;54:267–286. [Google Scholar]
- [23].Zhou S, Ingram LO. Synergistic hydrolysis of carboxymethyl cellulose and acid-swollen cellulose by two endoglucanases (CelZ and CelY) from Erwinia chrysanthemi. J Bacteriol. 2000;182:5676–5682. doi: 10.1128/jb.182.20.5676-5682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dashtban M, Schraft H, Qin W. Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. Int J Biol Sci. 2009;5:578–595. doi: 10.7150/ijbs.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Guerra A, Mendonca R, Ferraz A, Lu F, Ralph J. Structural characterization of lignin during Pinus taeda wood treatment with Ceriporiopsis subvermispora. Appl Environ Microbiol. 2004;70:4073–4078. doi: 10.1128/AEM.70.7.4073-4078.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Arora DS, Sharma RK. Enhancement in in vitro digestibility of wheat straw obtained from different geographical regions during solid state fermentation by white rot fungi. BioResour. 2009;4:909–920. [Google Scholar]
- [27].Fackler K, Gradinger C, Hinterstoisser B, Mess-ner K, Schwanninger M. Lignin degradation by white rot fungi on spruce wood shavings during short-time solid-state fermentations monitored by near infrared spectroscopy. Enzyme and Microbial Technology. 2006;39:1476–1483. [Google Scholar]
- [28].Hilden K, Hakala TK, Maijala P, Lundell TK, Hatakka A. Novel thermotolerant laccases produced by the white-rot fungus Physisporinus rivulosus. Appl Microbiol Biotechnol. 2007;77:301–309. doi: 10.1007/s00253-007-1155-x. [DOI] [PubMed] [Google Scholar]
- [29].Tanaka H, Itakura S, Enoki A. Hydroxyl radical generation by an extracellular low-molecular -weight substance and phenol oxidase activity during wood degradation by the white-rot basidiomycete Trametes versicolor. J Biotechnol. 1999;75:57–70. doi: 10.1016/s0168-1656(99)00138-8. [DOI] [PubMed] [Google Scholar]
- [30].Daniel G, Asiegbu F, Johansson M. The saprotrophic wood-degrading abilities of Het-erobasidium annosum intersterility groups P and S. Mycol Res. 1998;102:991–997. [Google Scholar]
- [31].Xu C, Ma F, Zhang X. Lignocellulose degradation and enzyme production by Irpex lacteus CD2 during solid-state fermentation of corn stover. J Biosci Bioeng. 2009;108:372–375. doi: 10.1016/j.jbiosc.2009.04.023. [DOI] [PubMed] [Google Scholar]
- [32].Anderson WF, Akin DE. Structural and chemical properties of grass lignocelluloses related to conversion for biofuels. J Ind Microbiol Biotechnol. 2008;35:355–366. doi: 10.1007/s10295-007-0291-8. [DOI] [PubMed] [Google Scholar]
- [33].Mai C, Kues U, Militz H. Biotechnology in the wood industry. Appl Microbiol Biotechnol. 2004;63:477–494. doi: 10.1007/s00253-003-1411-7. [DOI] [PubMed] [Google Scholar]
- [34].Blanchette RA, Burnes TA, Eerdmans MM, Akhtar M. Evaluating Isolates of Phanerochaete chrysosporium and Ceriporiopsis subvermispora for Use in Biological Pulping Processes. Holzforschung. 1992;46:109–115. [Google Scholar]
- [35].Lozovaya VV, Lygin AV, Zernova OV, Ulanov AV, Li S, Hartman GL, Widholm JM. Modification of phenolic metabolism in soybean hairy roots through down regulation of chalcone synthase or isoflavone synthase. Planta. 2007;225:665–679. doi: 10.1007/s00425-006-0368-z. [DOI] [PubMed] [Google Scholar]
- [36].Wesenberg D, Kyriakides I, Agathos SN. White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv. 2003;22:161–187. doi: 10.1016/j.biotechadv.2003.08.011. [DOI] [PubMed] [Google Scholar]
- [37].Singh D, Chen S. The white-rot fungus Phanerochaete chrysosporium: conditions for the production of lignin-degrading enzymes. Appl Microbiol Biotechnol. 2008;81:399–417. doi: 10.1007/s00253-008-1706-9. [DOI] [PubMed] [Google Scholar]
