Abstract
Angiogenesis is one of the crucial events for cancer development and growth and vascular endothelial growth factor (VEGF) family plays an essential role in this biological phenomenon. The members of VEGF family mainly involved in angiogenesis are VEGF-A, VEGF-B and placental growth factor (PlGF), which exert their activity through the binding and activation of two VEGF receptors, VEGFR-1 and VEGFR-2. Human VEGF-A and PlGF are expressed in different isoforms and have the peculiarity to form heterodimer if co-expressed in the same cell. The difference of two main human PlGF isoforms, PlGF1 and PlGF2, consist in the exclusive ability of PlGF2 to bind heparin and Neuropilin receptors. As previously reported for PlGF1 isoform, we have generated a PlGF2 variant named PlGF2 -DE, in which the residues D72 and E73 were substituted with alanine, that is unable to bind and activate VEGFR-1 but is still able to heterodimerize with VEGF. Here we report that overexpression in VEGF-A producing human tumor cell line derived from ovarian carcinoma (A2780) of PlGF2-DE variant by stable transfection, significantly reduces the production of VEGF-A homodimer via heterodimerization, determining a strong inhibition of xenograft tumor growth and associated neoangiogenesis, as well as significant reduction of monocyte-macrophage infiltration. Conversely, the overexpression of PlGF2wt, also reducing the VEGF-A homodimer production comparably to PlGF2-DE variant through the generation of VEGF-A/PlGF2 heterodimer, does not inhibit tumor growth and vessel density compared to control, but induces increase of monocyte-macrophage infiltration. Interestingly the comparison of PlGF2wt with PlGF1wt overexpression evidences a significant reduction of monocyte-macrophages recruitment as unique difference among the activity of the two PlGFwt isoforms. Therefore, the ‘less soluble’ PlGF2 shows a limited potential in monocyte-macrophages recruitment. In conclusion data here reported demonstrate that PlGF-DE variant acts as ‘dominant negative’ of VEGF-A independently from the PlGF isoform utilized, that the expression of active PlGF2 homodimer and VEGF-A/PlGF2 heterodimer is sufficient to rescue pro-angiogenic activity lost for reduction of VEGF-A due to heterodimerization mechanism, and that PlGF2 shows lower activity into recruitment of monocyte-macrophage cells compared to PlGF1 isoform.
Keywords: Angiogenesis, VEGF family, PlGF, VEGF/PlGF heterodimer, ovarian carcinoma, CD31, F4/80
Introduction
Angiogenesis is one of the major pathological changes associated with cancer growth and with a number of complex diseases like, atherosclerosis, arthritis, diabetic retinopathy and age-related macular degeneration [1]. Among the several molecular and cellular players involved in angiogenesis, some members of the vascular endothelial growth factor (VEGF) family and related receptor tyrosine kinases (VEGFR) play a decisive role. The VEGF family members mainly involved in angiogenesis are VEGF-A, VEGF-B and placental growth factor (PlGF), that exert their activity through the binding and activation of the two receptors VEGFR-1 (also known as Flt-1), recognized by all three VEGF members, and VEGFR-2 (also known as Flk-1 in mice and KDR in human), specifically recognized by VEGF-A [2, 3]. In addition to binding to receptor tyrosine kinases, certain VEGF family isoforms also interact with Neuropilin (NP) receptors 1 and 2, which serve as co-receptors of the VEGFR1 and VEGFR2 modulating the vascular functions mediated by VEGF receptors that results critical for tumor growth [4, 5].
PlGF, the second member of the family identified [6], differently from VEGF-A is essentially involved in pathological conditions and is able to stimulate growth, migration and survival of endothelial cells [7-9]. Moreover, thanks to the presence of its specific receptor Flt-1 in a wide spectrum of cells playing an important role in angiogenesis, PlGF/Flt-1 axis has also been implicated in the activation and recruitment of bone-marrow progenitors, inflammatory cells, smooth muscle cells and dendritic cells [10-13].
In human, four isoforms of PlGF (PlGF 1-4) generated by alternative splicing have been identified. PlGF3 is specifically expressed in placenta [14], PlGF4 has been identified again in placenta and in umbilical vein endothelial cells (HUVEC) [15]. The expression of the two main isoforms, PlGF1 and PLGF2, has been demonstrated in placenta and in other several tissues [16]. The main functional difference between PlGF1 and PlGF2 is represented by the ability of isoform 2 to bind heparin and NP receptors respect to isoform 1 that represents the fully soluble PlGF isoform [17]. Interestingly in mouse only the isoform PlGF2 has been identified [18].
