Abstract
Receptor EphA2 over-expression is associated with the aggressive nature of growth in malignant mesothelioma (MM) and silencing EphA2 with interference RNA suppressed MM proliferation. The mechanisms associated with targeting the EphA2 gene in MM were not clear. We sought to determine whether silencing EphA2 induces apoptosis in MM cells by either extrinsic or intrinsic pathways. The receptor EphA2 signaling pathway may provide attractive therapeutic strategy for MM. Apoptosis was determined by Cell Death ELISA in MM Cells transfected with siRNA-EphA2 and control siRNA. The gene expression profile of apoptosis pathways were analyzed by GEArray. Selected genes were further studied by quantitative PCR, Western analysis, and immunofluorescence. Caspases activities were measured by fluorescence spectrometer. Silencing EphA2 expression induced apoptosis in MMC. Apoptosis was characterized by FADD expression, activated caspase-8, caspase-3 and induction of Bax, Bak, and Bid as revealed by GEArray and protein fractionation assays. The expression of FADD, Bid, caspase-8, cytochrome-c and apaf-1 were significantly higher in the cytosolic fractions of EphA2-siRNA transfected cells. Furthermore, blocking the expression of caspase-8 by an inhibitor blunted FADD expression, indicating that caspase-8 is implicated in EphA2-siRNA induced apoptosis in MMC. Our data indicates that targeting the EphA2 gene by siRNA induced both extrinsic and intrinsic apoptotic pathways in MM Cells. Receptor EphA2 inhibition may be an effective approach for inhibiting MM growth and a promising direction for MM therapy.
Keywords: Receptor EphA2, malignant mesothelioma, interference RNA, siRNA, apoptosis, therapy
Introduction
Malignant mesothelioma (MM), an aggressive tumor of the pleura, is resistant to conventional therapies [1]. Approximately 3,000 new cases of malignant mesothelioma (MM) are diagnosed annually, in United States [2]. Currently, despite multimodality interventions, the overall survival of MM patients is less than 6% [3, 4]. Because MM fails to respond to conventional therapies, there is a pressing need for new therapeutic approaches such as identifying novel molecular signatures that inhibit tumor growth. MM cell (MMC) growth is characterized by a dysregulated death signaling pathway [5]. In previous studies, we demonstrated that receptor EphA2 is over-expressed in MM and blockade of the receptor EphA2 decreased proliferation and migration [6]. However, the mechanisms of EphA2 mediated tumor growth inhibition in MMC are not clear.
Eph receptors are the largest family of trans-membrane proteins with an extracellular domain capable of recognizing signals from the cells microenvironment and influencing cell-cell interaction, growth, and migration. Death receptor mediated (TNF-α or FAS) and mitochondria l dependent apoptosis pathways are regulated by many protein kinases such as protein kinase A (PKA), phosphatidylinositol-3 kinase, and members of mitogen-activated protein kinases. However, it is not known if receptor EphA2 signaling plays any role in apoptosis signaling pathways in MMC.
Apoptosis is an important process for the normal development and suppression of oncogenesis. It can proceed through two distinct pathways, a cell death receptor pathway (extrinsic) or a mitochondrial pathway (intrinsic) [7]. The extrinsic pathway involves the activation of adaptor protein such as FADD, which plays a crucial role in receiving and transmitting key signals from the membrane receptor tyrosine kinases (RTKs) that control cell growth, and differentiation [8]. The intrinsic pathway involves pro-apoptotic Bcl2 family members such as Bid, Bax and Bak that induce permeability of the mitochondrial outer membrane, permitting release of cytochrome-c [7, 9]. The extrinsic and intrinsic pathways can crosstalk via caspase-8 mediated cleavage of Bid, which then triggers the release of mitochondrial proteins [10, 11]. Bid, a 26 KDa protein is cleaved by caspase-8 into a fragment called truncated-Bid (t-Bid) which translocates to the mitochondria. Subsequently, t-Bid interacts with Bcl-xl which is an anti-apoptotic member of Bcl2 family and prevents the formation of the anti-apoptotic complex. The mitochondrial outer membrane becomes permeable either directly or indirectly through cleavage of Bid. The Apaf-1 apoptosome accordingly activate effector caspases and induce apoptosis [12-14]. The adaptor molecules relay and amplify the signal. However, the function of these molecules which are regulated by receptor EphA2 in MMC is yet to be defined.
In the present study we analyzed the profile of apoptotic signaling pathway genes using gene microarray and report that silencing receptor EphA2 inhibits tumor growth by inducing apoptosis in MMC via an FADD mediated apoptotic pathway.
Material and methods
MM cell culture and transfection
MM cell line (CRL-2081) was maintained as reported previously [6, 15]. In brief, the malignant mesothelioma cell line (CRL-2081) was obtained from American Type Culture Collection (ATCC, Manassas, VA). MMC were grown to a 70% confluence in culture dishes in RPMI-1640 medium (GIBCO-BRL, Baltimore, MD), containing 10% FBS (Atlanta Biological), Penicillin (100 Units/ml), and Streptomycin (100 µg/ml). The cells were plated in 75 cm2 culture flasks (Corning Costar Corporation, MA) and incubated at 37°C in 5% CO2 and 95% air. The media was changed on alternate days. The transient transfection was performed with specific stealth small interference RNA (siRNA) against EphA2, or control siRNA for overnight using Lipofectamine-2000 (Invitrogen; Carlsbad, CA), according the manufacturer's instructions. The transfection medium was changed with culture medium containing 5% FBS. The siRNA sequences were designed using RNAi designer software program, Oligoperfect (Invitrogen, Carlsbad, CA). The EphA2 (Accession number: NM_004431), target sequences used are as follows: siRNA-EphA2, Sense: CGCAAGAAGGGAGACUCCAACAG CU, Antisense: AGCUGUUGGAGUCUCCCUUCUUG CG; siRNA - control, Sense: CGAGAGAGUCGG-CGACUAUUGGUCA, Antisense: UGACCAAUAGUC GCCGACUCUC UCG. The transfection medium was changed with culture medium containing 5% FBS. The assays were carried out 36 hours post transfection.