- [38].Martinez D, Larrondo LF, Putnam N, Gelpke MD, Huang K, Chapman J, Helfenbein KG, Ra-maiya P, Detter JC, Larimer F, Coutinho PM, Henrissat B, Berka R, Cullen D, Rokhsar D. Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat Biotechnol. 2004;22:695–700. doi: 10.1038/nbt967. [DOI] [PubMed] [Google Scholar]
- [39].Kersten PJ. Glyoxal oxidase of Phanerochaete chrysosporium: its characterization and activation by lignin peroxidase. Proc Natl Acad Sci U S A. 1990;87:2936–2940. doi: 10.1073/pnas.87.8.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang L, Yan W, Chen J, Huang F, Gao P. Function of the iron-binding chelator produced by Coriolus versicolor in lignin biodegradation. Sci China C Life Sci. 2008;51:214–221. doi: 10.1007/s11427-008-0033-9. [DOI] [PubMed] [Google Scholar]
- [41].Moredo N, Lorenzo M, Domínguez A, Moldes D, Cameselle C, Sanroman A. Enhanced ligni-nolytic enzyme production and degrading capability of Phanerochaete chrysosporium and Trametes versicolor. World J Microb Biotechnol. 2003;19:665–669. [Google Scholar]
- [42].Srebotnik E, Messner K, Foisner R. Penetrability of White Rot-Degraded Pine Wood by the Lignin Peroxidase of Phanerochaete chrysosporium. Appl Environ Microbiol. 1988;54:2608–2614. doi: 10.1128/aem.54.11.2608-2614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tanaka H, Itakura S, Enoki A. Hydroxyl radical generation by an extracellular low-molecular -weight substance and phenol oxidase activity during wood degradation by the white-rot basidiomycete Phanerochaete chrysosporium. Holzforschung. 1999;53:21–28. doi: 10.1016/s0168-1656(99)00138-8. [DOI] [PubMed] [Google Scholar]
- [44].Claus H. Laccases: structure, reactions, distribution. Micron. 2004;35:93–96. doi: 10.1016/j.micron.2003.10.029. [DOI] [PubMed] [Google Scholar]
- [45].Baldrian P. Fungal laccases - occurrence and properties. FEMS Microbiol Rev. 2006;30:215–242. doi: 10.1111/j.1574-4976.2005.00010.x. [DOI] [PubMed] [Google Scholar]
- [46].Thurston CF. The structure and function of fungal laccases. Microbiol (UK) 1994;140:19–26. [Google Scholar]
- [47].Messerschmidt A, Huber R. The blue oxi-dases, ascorbate oxidase, laccase and ceru-loplasmin. Modelling and structural relationships. Eur J Biochem. 1990;187:341–352. doi: 10.1111/j.1432-1033.1990.tb15311.x. [DOI] [PubMed] [Google Scholar]
- [48].Ferraroni M, Myasoedova NM, Schmatchenko V, Leontievsky AA, Golovleva LA, Scozzafava A, Briganti F. Crystal structure of a blue laccase from Lentinus tigrinus: evidences for intermediates in the molecular oxygen reductive splitting by multicopper oxidases. BMC Struct Biol. 2007;7:60. doi: 10.1186/1472-6807-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mayer AM, Staples RC. Laccase: new functions for an old enzyme. Phytochemistry. 2002;60:551–565. doi: 10.1016/s0031-9422(02)00171-1. [DOI] [PubMed] [Google Scholar]
- [50].Yoshida H. Chemistry of lacquer (urushi) J Chem. Soc. 1883;43:472–486. [Google Scholar]
- [51].Pozdniakova NN, Turkovskaia OV, ludina EN, Rodakiewicz-Nowak Y. [Yellow laccase from the fungus Pleurotus ostreatus D1: purification and characterization] Prikl Biokhim Mikrobiol. 2006;42:63–69. [PubMed] [Google Scholar]
- [52].Michniewicz A, Ullrich R, Ledakowicz S, Hofrichter M. The white-rot fungus Cerrena unicolor strain 137 produces two laccase iso-forms with different physico-chemical and catalytic properties. Appl Microbiol Biotechnol. 2006;69:682–688. doi: 10.1007/s00253-005-0015-9. [DOI] [PubMed] [Google Scholar]