All members of VEGF family naturally exist as dimeric glycoproteins in order to interact and induce the dimerization of their specific receptors. PlGF and VEGF-A share a strict biochemical and functional relationship since, besides having VEGFR-1 as common receptor they can form heterodimer if co-expressed in the same cell [19]. In terms of receptor binding, the VEGF-A/PlGF heterodimer may induce VEGFR-1 homodimerization but not VEGFR-2 homodimerization, and like VEGF-A homodimer, may induce VEGFR-1/VEGFR-2 heterodimerization on cells expressing both the receptors, like the endothelial cells [20].
We have recently demonstrated that the ability of PlGF and VEGF-A to generate heterodimer may represent a successful strategy for the inhibition of VEGFs dependent angiogenesis associated to tumor growth. Indeed, the overexpression of a PlGF variant named PlGF1-DE, generated by the mutation of the residues D72 and E73 in alanine that is unable to bind and activate Flt-1 receptor but retains the ability to heterodimerize with VEGF-A [21], determined a strong and significant inhibition of tumor growth and neo-angiogenesis, accompanied by a strong inhibition of recruitment of bone marrow derived cells. Interestingly, the overexpression of PlGF1wt did not alter tumor growth and vessel density but determined a strong recruitment of bone marrow derived cells [20].
Due to the different properties of PlGF1 and PlGF2 isoforms, and to the peculiarity regarding the exclusive expression of PlGF2 isoform in mouse, we here report results on the impact of the overexpression in tumor cells of human PlGF2 isoform both in mutated (PlGF2-DE variant) and in wild type forms, in terms of tumor growth, tumor neovascularization and recruitment of cells crucial for correct angiogenesis process.
Material and methods
Plasmids
The human PlGF2 variant D72→A-E73→A was generated by site direct mutagenesis, using the oligonucleotides previously used for the generation of the same mutant of the human PlGF1 isoform, following the protocol already described [21]. The expression vector pCDNA3 carrying the full-length cDNA for PlGF2wt (pPlGF2wt), or for the variant PlGF2-DE (pPlGF2-DE), were used for the generation of stable cell lines and for transient transfections. pPlGF1wt and pPlGF1-DE were generated as previously described [21].
Cell culture and tumor stable clones generation
HEK 293T cells were grown in Dulbecco Modified Eagle Medium (DMEM), supplemented with 10 % inactivated Fetal Bovine Serum (FBS), 2 mM glutamine and standard concentration of antibiotics. The stable cell line overexpressing the Flt-1 receptor, named 293-Flt-1 [21], was grown in the same medium supplemented with 0.2 mg/ml of Geneticin. Transient transfections were performed in the HEK 293T cell line with pPlGF2wt, pPlGF2-DE, pPlGF1wt, pPlGF1-DE and the empty vector using the calcium phosphate precipitation technique. Cells were exposed to DNA precipitate for 16 hours. The precipitate was removed and fresh medium was added. After 24 hrs the medium was replaced with serum free medium followed by another 24 hrs of incubation, after which the medium was recovered, centrifuged to eliminate cell debris, concentrated and stored at -80°C for further analysis. Human tumor cell line A2780 (ECACC cat. n. 93112519, from ovarian carcinoma) was grown in RPMI 1640 medium containing 10% inactivated FBS, 2 mM glutamine, and standard concentration of antibiotics. To generate tumor stable cell lines, 1×107 A2780 cells were electroporated (Gene Pulser II System, 250 V/cm and 975 μF, Bio-Rad, Hercules, CA) with 50 μg of pPlGF2wt or pPlGF2-DE. Two days later, culture medium was supplemented with 0.8 mg/ml of Geneticin. After 2 weeks, the G418-resistant clones were picked, amplified and screened by ELISA to determine the PlGF2 concentration in the medium. For each transfection, the three clones expressing the highest amount of PlGF2 were mixed to avoid clonal effects.
ELISA assays
To quantify PlGF and VEGF-A dimers in the cell culture medium or tumor extracts, we used the protocols described elsewhere [22-24] with the following modifications. All the reagents used in ELISA were from R&D Systems (Minneapolis, MN). To avoid the interference of the heterodimer in the quantification of PlGF and VEGF-A homodimers, for PlGF determination samples were pre-incubated with anti-VEGF-A antibody coated on ELISA plate at 1 μg/ml, while for VEGF-A determination, samples were pre-incubated with anti-PlGF antibody, coated at 1 μg/ml. To quantify the VEGF-A/PlGF heterodimer, antibody anti-PlGF was coated on ELISA plate, while biotinylated antibody anti-VEGF-A was used in solution. As reference, human recombinant VEGF-A, PlGF1 and VEGF-A/ PlGF1 dimers were used. PlGF, VEGF-A and VEGF-A/PlGF concentrations were determined by interpolation with the relative standard curves, using linear regression analysis.