Tumor growth analysis
MM tumor growth was measured by matrigel assay as reported earlier [16]. Briefly, MMC (5 × 103) were cultured in 24 well culture plate previously coated with 200 µl of matrigel per well followed by polymerization for 30 min at 37°C. MMC transfected with EphA2 siRNA or scrambles siRNA (control) in serum free RPMI 1640 for 24 hours and seeded into the matrigels. Culture media were changed every 48 hours and the number of colonies formed was recorded after 12 days of incubation. Four to six randomly chosen fields from each test sample were photographed at 100× magnification.
Determination of apoptosis
The cytoplasmic histone-associated DNA fragments (mono and oligonucleosomes) were detected using a cell death ELISA kit (Roche Applied Science, Mannheim, Germany) according to the instructions. Briefly, MMC (5 × 104) were seeded in 24 well plates and incubated for 16 hours at 37°C. Cells were lysed in buffer for 30 min at 25°C and 20 µl of supernatant was transferred into the streptavidin-coated plate. 80 µl of immunoreagent containing 4 µl anti-histone-biotin and 4 µl anti-DNA-POD were added to each well and the plate was shaken for 2 hour at room temperature to detect the mono- and oligonucleosomes in the cell lysates. The well received 100 µl of 2, 2′-azino-di(3-ethylbenz-thiazolinesulfonate) (ABTS) solution for 20 min at room temperature. The absorbance was read immediately in a microplate reader at 405 nm (reference wavelength 540 nm). In some cultures Camptothecin (CMT) was used as a positive control.
Oligo GEArray
The expression of genes involved in apoptotic signaling pathway was determined by using Oligo GEArray following manufacturer's instructions (SuperArray, Frederick, MD) as reported earlier [17]. In brief, total RNA was isolated using TRIzol (Invitrogen) as reported earlier [6]. The RNA (4 µg) was converted to biotinylated cDNA fragments using GEArray AmpoLabelling-LPR Kit (SuperArray, Frederick, MD). The labeled cDNA was hybridized to GEArray membrane overnight. The relative expression levels of multiple genes were detected on the basis of chemiluminsecence signal by using the ECL kit (ECL, Amersham Pharmacia Biotech). Data analysis was done using GEArray expression Analysis Suite Software (SABioscience, Frederick, MD). The online software program converted the images into numerical data. Raw signal intensities were corrected for background by subtracting the signal intensity of the average between two sets of housekeeping genes, β-actin, glyceraldehyde-phosphatase dehydrogenase, or blank. The normalized signals were used to determine the relative fold change of particular transcripts in siRNA-EphA2 trans-fected or untransfected MMC.
Preparation of protein fractions
MMC were transfected with siRNA-EphA2 or control siRNA or left alone in SFM (serum free medium). Subsequently, they were harvested and washed in phosphate-buffered saline (PBS). The cell pellets were then subjected to stepwise extraction of cytosolic and membrane bound proteins using ProteoExtract sub-cellular proteome extraction kit following the manufacturer's instructions (S_PEK, Calbiochem, San Diego, CA). Protein concentrations were determined using Bio-Rad Protein assay kit. The samples were subjected to Western blot Analysis.
Western blot analysis
Western blots were performed as reported earlier [6]. Equal amounts of protein fractions (30 mg/lane) were loaded on 10.0 % polyacrylamide gels (Bio-Rad), and transferred electrophoretically onto polyvinylidene difluoride membrane (Immobilon-P, Millipore) as reported earlier. The blots were blocked overnight at 4°C with BSA and were incubated with the primary antibodies, at 1:500 – 1:000 for 1 hour at room temperature. The second antibody (horseradish peroxidase-conjugated anti-mouse IgG Ab) at a dilution of 1:2000 for 1 hour was used. The target proteins were detected by enhanced chemiluminsecence (ECL, Amersham Pharmacia Biotech). Prestained protein markers were included for molecular mass determination (Bio-Rad).
The primary antibodies used were rabbit polyclonal anti-FADD, monoclonal anti-caspase-3, monoclonal anti-caspase-8, monoclonal anti-Apaf-1, rabbit polyclonal anti-Bid (Chemicon, Temecula, CA); monoclonal anti-cytochrome-c antibody (R & D systems, Minneapolis, MN).
Quantitative RT-PCR
Total RNA was reverse transcribed into cDNA and PCR was performed using SYBR Green JumpStart Taq ReadyMix (Sigma, St. Louis) as reported earlier [18]. The primers used are given in Table 1. Total cellular RNA was isolated from MMC transfected with EphA2 siRNA or control siRNA or untransfected MMC using RNeasy kit (QIAGEN, Maryland) according to manufacturer's recommendations as reported earlier [6]. 100 ng/µl, of RNA was reverse transcribed into cDNA. After reverse transcription, 80 µl RNase-free water were added to each sample. 10 µl of diluted cDNA product was mixed with 25 µl of SYBR Green JumpStart Taq ReadyMix, 0.5 µl of Internal Reference Dye, and 14.5 µL of specific oligonucleotide primers (80nM final concentration) to total 50 µl volume for quantification real-time PCR. The primers used are given in Table 1. The quantification of real-time PCR were performed by SYBR Green method using the Applied Biosystems 7500 Real Time PCR System with the following profile: 1 cycle at 94°C for 2 min, 40 cycles at 94°C 15 sec, 60°C 1 min, 72°C 1 min, and the acquire the fluorescent signal from the elongation step. The real time PCR products were confirmed by electrophoresis on a 2% agarose gel. Data analysis was carried out by the ABI sequence detection software using the relative quantification. The threshold cycle (Ct), which is defined as the cycle at which PCR amplification reaches a significant value, is given as the mean value. The relative expression of each mRNA was calculated by the ΔCt method. The amount of the target genes relative to the β-actin mRNA was expressed as 2-(ΔCt).
Table 1.
