Abstract
Cadherin-like protein 22 (CDH22) is a transmembrane glycoprotein implicated in cell-cell adhesion and cancer metastasis. The expression of CDH22 has been shown to be increased in colorectal cancers. However, the role of CDH22 in melanomagenesis is not known. To investigate the role of CDH22 in melanoma progression, we examined the expression of CDH22 in melanocytic lesions at different stages and analysed the correlation between CDH22 expression and clinicopathlogic parameters and patient survival. Using tissue microarray and immunohisto-chemistry, we evaluated CDH22 staining in 76 dysplastic nevi, 247 primary melanomas, and 143 metastatic melanomas. We found that metastatic melanomas had a significantly higher percentage of negative CDH22 staining than dysplastic nevi (P = 0.012) and primary melanomas (P = 0.038). CDH22 expression was also reduced in thick (≥2 mm) and ulcerative melanomas (P = 0.003 and 0.022, respectively). Melanomas of AJCC stage II, III, and IV had a higher percentage of negative CDH22 staining than AJCC stage I melanomas (P = 0.004, P < 0.0001, and P = 0.009, respectively). Melanomas with negative CDH22 expression had significantly poorer disease-specific 5-year survival than those with positive CDH22 staining. Additionally, CDH22 expression depended on AJCC stage to predict patient survival. These data indicate that reduced CDH22 expression is associated with melanoma metastasis and poor patient prognosis.
Keywords: CDH22, melanoma, tissue microarray, immunohistochemistry
Introduction
Melanoma incidence is rising in most parts of the world and is highest in Caucasian Australian, New Zealand, and Swedish populations [1-2]. Incidence of malignant melanoma has rapidly increased in the past several decades [2-3]. Although melanoma makes up a small percentage of dermatological cancers, it is responsible for 80% of skin cancer-related deaths [1,4-5]. This is because melanoma has a greater potential to spread to distant body sites compared to other forms of skin cancer. Thick, ulcerated melanomas are “high-risk” and have the greatest metastatic potential [6-7]. Upon progression to metastatic disease, the survival rate is very low with the average survival of 6 to10 months for metastatic melanoma [8]. Currently, there is no effective treatment for late-stage metastatic melanoma. Therefore the identification of bio-markers, and specifically biomarkers predisposing patients to melanoma metastasis, would provide useful prognostic markers and potential therapeutic targets [4,9].
The cadherin family of proteins represents one such potential cancer biomarker, especially with respect to metastatic potential prediction. Cad-herins are Ca2+-dependent, single transmem-brane-domain glycoproteins important for cell adhesion [10-11]. They have unique repeating extracellular domain motifs and their cytoplas-mic domains interact with cytoskeletal proteins via α-catenin, and are involved in tyrosine phos-phorylation via β-catenin [11]. Cadherin expression dictates an “adhesive code,” providing tissue functional subdivisions through homophilic adhesion [10]. Loss of the prototypic E-cadherin is associated with increased metastatic capacity in most cancers [12]. The cadherin-like 22 (CDH22) gene, located in chromosome 20q13.1, is the human ortholog of rat pituitary-brain (PB) cadherin. PB cadherin was characterized in 1996 by Sugimoto and colleagues and is structurally similar to typical classical cadherins and its extracellular, transmembrane, and intra-cellular domains resemble those of E-cadherin [10]. It has two variants based on alternative splicing: long-type PB cadherin (LTPB-C), and short-type PB cadherin (STPB-C). STPB-C is important for the enhanced self-renewal of spermatogonial cells in rat testis, mediated by activating the JAK-STAT and PI3-K/Akt pathways and blocking TGF-β1 signaling [13].
In 2009, Zhou and colleagues examined CDH22 expression in 68 cases of primary colorectal cancer, 35 lymph node metastases, and 68 samples of adjacent normal colonic mucosa [14]. Strong CDH22 expression was detected in the primary tumours as well as lymph node metastases, whereas CDH22 was undetectable in the normal mucosa. In fresh colorectal cancer samples, CDH22 mRNA overexpression was positively correlated with metastasis and clinical stage. Also, CDH22 knockdown inhibited SW480 cell colony formation, and mice injected with CDH22 knockdown cells had decreased tumour growth compared to controls.