The procedure to evaluate the binding of PlGF and VEGF-A dimers present in the culture medium of A2780-PlGF2-DE stable clone was performed as recently described [20, 21]. The culture medium was opportunely concentrated and 2.5 to 10 ng/ml of VEGF-A was used. Consequently the quantity of PlGF2-DE/VEGF-A and PlGF2-DE analyzed were between 7,5 and 30 or between 81.25 and 325 ng/ml, respectively (Table 1). Antibody anti-VEGF-A was used to detect the VEGF-A/VEGFR-1 binding, whereas antibody anti-PlGF was used to detect the binding of PlGF2-DE and PlGF2-DE/VEGF dimers. Binding of active PlGF2 dimers produced by A2780-PlGF2wt was confirmed with similar assay (not shown). For all ELISAs, each point was carried out in triplicate and each experiment was repeated twice.
Table 1.
Quantification of human PlGF and VEGF-A dimers secreted by A2780 stable clones or detected in A2780 xenograft tumor extracts
| VEGF-A | PlGF/VEGF-A | PlGF | ||
|---|---|---|---|---|
| Cell lines | A2780-pCDNA3 | 2.0 ±0.2 | ND | ND |
| A2780-PlGF1wt | 0.9 ±0.1* | 1.9 ±0.2 | 24.4 ±0.4 | |
| A2780-PlGF1-DE | 1.0 ±0.1* | 2.0 ±0.1 | 30.3 ±0.6 | |
| A2780-PlGF2-wt | 0.9 ±0.1* | 2.6 ±0.2 | 36.1 ±1.1 | |
| A2780-PlGF2-DE | 0.8 ±0.2 * | 2.4 ±0.3 | 26.0 ±2.1 | |
| Xenograft tumors | A2780-pCDNA3 | 9.2 ±0.7 | ND | ND |
| A2780-PlGF1wt | 5.1 ±0.4# | 9.1 ±0.4 | 29.5 ±1.9 | |
| A2780-PlGF1-DE | 4.9 ±0.6# | 10.6 ±0.9 | 32.3 ±3.1 | |
| A2780-PlGF2-wt | 6.0 ±0.5# | 8.8 ±0.6 | 33.7 ±2.3 | |
| A2780-PlGF2-DE | 5.7 ±0.6# | 9.3 ±0.4 | 29.6 ±2.9 |
The values, expressed as ng/1×106 cells for cell lines and as ng/mg for tumor extracts, represent the average ± SEM of two independent experiments, in which each sample was analyzed in triplicate. ND: not detectable.
p <0.002
p<0.005 vs A2780-pCDNA3.
Cell proliferation assay
The growth rate of generated stable clones was evaluated using the CellTiter Aqueous One Cell Proliferation Assay (Promega, Madison, WI), following the manufacturer's procedure. Cells were seeded at different densities and the growth was evaluated each 24 hours up to 72 hours. Each point was done in triplicate and the experiment was repeated twice. The absorbance at 490 nm was measured on a microplate reader (BenchMark, Bio-Rad, Hercules, CA).
Receptor phosphorylation assays
Concentrated conditioned culture media from transient transfection performed with expression vectors for PlGF2wt, PlGF2-DE, PlGF1wt, PlGF1-DE, and as control with pCDNA3 empty vector, were used for receptor phosphorylation assays. 293-hFlt-1 cells were starved for 16 hours in absence of FBS. To induce Flt-1 receptor activation, cells were exposed to 20 ng/ml of PlGFwt isoforms or 100 ng/ml of PlGF-DE mutants for 10 minutes. To detect phosphorylated form of Flt-1 receptor in western blot experiments, performed following standard procedures, antibodies anti-p-Flt-1 (R&D Systems, Minneapolis, MN) diluted 1:500 was used. Normalization was performed using anti-Flt-1 antibody diluted 1:500 (Sigma-Aldrich) [20].
Xenograft tumors growth
For xenograft tumor experiments, 8-week-old CD1 male nude athymic mice (Charles River, Chatillon-sur-Chalaronne, France) were used (n=8 per group). Exponentially growing A2780 tumor cells stably transfected with PlGF2wt, PlGF2-DE, PlGF1wt, PlGF1-DE and as control with empty pCDNA3 vector, were injected sub-cutaneously (3×106 cells/flank). Tumor growth was followed by biweekly measurements of tumor diameters with a caliper and tumor volume (TV) was calculated according to the formula: TV (mm3)= d2*D/2, where d and D are the shortest and the longest diameters, respectively. For ethical reasons mice were sacrificed when control tumors reached a volume of 1500-2000 mm3. The care and husbandry of mice and xenograft tumor experimental procedures were in accordance with the European Directives no. 86/609, and with Italian D.L. 116. All the experiments were approved by the Institute of Genetics and Biophysics veterinarian.
