Abstract
The papillomavirus (PV) E2 proteins have been shown to exert many functions in the viral cycle including pivotal roles in transcriptional regulation and in viral DNA replication. Besides these historical roles, which rely on their aptitude to bind to specific DNA sequences, E2 has also been shown to modulate the host cells through direct protein interactions mainly through its amino terminal transactivation domain. We will describe here some of these new functions of E2 and their potential implication in the HPV-induced carcinogenesis. More particularly we will focus on E2-mediated modulation of the host cell cycle and consequences to cell transformation. In all, the HPV E2 proteins exhibit complex functions independent of transcription that can modulate the host cells in concert with the viral vegetative cycle and which could be involved in early carcinogenesis.
Keywords: HPV, E2, transformation, transcription, chromatin, replication, cell cycle, apoptosis, cervical carcinoma
Introduction
E2 is a transcription factor
The function of bovine papillomavirus E2 protein as a transcriptional activator was first demonstrated in 1985 by B. Spalholz in a seminal work [1]. This work assigned E2 as the main activator of the viral transforming proteins E6 and E7, thus playing a critical role in BPV1-induced carcinogenesis in bovine. Later, it was shown that indeed, E2 is a DNA binding protein which can interact specifically with many sequences present in the BPV1 genome and more specifically with the regulatory region of E6/E7 transcription which also contains the origin of replication [2, 3]. Binding of E2 to DNA palindromic sequences was associated with dimerization of the protein through a dimeric interface which was new for transcription factors at that time. The crystal structure of the DNA binding domain of BPV1 E2 provided an interesting example of a new type of structure formed by a compact beta-barrel dimer of beta-sheets containing only two small alpha-helices, one of which being responsible for direct interaction with the major grove of the DNA [4].
HPV E2 contains a conserved transactivation domain in its amino-terminal part [5]. Transactivation by E2 could be detected in artificial systems where E2 binding sites were cloned in enhancer position in front of minimal promoters such as the thymidine kinase (tk) promoter in CAT reporter plasmids [6]. E2 transactivation was shown to be cooperative requiring more than one binding sites to be most efficient and to occur over large distances [6, 7]. These characteristics of E2 transactivation were shown to be shared by BPV1 and high and low-risk HPV E2 proteins [8]. However, while this seemed appropriate for BPV1 E2 which has been shown to be a transactivator of BPV1 oncogenic expression, it was less understandable for the HPV E2 proteins which are not activators but repressors of E6/E7 transcription as detailed below.
E2 is a repressor of E6 and E7 transcription in the HPV context
At the forefront of human papillomaviruses research, one decisive result has been demonstration of the presence of integrated HPV16 genome in the cellular DNA of cervical carcinoma cell lines by Elisabeth Schwarz in zur Hausen's lab in 1985 [9]. This integrated genome actively transcribed mRNAs that encode for the two E6 and E7 proteins thus suggesting their transforming capabilities. Interestingly, the integrated viral genome was truncated, containing the regulatory region and E6 and E7 open reading frames (ORFs), while the downstream E1 and or E2 ORFs were usually disrupted. The regulatory region of the HPV18 genome was then isolated and sequenced and its role as a transcriptional control region for transcription of the E6 and E7 early gene products was first demonstrated in 1987 [10]. Soon after, the same group showed that the BPV1 E2 protein was not an activator for the HPV18 genome but rather a repressor and that the transcriptional repression was due to binding of E2 to two E2 binding sites close to the E6/E7 transcriptional promoter in the HPV18 and HPV16 genomes [11-13]. Around the same time it became evident that integration of the viral genome of high risk HPV16 and 18 usually lead to disruption of the E1 and E2 ORFs thus allowing unregulated transcription of E6 and E7 in cervical carcinoma cells [9, 14, 15]. In the 1990s the genomes of HPV16 and 18 were shown to immortalize primary keratinocytes in vitro [16, 17], results that were rapidly followed by the discovery that the E6 and E7 ORFs were necessary and sufficient for this phenotype, thus establishing them as bona fide oncogenes [18, 19]. Altogether these findings thus established E2 as a transcriptional repressor of the viral oncogenes for the high risk HPVs.
The role of E2 as a viral tumor suppressor was then confirmed by its absence in many cell lines established from cervical cancer and expressing the viral oncogenes [20]. In addition, reintroduction of E2 in cervical carcinoma cell lines was shown to be detrimental to the cell proliferation and to induce an arrest of the cell cycle in G1 through repression of the endogenous E6/E7 expression as well as cellular senescence and apoptosis [21-25]. Interestingly the transcriptional repression by E2 was also shown to occur through binding of E2 to DNA sequences that were the same used for viral DNA replication, thus linking the two phenomenon and implying that during viral DNA replication, probably E6 and E7 transcription were repressed, reviewed in [26].
To date, the viral gene(s) activated during the vegetative cycle by E2 have not been described in HPV models and this issue remains unresolved. There is however some evidence to explain the conservation of a transactivation domain (TAD) in E2 proteins, relying on their interaction with elements of the cellular chromatin that are involved in various functions (See Table 1). Specifically interaction of the E2 TAD with BRD4 has been shown to play a role in segregation of the viral genome between daughter cells during mitosis [27, 28]. This activity however is more multifunctional than previously thought since these interactions of BRD4 with the E2 TAD are also involved in transcriptional activation and repression as well as in E2 stability [29-32] as described below and in Table 1.
Table 1.
Published cellular proteins interacting with papillomavirus E2 proteins
| PV types | Interacting proteins with E2 | References | |
|---|---|---|---|
| HPV proteins | |||
| 1 | BPV1 | E1 | [67, 68, 69] |
| 2 | HPV16 | E1^E4 | [70] |
| 3 | HPV16 | E7 | [71, 72] |
| Transcription Repression/activation | |||
| 1 | BPV1, HPV16 | SMCX, EP400 | [31] |
| 2 | BPV1 | BRD4 | [27, 29, 31, 36, 40] |
| 3 | HPV16 | TopBP1 | [55, 57] |
| 4 | HPV 18 | hSNF5 (SWI/SNF component) | [61] |
| 5 | HPV16 | TAF1 | [53] |
| 6 | BPV1, HPV8 | TBP, TFIID and TFIIB | [46-48, 128] |
| 7 | HPV-11 | topoisomerase I | [54] |
| 8 | HPV 18 | BRCA1 | [51] |
| 9 | HPV 18 | p/CAF | [58] |
| 10 | HPV 18 | CBP | [130] |
| 11 | HPV 18 | PARP (poly(ADP-ribose) polymerase 1) | [52] |
| 12 | HPV8, BPV1 | P300 | [50, 59] |
| 13 | BPV1, HPV 18, HPV8 | Sp1 | [131, 132] |
| 14 | BPV1 | AMF-1/Gps2 | [49, 50] |
| 15 | BPV1, HPV8, HPV 18 | hNAP-1 (human nucleosome assembly protein 1) | [62] |
| 16 | BPV1 | BRM | [60] |
| 17 | HPV16 | Mdm2 | [133] |
| RNA processing | |||
| 1 | HPV 5 | SR proteins (ASF/SF2, SC35, U1-70K, U5-100kD) | [134] |
| Apoptosis | |||
| 1 | HPV16 | c-FLIP | [85] |
| 2 | HPV16 | P53 | [87, 135] |
| 3 | HPV 18 | Caspase 8 | [84] |
| Cell cycle/Mitosis | |||
| 1 | HPV 18 | Cdc20, Cdh1 | [98] |
| 2 | HPV5 | PLK | [136] |
| 3 | BPV1 | ChlR1 | [137] |
| E2 Protein degradation | |||
| 1 | BPV1, HPV16 | Cullin3 | [40] |
| 2 | HPV 18, HPV16, BPV1 | TAXBP1 | [138] |
| 3 | HPV 18 | Skp2 | [118] |
| Miscellaneous functions (Yeast Two-Hybrid targets) | |||
| 1 | HPV16 | TopBP1, RACK1, POMP, p27 (BBP), ODC antizyme, and Delta-adaptin. | [56] |
| 2 | HPV16 | BTBD1, BTBD2, CCHCR1, BRD4, TAF1, NR4A1, TMF1, HOXC9, SF1. | [139] |
The historical background presented in this introduction describes E2 proteins as essentially transcription and replication factors acting through binding to specific sequences of the viral regulatory region. These proteins are thus mainly expressed in the nucleus of infected cells and are only required for the early stages of the viral vegetative cycle. However, results published since the last decade point to a more complex figure suggesting involvement of E2 in many different cellular processes that go beyond regulation of transcription and replication. We will describe here some of these new characteristics of E2 and how they lead to the provocative idea that the viral protein could play a decisive role in carcinogenic progression in cervical cancer.