The primer sequences used for the present study are listed below. The primer sequences are designed using Primer3 and Blast software (NCBI)
| Gene | Genebank Accession no. | Primer sequence |
|---|---|---|
| BAK | NM_001188 | Forward: 5′- TCCTAGGCCAGGTGACCCGC -3′ |
| Reverse: 5′- GCTGAGGGAGGAGACGGCCA -3′ | ||
| BAX | NM_004324.3 | Forward: 5′- GCCGCCGTGGACACAGACTC -3′ |
| Reverse: 5′- AGGCCCAGGGTCCCAGAGGA -3′ | ||
| BID | NM_001196.2 | Forward: 5′- CTGGAGGGTGGTCGCCACTG -3′ |
| Reverse: 5′- TCACCAGGCCCGGAGGGATG -3′ | ||
| CASP-2 | NM_032982 | Forward: 5′- CGGTGTGTGAGTCCTGTCCCCT -3′ |
| Reverse: 5′- CGCCCTCCACACCATGCGAG -3′ | ||
| CASP-6 | NM_032992 | Forward: 5′- GGCACCCGGCAGTGTCAACT -3′ |
| Reverse: 5′- GCTCCAGCAGGCAGCGTGTA -3′ | ||
| CASP-7 | NM_001227 | Forward: 5′- CGCCGTGGGAACGATGGCAG -3′ |
| Reverse: 5′- CACTCGGTCCCGGGTGGTCT -3′ | ||
| FADD | NM_003824.3 | Forward: 5′- CCGCCATCCTTCACCAGA -3′ |
| Reverse: 5′- CAATCA CTCATCAGCACCTCA -3′ | ||
| FAS | NM_000043 | Forward: 5′- TTCTCCCGCGGGTTGGTGGA -3′ |
| Reverse: 5′- GCAGGGCACGCAGTCTGGTT -3′ | ||
| FAS-L | NM_000639 | Forward: 5′- GTTTGCTGGGGCTGGCCTGA -3′ |
| Reverse: 5′- GAGGGGCCCAGGGAGAGCTG -3′ | ||
| β-actin | NM_001101 | Forward: 5′- AGAGCTACGAGCTGCCTGAC -3′ |
| Reverse: 5′- AAAGCCATGCCAATCTCATC -3′ |
Immunofluorescence microscopy
Expression of Bid in MMC was analyzed by confocal microscopy (Zeiss, Thornwood, NY) as reported earlier [6]. In brief, the cells were cultured to confluence on gelatinized glass cover slips and fixed in 5% paraformaldehyde (with 50 mM phosphate buffer) in 50% Tris wash buffer (TWB). The glass cover slips were rinsed three times and permeabilized with 1.2% Triton X-100 for 5 minutes, rinsed three times, incubated with 1% BSA in 100% TWB for 1 h, then stained for the expression Bid using primary antibody rabbit anti-Bid polyclonal at (1:150) dilution and secondary antibody Goat anti-rabbit IgG conjugated with Rhodamine (Millipore, San Francisco, CA), and Dapi was used as nuclear stain.
Measurement of caspases activity
Caspases activities were estimated as reported earlier [19], by CytoFluor according to manufacturer's protocol (BD Pharmingen, CA). Enzymatic caspase activity was estimated according to manufacturer's protocol. In brief, MMC (3 × 106 cells) transfected with siRNAEphA2 or control siRNA or untransfected siRNA was collected by centrifugation and washed with buffer and lysed by 4 cycles of freeze-thawing as previously described [19]. The presence of active caspases was determined by AFC (7-amino -4-trifluoromethyl coumarin) assay using a specific substrate. Lysates were incubated with 20 µM DEVD-AFC for caspase-3 and IETD-AFC for Caspase-8 in a caspase assay buffer(10% glycerol, 50 mM PIPES pH 7.0, and 1 mM EDTA) containing 1 mM DTT in a final volume of 200uL. The release of free AFC was determined after 4 hours using a fluorescence spectrometer (Perkin Elmer Life Science, Boston, MA) at the 400 nm for excitation, 505 nm for emission. The signals representing caspase activities were corrected for the background.
Statistical analysis
The statistical analyses were performed by using SigmaStat 3.5 (SYSTAT Software, Inc. San Jose, CA). Results are expressed as mean ± SEM. Comparisons were made using one way ANOVA followed by Student-Newman-Keuls test for multiple comparisons. Differences were considered significant when P values were <0.05.
Results
Inhibition of receptor EphA2 expression attenuated MM tumor growth
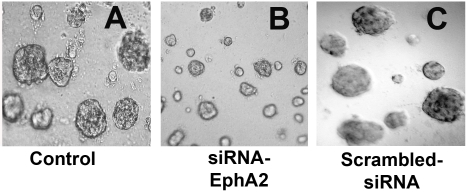
We measured MM tumor growth in vitro. MMC when cultured in matrigel, solid tumors were developed after one week of incubation. Inhibition of EphA2 receptor expression by siRNA significantly attenuated the tumor growth compared to control (Figure 1). Tumor growth in scrambled siRNA transfected cultures were unaffected and were comparable to control. This indicates that tumor growth inhibition was specific to silencing EphA2 receptor expression.
Figure 1.
Silencing the EphA2 expression resulted in attenuation of MM tumor growth. A. MMC were incubated in serum free medium (control); B. MMC transfected with 100 nM siRNA sequence to EphA2; C. MMC transfected with 100 nM of scrambled-siRNA sequence; Data presented was a representative of three independent obervations. Magnifiation at 100×.
Silencing EphA2 expression induced apoptosis in MMC
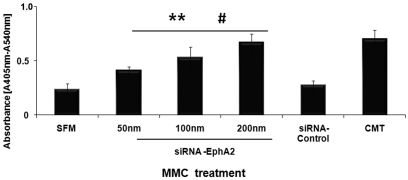
We determined EphA2-siRNA mediated apoptosis in MMC by cell death ELISA. When MMC were transfected with siRNA EphA2 (50nm, 100nm, 200nm), a significant increase in DNA fragmentation was noticed when compared to siRNA-control and untransfected MMC (Figure 2). Silencing EphA2 resulted in increased histone bound DNA fragments formation in the cytoplasm, the characteristic aftermath of apoptosis. Furthermore, the increased apoptosis in MMC was dependent on siRNA-EphA2 concentration. In subsequent assays 100nm of siRNA-EphA2 was used unless otherwise indicated. MM cultures activated with Camptothecin showed increased apoptosis (positive control). These results demonstrate that silencing EphA2 receptor expression induces cell death in MMC.