Expression of members of the cadherin family including CDH22 may affect tumourigenesis or metastasis of various cancers, and these proteins may serve as important biomarkers for cancer potential. Since the expression of CDH22 in melanoma has not been investigated, we used immunohistochemistry (IHC) and a tissue microarray (TMA) with biopsies from 466 melanocytic lesions to examine the differences in the expression profile of CDH22. We also analysed the correlation between CDH22 expression and patient survival or other clinicopathological features. CDH22 protein expression was lost in a greater percentage of metastatic melanoma samples compared to dysplastic nevi and primary melanoma, and patients with no CDH22 expression were more likely to die after 5 years.
Materials and methods
Cell culture and Western blot analysis
Normal human melanocytes (NHM), human fibroblasts (HFB), and seven melanoma cell lines (Sk-mel-3, Sk-mel-24, Sk-mel-28, Sk-mel-110, PMWK, MMAN, and MMLH) were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum at 37 °C in 5% CO2. Cells were grown on 100 mm plates, washed with PBS, harvested by scraping, and pelleted by centrifugation at 2,000 g for 2 minutes. Pellets were lysed with RIPA buffer and the samples were sonicated, incubated on ice for 15 minutes and the supernatants collected. Protein concentration was determined by Bradford assay and the samples (45 μg/lane) were separated on a 12% SDS-polyacrylamide gel. Western blot analysis was conducted by blotting onto polyvinlyidene difluoride membranes (Bio-Rad, Hercules, CA). Blots were blocked with 5% skim milk for 30 minutes at room temperature, incubated with the primary antibody (anti-CDH22, 1:500 or anti β-actin, 1:5000, both from Sigma-Alrich, St. Louis, MO), and washed three times with PBS containing 0.04% Tween-20. Blots were incubated with infrared dye-labeled secondary antibody (1:20000, LI-COR Biosciences, Lincoln, NE) for 1 hour and signals were detected with the Odyssey Infrared Imaging System (LI-COR Biosciences).
TMA and immunohistochemistry
For TMA construction, formalin-fixed, paraffin-embedded tissues were obtained from the Department of Pathology at the Vancouver General Hospital between the years 1990 and 2010. Ethics approval for the study was provided by the Clinical Research Ethics Board of the University of British Columbia and use of human tissues was in accordance with the Declaration of Helsinki guidelines. A tissue array instrument (Beecher Instruments, Silver Spring, MD) was used to assemble TMA after selecting the most representative tumour areas which do not contain necrotic tissues on the hematoxylin and eosin-stained (H&E) slides. Duplicate 0.6mm-thick cores were taken from the specimens, and 4-μm sections were cut using a microtome (Leica Microsystems Inc, Bannockburn, IL) and transferred to adhesive-coated slides. One section was stained with H&E and the others were kept for immunohistochemstry.
Immunohistochemical staining was performed using the primary anti-CDH22 antibody at a dilution of 1:35 (Sigma-Alrich), as described previously [15]. A universal biotin-labeled secondary antibody was used (DAKO Diagnostics, Glostrup, Denmark), and negative controls without the primary antibody incubation were included. A blinded dermatopathologist and two independent observers scored the TMA for CDH22 staining. The cells are considered CDH22 positive when a clear brown staining is observed. The expression of CDH22 was considered positive when ≥5% of tumour cells showed immunopositivity, and negative when less than 5% of tumour cells showed immunopositivity.
Statistical analysis
For correlation of CDH22 expression to melanoma progression, as well as to the various clinicopathological characteristics, χ2 tests were used. Kaplan-Meier and log-rank tests were used for overall and disease-specific 5-year survival analyses. Multivariate Cox regression analysis was used to determine status of prognostic relevance for CDH22 and other variables. Cox regression analysis was also used to generate hazard risk values. All analyses were completed with SPSS version 11.5 (SPSS Inc, Chicago, IL) software.