The E2 interactome
Using current proteomic tools such as yeast two-hybrid screening, GST pull-down, Tandem affinity purification (Taptag) mass spectrometry and immunoprecipitation experiments, novel inter-actors for PV E2 proteins were identified. We will describe here the published data regarding direct interaction of E2 with viral and cellular proteins. These proteins were usually associated with known functions of E2 in transcription, replication, apoptosis and cell cycle as described later (Table 1). Interaction of E2 with other HPV proteins such as E1, E1^E4 and E7 indicates that it may be acting in cooperation with other viral proteins and therefore may share some of their functions. Alternative splicing using part of the E2 ORF or overlapping E4, such as E8^E2C and E1^E4 indicate even more complexity in the E2 functions and its relationship with other viral proteins [33, 34]. Analyses of the E2 interactome may provide insights into novel functions of E2 and its roles in cervical cancer. The development of inhibitory molecules to these interactions could lead to new therapeutic approaches in cervical cancer.
Interaction of E2 with elements of the transcription machinery
More than half of the cellular proteins interacting with E2 are reported to modulate its transcriptional activity and belong to the transcription machinery or to the chromatin remodeling complexes (Table 1). One of the best known of these chromatin elements is Brd4 that was identified as a partner for most, if not all, of the papillomavirus E2 proteins [27, 29, 30, 35, 36]. This binding is mediated through the aminoterminal transactivation domain (TAD) of E2 and the carboxy-terminal domain of Brd4 [27]. Dimerization of E2 protein is necessary for Brd4 binding [37]. Two point mutants of the TAD domain in HPV16 E2, R37A and I73A, that are defective in transcriptional activation, have been reported to disrupt the binding to Brd4 [32]. In all, E2-Brd4 interaction has been shown to be implicated in tethering of viral episomes to cellular chromosomes during mitosis [27], transcriptional activation [38], maintenance of viral genome in active conformation for transcription [39], interference with E2 protein degradation [40, 41] and transcriptional repression [29, 31].
However, roles of HPV E2-Brd4 interaction in transcriptional repression of HPV LCR still remain controversial since two reports using comparable systems gave opposite results. Wu et. al. showed that Brd4 significantly enhanced E2-mediated repression of the LCR in a dose-dependent manner depending on the presence of the E2 amino terminal domain [29]. They hypothesized that the binding of Brd4 to E2 is able to displace transcription activators such as AP1 [29, 42], TBP [43, 44] TFIID and PolII leading to transcriptional repression. Originally, in similar experiments, Schweiger et al could not demonstrate any influence of BRD4 binding to E2 repression [30]. However, more recently the same group confirmed the role of E2-Brd4 interaction on E2-dependent repression of the HPV LCR using genome-wide siRNA screen. They found that the siRNA-mediated knock down of Brd4 resulted in partial alleviation of repression of the HPV18 LCR [31]. Taken together, these studies suggest that indeed, E2-mediated transcriptional repression is Brd4-dependent, as supported by a recently published work showing that E2-dependent abrogation of BRD4 interaction with the positive transcription elongation factor TEFb contributes to E2-dependent repression of the viral oncogenes [45].
Interactions of E2 with other components of the transcriptional machinery or of the chromatin have been less well characterized although they are usually participating to its transcriptional activation (Table 1). Among the published interaction of the E2 protein are components of the basal transcriptional machinery such as TFIIB and TFIID [46-48], coactivators such as AMF-1 [49, 50], BRCA1 [51, 129], PARP1 [52], TAF1 [53] topoisomerase 1 [54], TopBP1 [55-57], and chromatin remodeling components p/CAF [58], p300/CBP [59], BRM [60], hSNF5 [61] and NAP-1 [62]. It has been proposed that E2 represses the HPV LCR by steric hindrance of the recruitment of TFIID and Sp1 [43, 44, 63, 64] as well as of coactivators such as SMCX , BRD4 and EP400 [31].
Interaction of E2 with PV proteins
HPV E2 is among the first early proteins expressed from the HPV genome involved in viral transcription and replication [65, 66]. E1 and E2 form a complex with the viral origin of replication and recruit cellular DNA replication machinery such as DNA polymerases, replication protein A (RPA), replication protein C (RPC), topoisomerase I/II and proliferating-cell nuclear antigen (PCNA) to facilitate viral DNA replication [65, 67, 68]. Binding of BPV1 TAD domain of E2 to E1 is required for viral DNA replication. Papillomavirus E2 thus interacts with E1 through the third alpha-helix of its TAD domain [68, 69] contrary to its interaction with Brd4 which occurs across the three alpha-helices [36] as revealed by co-crystal structures, thus suggesting that interaction with the two proteins can concurrently occur.
Interaction of E2 with other viral proteins include E1^E4 [70] and E7 [71]. It is reported that the N-terminal domain of E7 interacts with the C-terminal domain of E2 in HPV16 [71, 72] and form different sizes of complexes/oligomers which could sequester E2, leading to uncontrolled E7 expression. E2 is reported to be the first HPV protein interacting directly with E1^E4 in vitro and in vivo and this leads to the increased stability of E2 protein. Interaction of the N-terminus of E2 to E1^E4 is able to relocalize E2 into the cytoplasm and stabilize it in the cytoplasmic fraction [70]. The physiological functions implicated by these interactions still remain unknown.
New data on expression and localization of HPV E2 in vivo
What are the physiological levels of expression of the HPV16 E2 protein, and where is the protein expressed in HPV lesions?
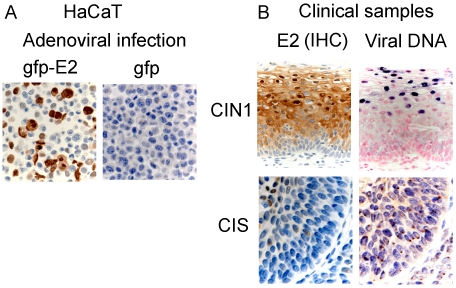
A crucial path in E2 research retrospectively appeared to have been the use of recombinant adenoviruses to express the viral proteins for the past 10 years. These expression vectors are extremely versatile and could be used to express proteins in many different cell lines and at various levels of expression more sustained in time than transfection. This proved to be a powerful tool to study this viral protein in vitro, close to the physiological conditions. It should be recalled at this stage that there is only one cell line containing the episomal viral DNA and expressing E2 together with E6 and E7, established from a low grade HPV16-associated lesion [73]. All other cell lines, either established in vitro with the viral genome, or grown from cervical cancers, do not express E2. In addition, no detection of the HPV E2 protein has ever been reported in the in vitro keratinocytes raft cultures expressing HPV16 or HPV18. Consequently and for almost two decades, it was difficult to guess what physiological levels of E2 could be and how the viral protein was expressed in the HPV context. Detection of HPV16 E2 by immunohistochemistry in clinical samples was first reported by Maitland's group in 1998 and 2000 to be mostly in the intermediate layers of low grade HPV16-associated CIN lesions where the viral DNA replicates [74, 75]. Then, an extensive study of E2 expression with a newly produced rabbit polyclonal antiserum raised against the carboxy-terminal domain of the HPV16 E2 protein, in several low and high grades lesions associated with HPV16, was published [76]. According to this work, E2 happens to be expressed at relatively high levels especially in differentiated cells of the intermediate layers of CIN lesions, while its expression is decreased with progression of the lesions to be totally absent in most of the cancers in situ (CIS) (Figure 1). In addition, and in accordance with its role as repressor of the viral oncogenes, its expression is usually inversely correlated with expression of E7, as detected by its surrogate marker p16INK4 [76].
Figure 1.
Detection of HPV16 E2 expression by immunohistochemistry. Expression of E2 is detected as brown staining by IHC in three different samples: A. HaCaT cells infected by adenovirus at multiplicity of infection (m.o.i.) 200, expressing the GFP-HPV16 E2 protein compared to HaCaT cells infected by the control adenovirus expressing GFP only. B. Low grade CIN1 HPV16 lesion and high grade HPV16-associated cancer in situ, as indicated. HPV DNA is detected by in situ hybridization as a blue uniform nuclear staining of adjacent serial sections indicating episomal replicating viral DNA in the low grade CIN1 lesion concomitant with E2 expression. In contrast, in CIS, very low E2 expression is correlated with integrated HPV genome identified as brown speckles in the nuclei.
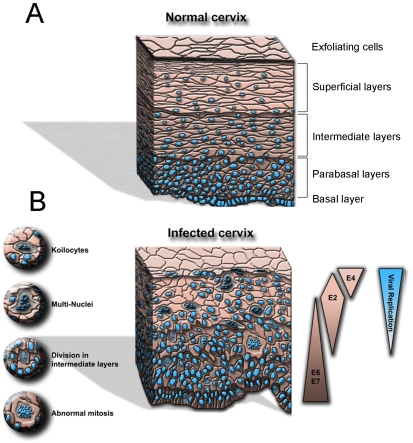
One surprising result here was that E2 appeared highly expressed in differentiated cells expressing keratin 13 for instance, but not in the proliferative cells expressing p63 or ki67. In addition, expression of E2 was both nuclear and cytoplasmic as expected from previous data obtained in tissue cultures in vitro [77]. These results indicated that E2 could be expressed at relatively high levels in differentiated cells in low and intermediate grade lesions and provided a definition of physiological levels of E2 expression for in vitro experiments as shown in Figure 1 where expression of E2 is compared in adeno-virus infected HaCaT cell and in CIN1. A summary of HPV proteins expression in HPV infected epithelium is given in Figure 2 showing expression of E2 increased together with differentiation.
Figure 2.