Figure 2.
Inhibition of receptor-EphA2 with siRNA induced apoptosis in MMC. MMC were transfected with siRNA EphA2 (50nm, 100nm, and 200nm) or siRNA control, or Camptothecin (CMT) and apoptosis was detected by cell-death ELISA. The absorbance value at 405 nm represents the mono- and oligonu-cleosomes in the cytoplasmic fraction of cell lysates. The data presented are mean ± SEM of three independent experiments. ** P < 0.05 compared with MMC cultured in serum free medium (SFM); #P < 0.05 compared with MMC transfected with siRNA-Control.
SiRNA-EphA2 induced apoptotic signaling genes in MMC
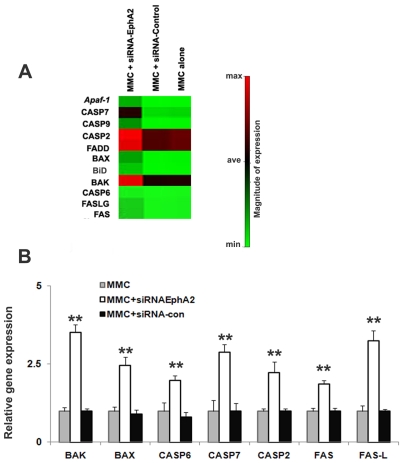
To gain insight into the gene expression profile underlying siRNA-EphA2 induced apoptosis in MMC, MMC were transfected with siRNA-EphA2, control siRNA or un-transfected and examined the gene expression profile using oligo GEAr-rayÒ, microarray that contains 112 genes as described in materials and methods. Figure 3A is a clustergram that demonstrates increased expression of key apoptotic genes in MMC transfected with siRNA-EphA2 when compared to control-siRNA and untransfected MMC. The list of selected genes which showed a greater than 1.5 fold increase in the expression when compared to untransfected MMC was given in Table 2. At least 11 differentially expressed genes involved in Bcl2 family members, death domain genes, caspases, TNF-receptor family, and genes involved in DNA damage response pathway were analyzed. The expression of these genes was also validated by real time-PCR, Figure 3B. Furthermore, in order to elucidate the mechanisms of siRNA-EphA2 mediated apoptosis signaling in MMC, translational expression of selected genes was analyzed by performing protein fractionation and immune blot assays.
Figure 3.
Silencing receptor EphA2 induces the expression of pro-apoptotic genes in MMC. A. Clustergram demonstrating the selected genes that showed expression of apoptotic genes in MMC when transfected with siRNA-EphA2 or siRNA-Control, less than 2 fold increase (Min= minimum), greater than 2 fold increases (Ave = average), greater than 4 fold increase (Max= maximum) compared to untransfected MMC. B. Relative mRNA expression for the selected genes was determined by quantitative real time PCR. The data presented are mean ±SEM of three independent experiments. ** P < 0.05 compared with MMC only.
Table 2.
Apoptotic genes upregulated by silencing interference RNA EphA2 in MMC
| Gene | Fold increase |
|---|---|
| Apaf-1 | 2.61 ± 0.265 |
| BAX | 2.92 ± 0.158 |
| BAK | 3.94 ± 0.301 |
| BID | 2.02 ± 0.284 |
| CASP6 | 1.67 ± 0.065 |
| CASP7 | 4.51 ± 0.372 |
| CASP9 | 3.93 ± 0.286 |
| CASP2 | 4.15 ± 0.561 |
| FADD | 4.49 ± 0.432 |
| FAS | 1.53 ± 0.155 |
| FASL | 2.12 ± 0.202 |
Expression profiles of selected genes with a fold change of > 1.5 when compared to untransfected control. The data represents densitometry values of autoradiography analyzed usingGEArray suite software (SABioscience, Fredrick, CA), Values represent mean ± SE of three independent experiments.
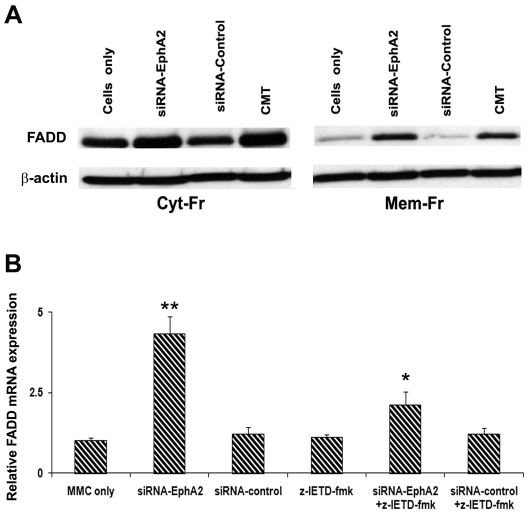
Silencing receptor EphA2 induced FADD expression in MMC
FADD plays an essential role as an adaptor molecule in Fas mediated apoptosis. The expression of FADD was evaluated in protein fractions from MMC transfected with siRNA-EphA2, siRNA-Control and untransfected. FADD expression was increased in siRNA-EphA2 transfected MMC in both cyto-solic and membrane fractions when compared to scrambled siRNA transfected or untransfected MMC. Camptothecin (CMT) was used as a positive control which showed a strong expression of FADD compared to untransfected MMC (Figure 4A). Our data indicates that inhibition of receptor EphA2 induces FADD in MMC. The FADD gene expression was also determined at the transcriptional level by quantitative-PCR analysis. We noticed significantly increased expression of FADD mRNA in siRNA-EphA2 transfected MMC when compared to scrambled (control)-siRNA transfected and untransfected MMC. To understand the association between FADD and caspase-8, an inhibitor for caspase-8 was used. MMC was pretreated with caspase-8 inhibitor (z-IETD-fmk) before transfection with siRNA-Eph2 or control-siRNA and FADD expression was determined. FADD mRNA expression was decreased in the caspase-8 inhibitor treated MMC suggesting that caspase-8 activation is associated with FADD recruitment in MMC (Figure 4B).