Results
CDH22 expression is reduced in melanoma cell lines and is correlated with melanoma progression
We compared CDH22 protein expression in seven melanoma cell lines to that of normal melanocytes (Figure 1). Expression was reduced by over 90% in Skmel-110 and MMAN cell lines, and was completely lost in Sk-mel-3, Sk-mel-24, Sk-mel-28, PMWK, and MMLH. Additionally, CDH22 expression was undetectable in human dermal fibroblasts (Figure 1).
Figure 1.

CDH22 protein expression is decreased in melanoma cell lines. Western blot analysis of CDH22 in whole cell extracts from human melanocytes, dermal fibroblasts, and melanoma cell lines.
A TMA analysis was conducted using 247 primary melanoma, 143 metastatic melanoma, and 76 dysplastic nevi. Of the 390 melanoma cases, 231 were male and 159 were female, with age ranging from 7 to 95 years (median age of 60; Table 1). Of the primary melanomas, 8 were acrolentigous, 36 were lentigous, 48 were nodular, and 83 were superficial spreading. Additionally, 162 cases of primary melanoma were located in sun-protected areas (arm, back, leg, trunk, and vulva) while 82 were located in sun-exposed areas (face, head, and neck). CDH22 expression was not correlated with age, gender, tumour subtype, or tumour site in primary melanoma, and was not correlated with age or gender in metastatic melanoma (Table 1).
Table 1.
CDH22 staining and clinicopathological characteristics of melanoma patients
| CDH22 staining | ||||
|---|---|---|---|---|
| Variables | Negative (%) | Positive (%) | Total | P valuea |
| Primary Melanoma | ||||
| Age | ||||
| <60 | 15(13.5%) | 96(86.5%) | 111 | 0.81 |
| ≥60 | 17(12.5%) | 119(87.5%) | 136 | |
| Gender | ||||
| Male | 17(12.3%) | 121(87.7%) | 138 | 0.74 |
| Female | 15(13.8%) | 94(86.2%) | 109 | |
| Tumour subtype | ||||
| Acrolentigous | 0(0%) | 8(100%) | 8 | 0.60 |
| Lentigous | 3(8.3%) | 33(91.7%) | 36 | |
| Nodular | 6(12.5%) | 42(87.5%) | 48 | |
| Superficial spreading | 11(13.3%) | 72(86.7%) | 83 | |
| Other | 12(16.7%) | 60(83.3%) | 72 | |
| Siteb | ||||
| Sun-protected | 22(13.6%) | 140(86.4%) | 162 | 0.70 |
| Sun-exposed | 9(11%) | 73(89%) | 82 | |
| Unspecified | 0(0) | 3(100%) | 3 | |
| Metastatic Melanoma | ||||
| Age | ||||
| <60 | 21(26.9%) | 57(73.1%) | 78 | 0.10 |
| ≥60 | 10(13.4%) | 55(84.6%) | 65 | |
| Gender | ||||
| Male | 16(17.2%) | 77(82.8%) | 93 | 0.08 |
| Female | 15(30%) | 35(70%) | 50 | |
χ2 test
Sun-protected sites: arm, back, leg, trunk, and vulva; Sun-exposed sites: face, head, and neck.
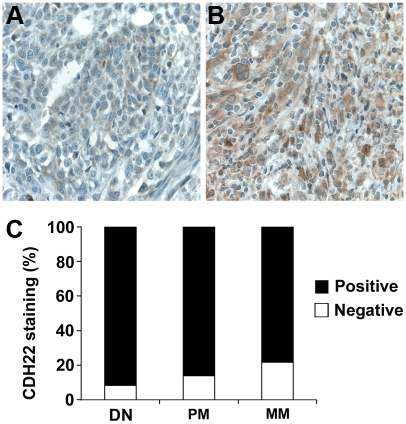
Loss of CDH22 expression was correlated with melanoma progression (Figure 2). Negative CDH22 staining was observed in 9% of dysplastic nevi, 16% of primary melanoma, and 28% of metastatic melanoma. A significantly greater percentage of metastatic melanomas had negative CDH22 staining compared to dysplastic nevi (P = 0.012), and compared to primary melanoma (P = 0.038). There was no significant difference in CDH22 staining between dysplastic nevi and primary melanoma (P = 0.20).