Schematic representation of the comparative structures of normal vs infected cervical epithelium. A. Representation of a normal epithelium with the nuclei in blue and the different layers of differentiation indicated on the right. B. Representation of an HPV infected epithelium or low grade HPV infected lesion with the different types of cells described on the left and expression of the viral proteins and viral DNA replication along with the tissue differentiation, depicted on the right.
E2 is an unstable protein expressed in both the nuclei and cytoplasm of infected cells
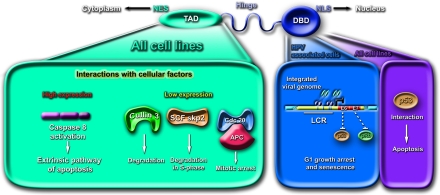
One of the first example of degradation of proteins by the proteasome through their interaction with a specific ubiquin ligase was provided by the discovery of the E6-dependent degradation of p53 through the E6AP ubiquitin ligase in cervical cancer by Martin Scheffner in 1990 [78]. Since then, degradation of regulatory proteins and more specifically of transcription factors has been documented in many systems leading to the idea that proteasomal degradation is one of the major ways of regulating gene expression in the cell. It was therefore not totally unexpected to discover that E2 proteins from the bovine papillomavirus [79], and from HPV [77, 80] are highly unstable and are degraded through the proteasome. The experiments aiming at understanding the regulation of E2 stability led to an unexpected finding that the E2 proteins from high-risk HPV16 and HPV18 are both nuclear and cytoplasmic and that, when cytoplasmic, they are expressed as heterogenous speckles. Interestingly no apparent cytoplasmic expression of the E2 proteins from low-risk HPV6 and HPV11 was demonstrated in comparable experimental conditions [77]. These differences in behavior could be linked to the number of NLS sequences present in the E2 proteins and to an NES sequence lying in the TAD amino terminal domain of E2, overlapping almost exactly with the second alpha-helix of the TAD domain (Figure 3). We also could link this same sequence with stability of the protein and could show that it was required for efficient E2 degradation although, since the E2 protein deleted of this sequence is mainly nuclear, it is possible that E2 stability is also dependent on its subcellular localization.
Figure 3.
Schematic representation of the structure and functions of E2 involved in cell cycle and apoptosis. The three domains of E2 are represented as DNA Binding Domain (DBD) at the carboxy-terminus, the flexible hinge in the middle and the Transactivation Domain (TAD) at the amino-terminal part of the protein. The DBD contains an NLS and is therefore localized in the nuclei while the TAD contains an NES and is localized in the cytoplasm. DNA binding of E2 to the promoter sequences is required fortranscriptional repression of E6 and E7 while interactions of cellular factors occur mainly with the TAD alone and are responsible for cell cycle modulation and apoptosis except for p53 interacting with the DBD.
E2 and apoptosis
Induction of apoptosis by E2 in vitro
Induction of apoptosis is another property of E2, together with E6/E7 repression, which could contribute to its classification as a viral anti-oncogene. Although the mechanism is controversial, the apoptotic induction is well documented in cells over expressing E2. The first suggestion of a direct induction of apoptosis by E2, independent of E6 and E7, was obtained in 1997 by two independent articles. Frattini et al. demonstrated cell death in human foreskin keratinocytes infected by an adenovirus expressing HPV31 E2 [81] while Desaintes et al. showed induction of apoptosis in HeLa cells with the full-length E2 protein but not with a protein deleted of its transactivation domain [25]. As both proteins can repress E6/E7 transcription, this indicated that apoptosis does not occur trough repression of the viral oncogenes [25]. This was confirmed by induction of apoptosis in HPV negative cells in 2000 by Webster et al. using HPV16 E2 [82]. They could demonstrate that cell death necessitates functional E2 amino-terminal domain confirming previous results. The involvement of the E2 amino-terminal domain in apoptosis was definitely established in 2003 by Demeret et al. who showed that expression of the amino-terminal domain alone was sufficient to induce apoptosis and furthermore that this occurred independently of transcriptional activity [83]. Interestingly, this E2-induced apoptosis involves activation of caspase 8 through the extrinsic pathway, although the mechanism remained unclear at that time [77, 83]. In 2008, Thierry et al. could show a direct interaction of caspase 8 with HPV18 E2, leading to further activation of the caspase, independently of the adaptor proteins involved in the classical extrinsic pathway of apoptosis (FAS-dependent pathway) [84]. Involvement of caspase 8 in E2-induced apoptosis was also demonstrated for HPV16 where E2 induces apoptosis through direct interaction with c-FLIP, abrogating its inhibitory function [85]. Since c-FLIP and caspase 8 are known to interact with each other, see [86] for review, it is possible that the high risk HPV E2 proteins form a tripartite complex involving c-FLIP and caspase 8, where c-FLIP would be inhibited and caspase 8 activated, leading to induction of apoptosis in vitro. This apoptosis seems specific of E2 from high-risk HPV genotypes since the low-risk HPV6 and HPV11 E2 proteins do not induce cell death in comparable experiments [77]. This specificity was further confirmed by Parish et al., who found induction of apoptosis in transiently transfected HeLa cells by high risk E2 proteins only [87].
Role of p53 in E2-induced apoptosis
As described above, E2 has been shown to induce apoptosis through activation of the extrinsic pathway and caspase 8, thus not involving p53 which mostly acts through the intrinsic pathway and activation of caspase 9. However, the role of p53 in E2-induced apoptosis is controversial. In 1999, Desaintes et al. published that E2-induced apoptosis is independent of p53 in transfected HeLa cells and that the stabilization of p53, due to E2 repression of E6 transcription, induces a cell cycle growth arrest in G1 but not apoptosis [88]. Later on, the same group found that, indeed E2 induces apoptosis in cells that do not express p53 and that this induction is only dependent on the transactivation domain of E2 [83]. Moreover the same group hypothesized that the specific induction of apoptosis by high-risk E2 proteins was not due to intrinsic effectors' properties, but rather correlates with their subcellular localization in the cytoplasm [77]. In contrast, Gaston's group claimed that the ability of high-risk HPV E2 proteins to induce apoptosis is due to their interaction with p53 through their carboxy-terminal domain. To sustain this hypothesis, they could show that high-risk E2 mutants, as well as low-risk HPV-E2 proteins, which were not able to bind p53, were both unable to induce cell death [87]. However, if E2-induced apoptosis were only p53-dependent, the amino-terminal domain of E2 alone should not be able to induce apoptosis while the carboxy-terminal domain could do it, which is in strong contradiction with other published data [83]. One way to reconcile these data is to hypothesize that there could be 2 independent mechanisms of E2-induced cell death. One p53-dependent would necessitate the binding of p53 to the C-terminal part of E2, and would occur in p53-positive cells only, while the other one (p53-independent but caspase-8 dependent) would be mediated by the E2 amino-terminal domain in both p53-positive and negative cell lines (Figure 3). In summary, E2 seems to be able to induce apoptosis in many cell lines (HPV-positive or negative), through p53-dependent and independent mechanisms including direct interaction with members of the extrinsic pathway of apoptosis leading to caspase 8 activation (Figure 3). As for the physiological relevance of this apoptosis in vivo, one can only speculate that E2 would help releasing the newly formed viral particles by killing productive cells.
An alternative role for caspase 8 activation
There is however an alternative hypothesis for E2-induced caspase 8 activation in vivo coming from recently published data that describe a crucial role of this caspase, independent of apoptosis, in skin differentiation. It is therefore possible that activation of caspase 8 by E2 could play a role in keratinocyte differentiation rather than in cell death in vivo. The prominent role of caspase 8 in skin homeostasis was recently described in mouse models where caspase 8 deficiency gave rise to a chronic inflammatory disease associated with hyper proliferative state of the epidermis [89]. According to these authors, the main driver of this phenotype is paracrine signaling through induction of IL-1α. However, another work describes the same phenotype in caspase 8 deficient epidermis but with a different underlying mechanism. These authors present evidence that the IFN regulatory pathway is constitutively activated in caspase 8-deficient epidermis, thus leading to increased inflammation as a result of constitutive activation of the response to foreign nucleic acids [90]. Interestingly however, in both cases, caspase 8 disruption induces thickening of the skin with no modulation of the terminal differentiation as observed in HPV benign lesions. In an independent set of data, caspase 8 has been shown to be involved in tumor cell motility and metastasis ([91] and references therein). Together with the fact that E2 is expressed in the intermediate differentiated layers of the HPV infected lesions, we can speculate that modulation of caspase 8 by E2 in vivo might play a role in the formation of wart, although with unknown underlying mechanisms.
E2 interaction with cell cycle checkpoints
E2, G1/S transition and re-replication
The best characterized effect of E2 on the cell cycle has been the G1 growth arrest induced by its reintroduction in HPV-positive cells [21, 23-25]. This was due to repression of endogenous E6/E7 transcription and reactivation of the p53 and pRB pathways through binding of E2 to the E6/E7 promoter sequences and was therefore not an intrinsic function of the E2 proteins [13, 21, 23-25, 92, 93].