Figure 4.
Western blot and mRNA expression analysis for FADD in MMC. A. The cytosolic and membrane bound lyastes after transfection of MMC with siRNA-EphA2 or siRNA-Control or MMC alone were subjected to Western analysis. CMT is Camptothecin (10µM/L) was used as a positive control. β-actin served as a loading control. B. FADD mRNA expression in MMC was determined by quantitative real time PCR. The data presented are mean ± SEM of three independent experiments. ** P < 0.05 compared with MMC only; * P < 0.05 compared to siRNA-EphA2 versus siRNA-EphA2 + z-IETD-fmk; Cyt-Fr = cytoplasmic fraction; Mem-Fr = membrane fraction; z-IETD-fmk = caspase-8 inhibitor.
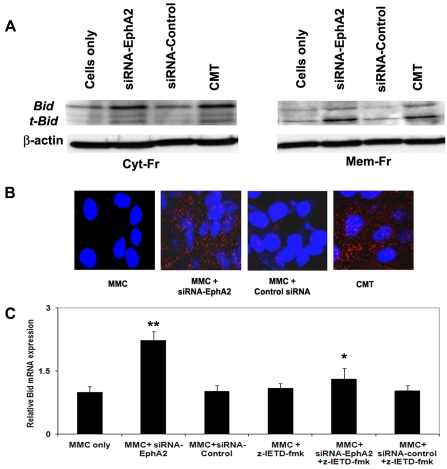
Silencing receptor EphA2 induced the expression of Bid
The knockdown of receptor EphA2 gene activated the intrinsic pathway in MMC by inducing the expression of Bid which promotes cell death. Bid expression was increased in siRNA-EphA2 transfected MMC in both cytosolic and membrane bound fractions when compared to control-siRNA or untransfected MMC. Interestingly, the membrane bound fraction showed strong expression of t-Bid in siRNA-EphA2 transfected MMC (Figure 5A). The translocation of t-Bid to mitochondria promotes the release of cytochrome-c. The immunofluorescence staining for Bid showed diffuse staining in the cytoplasm in siRNA-EphA2 transfected MMC when compared to control-siRNA. MMC treated with CMT showed strong staining for Bid (Figure 5B). The expression of Bid increased significantly in siRNA-EphA2 treated MMC when compared to control-siRNA at the transcriptional levels. In order to understand if Bid activation is dependent on caspase-8, MMC were treated with caspase-8 inhibitor (z-IETD-fmk) before transfection with siRNA-Eph2 or control-siRNA and the expression of Bid was determined. Decreased expression of Bid was noted in the MMC treated with caspase-8 inhibitor suggesting that caspase-8 activation is associated with Bid (Figure 5C). These data indicate that knockdown of receptor EphA2 gene induced Bid expression and the expression of Bid is dependent on the activation of caspase-8 in FADD mediated MMC apoptosis.
Figure 5.
Bid gene expression was upregulated in MMC trans-fected with siRNA-EphA2. A. Western blot of cytosolic and membrane lyastes of MMC after transfection with siRNA-EphA2 or siRNA-Control or un-transfected, CMT = Camptothecin treated MMC. B. Bid expression in MMC as visualized by fluorescence staining. The red fluorescence in the cytoplasmic region indicates Bid and nuclei are stained blue using DAPI. C. Relative mRNA expression for Bid. The data presented are mean ± SEM from three independent experiments. **, * P < 0.05, ** compared with MMC only; * siRNA-EphA2 versus siRNA-EphA2 + z-IETD-fmk; Cyt-Fr = cytoplasmic fraction; Mem-Fr = membrane fraction; z-IETD-fmk = a caspase-8 inhibitor.
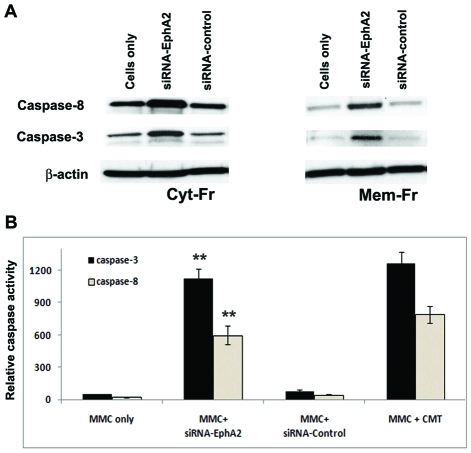
Silencing receptor EphA2 induced the expression of Caspase- 8 and Caspase-3
Caspase-8 is a pro-apoptotic protease which initiates the extrinsic apoptotic pathway signaling in MMC. The expression of caspase-8 was evaluated in cytosolic and membrane bound lysate protein fractions from MMC transfected with siRNA-EphA2 or control-siRNA or untransfected. Caspase-8 expression was increased in siRNA-EphA2 transfected MMC in both cytosolic and membrane bound fractions when compared to control-siRNA transfected or untransfected MMC (Figure 6A). The activity of caspase-8 was also evaluated by fluorescence analysis using the substrate Ac-IETD-AFC in MMC. The level of caspase-8 activity was found to be higher in MMC transfected with siRNA-EphA2 when compared to control siRNA (Figure 6B). These data together indicate that knockdown of receptor EphA2 gene induced the expression of caspase-8, and activation of caspase-8 may in turn cleave the Bid to the truncated form of Bid, t-Bid, in MMC.
Figure 6.
Caspase-8 and caspases-3 expression was increased in MMC transfected with siRNA-EphA2. A. Western blot showing caspase-8 and caspase-3 specific bands in cytosolic and membrane lyastes after trans-fection of MMC with siRNA-EphA2 or control siRNA or MMC alone; B. Caspase-8 and caspases-3 specific activities measured by fluorescence analysis. MMC transfected with siRNA-EphA2 or siRNA-control, 50 µg of cellular proteins (cytosolic and membrane bound) were allowed to react with 20µmol/L of substrates Ac-IETD-AFC for caspase-8 and Ac-DEVE-AFC for caspase-3, respectively. CMT served as a positive control. The AFC liberated from the substrates were measured at excitation wavelength of 400nm and emission of 500nm. The data presented are mean ± SEM of three independent experiments. ** P < 0.05 compared with MM Cells only.