- [53].Necochea R, Valderrama B, Diaz-Sandoval S, Folch-Mallol JL, Vazquez-Duhalt R, Iturriaga G. Phylogenetic and biochemical characterisation of a recombinant laccase from Trametes versicolor. FEMS Microbiol Lett. 2005;244:235–241. doi: 10.1016/j.femsle.2005.01.054. [DOI] [PubMed] [Google Scholar]
- [54].Xiao YZ, Tu XM, Wang J, Zhang M, Cheng Q, Zeng WY, Shi YY. Purification, molecular characterization and reactivity with aromatic compounds of a laccase from basidiomycete Trametes sp. strain AH28-2. Appl Microbiol Biotechnol. 2003;60:700–707. doi: 10.1007/s00253-002-1169-3. [DOI] [PubMed] [Google Scholar]
- [55].Shleev S, Nikitina O, Christenson A, Reimann CT, Yaropolov Al, Ruzgas T, Gorton L. Characterization of two new multiforms of Trametes pubescens laccase. Bioorg Chem. 2007;35:35–49. doi: 10.1016/j.bioorg.2006.08.001. [DOI] [PubMed] [Google Scholar]
- [56].Salony, Mishra S, Bisaria VS. Production and characterization of laccase from Cyathus bulleri and its use in decolourization of recalcitrant textile dyes. Appl Microbiol Biotechnol. 2006;71:646–653. doi: 10.1007/s00253-005-0206-4. [DOI] [PubMed] [Google Scholar]
- [57].Lee KH, Wi SG, Singh AP, Kim YS. Micro-morphological characteristics of decayed wood and laccase produced by the brown-rot fungus Coniophora puteana. J Wood Sci. 2004;50:281–284. [Google Scholar]
- [58].Hakulinen N, Kruus K, Koivula A, Rouvinen J. A crystallographic and spectroscopic study on the effect of X-ray radiation on the crystal structure of Melanocarpus albomyces laccase. Biochem Biophys Res Commun. 2006;350:929–934. doi: 10.1016/j.bbrc.2006.09.144. [DOI] [PubMed] [Google Scholar]
- [59].Ishigami T, Yamada Y. Purification and properties of polyphenol oxidase from Chae-tomium thermophile, a thermophilic fungus. Journal of General and Applied Microbiology. 1986;32:293–301. [Google Scholar]
- [60].Iyer G, Chattoo BB. Purification and characterization of laccase from the rice blast fungus, Magnaporthe grisea. FEMS Microbiol Lett. 2003;227:121–126. doi: 10.1016/S0378-1097(03)00658-X. [DOI] [PubMed] [Google Scholar]
- [61].Sulistyaningdyah WT, Ogawa J, Tanaka H, Maeda C, Shimizu S. Characterization of alkaliphilic laccase activity in the culture supernatant of Myrothecium verrucaria 24G-4 in comparison with bilirubin oxidase. FEMS Microbiol Lett. 2004;230:209–214. doi: 10.1016/S0378-1097(03)00892-9. [DOI] [PubMed] [Google Scholar]
- [62].Germann UA, Muller G, Hunziker PE, Lerch K. Characterization of two allelic forms of Neurospora crassa laccase. Amino- and carboxyl-terminal processing of a precursor. J Biol Chem. 1988;263:885–896. [PubMed] [Google Scholar]
- [63].Pointing SB, Pelling AL, Smith GJ, Hyde KD, Reddy CA. Screening of basidiomycetes and xylariaceous fungi for lignin peroxidase and laccase gene-specific sequences. Mycol Res. 2005;109:115–124. doi: 10.1017/s0953756204001376. [DOI] [PubMed] [Google Scholar]
- [64].Durao P, Bento I, Fernandes AT, Melo EP, Lind-ley PF, Martins LO. Perturbations of the T1 copper site in the CotA laccase from Bacillus subtilis: structural, biochemical, enzymatic and stability studies. J Biol Inorg Chem. 2006;11:514–526. doi: 10.1007/s00775-006-0102-0. [DOI] [PubMed] [Google Scholar]
- [65].Endo K, Hayashi Y, Hibi T, Hosono K, Beppu T, Ueda K. Enzymological characterization of EpoA, a laccase-like phenol oxidase produced by Streptomyces griseus. J Biochem. 2003;133:671–677. doi: 10.1093/jb/mvg086. [DOI] [PubMed] [Google Scholar]
- [66].Koschorreck K, Richter SM, Ene AB, Roduner E, Schmid RD, Urlacher VB. Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids. Appl Microbiol Biotechnol. 2008;79:217–224. doi: 10.1007/s00253-008-1417-2. [DOI] [PubMed] [Google Scholar]