Figure 2.
CDH22 expression is lost in a greater proportion of human metastatic melanoma biopsies compared to dysplastic nevi and primary melanoma as determined by immunohistochemical staining of melanocytic lesions. (A) Negative CDH22 staining in metastatic melanoma. (B) Positive CDH22 staining in dysplastic nevi. (C) The percentage of negative CDH22 expression was significantly increased in metastatic melanoma compared to both dysplastic nevi (P = 0.012, χ2 test), and primary melanoma (P = 0.038, χ2test).
Loss of CDH22 is correlated with melanoma thickness, ulceration, and AJCC stage
For primary melanoma, there were 122 thick (≥2 mm) and 125 thin (<2 mm) tumours. We found that thick melanoma had a significantly higher percentage of negative CDH22 staining than thin melanoma (P = 0.003; Figure 3a). Ulceration was observed in 54 cases of primary melanoma and was absent in 193 cases. Primary melanomas positive for ulceration had significantly more cases with negative CDH22 staining than melanomas negative for ulceration (P = 0.022; Figure 3b). Also, primary and metastatic melanomas were assigned an AJCC stage from I to IV. 116 cases were at stage I, 126 cases stage II, 63 cases stage III, and 85 cases stage IV. AJCC stage II, III, and IV all had significantly more cases negative for CDH22 staining compared to AJCC I (P = 0.004, P < 0.0001, and P = 0.009, respectively; Figure 3c). There were no significant differences in CDH22 staining between stages II, III, or IV.
Figure 3.
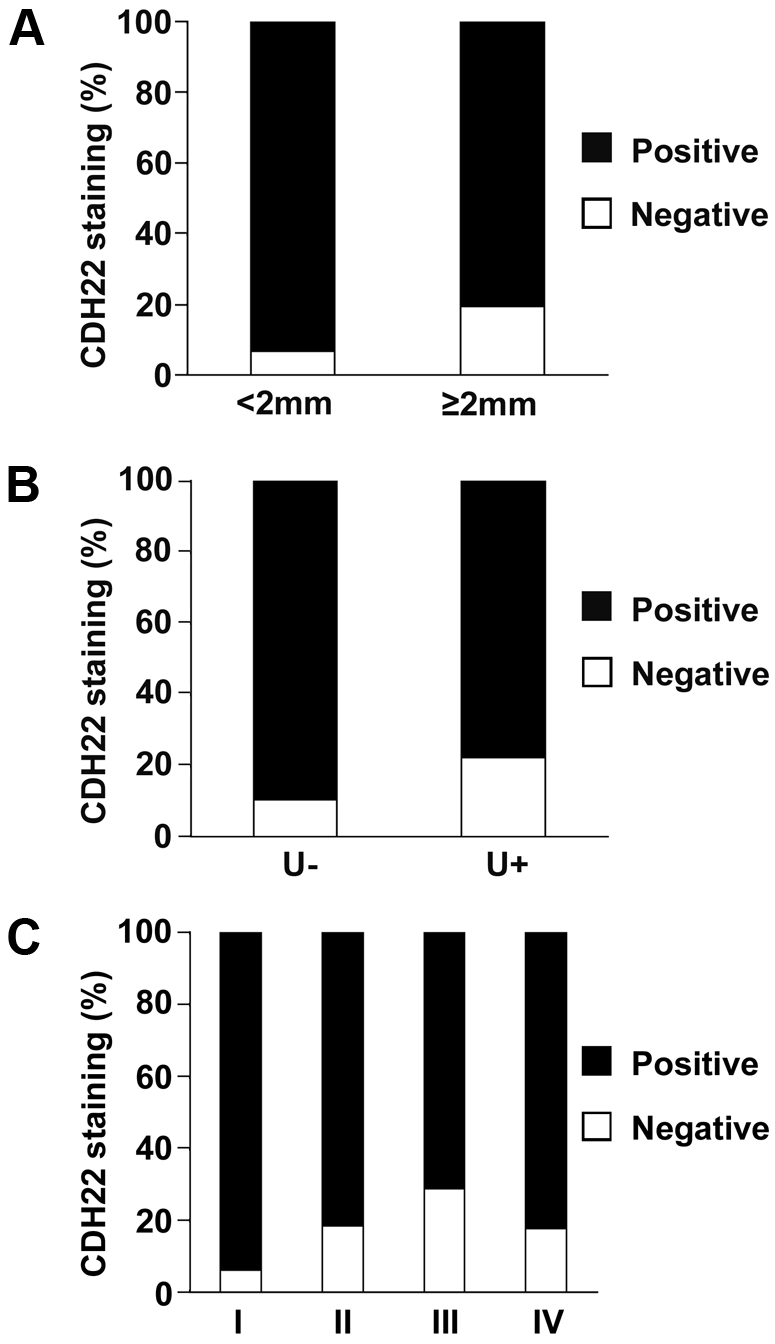
Correlation between CDH22 staining and tumour thickness, ulceration, and AJCC stage. (A) CDH22 expression is lost in a higher percentage of thick primary melanoma biopsies (P = 0.003). (B) CDH22 expression is lost in a higher percentage of primary melanoma patients with tumour ulceration (P = 0.022). (C) Melanomas at AJCC stage I had significantly more positive CDH22 staining than stages II, III, and IV (P = 0.004, P < 0.0001, and P = 0.009, respectively).
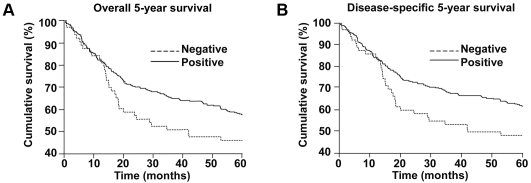
CDH22 protein expression is correlated with patient survival
The associations between CDH22 staining and both overall and disease-specific survival for all melanoma patients are presented in Figure 4. Kaplan-Meier analysis showed that primary and metastatic melanoma patients with negative CDH22 staining had a significantly worse disease-specific 5-year survival than those with positive CDH22 staining (P = 0.036). A similar trend was observed for overall 5-year survival, although not statistically