Caspase-3 expression was increased in siRNA-EphA2 transfected MMC in cytosolic fraction when compared to control siRNA or untrans-fected MMC (Figure 6A). Caspase-3 activity was measured by fluorescence analysis in the cytosol lysates from MMC transfected with siRNA-EphA2 or control-siRNA using Ac-DEVD-AFC as the caspase-3 substrate. When caspase-3 is activated in the cytosol, it can resolve the fluorescent substrate and the activity can be analyzed by a fluorescence analyzer. The caspase-3 activity was higher in siRNA-EphA2 transfected MMC when compared to control-siRNA or untransfected MMC (Figure 6B). Induction of Caspase-3 activity leads to apoptosis in MMC.
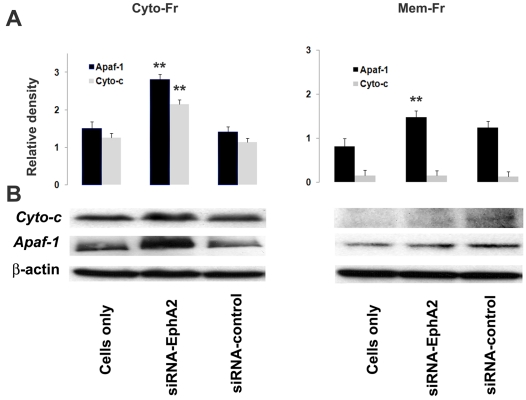
Silencing receptor EphA2 induced the expression of cytochrome-c, and Apaf-1
Cytochrome-c is an important mediator of apoptosis. The activation of cell surface death receptor, FADD results in release of cytochrome-c into the cytosol from mitochondria. Cytochrome-c, and Apaf-1 expression was increased in siRNA-EphA2 transfected MMC in cytosolic fraction when compared to control siRNA or untrans-fected MMC (Figure 7A and B). However, the expression of cytochrome-c was modest in membrane bound fractions, which indicates that the cytochrome-c is released into the cytoplasm. The release of cytochrome-c into cytoplasm results in association of cytochrome-c with Apaf-1 and leads to the formation of the apoptosome complex. The cytochrome-c release also triggers the activation of caspases, particularly caspase-9. These results indicate that receptor EphA2 gene regulates the activity of cytochrome-c in MMC.
Figure 7.
Cytochrome-c and Apaf-1 expression in MMC. A. Relative densitometry value as obtained by Bio Rad Imaging densitometer using Quality one software program. B. Silencing receptor EphA2 expression induced cytochrome-c and Apaf-1 expression in cytosolic and membrane lyastes of MMC as determined by Western blot analysis. The data presented are mean ± SEM of three independent experiments and values are considered significant if p < 0.05, **comparison, siRNA-EphA2 versus MM Cells only.
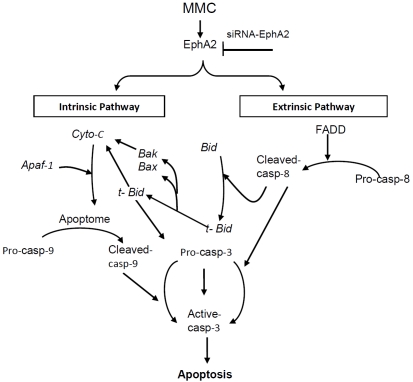
Taken together these results indicate that knockdown of receptor EphA2 expression induces the activation of death receptor FADD resulting in the activation of caspase-8. The cleavage of Bid into t-Bid indicated that intrinsic pathway is turned on in MMC upon silencing expression of receptor EphA2. The schematic diagram (Figure 8) summarizes the cascade of events that are prominent in siRNA-EphA2 mediated apoptosis signaling pathway in MMC. This is the first study to demonstrate that silencing receptor EphA2 expression induces apoptosis in MMC via extrinsic and intrinsic pathway.
Figure 8.
Schematic Model of apoptotic signaling induced by targeting EphA2 gene by siRNA in MMC. The extrinsic pathway is initiated with FADD activation which affects caspase-8 activity. The activated caspase-8 cleaves Bid to t-Bid which transloactes to the mitochon-drial compartment that indicates the onset of intrinsic pathway. The various intermediate molecules studied were listed in the diagram. Cyto-c = cytochrome-c; casp-8 = caspase-8; casp-9 = caspase-9; casp-3 = caspase-3; Apaf-1 = apoptotic activating factor -1. Arrows indicates activation, and inverted ‘T’ sign indicate inhibition.
Discussion
MM is a fatal malignancy with almost 100% mortality. Unlike other cancers, MM is associated with dysregulated growth and the tumor is resistant to conventional anti-cancer treatment modalities [20, 21]. One of the striking features of MM is its aggressive nature which promotes oncogenic progression. MM cells are resistant to apoptotic stimuli and this inhibition of apoptosis facilitate to enhance tumor growth and development [22, 23]. It is well established that drugs used for conventional cancer chemotherapy often exhibit serious nonspecific cytotoxicity that kills healthy tissues as well as cancer cells. Therefore, novel treatment strategies selectively targeting tumor tissue are in great demand. The strategy proposed in this study was based on selective inhibition of target gene sparing normal tissues. Receptor EphA2 is one such candidate gene highly expressed in MM cells and is involved in oncongenic progression of MM. In earlier studies, we reported that receptor EphA2 is over expressed in MMC with little or no expression in normal mesothelial cells, and inhibition of EphA2 expression attenuated proliferation and haptotaxis in MMC[6]. However, the mechanisms of cell death were not clear. In the present study we have demonstrated that inhibition of receptor EphA2 expression attenuated tumor growth due to induction of apoptosis via FADD activity in MMC. We report that silencing the receptor EphA2 induces apoptosis via extrinsic and intrinsic pathways in MMC.