Tumor protein extracts
Frozen tumor samples were disrupted with a Tissue-Lyser (Qiagen, Milan, Italy) in a lysis buffer composed by 10 mM Tris-HCl pH 8, 150 mM NaCl, 1% Triton-X 100, 0.1% SDS, 0.5% Na-Deossycolate, 0.2% NaN3, and a mixture of protease inhibitors, 300 μl / 100 mg of tissue, for 5 minutes at 3000 rpm. Samples were then incubated under agitation for 1 hour at 4°C and centrifuged at 12,000 × g for 15 minutes. The supernatants were recovered, aliquoted and stored at -80°C. The protein concentration was determined by the Bradford method (BioRad reagent). 200 μg of extract was used in ELISAs for determination of VEGF and PlGF dimers.
Immunohistochemical analyses
Four μm-thick deparaffined tumor sections were incubated ON at 4°C with the following primary antibodies: rat anti-mouse PECAM-1 (anti-CD31; BD Pharmingen, San Jose, CA) 1:1000, rat anti-mouse F4/80 (Serotec, Oxford, UK) 1:50. The staining procedure was continued using specific secondary biotinylated antibody (all from DAKO, Glostrup, Denmark). Slides were counterstained with hematoxylin. Images were recorded with a digital camera Leica DC480 (Milano, Italy). Vessel density was manually measured. Densitometric analysis for F4/80 staining was performed with QwinPro software (Leica).
Statistical analysis
Data are expressed as mean±SEM, with p<0.05 considered statistically significant. Differences among groups were tested by one-way ANOVA; Tukey HD test was used as post hoc test to identify which group differences account for the significant overall ANOVA. All calculations were carried out using SPSS statistical package (vers12.1, Chicago, IL).
Results
Generation and characterization of A2780 stable clones overexpressing PlGF2wt and PlGF2-DE variant
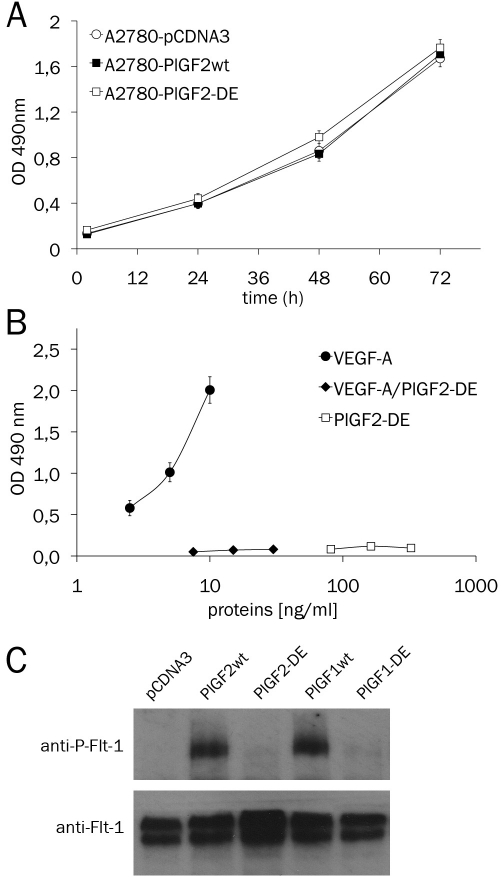
The human PlGF2-DE variant was generated by site direct mutagenesis using the same procedure previously applied for the generation of human PlGF1-DE variant [21]. The cDNA for human PlGF2wt and PlGF2-DE variant cloned in the pCDNA3 expression vector, were used to stably transfect the VEGF-producing PlGF-non-producing human tumor cell line A2780 (ovarian carcinoma). To avoid clonal effects for each cell line the three clones expressing the highest amount of PlGF2, as determined by sandwich ELISA assay, were mixed. The stable cell lines A2780-PlGF2wt and A2780-PlGF2-DE obtained were characterized for the production of secreted VEGF-A and PlGF2 homodimers and VEGF-A/PlGF2 heterodimer. As control, stable cell lines previously established by transfection of PlGF1wt, PlGF1-DE or empty vector, were used [20]. For the new cell lines generated we observed a similar and significant reduction of secreted VEGF-A homodimer (∼50%) to which corresponded the appearance of VEGF-A/PlGF2 heterodimer (Table 1). Importantly, the overexpression of PlGF2-DE or PlGF2wt did not affect the growth of stable cell lines in vitro (Figure 1A).
Figure 1.