In contrast, a direct role of E2 in regulating the cell cycle, independently of E6 and E7, was first described by Frattini et al., who showed that HPV31 E2 could enhance cellular DNA replication, which the authors attributed to abrogation of a mitotic checkpoint [81]. At that time, very little was known about the regulation of the mitotic checkpoint (today known as the SAC, Spindle Assembly Checkpoint), and these results could therefore be reinterpreted in light of more recent data. The authors showed a massive S phase-block induced by E2, followed by cellular DNA re-replication characterized by appearance of an aneuploid population of cells with >4N DNA content. They correlated this with a decreased p53 level which was detected in E2-expressing cells compared to controls. Since at that time p53 was thought to be involved in the mitotic checkpoint induced in response to microtubules drugs like nocodazole [94, 95], the authors concluded from their data that E2 abrogated a p53-dependent mitotic checkpoint, thus allowing re-replication. However, we know now that p53 is not involved in the SAC, but in the post-mitotic checkpoint preventing replication of 4N G1 cells. Therefore these authors probably detected abrogation of the post-mitotic checkpoint after E2 expression due to decrease of p53. However, even though loss of p53 is known to lead to re-replication, this is not sufficient since re-replication occurs in p53-negative cells only if they have previously been treated by microtubules inhibitors to activate the SAC [96]. This means that in these experiments E2 should somehow have been able to mimic the effect of prior microtubules drugs treatment. This conclusion implies: first, that E2 is able to activate the SAC, and second that E2 can induce polyploidy.
E2 is a modulator of mitosis
If E2 can activate the SAC, it implies that it should be able to block the cell cycle in G2/M. Although Frattini et al. mentioned a G2/M arrest induced by HPV31 E2 prior re-replication [81], they did not elaborate on this data. Two years later in 1999, Fournier et al. described a delay in the onset of anaphase as well as a block in G2/M, correlated with a delay in activation of the cdc2 kinase (homolog of the human Cdk1), in the yeast S. pombe expressing HPV16 E2 [97]. These authors did not find any re-replication associated with this arrest, probably due to regulatory differences between yeast and mammals. More recently, Bellanger et al. described a massive G2/M arrest induced by both HPV16 and HPV18 E2 proteins in HPV-negative cells [98]. Analyzing this phenotype, they could show that these cells were delayed in prophase and arrested in metaphase characterized by elevated levels of cyclin B/Cdk1 activity and extensive histone H3 phosphorylation. A small percentage of cells would either encounter “mitotic slippage” giving rise to only one daughter cell, or complete mitosis with abnormal cytokinesis giving rise to more than 2 sister cells.
The mitotic arrest is dependent on interaction of E2 with Cdc20 and/or Cdh1, 2 subunits of the Anaphase Promoting Complex/Cyclosome (APC/ C), the ubiquitin-ligase responsible for metaphase-to-anaphase transition. Since this arrest is associated with stabilization of cyclin B, a well-known APC/C substrate, the authors raised the hypothesis that E2 could inhibit the APC/C through interaction with Cdc20 and/or Cdh1, leading to stabilization of its substrates, meta-phase arrest and mitotic abnormalities. At that time the SAC was not well characterized while it is now established that inhibition of APC/C in metaphase is achieved through activation of the SAC (which leads to inactivation of Cdc20 through interaction with BubR1 and Mad2), so that the cells do not undergo anaphase before complete attachment of the kinetochores to the metaphasic plate [99-102]. In light of these more recent concepts, and combining Bellanger et al. and Frattini et al. results, we now can raise the hypothesis that E2 could inactivate APC/C through activation of the SAC, leading to metaphasic arrest and “mitotic slippage” followed by re-replication. This hypothesis would create an exciting link between the recent literature and the E2-mediated mitotic phenotypes.
Potential role of abnormal mitoses induced by E2 in HPV-associated carcinogenesis
Over the past few years, mitosis has emerged has a potential crucial event in the initiation of carcinogenesis. Indeed, abnormal mitoses, especially abnormal chromosome segregation after anaphase, leads to aneuploidy or DNA breaks as demonstrated in many in vivo and in vitro models. This seems to be mainly due to malfunction of the SAC, either through inhibition or over-activation. For example over-expression of Mad2, a main component of the SAC, induces hyper-activation of the SAC, leading to mitotic slippage followed by accumulation of abnormal mitoses, thereby promoting tumorigenesis in mice [103]. Moreover, members of the SAC, such as BubR1, are overexpressed in many cancers [104-108], including HPV-associated cancers [109], pointing out to a critical role of mis-regulation of this checkpoint in carcinogenesis.
Another interesting result was that only E2 proteins from high-risk HPVs could induce abnormal mitotic phenotypes in contrast to the E2 proteins from the low-risk HPV11 and 6 which were inactive [98]. This could obviously be correlated with a potential role of high-risk E2 proteins in HPV-induced carcinogenesis. This idea was recently sustained by in vivo data showing that the high-risk skin type HPV8 E2 protein alone, in the absence of other viral proteins, exhibits transforming abilities since it is sufficient to induce skin tumors in mice [110], whereas the low-risk HPV11 E2 does not [111]. E2 has been shown to associate with mitotic cellular chromatin, presumably to help partitioning the viral genomes between the sister cells in mitosis [27, 28, 112-116]. However, it has been postulated that this association of E2 to the cellular chromatin could also interfere with kinetochores functions (which are known to be the primarily place of SAC activation) and lead to mitotic abnormalities, since HPV8 E2 transgenic mice exhibit mitotic abnormalities and nuclear atypia [117]. High risk E2-induced polyploidy through generation of abnormal mitoses could therefore be an important step in HPV-induced carcinogenesis, a provocative hypothesis that is yet to be demonstrated.
E2 induces over expression ofSkp2, a main regulator of the G0-G1/S transition
The stabilization of APC/C substrates by E2 impacts on the global quantity of these proteins in E2-expressing cells. Consequently stabilization of cyclin B [98], Aurora kinases (unpublished data), and Skp2 [118], which is a component of the SCFSkp2 ubiquitin-ligase involved in the G0-G1/S transition, were described in E2-expressing cells. Skp2 is an APC/C substrate in early and mid-G1 which is stabilized in late G1 following inactivation of APC/C [119, 120]. This stabilization of Skp2 activates the SCFSkp2, the ubiquitin-ligase which targets p21 and p27 for degradation, and enhances the G0-G1/S transition [121, 122]. Many cancers have been shown to express high levels of Skp2, which is now considered as an oncogene as its overexpression accelerates proliferation through activation of the G1/S transition [123, 124]. As an APC/C substrate, Skp2 was found to be stabilized by E2 [118]. Moreover, we could show that E2 itself is a substrate of the SCFSkp2 ubiquitin-ligase and is consequently degraded in late G1 and early S phase of the cell cycle [118]. Unlike degradation of E2 by Cullin 3, which can be inhibited by the chromatin component Brd4 [40, 41, 125], the Skp2-dependent degradation of E2 is not sensitive to interaction with Brd4. Altogether, these results imply that stabilization of Skp2 by E2 could accelerate the G1/S transition, but also, through a feedback mechanism, could lead to E2 degradation in S phase, allowing deregulated E6 and E7 expression. Although this hypothesis has not formally been demonstrated, these data altogether point out to an involvement of E2 in early stages of carcinogenesis progression.
Conclusion
The high-risk HPV-E2 proteins are replication and transcription factors negatively regulating E6/E7 transcription while activating viral replication through binding to the same sites in the viral regulatory region reviewed in [26, 66, 126]. More recently, the E2 proteins of high risk HPVs have been shown to exhibit additional intrinsic properties that could participate in cell transformation. These new oncogenic properties rely on their ability to induce abnormal mitoses, leading to either loss or excess of DNA, together with DNA breaks during anaphase. They could also be due to stabilization of oncogenes like Skp2, leading to acceleration of the G1/S transition. However, expression of E2 alone may not be sufficient for transformation since E2 expressing mice develop tumors more efficiently after irradiation, meaning that mutations of other pathway(s) are necessary [110]. Moreover E2 is absent from most human cervical carcinomas, meaning that its presence is not required for maintenance of carcinogenesis. However, it should be noted that E2 has been shown to be able to create chromosomal instability by inducing, together with E1, DNA replication of the integrated viral genome and formation of unstable regions of the chromosome containing onion skin structures [127]. E2 has been shown to bind to the viral episomes but, as described above, it also binds to the cellular chromatin [126]. One intriguing possibility is that binding of E2 to both viral and cellular DNA during the HPV vegetative cycle, could bring them close to each other and, through DNA breaks induced by E2 in mitosis, could then facilitate integration of the viral DNA into the cellular genome. As demonstrated before, integration then establishes conditions of cell transformation by enhancement of E6/E7 expression. This hypothesis also provides an explanation of why the low-risk HPVs DNA never integrates into the cellular chromatin. Indeed, low risk E2 proteins do not induce chromosomal instability and DNA breaks although they are able to bind both viral and cellular DNA. In summary, for high-risk HPVs, the E2 protein could be the actor of an hit-and-run mechanism, where its primarily expression would allow chromosomal instability followed by integration of the viral genome which has been documented as one of the major step leading to HPV-induced transformation.