- [67].Martins LO, Soares CM, Pereira MM, Teixeira M, Costa T, Jones GH, Henriques AC. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis en-dospore coat. J Biol Chem. 2002;277:18849–18859. doi: 10.1074/jbc.M200827200. [DOI] [PubMed] [Google Scholar]
- [68].Chang A, Scheer M, Grote A, Schomburg I, Schomburg D. BRENDA, AMENDA and FRENDA the enzyme information system: new content and tools in 2009. Nucleic Acids Res. 2009;37:D588–592. doi: 10.1093/nar/gkn820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Balakshin M, Chen C-L, Gratzla JS, Kirkmana AG, Jakob H. Biobleaching of pulp with di-oxygen in laccase-mediator system—effect of variables on the reaction kinetics. Journal of Molecular Catalysis B: Enzymatic. 2001;16:205–215. [Google Scholar]
- [70].Kulys J, Vidziunaite R. Amperometric biosensors based on recombinant laccases for phenols determination. Biosens Bioelectron. 2003;18:319–325. doi: 10.1016/s0956-5663(02)00172-0. [DOI] [PubMed] [Google Scholar]
- [71].Selinheimo E, Autio K, Kruus K, Buchert J. Elucidating the mechanism of laccase and ty-rosinase in wheat bread making. J Agric Food Chem. 2007;55:6357–6365. doi: 10.1021/jf0703349. [DOI] [PubMed] [Google Scholar]
- [72].Rodríguez Couto S, Hofer D, Sanromán MA, Gubitz GM. Production of laccase by Trametes hirsuta grown in an immersion bioreactor. Application to decolourisation of dyes from a leather factory. Eng Life Sci. 2004;4:233–238. [Google Scholar]
- [73].Nyanhongo GS, Couto SR, Guebitz GM. Coupling of 2,4,6-trinitrotoluene (TNT) metabolites onto humic monomers by a new laccase from Trametes modesta. Chemosphere. 2006;64:359–370. doi: 10.1016/j.chemosphere.2005.12.034. [DOI] [PubMed] [Google Scholar]
- [74].Mustafa R, Muniglia L, Rovel B, Girardin M. Phenolic colorants obtained by enzymatic synthesis using a fungal laccase in a hydroorganic biphasic system. Food Res Int. 2005;38:995–1000. [Google Scholar]
- [75].Rodriguez, Couto S, Toca Herrera JL. Industrial and biotechnological applications of laccases: a review. Biotechnol Adv. 2006;24:500–513. doi: 10.1016/j.biotechadv.2006.04.003. [DOI] [PubMed] [Google Scholar]
- [76].Lu L, Zhao M, Wang Y. Immobilization of laccase by alginate-chitosan microcapsules and its use in dye decolorization. World J Microbiol Biotechnol. 2007;23:159–166. [Google Scholar]
- [77].Piontek K, Smith AT, Blodig W. Lignin peroxidase structure and function. Biochem Soc Trans. 2001;29:111–116. doi: 10.1042/0300-5127:0290111. [DOI] [PubMed] [Google Scholar]
- [78].Glenn JK, Morgan MA, Mayfield MB, Kuwahara M, Gold MH. An extracellular H202-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Bio-phys Res Commun. 1983;114:1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- [79].Renganathan V, Miki K, Gold MH. Multiple molecular forms of diarylpropane oxygenase, an H202-requiring, lignin-degrading enzyme from Phanerochaete chrysosporium. Arch Biochem Biophys. 1985;241:304–314. doi: 10.1016/0003-9861(85)90387-x. [DOI] [PubMed] [Google Scholar]
- [80].Tien M, Kirk TK. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)0(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984;81:2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Johansson T, Welinder KG, Nyman PC. Isozymes of lignin peroxidase and manganese (II) peroxidase from the white-rot basidiomycete Trametes versicolor. II. Partial sequences, peptide maps, and amino acid and carbohydrate compositions. Arch Biochem Biophys. 1993;300:57–62. doi: 10.1006/abbi.1993.1008. [DOI] [PubMed] [Google Scholar]
- [82].Ghodake GS, Kalme SD, Jadhav JP, Govind-war SP. Purification and partial characterization of lignin peroxidase from Acinetobacter calcoaceticus NCIM 2890 and its application in decolorization of textile dyes. Appl Biochem Biotechnol. 2009;152:6–14. doi: 10.1007/s12010-008-8258-4. [DOI] [PubMed] [Google Scholar]