In vitro proliferation of A2780 stable clones and binding properties of PlGF2-DE. A. A2780 stable clones were seeded in 96-well plates (2000 cells/ cm2) and cell proliferation was evaluated using the CellTiter Aqueous One Cell Proliferation Assay (Promega) at indicated time. B. Binding of VEGF-A homodimer (2.5 to 10 ng/ml), VEGF-A/PlGF2-DE heterodimer (7.5 to 30 ng/ml) and PlGF2-DE homodimer (81.25 to 325 ng/ml) present in the supernatant of A2780-PlGF2-DE stable clone, to coated Flt-1 (0.5 μg/ml) in ELISA based assay. For both experiments each point was carried out in triplicate and the data are represented as the mean of two experiments ± SEM. (C) Western blot analysis of Flt-1 phosphorylation (anti-P-Flt-1) induced by 20 ng/ ml of PlGFwt isoforms or 100 ng/ml of PlGF-DE isoforms, on starved 293-Flt-1 cells. One hundred μg of the cell protein extracts were analyzed. Normalization was performed with ant-Flt-1 antibody.
Moreover we evaluated the loss of binding property of PlGF2 homodimer and VEGF-A/PlGF2 heterodimer carrying the mutations D72→A-E73→A to their specific receptor. These two dimers produced by A2780-PlGF2-DE cells lost the ability to bind to coated recombinant Flt-1 receptor in ELISA based assay, differently from wild type VEGF-A homodimer produced by the same cells (Figure 1B). Furthermore we investigated if to the inability of PlGF2-DE to interact with Flt-1 receptor corresponded a failure into activation of receptor phosphorylation. PlGF2-DE completely lost the capacity to activate receptor phosphorylation, despite it was used at concentration five times higher than that utilized for wild type isoforms (100 ng/ml vs 20 ng/ml). As expected, PlGF2wt and PlGF1wt were able to induce Flt-1 phosphorylation in a comparable manner (Figure 1C).
Overexpression of PlGF2-DE strongly inhibited xenograft tumor growth and neovascularization
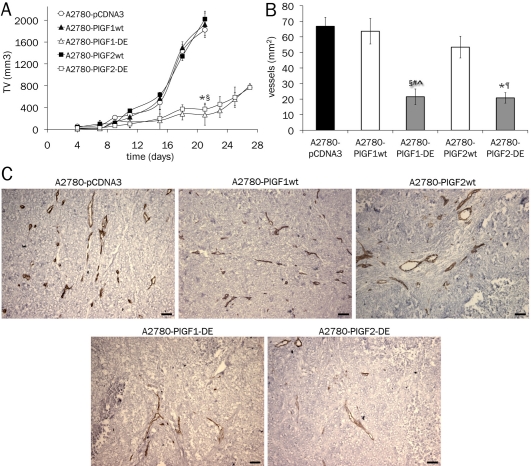
Xenograft tumors generated by subcutaneous injection of A2780-pCDNA3, A2780-PlGF1wt and A2780-PlGF2wt cells, showed a growth rate fully comparable with a mean volume after 21 days of ∼ 1.93 cm3. In contrast, tumors generated by A2780-PlGF2-DE cells, starting from day eleven showed a significant growth delay and at day 21 were significantly smaller than controls with a mean volume of 370 mm3 (p<0.0001) reaching at day 27 a mean volume of only 750 mm3 (Figure 2A). Interestingly, the growth rate of A2780-PlGF2-DE was similar to that of tumor cells overexpressing the PlGF1-DE variant.
Figure 2.
PlGF2-DE variant inhibits tumor growth and neoangiogenesis. A. Exponentially growing A2780 stable clones were subcutaneously injected into 8-week-old CD1 nude athymic mice (3×106 cells, n=8 per groups). Tumor volume (TV) was measured two times a week. The volume of tumors overexpressing the DE variant of both PlGF isoforms was strongly and significantly reduced at day 21 (*,§ p<0.0001 vs A2780-pCDNA3, A2780-PlGF2wt and A2780-PlGF1wt). Data are represented as the mean ± SEM. B. Vessel density was calculated analyzing five optical fields for each tumor, counting CD31 positive vessels. *,§p<0.0001 vs A2780-pCDNA3; ¶p<0.001 vs A2780-PlGF1wt and A2780-PlGF2wt; #p<0.001 vs A2780-PlGF1wt; ^p<0.005 vs A2780-PlGF2wt. Data are represented as the mean ± SEM. C. Representative pictures of CD31 staining of A2780 tumors. Scale bars represent 50 μm.
Tumors were thus characterized for the presence of human PlGF and VEGF-A dimers in tumor protein extracts, using ELISA assays that did not cross-react with endogenous proteins. As reported in Table 1, A2780 cells transfected with PlGF2 were able to produce VEGF-A/PlGF-2 heterodimer in vivo, showing significant reduction in VEGF-A homodimer production if compared to tumor generated with A2780-pCDNA3 cells.