References
- 1.Spalholz BA, Yang YC, Howley PM. Transactivation of a bovine papillomavirus transcriptional regulatory element by the E2 gene product. Cell. 1985;42:183–191. doi: 10.1016/s0092-8674(85)80114-8. [DOI] [PubMed] [Google Scholar]
- 2.McBride AA, Byrne JC, Howley PM. E2 polypeptides encoded by bovine papillomavirus type 1 form dimers through the common carboxyl-terminal domatransactivation is mediated by the conserved aminoterminal domain. Proc Natl Acad Sci USA. 1989;86:510–514. doi: 10.1073/pnas.86.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dostatni N, Thierry F, Yaniv M. A dimer of BPV-1 E2 containing a protease resistant core interacts with its DNA target. EMBO J. 1988;7:3807–3816. doi: 10.1002/j.1460-2075.1988.tb03265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegde RS, Grossman SR, Laimins LA, Sigler PB. Crystal structure at 1.7 A of the bovine papillomavirus-1 E2 DNA-binding domain bound to its DNA target. Nature. 1992;359:505–512. doi: 10.1038/359505a0. [DOI] [PubMed] [Google Scholar]
- 5.Giri I, Yaniv M. Structural and mutational analysis of E2 trans-activating proteins of papillomaviruses reveals three distinct functional domains. EMBO J. 1988;7:2823–2829. doi: 10.1002/j.1460-2075.1988.tb03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thierry F, Dostatni N, Arnos F, Yaniv M. Cooperative activation of transcription by bovine papillomavirus type 1 E2 can occur over a large distance. Mol Cell Biol. 1990;10:4431–4437. doi: 10.1128/mcb.10.8.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez-Ramon EE, Burns JE, Zhang W, Walker HF, Allen S, Antson AA, Maitland NJ. Dimerization of the human papillomavirus type 16 E2 N terminus results in DNA looping within the upstream regulatory region. J Virol. 2008;82:4853–4861. doi: 10.1128/JVI.02388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang CM, Dong G, Broker TR, Chow LT. Control of human papillomavirus type 11 origin of replication by the E2 family of transcription regulatory proteins. J. Virol. 1992;66:5224–5231. doi: 10.1128/jvi.66.9.5224-5231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314:111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- 10.Thierry F, Heard JM, Dartmann K, Yaniv M. Characterization of a transcriptional promoter of human papillomavirus 18 and modulation of its expression by simian virus 40 and adenovirus early antigens. J. Virol. 1987;61:134–142. doi: 10.1128/jvi.61.1.134-142.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romanczuk H, Thierry F, Howley PM. Mutational analysis of cis elements involved in E2 modulation of human papillomavirus type 16 P97 and type 18 P105 promoters. J. Virol. 1990;64:2849–2859. doi: 10.1128/jvi.64.6.2849-2859.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thierry F, Howley PM. Functional analysis of E2-mediated repression of the HPV18 P105 promoter. New Biol. 1991;3:90–100. [PubMed] [Google Scholar]
- 13.Bernard BA, Bailly C, Lenoir MC, Darmon M, Thierry F, Yaniv M. The human papillomavirus type 18 (HPV18) E2 gene product is a repressor of the HPV18 regulatory region in human keratinocytes. J. Virol. 1989;63:4317–4324. doi: 10.1128/jvi.63.10.4317-4324.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seedorf K, Krammer G, Dürst M, Suhai S, Rowenkamp WG. Human papillomavirus type 16 DNA sequence. Virology. 1985;145:181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- 15.Smotkin D, Wettstein FO. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur P, McDougall JK. Characterization of primary human keratinocytes transformed by human papillomavirus type 18. J Virol. 1988;62:1917–1924. doi: 10.1128/jvi.62.6.1917-1924.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCance DJ, Kopan R, Fuchs E, Laimins LA. Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:7169–7173. doi: 10.1073/pnas.85.19.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlegel R, Phelps WC, Zhang YL, Barbosa M. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J. 1988;7:3181–3187. doi: 10.1002/j.1460-2075.1988.tb03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinokurova S, Wentzensen N, Kraus I, Klaes R, Driesch C, Melsheimer P, Kisseljov F, Durst M, Schneider A, von Knebel Doeberitz M. Typedependent integration frequency of human papillomavirus genomes in cervical lesions. Cancer Res. 2008;68:307–313. doi: 10.1158/0008-5472.CAN-07-2754. [DOI] [PubMed] [Google Scholar]
- 21.Dowhanick JJ, McBride AA, Howley PM. Suppression of cellular proliferation by the papillomavirus E2 protein. J. Virol. 1995;69:7791–7799. doi: 10.1128/jvi.69.12.7791-7799.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodwin EC, Yang E, Lee CJ, Lee HW, DiMaio D, Hwang ES. Rapid induction of senescence in human cervical carcinoma cells. Proc Natl Acad Sci U S A. 2000;97:10978–10983. doi: 10.1073/pnas.97.20.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodwin EC, DiMaio D. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc Natl Acad Sci U S A. 2000;97:12513–12518. doi: 10.1073/pnas.97.23.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodwin EC, Naeger LK, Breiding DE, Androphy EJ, DiMaio D. Transactivation-competent bovine papillomavirus E2 protein is specifically required for efficient repression of human papillomavirus oncogene expression and for acute growth inhibition of cervical carcinoma cell lines. J. Virol. 1998;72:3925–3934. doi: 10.1128/jvi.72.5.3925-3934.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desaintes C, Demeret C, Goyat S, Yaniv M, Thierry F. Expression of the papillomavirus E2 protein in HeLa cells leads to apoptosis. Embo J. 1997;16:504–514. doi: 10.1093/emboj/16.3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desaintes C, Demeret C. Control of papillomavirus DNA replication and transcription. Seminars in Cancer biology. 1996;7:339–347. doi: 10.1006/scbi.1996.0043. [DOI] [PubMed] [Google Scholar]
- 27.You J, Croyle JL, Nishimura A, Ozato K, Howley PM. Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes. Cell. 2004;117:349–360. doi: 10.1016/s0092-8674(04)00402-7. [DOI] [PubMed] [Google Scholar]
- 28.Sekhar V, Reed SC, McBride AA. Interaction of the betapapillomavirus E2 tethering protein with mitotic chromosomes. J Virol. 2010;84:543–557. doi: 10.1128/JVI.01908-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu SY, Lee AY, Hou SY, Kemper JK, Erdjument-Bromage H, Tempst P, Chiang CM. Brd4 links chromatin targeting to HPV transcriptional silencing. Genes Dev. 2006;20:2383–2396. doi: 10.1101/gad.1448206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schweiger MR, Ottinger M, You J, Howley PM. Brd4-independent transcriptional repression function of the papillomavirus e2 proteins. J Virol. 2007;81:9612–9622. doi: 10.1128/JVI.00447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith JA, White EA, Sowa ME, Powell ML, Ottinger M, Harper JW, Howley PM. Genomewide siRNA screen identifies SMCX, EP400, and Brd4 as E2-dependent regulators of human papillomavirus oncogene expression. Proc Natl Acad Sci U S A. 2010;107:3752–3757. doi: 10.1073/pnas.0914818107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schweiger MR, You J, Howley PM. Bromodomain protein 4 mediates the papillomavirus E2 transcriptional activation function. J Virol. 2006;80:4276–4285. doi: 10.1128/JVI.80.9.4276-4285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ammermann I, Bruckner M, Matthes F, Iftner T, Stubenrauch F. Inhibition of transcription and DNA replication by the papillomavirus E8-E2C protein is mediated by interaction with corepressor molecules. J Virol. 2008;82:5127–5136. doi: 10.1128/JVI.02647-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doorbar J, Campbell D, Grand RJ, Gallimore PH. Identification of the human papilloma virus-1a E4 gene products. Embo J. 1986;5:355–362. doi: 10.1002/j.1460-2075.1986.tb04219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baxter MK, McPhillips MG, Ozato K, McBride AA. The mitotic chromosome binding activity of the papillomavirus E2 protein correlates with interaction with the cellular chromosomal protein, Brd4. J Virol. 2005;79:4806–4818. doi: 10.1128/JVI.79.8.4806-4818.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbate EA, Voitenleitner C, Botchan MR. Structure of the papillomavirus DNA-tethering complex E2:Brd4 and a peptide that ablates HPV chromosomal association. Mol Cell. 2006;24:877–889. doi: 10.1016/j.molcel.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Cardenas-Mora J, Spindler JE, Jang MK, McBride AA. Dimerization of the papillomavirus E2 protein is required for efficient mitotic chromosome association and Brd4 binding. J Virol. 