FADD is essential for signal transduction of death receptor signaling. Over-expression of FADD induces cell death in adenocarcinoma cells [24]. FADD forms an essential component of the death inducing signaling complex (DISC) and in addition, increased expression of FADD causes cell death even in the absence of ligand binding [25-27]. In MMC, inhibition of the EphA2 receptor enhanced the expression of FADD in the cytosolic fraction whereas the membrane fraction showed moderate expression. The increased expression of FADD indicates that silencing receptor EphA2 induces apoptosis in MMC via a death receptor signaling pathway. Interestingly, the levels of FADD significantly decreased when MMC were pretreated with caspase-8 inhibitor before silencing receptor EphA2 expression. Stupack et al., 2006, have demonstrated that increased expression of caspase-8 suppresses the invasion of tumor cells via induction of apoptosis in neuroblastoma [28]. Activation of caspases is one of the key events in apoptosis process initiation. Caspase-8 along with caspase-2 and caspase-9 are associated with initiation of apoptosis. Pro-caspase-8 is activated to its active form upon interaction with FADD. Therefore it is possible that in MMC over-expression of receptor EphA2 turns off the FADD mediated pathway while directing the downstream signals to promote tumor growth. Whereas silencing of EphA2 expression turns on the death receptor pathway via it's up regulation and subsequently activates caspase-8. Indeed, caspase-8 is an initiator caspase in the extrinsic pathway, which then cleaves effector caspase, caspase-3.
In earlier studies we noticed increased expression of active caspase-9 upon silencing EphA2 expression by siRNA in MMC [6]. In the present study we further report that silending EphA2 gene also induced the expression of Bid, active caspase-8, cytochrome-c, and Apaf-1 in MMC. Others have noticed that cellular stress such as silencing of the gene receptor EphA2 up stream of mitochondria induces the expression of distinct caspases [29]. The initiator caspases such as caspase-8, caspase-9 are known to be activated during early stage of apoptosis. Caspase-8 initiates downstream effector caspases including caspase-3 and eventually regulates apoptosis [30, 31]. Caspase-8 is activated following DNA damage. Caspase-8 stimulates mitochondrial outer membrane permeability either directly or indirectly through cleavage of Bid and relies upon the Apaf-1 apoptosome to activate effector caspases and induce apoptosis [12, 13]. The activation of caspase-8 by FADD leads to cleavage of Bid. Bid exists as a precursor in the cytosolic fraction of living cells that becomes activated upon cleavage by caspase-8. Bid is a specific proximal substrate for caspase-8 in the Fas mediated signaling pathway. Elimination of Bid by immuno-depletion has abolished cytochrome-c release suggesting Bid is essential for intrinsic pathway [10]. The full length of Bid is localized in the cytosol and the truncated Bid (t-Bid) is found in mitochondria. In MMC, silencing receptor EphA2 induced the expression of Bid in cytosolic fractions as well as membrane fractions. The expression of t-Bid was noticed more in the membrane fractions suggesting the cleavage of Bid and translocation to mitochondrial compartment. Hence in MMC, the apoptotic signal transduced from the cytosol to mitochondria initiates the cross-talk with the mitochondrial pathway.
The intrinsic pathway is complex and Bcl2 family proteins regulate apoptosis mediated by mitochondria. Bcl2 members monitor changes in the intracellular environment such as absence of an oncogenic signal and DNA damage which induces permeability of mitochondrial membrane [32]. The anti-apoptotic proteins Bak and Bax compromise the membrane integrity leading to the release of cytochrome-c. Silencing receptor EphA2 induced the expression of Bak, Bax and release of cytochrome-c in MMC. Cytochrome-c is normally found in the inter membrane space of mitochondria, which is released into the cytosol during apoptosis [33]. Thus, release of cytochrome-c triggers the activation of caspases through Apaf-1 [34]. In MMC silencing receptor EphA2 induced caspase-3 activity indicating the association between EphA2 and caspase-3. Studies from glioma cells indicate that inhibition of EphA2 by siRNAs increased caspase-3 activity [35, 36]. Caspase-3 is a key mediator of apoptosis which is responsible for cleavage of structural elements of the cytoplasm and nucleus. Cells become round in shape, and undergo nuclear fragmentation, a hallmark of apoptosis [37, 38]. Taken together our results demonstrate that silencing EphA2 gene induces activation of caspase-3 thereby resulting in apoptosis of MMC via the intrinsic pathway.
In conclusion, this study indicates that silencing EphA2 receptor is a novel and effective strategy for inhibition of MM tumor growth. Silencing EphA2 receptor expression results in MMC cell death due to the activation of both extrinsic and intrinsic apoptotic pathways. We conclude that this concept for silencing EphA2 may prove to be a new and effective therapeutic approach to the clinical management of patients with malignant mesothelioma.
Acknowledgments
This work was supported by NIR grant # 09KN-09; RC1 grant # 09KW-08 from Florida Department of Health to Nasreen Najmunnisa (2009). We thank Zita Burkhalter for technical assistance.