Vessel density was determined by immunostaining with anti-CD31 antibody and a significant reduction was observed in A2780-PlGF2-DE tumors if compared to tumors generated with cells stably transfected with PlGF2wt (p=0.0009), PlGF1wt (p=0.0003) or empty vector (p<0.0001). The observed reduction in terms of vessel density was similar to that obtained in A2780-PlGF1wt tumors. Conversely, tumors overexpressing PlGF2wt showed only a slight decrease of vessel density with respect to tumors overexpressing PlGF1wt or generated by cells stably transfected with empty vector, also if the reduction observed was not significant (Figure 2B, for representative pictures see Figure 2C).
Differences in monocyte-macrophage infiltration in tumors overexpressing PlGF2-DE or PlGF2wt
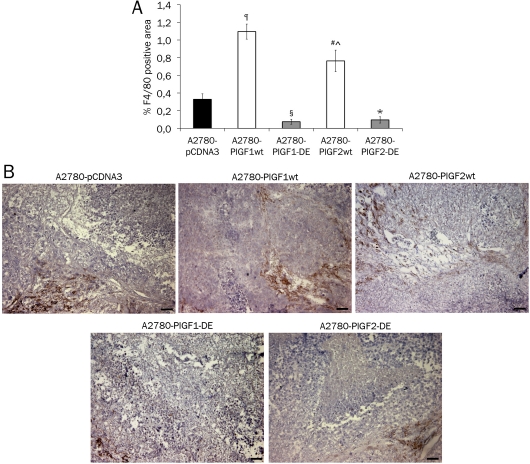
The overexpression of DE variant of the human PlGF isoforms 1 and 2, as well as of the two wild type isoforms, determined similar results in terms of tumor growth and vessel density. In order to assess which differences are generated in tumors overexpressing the wild type and mutated isoforms of PlGF, we evaluated the tumor monocyte-macrophage infiltration, due to well-established role of VEGF-A and PlGF dimers in their recruitment [10, 20, 25]. The immunostaining with anti-F4/80 antibody evidenced as A2780-PlGF2-DE tumors, like the A2780-PlGF1-DE tumors, showed a similar significant reduction of F4/80 positive area, compared to A2780-pCDNA3 tumor infiltrate (p=0.0060 and 0.0025 respectively). As expected tumors overexpressing PlGF1wt showed a strong and significant increase of F4/80 positive area compared to A2780-pCDNA3 tumor (p<0.0001). Interestingly also tumors overexpressing PlGF2wt showed a significant increase of F4/80 positive area compared to A2780-pCDNA3 tumors (p=0.0068) but also a significant decrease if compared to A2780-PlGF1-wt tumors (p=0.0424) (Figure 3A, for representative pictures see Figure 3B), indicating how the overexpression of wild type human PlGF isoforms produced a diverse affect of the recruitment of F4/80 positive cells.
Figure 3.
Monocyte-macrophage infiltration in tumors overexpressing PlGF2-DE or PlGF2wt. The area of monocyte-macrophage infiltration in the xenograft tumors was evaluated by immunostaining with anti-F4/80 antibody analyzing five optical fields for each tumor and data are represented as the mean ± SEM. A. A2780-PlGF2-DE tumors showed a reduced F4/80 positive area compared to A2780-pCDNA3 tumors (*p=0.0060) and similar to that observed in A2780-PlGF1-DE tumors (§p=0.0025 vs A2780-pCDNA3). Conversely, both A2780-PlGF2wt and A2780-PlGF1wt tumors showed an increase of F4/80 positive cells area if compared to A2780-pCDNA3 (#p=0.0068 and ¶p<0.0001 respectively). The F4/80 positive area in A2780-PlGF2wt tumors resulted significantly reduced if compared to A2780-PlGF1wt tumors (^p=0.0424). B. Representative pictures of F4/80 staining of A2780 tumors. Scale bars represent 50 μm.
Discussion
In this report we have confirmed that the property of VEGF-A and PlGF to form heterodimer when coexpressed in the same cell represents a successful strategy to reduce the production by tumor cells of active VEGF-A with consequent potent inhibition of VEGF-A-dependent angiogenesis. This inhibition has been obtained using a mutant of human PlGF2 in which the two residues D72 and E73, essential for Flt-1 recognition and binding, were changed in alanine. PlGF2-DE mutant lost the ability to bind and activate Flt-1 receptor but was still able to heterodimerize with VEGF-A. The overexpression of PlGF2-DE variant in tumor VEGF-A producing cells, besides determining the reduction of active VEGF-A by heterodimerization process, produced two inactive dimers: PlGF2-DE homodimer and PlGF2-DE/VEGF-A heterodimer. As result, the human A2780 tumor cell lines derived from ovarian carcinoma stably transfected with PlGF2-DE, showed severe growth impairment and reduced neoangiogenesis when grafted in vivo, respect to cells transfected with empty vector or with PlGF1wt or PlGF2wt. The observed inhibitions were similar to that obtained with the overexpression of PlGF1-DE.