- [83].Gottschalk LM, Bon EP, Nobrega R. Lignin peroxidase from Streptomyces viridosporus T7A: enzyme concentration using ultrafiltration. Appl Biochem Biotechnol. 2008;147:23–32. doi: 10.1007/s12010-007-8081-3. [DOI] [PubMed] [Google Scholar]
- [84].Asgher M, Bhatti HN, Ashraf M, Legge RL. Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation. 2008;19:771–783. doi: 10.1007/s10532-008-9185-3. [DOI] [PubMed] [Google Scholar]
- [85].Hofrichter M, Ziegenhagen D, Vares T, Friedrich M, Jager MG, Fritsche W, Hatakka A. Oxida-tive decomposition of malonic acid as basis for the action of manganese peroxidase in the absence of hydrogen peroxide. FEBS Lett. 1998;434:362–366. doi: 10.1016/s0014-5793(98)01023-0. [DOI] [PubMed] [Google Scholar]
- [86].Glenn JK, Gold MH. Purification and characterization of an extracellular Mn(ll)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985;242:329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- [87].Lisov AV, Leontievsky AA, Golovleva LA. Hybrid Mn-peroxidase from the ligninolytic fungus Panus tigrinus 8/18. Isolation, substrate specificity, and catalytic cycle. Biochemistry (Mosc) 2003;68:1027–1035. doi: 10.1023/a:1026072815106. [DOI] [PubMed] [Google Scholar]
- [88].Hoshino F, Kajino T, Sugiyama H, Asami O, Takahashi H. Thermally stable and hydrogen peroxide tolerant manganese peroxidase (MnP) from Lenzites betulinus. FEBS Lett. 2002;530:249–252. doi: 10.1016/s0014-5793(02)03454-3. [DOI] [PubMed] [Google Scholar]
- [89].de la Rubia T, Linares A, Perez J, Munoz-Dorado J, Romera J, Martinez J. Characterization of manganese-dependent peroxidase isoenzymes from the ligninolytic fungus Phanerochaete flavido-alba. Res Microbiol. 2002;153:547–554. doi: 10.1016/s0923-2508(02)01357-8. [DOI] [PubMed] [Google Scholar]
- [90].Lankinen VP, Bonnen AM, Anton LH, Wood DA, Kalkkinen N, Hatakka A, Thurston CF. Characteristics and N-terminal amino acid sequence of manganese peroxidase from solid substrate cultures of Agaricus bisporus. Appl Microbiol Biotechnol. 2001;55:170–176. doi: 10.1007/s002530000509. [DOI] [PubMed] [Google Scholar]
- [91].Palma C, Martinez AT, Lema JM, Martinez MJ. Different fungal manganese-oxidizing per-oxidases: a comparison between Bjerkandera sp. and Phanerochaete chrysosporium. J Biotechnol. 2000;77:235–245. doi: 10.1016/s0168-1656(99)00218-7. [DOI] [PubMed] [Google Scholar]
- [92].Hilden KS, Bortfeldt R, Hofrichter M, Hatakka A, Lundell TK. Molecular characterization of the basidiomycete isolate Nematoloma fro-wardii bl9 and its manganese peroxidase places the fungus in the corticioid genus Phlebia. Microbiology. 2008;154:2371–2379. doi: 10.1099/mic.0.2008/018747-0. [DOI] [PubMed] [Google Scholar]
- [93].Chagas EP, Durrant LR. Decolorization of azo dyes by Phanerochaete chrysosporium and Pleurotus sajorcaju. Enzyme Microb Technol. 2001;29:473–477. [Google Scholar]
- [94].Christian VV, Shrivastava R, Novotny C, Vyas BR. Decolorization of sulfonphthalein dyes by manganese peroxidase activity of the white-rot fungus Phanerochaete chrysosporium. Folia Microbiol (Praha) 2003;48:771–774. doi: 10.1007/BF02931512. [DOI] [PubMed] [Google Scholar]
- [95].Xu H, Scott GM, Jiang F, Kelly C. Recombi-nant manganese peroxidase (rMnP) from Pichia pastoris. Part 1: Kraft pulp delignification. Hol-zforschung. 2010;64:137–143. [Google Scholar]