significant (P = 0.070). To determine whether CDH22 staining is an independent prognostic factor for melanoma 5-year survival, we performed a multivariate Cox regression analysis (Table 2). AJCC stage, age, gender, and CDH22 staining were included in the analysis of both primary and metastatic melanoma cases. Since the differences in AJCC stage with respect to CDH22 staining were observed between stage I and the other three stages, stages II, III, and IV were grouped together. CDH22 is not an independent prognostic factor for overall or disease-specific 5-year survival (P = 0.70 and 0.55, respectively). As expected, AJCC stage, which takes into account for tumour thickness, ulceration, and metastasis, independently predicted disease-specific 5-year survival of melanoma patients (HR = 8.08, 95% CI = 4.57-14.27, P < 0.001).
Figure 4.
CDH22 expression is correlated to 5-year survival of all melanoma patients. (A) Patients with negative CDH22 staining have a worse overall 5-year survival than patients with positive CDH22 staining (P = 0.070). (B) Patients with negative CDH22 staining have a worse disease-specific 5-year survival than patients with positive CDH22 staining (P = 0.036).
Discussion
Since CDH22 expression in melanoma had not been previously studied, we examined CDH22 expression in different stages of melanocytic lesions. The results from our Western blot analysis of melanoma cell lines as well as the TMA suggest that CDH22 expression is reduced with an increasingly metastatic phenotype. CDH22 expression was greatly reduced in two of seven melanoma cell lines, and lost completely in the remaining five. Additionally, significantly fewer metastatic melanomas expressed CDH22 than primary melanomas (P = 0.038), but no significant difference was found between dysplasticnevi and primary melanomas (P = 0.20). This suggests that CDH22 expression is more important for melanoma invasion and metastasis than for cancer initiation.
CDH22 expression was also related to the development of “high-risk” melanoma as well as 5-year patient survival. Thick melanoma, tumours with ulceration, and cases with AJCC stages II-IV had a greater percentage of negative CDH22 staining than thin, non-ulcerative, AJCC stage I cancers. Also, more patients with positive CDH22 staining had tumour-free survival after 5 years than patients with negative CDH22 staining (P = 0.036). However, CDH22 is not an independent prognostic marker as its expression is closely correlated with tumour thickness, ulceration, and AJCC stage.