2008;82:7298–7305. doi: 10.1128/JVI.00772-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McPhillips MG, Oliveira JG, Spindler JE, Mitra R, McBride AA. Brd4 is required for e2- mediated transcriptional activation but not genome partitioning of all papillomaviruses. J Virol. 2006;80:9530–9543. doi: 10.1128/JVI.01105-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jang MK, Kwon D, McBride AA. Papillomavirus E2 proteins and the host BRD4 protein associate with transcriptionally active cellular chromatin. J Virol. 2009;83:2592–2600. doi: 10.1128/JVI.02275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng G, Schweiger MR, Martinez-Noel G, Zheng L, Smith JA, Harper JW, Howley PM. Brd4 regulation of papillomavirus protein E2 stability. J Virol. 2009;83:8683–8692. doi: 10.1128/JVI.00674-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gagnon D, Joubert S, Senechal H, Fradet-Turcotte A, Torre S, Archambault J. Proteasomal degradation of the papillomavirus E2 protein is inhibited by overexpression of bromodomain-containing protein 4. J Virol. 2009;83:4127–4139. doi: 10.1128/JVI.02468-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thierry F, Spyrou G, Yaniv M, Howley PM. Two AP1 sites binding JunB are essential for HPV18 transcription in keratinocytes. J. Virol. 1992;66:3740–3748. doi: 10.1128/jvi.66.6.3740-3748.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dostatni N, Lambert PF, Sousa R, Ham J, Howley PM, Yaniv M. The functional BPV1 E2 trans-activating protein can act as a repressor by preventing formation of the initiation complex. Genes Dev. 1991;5:1657–1671. doi: 10.1101/gad.5.9.1657. [DOI] [PubMed] [Google Scholar]
- 44.Hou SY, Wu SY, Zhou T, Thomas MC, Chiang CM. Alleviation of human papillomavirus E2- mediated transcriptional repression via formation of a TATA binding protein (or TFIID)-TFIIBRNA polymerase II-TFIIF preinitiation complex. Mol Cell Biol. 2000;20:113–125. doi: 10.1128/mcb.20.1.113-125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan J, Li Q, Lievens S, Tavernier J, You J. Abrogation of the Brd4-positive transcription elongation factor B complex by papillomavirus E2 protein contributes to viral oncogene repression. J Virol. 2010;84:76–87. doi: 10.1128/JVI.01647-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao JM, Breiding DE, Androphy EJ. Functional interaction of the bovine papillomavirus E2 transactivation domain with TFIIB. Journal of Virology. 1998;72:1013–1019. doi: 10.1128/jvi.72.2.1013-1019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rank NM, Lambert PF. Bovine papillomavirus type 1 E2 transcriptional regulators directly bind two cellular transcription factors, TFIID and TFIIB. J Virol. 1995;69:6323–6334. doi: 10.1128/jvi.69.10.6323-6334.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enzenauer C, Mengus G, Lavigne A, Davidson I, Pfister H, May M. Interaction of human papillomavirus 8 regulatory proteins E2, E6 and E7 with components of the TFIID complex. Intervirology. 1998;41:80–90. doi: 10.1159/000024918. [DOI] [PubMed] [Google Scholar]
- 49.Breiding DE, Sverdrup F, Grossel MJ, Moscufo N, Boonchai W, Androphy EJ. Functional interaction of a novel cellular protein with the papillomavirus E2 transactivation domain. Molecular and Cellular Biology. 1997;17:7208–7219. doi: 10.1128/mcb.17.12.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng Y, Breiding DE, Sverdrup F, Richard J, Androphy EJ. AMF-1/Gps2 binds p300 and enhances its interaction with papillomavirus E2 proteins. J. Vrol. 2000;74:5872–5879. doi: 10.1128/jvi.74.13.5872-5879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J, Lee D, Gwan Hwang S, Hwang ES, Choe J. BRCA1 associates with human papillomavirus type 18 E2 and stimulates E2-dependent transcription. Biochem Biophys Res Commun. 2003;305:1008–1016. doi: 10.1016/s0006-291x(03)00880-5. [DOI] [PubMed] [Google Scholar]
- 52.Lee D, Kim JW, Kim K, Joe CO, Schreiber V, Menissier-De Murcia J, Choe J. Functional interaction between human papillomavirus type 18 E2 and poly(ADP-ribose) polymerase 1. Oncogene. 2002;21:5877–5885. doi: 10.1038/sj.onc.1205723. [DOI] [PubMed] [Google Scholar]
- 53.Centeno F, Ramirez-Salazar E, Garcia-Villa E, Gariglio P, Garrido E. TAF1 interacts with and modulates human papillomavirus 16 E2-dependent transcriptional regulation. Intervirology. 2008;51:137–143. doi: 10.1159/000141706. [DOI] [PubMed] [Google Scholar]
- 54.Clower RV, Hu Y, Melendy T. Papillomavirus E2 protein interacts with and stimulates human topoisomerase I. Virology. 2006;348:13–18. doi: 10.1016/j.virol.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 55.Boner W, Taylor ER, Tsirimonaki E, Yamane K, Campo MS, Morgan IM. A functional interaction between the HPV16 transcription/replication factor E2 and the DNA damage response protein TopBP1. J Biol Chem. 2002;4:4. doi: 10.1074/jbc.M202163200. [DOI] [PubMed] [Google Scholar]
- 56.Boner W, Morgan IM. Novel cellular interacting partners of the human papillomavirus 16 transcription/replication factor E2. Virus Res. 2002;90:113–118. doi: 10.1016/s0168-1702(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 57.Donaldson MM, Boner W, Morgan IM. TopBP1 regulates human papillomavirus type 16 E2 interaction with chromatin. J Virol. 2007;81:4338–4342. doi: 10.1128/JVI.02353-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee D, Hwang SG, Kim J, Choe J. Functional interaction between p/CAF and human papillomavirus E2 protein. J Biol Chem. 2002;277:6483–6489. doi: 10.1074/jbc.M105085200. [DOI] [PubMed] [Google Scholar]
- 59.Muller A, Ritzkowsky A, Steger G. Cooperative activation of human papillomavirus type 8 gene expression by the E2 protein and the cellular coactivator p300. J Virol. 2002;76:11042–11053. doi: 10.1128/JVI.76.21.11042-11053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar RA, Naidu SR, Wang X, Imbalzano AN, Androphy EJ. Interaction of papillomavirus E2 protein with the Brm chromatin remodeling complex leads to enhanced transcriptional activation. J Virol. 2007;81:2213–2220. doi: 10.1128/JVI.01746-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cha S, Seo T. hSNF5 Is Required for Human Papillomavirus E2-Driven Transcriptional Activation and DNA Replication. Intervirology. 2010;54:66–77. doi: 10.1159/000318871. [DOI] [PubMed] [Google Scholar]
- 62.Rehtanz M, Schmidt HM, Warthorst U, Steger G. Direct interaction between nucleosome assembly protein 1 and the papillomavirus E2 proteins involved in activation of transcription. Mol Cell Biol. 2004;24:2153–2168. doi: 10.1128/MCB.24.5.2153-2168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Demeret C, Yaniv M, Thierry F. The E2 transcriptional repressor can compensate for Sp1 activation of the human papillomavirus type 18 early promoter. J Virol. 1994;68:7075–7082. doi: 10.1128/jvi.68.11.7075-7082.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan SH, Gloss B, Bernard HU. During negative regulation of the human papillomavirus-16 E6 promoter, the viral E2 protein can displace Sp1 from a proximal promoter element. Nucleic Acids Res. 1992;20:251–256. doi: 10.1093/nar/20.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melendy T, Sedman J, Stenlund A. Cellular factors required for papillomavirus DNA replication. J Virol. 1995;69:7857–7867. doi: 10.1128/jvi.69.12.7857-7867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thierry F. Transcriptional regulation of the papillomavirus oncogenes by cellular and viral transcription factors in cervical carcinoma. Virology. 2009;384:375–379. doi: 10.1016/j.virol.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 67.Mohr IJ, Clark R, Sun S, Androphy EJ, MacPherson P, Botchan MR. Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science. 1990;250:1694–1699. doi: 10.1126/science.2176744. [DOI] [PubMed] [Google Scholar]
- 68.Berg M, Stenlund A. Functional interactions between papillomavirus E1 and E2 proteins. J. Virol. 1997;71:3853–3863. doi: 10.1128/jvi.71.5.3853-3863.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abbate EA, Berger JM, Botchan MR. The Xray structure of the papillomavirus helicase in complex with its molecular matchmaker E2. Genes Dev. 2004;18:1981–1996. doi: 10.1101/gad.1220104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davy C, McIntosh P, Jackson DJ, Sorathia R, Miell M, Wang Q, Khan J, Soneji Y, Doorbar J. A novel interaction between the human papillomavirus type 16 E2 and E1–E4 proteins leads to stabilization of E2. Virology. 2009;394:266–275. doi: 10.1016/j.virol.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 71.Gammoh N, Grm HS, Massimi P, Banks L. Regulation of human papillomavirus type 16 E7 activity through direct protein interaction with the E2 transcriptional activator. J Virol. 2006;80:1787–1797. doi: 10.1128/JVI.80.4.1787-1797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smal C, Wetzler DE, Dantur KI, Chemes LB, Garcia-Alai MM, Dellarole M, Alonso LG, Gaston K, de Prat-Gay G. The human papillomavirus E7-E2 interaction mechanism in vitro reveals a finely tuned system for modulating available E7 and E2 proteins. Biochemistry. 2009;48:11939–11949. doi: 10.1021/bi901415k. [DOI] [PubMed] [Google Scholar]