- [96].Harazono K, Kondo R, Sakai K. Bleaching of Hardwood Kraft Pulp with Manganese Peroxidase from Phanerochaete sordida YK-624 without Addition of MnS0(inf4) Appl Environ Microbiol. 1996;62:913–917. doi: 10.1128/aem.62.3.913-917.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gu L, Lajoie C, Kelly C. Expression of a Phanerochaete chrysosporium manganese peroxidase gene in the yeast Pichia pastoris. Biotechnol Prog. 2003;19:1403–1409. doi: 10.1021/bp025781h. [DOI] [PubMed] [Google Scholar]
- [98].Ruiz-Duenas FJ, Morales M, Garcia E, Miki Y, Martinez MJ, Martinez AT. Substrate oxidation sites in versatile peroxidase and other basidiomycete peroxidases. J Exp Bot. 2009;60:441–452. doi: 10.1093/jxb/ern261. [DOI] [PubMed] [Google Scholar]
- [99].Gomez-Toribio V, Martinez AT, Martinez MJ, Guillen F. Oxidation of hydroquinones by the versatile ligninolytic peroxidase from Pleurotus eryngii. H202 generation and the influence of Mn2+ Eur J Biochem. 2001;268:4787–4793. doi: 10.1046/j.1432-1327.2001.02405.x. [DOI] [PubMed] [Google Scholar]
- [100].Heinfling A, Ruiz-Duenas FJ, Martinez MJ, Bergbauer M, Szewzyk U, Martinez AT. A study on reducing substrates of manganese-oxidizing peroxidases from Pleurotus eryngii and Bjerkandera adusta. FEBS Lett. 1998;428:141–146. doi: 10.1016/s0014-5793(98)00512-2. [DOI] [PubMed] [Google Scholar]
- [101].Camarero S, Sarkar S, Ruiz-Duenas FJ, Martinez MJ, Martinez AT. Description of a versatile peroxidase involved in the natural degradation of lignin that has both manganese peroxidase and lignin peroxidase substrate interaction sites. J Biol Chem. 1999;274:10324–10330. doi: 10.1074/jbc.274.15.10324. [DOI] [PubMed] [Google Scholar]
- [102].Ruiz-Duenas FJ, Guillen F, Camarero S, Perez-Boada M, Martinez MJ, Martinez AT. Regulation of peroxidase transcript levels in liquid cultures of the ligninolytic fungus Pleurotus eryngii. Appl Environ Microbiol. 1999;65:4458–4463. doi: 10.1128/aem.65.10.4458-4463.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Cohen R, Hadar Y, Yarden O. Transcript and activity levels of different Pleurotus ostreatus peroxidases are differentially affected by Mn2+ Environ Microbiol. 2001;3:312–322. doi: 10.1046/j.1462-2920.2001.00197.x. [DOI] [PubMed] [Google Scholar]
- [104].Wang Y, Vazquez-Duhalt R, Pickard MA. Manganese-lignin peroxidase hybrid from Bjerkandera adusta oxidizes polycyclic aromatic hydrocarbons more actively in the absence of manganese. Can J Microbiol. 2003;49:675–682. doi: 10.1139/w03-091. [DOI] [PubMed] [Google Scholar]
- [105].Mester T, Field JA. Characterization of a novel manganese peroxidase-lignin peroxidase hybrid isozyme produced by Bjerkandera species strain B0S55 in the absence of manganese. J Biol Chem. 1998;273:15412–15417. doi: 10.1074/jbc.273.25.15412. [DOI] [PubMed] [Google Scholar]
- [106].Moreira PR, Almeida-Vara E, Sena-Martins G, Polonia I, Malcata FX, Cardoso Duarte J. Decolourisation of Remazol Brilliant Blue R via a novel Bjerkandera sp. strain. J Biotechnol. 2001;89:107–111. doi: 10.1016/s0168-1656(01)00320-0. [DOI] [PubMed] [Google Scholar]
- [107].Rodakiewicz-Nowak J, Jarosz-Wilkolazka A, Luterek J. Catalytic activity of versatile peroxidase from Bjerkandera fumosa in aqueous solutions of water-miscible organic solvents. Applied Catalysis A: General. 2006;308:56–61. [Google Scholar]
- [108].Camarero S, Bockle B, Martinez MJ, Martinez AT. Manganese-Mediated Lignin Degradation by Pleurotus pulmonarius. Appl Environ Microbiol. 1996;62:1070–1072. doi: 10.1128/aem.62.3.1070-1072.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Larrondo L, Gonzalez A, Perez Acle T, Cullen D, Vicuna R. The nop gene from Phanerochaete chrysosporium encodes a peroxidase with novel structural features. Biophys Chem. 2005;116:167–173. doi: 10.1016/j.bpc.2005.03.006. [DOI] [PubMed] [Google Scholar]