Furthermore a similar degree of inhibition of F4/80 positive cells recruitment was present in tumors overexpressing PlGF1-DE or PlGF2-DE compared to the control. This effect may be ascribed to the reduced availability of VEGF-A active homodimer [10] also if, almost in part, it must be considered that the strong inhibition of monocyte-macrophage infiltration observed may be due to the reduced neovascularization that di per se represents a limit to monocyte-macrophages infiltration.
Conversely to the mutated PlGF2, the overexpression of PlGF2wt did not inhibit tumor growth compared to the control, indicating how the effects produced by the reduction of VEGF-A homodimer via heterodimerization were completely abolished by the overexpression of active PlGF2wt homodimer and the generation of functional VEGF-A/PlGF2 heterodimer. The data on tumor volume and associated neoangiogenesis obtained grafting cells overexpressing PlGF1wt or PlGF2wt are comparable indicating that the two isoforms of human PlGF have a similar effect on tumor growth and neo-vessels formation, almost in the model and for the time of observation analyzed.
On the contrary, a significant difference on the ability to recruit F4/80 positive cells exists between PlGF1wt and PlGF2wt overexpressing tumors. As expected for the well documented ability mainly of PlGF in the recruitment of F4/80 positive cells [11, 25], both the tumors showed a significant increase of monocyte-macrophages cell recruitment compared to tumors generated with cells stably transfected with control vector, due to the overexpression of active PlGF. Surprisingly A2780-PlGF2wt tumors showed a significant reduced ability in F4/80 positive cells recruitment compared to A2780-PlGF1wt tumors.
This effect may be due to the different solubility properties of PlGF1 and PlGF2 isoforms. Indeed tumor overexpressing PlGF1wt or PlGF2wt produced similar amount of VEGF-A, as well as of VEGF-A/PlGF heterodimer and PlGF homodimer. The last two dimers have a different solubility depending on PlGF isoform utilized, because VEGF-A/PlGF2 heterodimer and PlGF2wt homodimer are less soluble than VEGF-A/PlGF1 heterodimer and PlGF1wt homodimer for the presence of heparin binding domain in the PlGF2 isoform. Consequently less PlGF homodimer and VEGF-A/PlGF heterodimer produced by A2780-PlGF2wt tumors may be available to recruit F4/80 positive cells distant from the neoangiogenic site.
To conclude we have reported that the PlGF2 isoform carrying the double mutation D72 and E73 in alanine is able to inhibit tumor growth and associated neoangiogenesis once co-expressed in VEGF-A producing tumor cells thanks to the ability to heterodimerize with VEGF-A. The inhibition was fully similar to that obtained with PlGF1 isoform indicating that the different properties of PlGF isoforms did not change the effect of PlGF-DE variant. At the same time the results here reported confirm the active role of wild type PlGF2 homodimer and VEGF-A/PlGF2 heterodimer in pathological angiogenesis, for their ability to rescue the decrease of VEGF-A concentration induced by heterodimerization mechanism. Interestingly the different solubility property of functional PlGFwt isoforms seems to play a role only in the recruitment of F4/80 positive cells, assigning to the more soluble PlGF1 isoform an increased ability in this biological function crucial for angiogenesis process.
Acknowledgments
The authors thank Onofrio Capasso, Vincenzo Mercadante, the IGB animal house facility staff and the IGB integrated microscopy facility for technical help and Anna Maria Aliperti for manuscript editing. This work was supported by AIRC (Associazione Italiana Ricerca sul Cancro, grant number 4840) and Telethon - Italy (grant number GGP08062) to S.D.F.