- 73.Stanley MA, Browne HM, Appleby M, Minson AC. Properties of a non-tumorigenic human cervical keratinocyte cell line. International Journal of Cancer. 1989;43:672–676. doi: 10.1002/ijc.2910430422. [DOI] [PubMed] [Google Scholar]
- 74.Maitland NJ, Conway S, Wilkinson NS, Ramsdale J, Morris JR, Sanders CM, Burns JE, Stern PL, Wells M. Expression patterns of the human papillomavirus type 16 transcription factor E2 in low- and high-grade cervical intraepithelial neoplasia. J Pathol. 1998;186:275–280. doi: 10.1002/(SICI)1096-9896(1998110)186:3<275::AID-PATH159>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 75.Stevenson M, Hudson LC, Burns JE, Stewart RL, Wells M, Maitland NJ. Inverse relationship between the expression of the human papillomavirus type 16 transcription factor E2 and virus DNA copy number during the progression of cervical intraneoplasia. J. Gen. virol. 2000;81:1825–1832. doi: 10.1099/0022-1317-81-7-1825. [DOI] [PubMed] [Google Scholar]
- 76.Xue Y, Bellanger S, Zhang W, Lim D, Low J, Lunny D, Thierry F. HPV16 E2 is an immediate early marker of viral infection, preceding E7 expression in precursor structures of cervical carcinoma. Cancer Res. 2010;70:5316–5325. doi: 10.1158/0008-5472.CAN-09-3789. [DOI] [PubMed] [Google Scholar]
- 77.Blachon S, Bellanger S, Demeret C, Thierry F. Nucleo-cytoplasmic shuttling of high risk human Papillomavirus E2 proteins induces apoptosis. J Biol Chem. 2005;280:36088–36098. doi: 10.1074/jbc.M505138200. [DOI] [PubMed] [Google Scholar]
- 78.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 79.Penrose K, McBride A. Proteasomemediated degradation of the papillomavirus E2-TA protein is regulated by phosphorylation and can modulate viral genome copy number. J. Virol. 2000;74:6031–6038. doi: 10.1128/jvi.74.13.6031-6038.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bellanger S, Demeret C, Goyat S, Thierry F. Stability of the human papillomavirus type18 E2 protein is regulated by a proteasome degradation pathway through its amino-terminal transactivation domain. J. Virol. 2001;75:7244–7251. doi: 10.1128/JVI.75.16.7244-7251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frattini MG, Hurst SD, Lim HB, Swaminathan S, Laimins LA. Abrogation of a mitotic checkpoint by E2 proteins from oncogenic human papillomaviruses correlates with increased turnover of the p53 tumor suppressor protein. Embo J. 1997;16:318–331. doi: 10.1093/emboj/16.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Webster K, Parish J, Pandya M, Stern PL, Clarke AR, Gaston K. The human papillomavirus (HPV) 16 E2 protein induces apoptosis in the absence of other HPV proteins and via a p53-dependent pathway. J Biol Chem. 2000;275:87–94. doi: 10.1074/jbc.275.1.87. [DOI] [PubMed] [Google Scholar]
- 83.Demeret C, Garcia-Carranca A, Thierry F. Transcription-independent triggering of the extrinsic pathway of apoptosis by human papillomavirus 18 E2 protein. Oncogene. 2003;22:168–175. doi: 10.1038/sj.onc.1206108. [DOI] [PubMed] [Google Scholar]
- 84.Thierry F, Demeret C. Direct activation of caspase 8 by the proapoptotic E2 protein of HPV18 independent of adaptor proteins. Cell Death Differ. 2008;15:1356–1363. doi: 10.1038/cdd.2008.53. [DOI] [PubMed] [Google Scholar]
- 85.Wang W, Fang Y, Sima N, Li Y, Li W, Li L, Han L, Liao S, Han Z, Gao Q, Li K, Deng D, Meng L, Zhou J, Wang S, Ma D. Triggering of death receptor apoptotic signaling by human papillomavirus 16 E2 protein in cervical cancer cell lines is mediated by interaction with c-FLIP. Apoptosis. 2010 doi: 10.1007/s10495-010-0543-3. [DOI] [PubMed] [Google Scholar]
- 86.Bagnoli M, Canevari S, Mezzanzanica D. Cellular FLICE-inhibitory protein (c-FLIP) signalling: a key regulator of receptor-mediated apoptosis in physiologic context and in cancer. Int J Biochem Cell Biol. 2010;42:210–213. doi: 10.1016/j.biocel.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 87.Parish JL, Kowalczyk A, Chen HT, Roeder GE, Sessions R, Buckle M, Gaston K. E2 proteins from high- and low-risk human papillomavirus types differ in their ability to bind p53 and induce apoptotic cell death. J Virol. 2006;80:4580–4590. doi: 10.1128/JVI.80.9.4580-4590.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Desaintes C, Goyat S, Garbay S, Yaniv M, Thierry F. Papillomavirus E2 induces p53-independent apoptosis in HeLa cells. Oncogene. 1999;18:4538–4546. doi: 10.1038/sj.onc.1202818. [DOI] [PubMed] [Google Scholar]
- 89.Lee P, Lee DJ, Chan C, Chen SW, Ch'en I, Jamora C. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature. 2009;458:519–523. doi: 10.1038/nature07687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kovalenko A, Kim JC, Kang TB, Rajput A, Bogdanov K, Dittrich-Breiholz O, Kracht M, Brenner O, Wallach D. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med. 2009;206:2161–2177. doi: 10.1084/jem.20090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barbero S, Mielgo A, Torres V, Teitz T, Shields DJ, Mikolon D, Bogyo M, Barila D, Lahti JM, Schlaepfer D, Stupack DG. Caspase-8 association with the focal adhesion complex promotes tumor cell migration and metastasis. Cancer Res. 2009;69:3755–3763. doi: 10.1158/0008-5472.CAN-08-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thierry F, Yaniv M. The BPV1-E2 transacting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J. 1987;6:3391–3397. doi: 10.1002/j.1460-2075.1987.tb02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Demeret C, Desaintes C, Yaniv M, Thierry F. Different mechanisms contribute to the E2- mediated transcriptional repression of human papillomavirus type18 viral oncogenes. J. Virol. 1997;71:9343–9349. doi: 10.1128/jvi.71.12.9343-9349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cross SM, Sanchez CA, Morgan CA, Schimke MK, Ramel S, Idzerda RL, Raskind WH, Reid BJ. A p53-dependent mouse spindle checkpoint. Science. 1995;267:1353–1356. doi: 10.1126/science.7871434. [DOI] [PubMed] [Google Scholar]
- 95.Minn AJ, Boise LH, Thompson CB. Expression of Bcl-xL and loss of p53 can cooperate to overcome a cell cycle checkpoint induced by mitotic spindle damage. Genes Dev. 1996;10:2621–2631. doi: 10.1101/gad.10.20.2621. [DOI] [PubMed] [Google Scholar]
- 96.Khan SH, Wahl GM. p53 and pRb prevent rereplication in response to microtubule inhibitors by mediating a reversible G1 arrest. Cancer Res. 1998;58:396–401. [PubMed] [Google Scholar]
- 97.Fournier N, Raj K, Saudan P, Utzig S, Sahli R, Simanis V, Beard P. Expression of human papillomavirus 16 E2 protein in Schizosaccharomyces pombe delays the initiation of mitosis. Oncogene. 1999;18:4015–4021. doi: 10.1038/sj.onc.1202775. [DOI] [PubMed] [Google Scholar]
- 98.Bellanger S, Blachon S, Mechali F, Bonne-Andrea C, Thierry F. High-risk but not lowrisk HPV E2 proteins bind to the APC activators Cdh1 and Cdc20 and cause genomic instability. Cell Cycle. 2005;4:1608–1615. doi: 10.4161/cc.4.11.2123. [DOI] [PubMed] [Google Scholar]
- 99.Musacchio A, Salmon ED. The spindleassembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 100.Choi E, Choe H, Min J, Choi JY, Kim J, Lee H. BubR1 acetylation at prometaphase is required for modulating APC/C activity and timing of mitosis. Embo J. 2009;28:2077–2089. doi: 10.1038/emboj.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kulukian A, Han JS, Cleveland DW. Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev Cell. 2009;16:105–117. doi: 10.1016/j.devcel.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nilsson J, Yekezare M, Minshull J, Pines J. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat Cell Biol. 2008;10:1411–1420. doi: 10.1038/ncb1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sotillo R, Hernando E, Diaz-Rodriguez E, Teruya-Feldstein J, Cordon-Cardo C, Lowe SW, Benezra R. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grabsch H, Takeno S, Parsons WJ, Pomjanski N, Boecking A, Gabbert HE, Mueller W. Overexpression of the mitotic checkpoint genes BUB1, BUBR1, and BUB3 in gastric cancer–association with tumour cell proliferation. J Pathol. 2003;200:16–22. doi: 10.1002/path.1324. [DOI] [PubMed] [Google Scholar]