- [110].Lisov AV, Leontievsky AA, Golovleva LA. Hybrid Mn-peroxidases from basidiomycetes. Applied Biochemistry and Microbiology. 2007;43:536–543. [Google Scholar]
- [111].Eibes GM, Lu-Chau TA, Ruiz-Duenas FJ, Feijoo G, Martinez MJ, Martinez AT, Lema JM. Effect of culture temperature on the heterologous expression of Pleurotus eryngii versatile peroxi-dase in Aspergillus hosts. Bioprocess Biosyst Eng. 2009;32:129–134. doi: 10.1007/s00449-008-0231-7. [DOI] [PubMed] [Google Scholar]
- [112].Mester T, Tien M. Engineering of a manganese-binding site in lignin peroxidase isozyme H8 from Phanerochaete chrysosporium. Bio-chem Biophys Res Commun. 2001;284:723–728. doi: 10.1006/bbrc.2001.5015. [DOI] [PubMed] [Google Scholar]
- [113].Timofeevski SL, Nie G, Reading NS, Aust SD. Addition of veratryl alcohol oxidase activity to manganese peroxidase by site-directed mutagenesis. Biochem Biophys Res Commun. 1999;256:500–504. doi: 10.1006/bbrc.1999.0360. [DOI] [PubMed] [Google Scholar]
- [114].Guillen F, Martinez AT, Martinez MJ. Substrate specificity and properties of the aryl-alcohol oxidase from the ligninolytic fungus Pleurotus eryngii. Eur J Biochem. 1992;209:603–611. doi: 10.1111/j.1432-1033.1992.tb17326.x. [DOI] [PubMed] [Google Scholar]
- [115].Guillen F, Martinez MJ, Munoz C, Martinez AT. Quinone redox cycling in the ligninolytic fungus Pleurotus eryngii leading to extracellular production of superoxide anion radical. Arch Biochem Biophys. 1997;339:190–199. doi: 10.1006/abbi.1996.9834. [DOI] [PubMed] [Google Scholar]
- [116].Henriksson G, Ander P, Pettersson B, Pet-tersson G. Cellobiose dehydrogenase (cellobiose oxidase) from Phanerochaete chrysosporium as a wood degrading enzymestudies on cellulose, xylan and synthetic lignin. Appl Microbiol Biotechnol. 1995;42:790–796. [Google Scholar]
- [117].Henriksson G, Johansson G, Pettersson G. A critical review of cellobiose dehydrogenases. J Biotechnol. 2000;78:93–113. doi: 10.1016/s0168-1656(00)00206-6. [DOI] [PubMed] [Google Scholar]
- [118].Suzuki MR, Hunt CG, Houtman CJ, Dalebroux ZD, Hammel KE. Fungal hydroquinones contribute to brown rot of wood. Environ Microbiol. 2006;8:2214–2223. doi: 10.1111/j.1462-2920.2006.01160.x. [DOI] [PubMed] [Google Scholar]
- [119].Call HP, Mūcke I. History overview and applications of mediated lignolytic systems, especially I a cease-media tor-systems (LignozymeO-process) J Biotechnol. 1997;53:163–202. [Google Scholar]
- [120].Wang W, Huang F, Mei Lu X, Ji Gao P. Lignin degradation by a novel peptide, Gt factor, from brown rot fungus Gloeophyllum trabeum. Biotechnol J. 2006;1:447–453. doi: 10.1002/biot.200500016. [DOI] [PubMed] [Google Scholar]
- [121].Baldrian P, Valaskova V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol Rev. 2008;32:501–521. doi: 10.1111/j.1574-6976.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- [122].Renganathan V, Usha SN, Lindenburg F. Cellobiose oxidizing enzymes from the lignocellulose-degrading basidiomycete Phanerochaete chrysosporium - interaction with microcrystal-line cellulose. Appl Microbiol Biotechnol. 1990;32:609–613. [Google Scholar]
- [123].Baminger U, Subramaniam SS, Renganathan V, Haltrich D. Purification and characterization of cellobiose dehydrogenase from the plant pathogen Sclerotium (Athelia) rolfsii. Appl Environ Microbiol. 2001;67:1766–1774. doi: 10.1128/AEM.67.4.1766-1774.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Yang W, Liu J, Wang W, Zhang Y, Gao P. Function of a low molecular peptide generated by cellulolytic fungi for the degradation of native cellulose. Biotechnol Lett. 2004;26:1799–1802. doi: 10.1007/s10529-004-4612-y. [DOI] [PubMed] [Google Scholar]