References
- 1.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109:227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2:re1. doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 5.Grunewald FS, Prota AE, Giese A, Ballmer-Hofer K. Structure-function analysis of VEGF receptor activation and the role of coreceptors in angiogenic signaling. Biochim Biophys Acta. 2010;1804:567–580. doi: 10.1016/j.bbapap.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziche M, Maglione D, Ribatti D, Morbidelli L, Lago CT, Battisti M, Paoletti I, Barra A, Tucci M, Parise G, Vincenti V, Granger HJ, Viglietto G, Persico MG. Placenta growth factor-1 is chemotactic, mitogenic, and angiogenic. Lab Invest. 1997;76:517–531. [PubMed] [Google Scholar]
- 8.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, Vandendriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 9.Adini A, Kornaga T, Firoozbakht F, Benjamin LE. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res. 2002;62:2749–2752. [PubMed] [Google Scholar]
- 10.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 11.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in mono-cyte activation and chemotaxis. J Biol Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 12.Sawano A, Iwai S, Sakurai Y, Ito M, Shitara K, Nakahata T, Shibuya M. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood. 2001;97:785–791. doi: 10.1182/blood.v97.3.785. [DOI] [PubMed] [Google Scholar]
- 13.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 14.Cao Y, Ji WR, Qi P, Rosin A. Placenta growth factor: identification and characterization of a novel isoform generated by RNA alternative splicing. Biochem Biophys Res Commun. 1997;235:493–498. doi: 10.1006/bbrc.1997.6813. [DOI] [PubMed] [Google Scholar]
- 15.Yang W, Ahn H, Hinrichs M, Torry RJ, Torry DS. Evidence of a novel isoform of placenta growth factor (PlGF-4) expressed in human trophoblast and endothelial cells. J Reprod Immunol. 2003;60:53–60. doi: 10.1016/s0165-0378(03)00082-2. [DOI] [PubMed] [Google Scholar]
- 16.Maglione D, Guerriero V, Viglietto G, Ferraro MG, Aprelikova O, Alitalo K, Del Vecchio S, Lei KJ, Chou JY, Persico MG. Two alternative mRNAs coding for the angiogenic factor, placenta growth factor (PlGF), are transcribed from a single gene of chromosome 14. Oncogene. 1993;8:925–931. [PubMed] [Google Scholar]
- 17.Migdal M, Huppertz B, Tessler S, Comforti A, Shibuya M, Reich R, Baumann H, Neufeld G. Neuropilin-1 is a placenta growth factor-2 receptor. J Biol Chem. 1998;273:22272–22278. doi: 10.1074/jbc.273.35.22272. [DOI] [PubMed] [Google Scholar]
- 18.DiPalma T, Tucci M, Russo G, Maglione D, Lago CT, Romano A, Saccone S, Della Valle G, De Gregorio L, Dragani TA, Viglietto G, Persico MG. The placenta growth factor gene of the mouse. Mamm Genome. 1996;7:6–12. doi: 10.1007/s003359900003. [DOI] [PubMed] [Google Scholar]
- 19.DiSalvo J, Bayne ML, Conn G, Kwok PW, Trivedi PG, Soderman DD, Palisi TM, Sullivan KA, Thomas KA. Purification and characterization of a naturally occurring vascular endothelial growth factor placenta growth factor heterodimer. J Biol Chem. 1995;270:7717–7723. doi: 10.1074/jbc.270.13.7717. [DOI] [PubMed] [Google Scholar]
- 20.Tarallo V, Vesci L, Capasso O, Esposito MT, Riccioni T, Pastore L, Orlandi A, Pisano C, De Falco S. A placental growth factor variant unable to recognize vascular endothelial growth factor (VEGF) receptor-1 inhibits VEGFdependent tumor angiogenesis via heterodimerization. Cancer Res. 2010;70:1804–1813. doi: 10.1158/0008-5472.CAN-09-2609. [DOI] [PubMed] [Google Scholar]
- 21.Errico M, Riccioni T, Iyer S, Pisano C, Acharya KR, Persico MG, De Falco S. Identification of placenta growth factor determinants for binding and activation of Flt-1 receptor. J Biol Chem. 2004;279:43929–43939. doi: 10.1074/jbc.M401418200. [DOI] [PubMed] [Google Scholar]
- 22.Tarsitano M, De Falco S, Colonna V, McGhee JD, Persico MG. The C. elegans pvf-1 gene encodes a PDGF/VEGF-like factor able to bind mammalian VEGF receptors and to induce angiogenesis. FASEB J. 2006;20:227–233. doi: 10.1096/fj.05-4147com. [DOI] [PubMed] [Google Scholar]
- 23.Ponticelli S, Braca A, De Tommasi N, De Falco S. Competitive ELISA-based screening of plant derivatives for the inhibition of VEGF family members interaction with vascular endothelial growth factor receptor 1. Planta Med. 2008;74:401–406. doi: 10.1055/s-2008-1034346. [DOI] [PubMed] [Google Scholar]
- 24.Ponticelli S, Marasco D, Tarallo V, Albuquerque RJ, Mitola S, Takeda A, Stassen JM, Presta M, Ambati J, Ruvo M, De Falco S. Modulation of angiogenesis by a tetrameric tripeptide that antagonizes vascular endothelial growth factor receptor 1. J Biol Chem. 2008;283:34250–34259. doi: 10.1074/jbc.M806607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pipp F, Heil M, Issbrucker K, Ziegelhoeffer T, Martin S, van den Heuvel J, Weich H, Fernandez B, Golomb G, Carmeliet P, Schaper W, Clauss M. VEGFR-1-selective VEGF homologue PlGF is arteriogenic: evidence for a monocytemediated mechanism. Circ Res. 2003;92:378–385. doi: 10.1161/01.RES.0000057997.77714.72. [DOI] [PubMed] [Google Scholar]