List of Abbreviations
- MM
Malignant mesothelioma
- MMC
Malignant mesothelioma cells
- RTKs
Receptor tyrosine kinase
- FADD
Fas associated death domain
- APAF1
Apoptotic protease activating factor 1
- RT-PCR
Reverse transcriptase polymerase chain reaction
References
- 1.Grondin SC, Sugarbaker DJ. Malignant mesothelioma of the pleural space. Oncology (Williston Park) 1999;13:919–26. discussion 26, 31-2. [PubMed] [Google Scholar]
- 2.Jaklitsch MT, Grondin SC, Sugarbaker DJ. Treatment of malignant mesothelioma. World J Surg. 2001;25:210–7. doi: 10.1007/s002680020021. [DOI] [PubMed] [Google Scholar]
- 3.Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet. 2005;366:397–408. doi: 10.1016/S0140-6736(05)67025-0. [DOI] [PubMed] [Google Scholar]
- 4.Becklake MR, Bagatin E, Neder JA. Asbestosrelated diseases of the lungs and pleura: uses, trends and management over the last century. Int J Tuberc Lung Dis. 2007;11:356–69. [PubMed] [Google Scholar]
- 5.Reed JC. Dysregulation of apoptosis in cancer. Cancer J Sci Am. 1998;4(Suppl 1):S8–14. [PubMed] [Google Scholar]
- 6.Nasreen N, Mohammed KA, Antony VB. Silencing the receptor EphA2 suppresses the growth and haptotaxis of malignant mesothelioma cells. Cancer. 2006;107:2425–35. doi: 10.1002/cncr.22254. [DOI] [PubMed] [Google Scholar]
- 7.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 8.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–82. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 9.Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–90. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 12.Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES. Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J Biol Chem. 2002;277:13430–7. doi: 10.1074/jbc.M108029200. [DOI] [PubMed] [Google Scholar]
- 13.Robertson JD, Gogvadze V, Kropotov A, Vakifahmetoglu H, Zhivotovsky B, Orrenius S. Processed caspase-2 can induce mitochondriamediated apoptosis independently of its enzymatic activity. EMBO Rep. 2004;5:643–8. doi: 10.1038/sj.embor.7400153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/ caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 15.Nasreen N, Mohammed KA, Dowling PA, Ward MJ, Galffy G, Antony VB. Talc induces apoptosis in human malignant mesothelioma cells in vitro. Am J Respir Crit Care Med. 2000;161(2 Pt 1):595–600. doi: 10.1164/ajrccm.161.2.9904123. [DOI] [PubMed] [Google Scholar]
- 16.Lai YM, Mohammed KA, Nasreen N, Baumuratov A, Bellew BF, Antony VB. Induction of cell cycle arrest and apoptosis by BCG infection in cultured human bronchial airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L393–401. doi: 10.1152/ajplung.00392.2006. [DOI] [PubMed] [Google Scholar]
- 17.Antony AB, Tepper RS, Mohammed KA. Cockroach extract antigen increases bronchial airway epithelial permeability. J Allergy Clin Immunol. 2002;110:589–95. doi: 10.1067/mai.2002.127798. [DOI] [PubMed] [Google Scholar]
- 18.Mohammed KA, Nasreen N, Hardwick J, Van Horn RD, Sanders KL, Antony VB. Mycobacteria induces pleural mesothelial permeability by down-regulating beta-catenin expression. Lung. 2003;181:57–66. doi: 10.1007/s00408-003-1006-1. [DOI] [PubMed] [Google Scholar]
- 19.Nasreen N, Mohammed KA, Sanders KL, Hardwick J, Van Horn RD, Sharma RK, et al. Pleural mesothelial cells modulate polymorphonuclear leukocyte apoptosis in empyema. J Clin Immunol. 2003;23:1–10. doi: 10.1023/a:1021958529763. [DOI] [PubMed] [Google Scholar]
- 20.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 21.Stewart DJ, Edwards JG, Smythe WR, Waller DA, O'Byrne KJ. Malignant pleural mesothelioma–an update. Int J Occup Environ Health. 2004;10:26–39. doi: 10.1179/oeh.2004.10.1.26. [DOI] [PubMed] [Google Scholar]
- 22.Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000;157:1415–30. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 24.Yin A, Jiang Y, Zhang X, Luo H. Overexpression of FADD enhances 5-fluorouracil-induced apoptosis in colorectal adenocarcinoma cells. Med Oncol. 2009 doi: 10.1007/s12032-009-9224-x. May 5. [DOI] [PubMed] [Google Scholar]
- 25.Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–12. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 26.Sandu C, Morisawa G, Wegorzewska I, Huang T, Arechiga AF, Hill JM, Kim T, Walsh CM, Werner MH. FADD self-association is required for stable interaction with an activated death receptor. Cell Death Differ. 2006;13:2052–61. doi: 10.1038/sj.cdd.4401966. [DOI] [PubMed] [Google Scholar]
- 27.Carrington PE, Sandu C, Wei Y, Hill JM, Morisawa G, Huang T, Gavathiotis E, Wei Y, Werner MH. The structure of FADD and its mode of interaction with procaspase-8. Mol Cell. 2006;22:599–610. doi: 10.1016/j.molcel.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Stupack DG, Teitz T, Potter MD, Mikolon D, Houghton PJ, Kidd VJ, Lahti JM, Cheresh DA. Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature. 2006;439:95–9. doi: 10.1038/nature04323. [DOI] [PubMed] [Google Scholar]
- 29.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–90. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 30.Wu GS, Burns TF, Zhan Y, Alnemri ES, El-Deiry WS. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosisinducing ligand (TRAIL) death receptor. Cancer Res. 1999;59:2770–5. [PubMed] [Google Scholar]
- 31.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosisinducing ligand in vivo. Nat Med. 1999;5:157–63. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 32.Gustafsson AB, Gottlieb RA. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol. 2007;292:C45–51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- 33.Reed JC. Cytochrome c: can't live with it–can't live without it. Cell. 1997;91:559–62. doi: 10.1016/s0092-8674(00)80442-0. [DOI] [PubMed] [Google Scholar]
- 34.Krippner A, Matsuno-Yagi A, Gottlieb RA, Babior BM. Loss of function of cytochrome c in Jurkat cells undergoing fas-mediated apoptosis. J Biol Chem. 1996;271:21629–36. doi: 10.1074/jbc.271.35.21629. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Z, Yuan X, Li Z, Tu H, Li D, Qing J, Wang H, Zhang L. RNA interference targeting EphA2 inhibits proliferation, induces apoptosis, and cooperates with cytotoxic drugs in human glioma cells. Surg Neurol. 2008;70:562–8. doi: 10.1016/j.surneu.2008.04.031. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 36.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene. 2004;23:1448–56. doi: 10.1038/sj.onc.1207247. [DOI] [PubMed] [Google Scholar]
- 37.Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–52. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 38.Blanc C, Deveraux QL, Krajewski S, Janicke RU, Porter AG, Reed JC, Jaggi R, Marti A. Caspase-3 is essential for procaspase-9 processing and cisplatin-induced apoptosis of MCF-7 breast cancer cells. Cancer Res. 2000;60:4386–90. [PubMed] [Google Scholar]