- [125].Enoki A, Hirano T, Tanaka H. Extracellular substance from the brown rot basidiomycete Gloeophyllum trabeum that produces and reduces hydrogen peroxide. Mater Organism. 1992;27:247–261. [Google Scholar]
- [126].Tanaka H, Yoshida G, Baba Y, Matsumura K, Wasada H, Murata J, Agawa M, Itakura S, Enoki A. Characterization of a hydroxyl-radical-producing glycoprotein and its presumptive genes from the white-rot basidiomycete Phanerochaete chrysosporium. J Biotechnol. 2007;128:500–511. doi: 10.1016/j.jbiotec.2006.12.010. [DOI] [PubMed] [Google Scholar]
- [127].Lundell TK, Makela MR, Hilden K. Lignin-modifying enzymes in filamentous basidiomycetes-ecological, functional and phylogenetic review. J Basic Microbiol. 50:5–20. doi: 10.1002/jobm.200900338. [DOI] [PubMed] [Google Scholar]
- [128].Bouws H, Wattenberg A, Zorn H. Fungal secretomes-nature's toolbox for white biotechnology. Appl Microbiol Biotechnol. 2008;80:381–388. doi: 10.1007/s00253-008-1572-5. [DOI] [PubMed] [Google Scholar]
- [129].Sato S, Feltus FA, Iyer P, Tien M. The first genome-level transcriptome of the wood-degrading fungus Phanerochaete chrysosporium grown on red oak. Curr Genet. 2009;55:273–286. doi: 10.1007/s00294-009-0243-0. [DOI] [PubMed] [Google Scholar]
- [130].Sticklen MB. Plant genetic engineering for bio-fuel production: towards affordable cellulosic ethanol. Nat Rev Genet. 2008;9:433–443. doi: 10.1038/nrg2336. [DOI] [PubMed] [Google Scholar]
- [131].Hilden K, Hakala TK, Lundell T. Thermotol-erant and thermostable laccases. Biotechnol Lett. 2009;31:1117–1128. doi: 10.1007/s10529-009-9998-0. [DOI] [PubMed] [Google Scholar]
- [132].Hammel KE, Cullen D. Role of fungal peroxi-dases in biological ligninolysis. Curr Opin Plant Biol. 2008;11:349–355. doi: 10.1016/j.pbi.2008.02.003. [DOI] [PubMed] [Google Scholar]
- [133].Sundaramoorthy M, Youngs HL, Gold MH, Poulos TL. High-resolution crystal structure of manganese peroxidase: substrate and inhibitor complexes. Biochemistry. 2005;44:6463–6470. doi: 10.1021/bi047318e. [DOI] [PubMed] [Google Scholar]
- [134].Baborova P, Moder M, Baldrian P, Cajthamlova K, Cajthaml T. Purification of a new manganese peroxidase of the white-rot fungus Irpex lacteus, and degradation of polycyclic aromatic hydrocarbons by the enzyme. Res Microbiol. 2006;157:248–253. doi: 10.1016/j.resmic.2005.09.001. [DOI] [PubMed] [Google Scholar]
- [135].Davila-Vazqueza G, Tinoco R, Pickard MA, Vazquez-Duhalt R. Transformation of halo-genated pesticides by versatile peroxidase from Bjerkandera adusta. Enzyme and Microbial Technology. 2005;36:223–231. [Google Scholar]
- [136].Kurek B, Gaudard F. Oxidation of spruce wood sawdust by MnO(2) plus oxalate: a biochemical investigation. J Agric Food Chem. 2000;48:3058–3062. doi: 10.1021/jf000015g. [DOI] [PubMed] [Google Scholar]
- [137].Makela MR, Hilden K, Hatakka A, Lundell TK. Oxalate decarboxylase of the white rot fungus Dichomitus squalens demonstrates a novel nzyme primary structure and non-induced expression on wood and in liquid cultures. Microbiology. 2009 doi: 10.1099/mic.0.028860-0. [DOI] [PubMed] [Google Scholar]
- [138].Brock BJ, Rieble S, Gold MH. Purification and Characterization of a 1,4-Benzoquinone Reductase from the Basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1995;61:3076–3081. doi: 10.1128/aem.61.8.3076-3081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Enoki A, Tanaka H, Itakura S. Physical and chemical characteristics of glycopeptide from wood decay fungi. In: Goodell B, Nicholas DD, Schultz TP, editors. Oxford University Press; Washington, DC: 2003. pp. 140–153. Wood Deterioration and Preservation. [Google Scholar]