- 105.Tanaka K, Mohri Y, Ohi M, Yokoe T, Koike Y, Morimoto Y, Miki C, Tonouchi H, Kusunoki M. Mitotic checkpoint genes, hsMAD2 and BubR1, in oesophageal squamous cancer cells and their association with 5-fluorouracil and cisplatin-based radiochemotherapy. Clin Oncol (R Coll Radiol) 2008;20:639–646. doi: 10.1016/j.clon.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 106.Liu AW, Cai J, Zhao XL, Xu AM, Fu HQ, Nian H, Zhang SH. The clinicopathological significance of BUBR1 overexpression in hepatocellular carcinoma. J Clin Pathol. 2009;62:1003–1008. doi: 10.1136/jcp.2009.066944. [DOI] [PubMed] [Google Scholar]
- 107.Ando K, Kakeji Y, Kitao H, Iimori M, Zhao Y, Yoshida R, Oki E, Yoshinaga K, Matumoto T, Morita M, Sakaguchi Y, Maehara Y. High expression of BUBR1 is one of the factors for inducing DNA aneuploidy and progression in gastric cancer. Cancer Sci. 2010;101:639–645. doi: 10.1111/j.1349-7006.2009.01457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pinto M, Vieira J, Ribeiro FR, Soares MJ, Henrique R, Oliveira J, Jeronimo C, Teixeira MR. Overexpression of the mitotic checkpoint genes BUB1 and BUBR1 is associated with genomic complexity in clear cell kidney carcinomas. Cell Oncol. 2008;30:389–395. doi: 10.3233/CLO-2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lira RC, Miranda FA, Guimaraes MC, Simoes RT, Donadi EA, Soares CP, Soares EG. BUBR1 expression in benign oral lesions and squamous cell carcinomas: correlation with human papillomavirus. Oncol Rep. 2010;23:1027–1036. doi: 10.3892/or_00000729. [DOI] [PubMed] [Google Scholar]
- 110.Pfefferle R, Marcuzzi GP, Akgul B, Kasper HU, Schulze F, Haase I, Wickenhauser C, Pfister H. The human papillomavirus type 8 E2 protein induces skin tumors in transgenic mice. J Invest Dermatol. 2008;128:2310–2315. doi: 10.1038/jid.2008.73. [DOI] [PubMed] [Google Scholar]
- 111.Leykauf K, Kabsch K, Gassler N, Gissmann L, Alonso A, Schenkel J. Expression of the HPV11 E2 gene in transgenic mice does not result in alterations of the phenotypic pattern. Transgenic Res. 2008;17:1–8. doi: 10.1007/s11248-007-9130-y. [DOI] [PubMed] [Google Scholar]
- 112.Poddar A, Reed SC, McPhillips MG, Spindler JE, McBride AA. The human papillomavirus type 8 E2 tethering protein targets the ribosomal DNA loci of host mitotic chromosomes. J Virol. 2009;83:640–650. doi: 10.1128/JVI.01936-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Skiadopoulos MH, McBride AA. Bovine papillomavirus type 1 genomes and the E2 transactivator protein are closely associated with mitotic chromatin. J Virol. 1998;72:2079–2088. doi: 10.1128/jvi.72.3.2079-2088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ilves I, Kivi S, Ustav M. Long-term episomal maintenance of bovine papillomavirus type 1 plasmids is determined by attachment to host chromosomes, which Is mediated by the viral E2 protein and its binding sites. J Virol. 1999;73:4404–4412. doi: 10.1128/jvi.73.5.4404-4412.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lehman CW, Botchan MR. Segregation of viral plasmids depends on tethering to chromosomes and is regulated by phosphorylation [see comments] Proc. Natl. Acad. Sci. USA. 1998;95:4338–4343. doi: 10.1073/pnas.95.8.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oliveira JG, Colf LA, McBride AA. Variations in the association of papillomavirus E2 proteins with mitotic chromosomes. Proc Natl Acad Sci U S A. 2006;103:1047–1052. doi: 10.1073/pnas.0507624103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McLaughlin-Drubin M, Munger K. The human papillomavirus type 8 E2 gene encodes a transforming activity sufficient for skin tumor formation in transgenic mice. J Invest Dermatol. 2008;128:2142–2144. doi: 10.1038/jid.2008.217. [DOI] [PubMed] [Google Scholar]
- 118.Bellanger S, Tan CL, Nei W, He PP, Thierry F. The human papillomavirus type 18 E2 protein is a cell cycle-dependent target of the SCFSkp2 ubiquitin ligase. J Virol. 2010;84:437–444. doi: 10.1128/JVI.01162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG., Jr Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–198. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- 120.Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–193. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- 121.Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem. 2003;278:25752–25757. doi: 10.1074/jbc.M301774200. [DOI] [PubMed] [Google Scholar]
- 122.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 123.Hershko DD. Oncogenic properties and prognostic implications of the ubiquitin ligase Skp2 in cancer. Cancer. 2008;112:1415–1424. doi: 10.1002/cncr.23317. [DOI] [PubMed] [Google Scholar]
- 124.Gstaiger M, Jordan R, Lim M, Catzavelos C, Mestan J, Slingerland J, Krek W. Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci U S A. 2001;98:5043–5048. doi: 10.1073/pnas.081474898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee AY, Chiang CM. Chromatin adaptor Brd4 modulates E2 transcription activity and protein stability. J Biol Chem. 2009;284:2778–2786. doi: 10.1074/jbc.M805835200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McBride AA, Oliveira JG, McPhillips MG. Partitioning viral genomes in mitosis: same idea, different targets. Cell Cycle. 2006;5:1499–1502. doi: 10.4161/cc.5.14.3094. [DOI] [PubMed] [Google Scholar]
- 127.Kadaja M, Isok-Paas H, Laos T, Ustav E, Ustav M. Mechanism of genomic instability in cells infected with the high-risk human papillomaviruses. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000397. e1000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Steger G, Ham J, Lefebvre O, Yaniv M. The bovine papillomavirus 1 E2 protein contains two activation domains: one that interacts with TBP and another that functions after TBP binding. EMBO J. 1995;14:329–340. doi: 10.1002/j.1460-2075.1995.tb07007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim S, Rimm D, Carter D, Khan A, Parisot N, Franco MA, Bale A, Haffty BG. BRCA status, molecular markers, and clinical variables in early, conservatively managed breast cancer. Breast J. 2003;9:167–174. doi: 10.1046/j.1524-4741.2003.09307.x. [DOI] [PubMed] [Google Scholar]
- 130.Lee D, Lee B, Kim J, Kim DW, Choe J. cAMP response element-binding protein-binding protein binds to human papillomavirus E2 protein and activates E2-dependent transcription. J Biol Chem. 2000;275:7045–7051. doi: 10.1074/jbc.275.10.7045. [DOI] [PubMed] [Google Scholar]
- 131.Li R, Knight JD, Jackson SP, Tjian R, Botchan MR. Direct interaction between Sp1 and the BPV enhancer E2 protein mediates synergistic activation of transcription. Cell. 1991;65:493–505. doi: 10.1016/0092-8674(91)90467-d. [DOI] [PubMed] [Google Scholar]
- 132.Steger G, Schnabel C, Schmidt HM. The hinge region of the human papillomavirus type 8 E2 protein activates the human p21(WAF1/ CIP1) promoter via interaction with Sp1. J Gen Virol. 2002;83:503–510. doi: 10.1099/0022-1317-83-3-503. [DOI] [PubMed] [Google Scholar]
- 133.Gammoh N, Gardiol D, Massimi P, Banks L. The Mdm2 ubiquitin ligase enhances transcriptional activity of human papillomavirus E2. J Virol. 2009;83:1538–1543. doi: 10.1128/JVI.01551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lai MC, Teh BH, Tarn WY. A human papillomavirus E2 transcriptional activator. The interactions with cellular splicing factors and potential function in pre-mRNA processing. J Biol Chem. 1999;274:11832–11841. doi: 10.1074/jbc.274.17.11832. [DOI] [PubMed] [Google Scholar]
- 135.Massimi P, Pim D, Bertoli C, Banks L. Interaction between the HPV-16 E2 transcriptional activator and p53. Oncogene. 1999;18:7748–7754. doi: 10.1038/sj.onc.1203208. [DOI] [PubMed] [Google Scholar]
- 136.Wang WS, Lee MS, Tseng CE, Liao IH, Huang SP, Lin RI, Li C. Interaction between human papillomavirus type 5 E2 and polo-like kinase 1. J Med Virol. 2009;81:536–544. doi: 10.1002/jmv.21404. [DOI] [PubMed] [Google Scholar]
- 137.Parish JL, Bean AM, Park RB, Androphy EJ. ChlR1 is required for loading papillomavirus E2 onto mitotic chromosomes and viral genome maintenance. Mol Cell. 2006;24:867–876. doi: 10.1016/j.molcel.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 138.Wang X, Naidu SR, Sverdrup F, Androphy EJ. Tax1BP1 interacts with papillomavirus E2 and regulates E2-dependent transcription and stability. J Virol. 2009;83:2274–2284. doi: 10.1128/JVI.01791-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Olejnik-Schmidt AK, Schmidt MT, Kedzia W, Gozdzicka-Jozefiak A. Search for cellular partners of human papillomavirus type 16 E2 protein. Arch Virol. 2008;153:983–990. doi: 10.1007/s00705-008-0061-6. [DOI] [PubMed] [Google Scholar]