Abstract
Drosophila melanogaster DNA has been cloned which encompasses the major part of the 20-OH-ecdysone inducible puff 74EF. One 20-OH-ecdysone responsive transcription unit was detected which gives rise to two alternative transcripts. The expression of one transcript in salivary glands of 3rd instar larvae is correlated with the 20-OH-ecdysone induced activity of puff 74EF. Corresponding cDNA analysis indicates that the two transcripts are translated into two different proteins which have alternative amino terminal ends. The carboxy terminal domain of the 74E proteins is similar to the carboxy terminal sequences of ets-oncoproteins suggesting that the 74E proteins represent alternative transcription factors. It is proposed that the activity of the 20-OH-ecdysone inducible puff 74EF leads to a switch in the synthesis of a transcription factor.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M. Patterns of puffing activity in the salivary gland chromosomes of Drosophila. VI. Induction by ecdysone in salivary glands of D. melanogaster cultured in vitro. Chromosoma. 1972;38(3):255–281. doi: 10.1007/BF00290925. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Sequential gene activation by ecdysone in polytene chromosomes of Drosophila melanogaster. I. Dependence upon ecdysone concentration. Dev Biol. 1973 Nov;35(1):47–61. doi: 10.1016/0012-1606(73)90006-7. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Sequential gene activation by ecdysone in polytene chromosomes of Drosophila melanogaster. II. The effects of inhibitors of protein synthesis. Dev Biol. 1974 Jul;39(1):141–157. doi: 10.1016/s0012-1606(74)80016-3. [DOI] [PubMed] [Google Scholar]
- BECKER H. J. [The puffs of salivary gland chromosomes of Drosophilia melanogaster. Part 1. Observations on the behavior of a typical puff in the normal strain and in two mutants, giant and lethal giant larvae]. Chromosoma. 1959;10:654–678. doi: 10.1007/BF00396591. [DOI] [PubMed] [Google Scholar]
- Baumann A., Krah-Jentgens I., Müller R., Müller-Holtkamp F., Seidel R., Kecskemethy N., Casal J., Ferrus A., Pongs O. Molecular organization of the maternal effect region of the Shaker complex of Drosophila: characterization of an I(A) channel transcript with homology to vertebrate Na channel. EMBO J. 1987 Nov;6(11):3419–3429. doi: 10.1002/j.1460-2075.1987.tb02665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bhat N. K., Fisher R. J., Fujiwara S., Ascione R., Papas T. S. Temporal and tissue-specific expression of mouse ets genes. Proc Natl Acad Sci U S A. 1987 May;84(10):3161–3165. doi: 10.1073/pnas.84.10.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialojan S., Falkenburg D., Renkawitz-Pohl R. Characterization and developmental expression of beta tubulin genes in Drosophila melanogaster. EMBO J. 1984 Nov;3(11):2543–2548. doi: 10.1002/j.1460-2075.1984.tb02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Boulukos K. E., Pognonec P., Begue A., Galibert F., Gesquière J. C., Stéhelin D., Ghysdael J. Identification in chickens of an evolutionarily conserved cellular ets-2 gene (c-ets-2) encoding nuclear proteins related to the products of the c-ets proto-oncogene. EMBO J. 1988 Mar;7(3):697–705. doi: 10.1002/j.1460-2075.1988.tb02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworniczak B., Seidel R., Pongs O. Puffing activities and binding of ecdysteroid to polytene chromosomes of Drosophila melanogaster. EMBO J. 1983;2(8):1323–1330. doi: 10.1002/j.1460-2075.1983.tb01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Golay J., Introna M., Graf T. A single point mutation in the v-ets oncogene affects both erythroid and myelomonocytic cell differentiation. Cell. 1988 Dec 23;55(6):1147–1158. doi: 10.1016/0092-8674(88)90259-0. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H., Pongs O. Localization of ecdysterone on polytene chromosomes of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2108–2112. doi: 10.1073/pnas.77.4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlson P. Ecdyson, das Häutungshormon der Insekten. Naturwissenschaften. 1966 Sep;53(18):445–453. doi: 10.1007/BF00601742. [DOI] [PubMed] [Google Scholar]
- Lin H. C., Lei S. P., Wilcox G. An improved DNA sequencing strategy. Anal Biochem. 1985 May 15;147(1):114–119. doi: 10.1016/0003-2697(85)90016-8. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
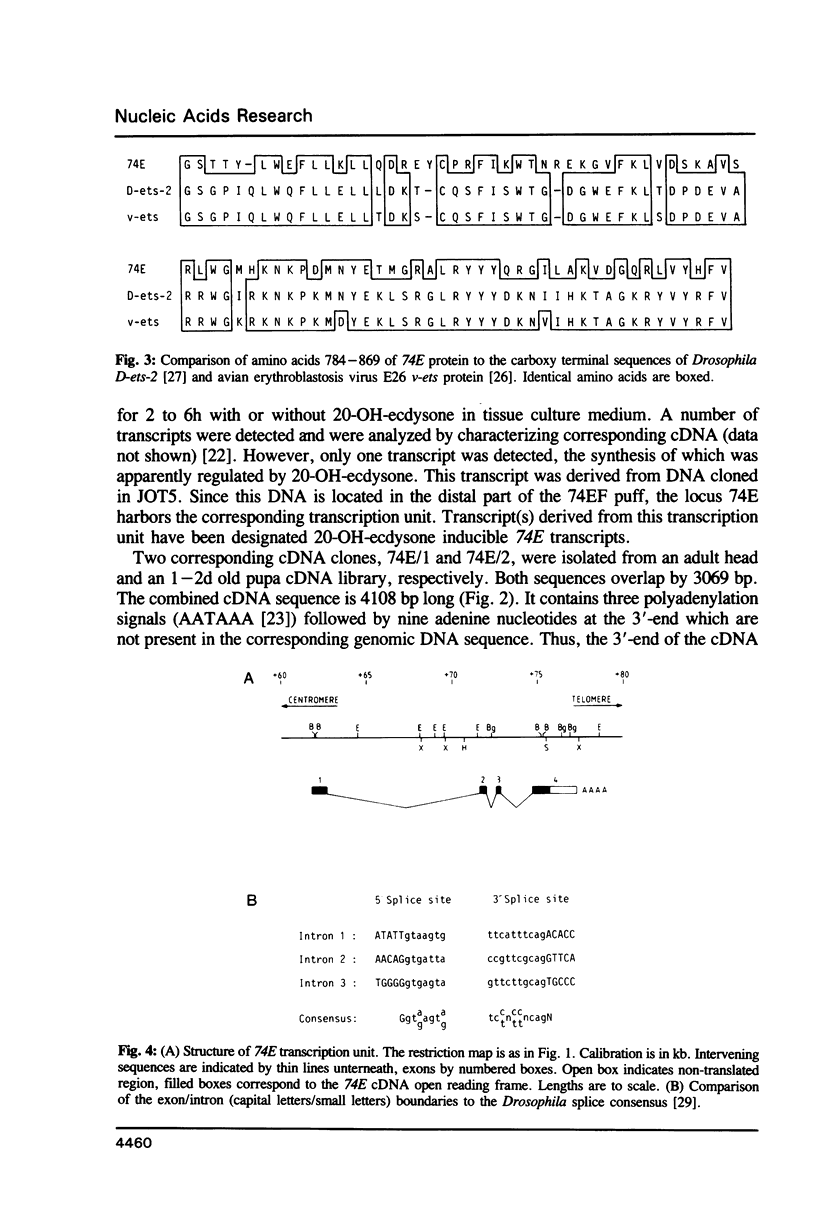
- Möritz T., Edström J. E., Pongs O. Cloning of a gene localized and expressed at the ecdysteroid regulated puff 74EF in salivary glands of Drosophila larvae. EMBO J. 1984 Feb;3(2):289–295. doi: 10.1002/j.1460-2075.1984.tb01798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn M. F., Seeburg P. H., Moscovici C., Duesberg P. H. Tripartite structure of the avian erythroblastosis virus E26 transforming gene. Nature. 1983 Nov 24;306(5941):391–395. doi: 10.1038/306391a0. [DOI] [PubMed] [Google Scholar]
- Pongs O. Ecdysteroid-regulated gene expression in Drosophila melanogaster. Eur J Biochem. 1988 Aug 1;175(2):199–204. doi: 10.1111/j.1432-1033.1988.tb14184.x. [DOI] [PubMed] [Google Scholar]
- Pribyl L. J., Watson D. K., McWilliams M. J., Ascione R., Papas T. S. The Drosophila ets-2 gene: molecular structure, chromosomal localization, and developmental expression. Dev Biol. 1988 May;127(1):45–53. doi: 10.1016/0012-1606(88)90187-x. [DOI] [PubMed] [Google Scholar]
- Richards G. The radioimmune assay of ecdysteroid titres in Drosophila melanogaster. Mol Cell Endocrinol. 1981 Mar;21(3):181–197. doi: 10.1016/0303-7207(81)90013-7. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaltmann K., Pongs O. Identification and characterization of the ecdysterone receptor in Drosophila melanogaster by photoaffinity labeling. Proc Natl Acad Sci U S A. 1982 Jan;79(1):6–10. doi: 10.1073/pnas.79.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Hunkapiller M., Yuen D., Silvert D., Fristrom J., Davidson N. Cuticle protein genes of Drosophila: structure, organization and evolution of four clustered genes. Cell. 1982 Jul;29(3):1027–1040. doi: 10.1016/0092-8674(82)90466-4. [DOI] [PubMed] [Google Scholar]
- Walker V. K., Ashburner M. The control of ecdysterone-regulated puffs in Drosophila salivary glands. Cell. 1981 Oct;26(2 Pt 2):269–277. doi: 10.1016/0092-8674(81)90309-3. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Yedvobnick B., Finnerty V. G., Artavanis-Tsakonas S. opa: a novel family of transcribed repeats shared by the Notch locus and other developmentally regulated loci in D. melanogaster. Cell. 1985 Jan;40(1):55–62. doi: 10.1016/0092-8674(85)90308-3. [DOI] [PubMed] [Google Scholar]