The relationships between CDH22 expression and melanoma progression are opposite to those observed in colorectal cancer [14]. This may be explained by the two potential functions of cell adhesion molecules. Once cells have broken off from the primary tumour, increased cadherin expression could allow for increased anchorage to other cells at distant locations in the body, enhancing metastatic potential. On the other hand, higher expression of cadherins might function to increase cell-cell adhesion at the site of the primary tumour, preventing cells from breaking off and metastasizing. Epithelial cancers often lose expression of E cadherin as they become malignant [12]. However, in certain cancer types including ovarian and inflammatory breast cancer, E-cadherin expression is essential for tumourigenesis [16-17]. Since PB cadherin closely resembles the classical cadherins, including E-cadherin, it is also likely that the role of CDH22 in cancer development and metastasis is tissue type-specific.
The cause of CDH22 downregulation in melanoma remains unclear. However, in 2009, Liu and colleagues identified a potential upstream regulator of CDH22 in colorectal cancer cell lines [18]. They found that PRL-3 (phosphatase of regenerating liver 3) promoted epithelial mesenchymal transition (EMT) in colorectal cancer cells by downregulating E-cadherin, and PRL-3 additionally promoted CDH22 downregulation. Since PRL-3 expression is upregulated in melanoma and its expression enhances melanoma metastatic potential, perhaps CDH22 loss in melanoma is similarly dependent on PRL-3 status and related to EMT [19-22]. Melanocytes are neural-crest derived [23-24] and loss of CDH22 may give cells a more “mesenchymal” phenotype. CDH22 should be expressed to a greater extent in neural tissue since it is the ortholog of rat PB cadherin. Additionally, other mechanisms such as mutation, epigenetic silencing, and increased proteolysis may also apply to loss of CDH22 expression [12].
Overall, perturbations in CDH22 affect the ability of melanoma to metastasize, and the loss of CDH22 may result in EMT through promoting the development of a “mesenchymal” phenotype. Higher expression of CDH22 might function to increase cell-cell adhesion at the site of the primary tumour, resulting in cells less capable of breaking off and metastasizing. Further investigation is necessary to determine the cause of reduced CDH22 expression in metastatic melanoma.
Acknowledgments
This research was supported by the Canadian Institutes of Health Research (MOP-84559 and MOP-93810) and Canadian Dermatology Foundation (G.L.), Canadian Institutes of Health Research Skin Research Training Centre PhD Scholarship (B.P.), and Natural Sciences and Engineering Research Council of Canada (S.K.).
References
- 1.MacKie RM. Long-term health risk to the skin of ultraviolet radiation. Prog Biophys Mol Biol. 2006;92:92–96. doi: 10.1016/j.pbiomolbio.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Erickson C, Driscoll MS. Melanoma epidemic: Facts and controversies. Clinics in Dermatol. 2010;28:281–286. doi: 10.1016/j.clindermatol.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Jennings L, Murphy G. Predicting outcome in melanoma: Where are we now? Br J Dermatol. 2009;161:496–503. doi: 10.1111/j.1365-2133.2009.09324.x. [DOI] [PubMed] [Google Scholar]
- 4.Garate M, Wani AA, Li G. The NAD(P) H:Quinone oxidoreductase 1 induces cell cycle progression and proliferation of melanoma cells. Free Rad Biol Med. 2010;48:1601–1609. doi: 10.1016/j.freeradbiomed.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Miller AJ, Mihm MC. Melanoma. New Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 6.Hersey P. Adjuvant therapy for high-risk primary and resected metastatic melanoma. Int Med J. 2003;33:33–43. doi: 10.1046/j.1445-5994.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 7.Rigel DS, Russak J, Friedman R. The evolution of melanoma diagnosis: 25 years beyond the abcds. CA: Cancer J Clin. 2010;60:301–316. doi: 10.3322/caac.20074. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Thomas A, Murray T, Thomas A. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 9.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 10.Kitajima K, Koshimizu U, Nakamura T. Expression of a novel type of classic cadherin, PB-cadherin in developing brain and limb buds. Dev Dynam. 1999;215:206–214. doi: 10.1002/(SICI)1097-0177(199907)215:3<206::AID-AJA3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 11.Sugimoto K, Honda S, Yamamoto T, Ueki T, Monden M, Kaji A, Matsumoto K, Nakamura T. Molecular cloning and characterization of a newly identified member of the cadherin family, PB-cadherin. J Biol Chem. 1996;271:11548–11556. doi: 10.1074/jbc.271.19.11548. [DOI] [PubMed] [Google Scholar]
- 12.van Roy F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol. 2009;1:1–27. doi: 10.1101/cshperspect.a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Zhang Y, Tian GG, Zou K, Lee CM, Yu Q, Yuan Z. Short-type PB-cadherin promotes self-renewal of spermatogonial stem cells via multiple signaling pathways. Cell Signal. 2008;20:1052–1060. doi: 10.1016/j.cellsig.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Chen J, Liu Y, Gao W, Ding Y. Over-expression of CDH22 is associated with tumor progression in colorectal cancer. Tumor Biol. 2009;30:130–140. doi: 10.1159/000225242. [DOI] [PubMed] [Google Scholar]
- 15.Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: A clinicopathologic study of 292 cases. J Clin Oncol. 2005;23:1473–1482. doi: 10.1200/JCO.2005.07.168. [DOI] [PubMed] [Google Scholar]
- 16.Sundfeldt K. Cell-cell adhesion in the normal ovary and ovarian tumors of epithelial origin; an exception to the rule. Mol Cell Endocrinol. 2003;202:89–96. doi: 10.1016/s0303-7207(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 17.Kleer CG, van Golen KL, Braun T, Merajver SD. Persistent E-cadherin expression in inflammatory breast cancer. Mod Pathol. 2001;14:458–464. doi: 10.1038/modpathol.3880334. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Chen J, Gao W, Le Y, Ding Y, Li J. Prl-3 promotes epithelial mesenchymal transition by regulating cadherin directly. Cancer Biol Ther. 2009;8:1352–1359. doi: 10.4161/cbt.8.14.8695. [DOI] [PubMed] [Google Scholar]
- 19.Kim SH, Kim M, Kim DS, Lee SC, Chi SW, Lee do H, Park SG, Park BC, Bae KH, KangS Comparative proteomic analysis of mouse melanoma cell line B16, a metastatic descendant B16F10, and Bl6 overexpressing the metastasis-associated tyrosine phosphatase Prl-3. Oncol Res. 2009;17:601–612. doi: 10.3727/096504009789745494. [DOI] [PubMed] [Google Scholar]
- 20.Song R, Li YP, Sheng S, Cao SX, Xu Q. Phosphatase of regenerating liver-3 localizes to cyto-membrane and is required for B16F1 melanoma cell metastasis in vitro and in vivo. PLoS One. 2009;4:e4450. doi: 10.1371/journal.pone.0004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian F, Sheng X, Zhang ZC, Song R, Dong W, Cao SX, Hua ZC, Xu Q. Prl-3 siRNA inhibits the metastasis of B16-Bl6 mouse melanoma cells in vitro and in vivo. Mol Med. 2007;13:151–159. doi: 10.2119/2006-00076.Qian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, Zhang X, Zhao Y, Sha H, Ge X, Zhang M, Gao X, Xu Q. Phosphatase of regenerating liver-3 promotes motility and metastasis of mouse melanoma cells. Am J Pathol. 2004;164:2039–2054. doi: 10.1016/S0002-9440(10)63763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernfors P. Cellular origin and developmental mechanisms during the formation of skin melanocytes. Exp Cell Res. 2010;316:1397–1407. doi: 10.1016/j.yexcr.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 24.Adameyko I, Lallemend F. Glial versus melanocyte cell fate choice: Schwann cell precursors as a cellular origin of melanocytes. Cell Mol Life Sci. 2010;67:3037–3055. doi: 10.1007/s00018-010-0390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]