Abstract
Adrenocortical carcinoma (ACC) is a very aggressive tumor with a poor prognosis. Available treatments for this type of cancer are far from being satisfactory. The IGF signalling pathway represents an important mechanism for ACT growth and constitutes a relevant therapeutic target. We investigated the effect of picropodophyllin (PPP), a member of the cyclolignan family and a new inhibitor of IGF-1R, on proliferation of human adrenocortical cell lines H295R and SW-13. PPP inhibits proliferation and induces an important accumulation in G2/M phase and apoptosis of H295R and SW-13 cells. Our data suggest that PPP may be a promising candidate for drug development for adrenocortical carcinoma.
Keywords: Adrenal cortex, cancer, IGF receptor, cell lines
Introduction
Adrenocortical carcinoma (ACC) is a rare and very aggressive tumor with an incidence of approximatively 1-2 per million people per year [1, 2]. Its poor prognosis depends mainly upon a limited number of therapeutic tools. A complete surgical resection after an early diagnosis is the most valuable option for treatment. The use of the adrenolytic agent, mitotane (o,p-DDD), associated or not with DNA-damaging drugs, is the only medical therapy available up to date [3].
The molecular mechanisms of tumorigenesis of the adrenal cortex are to be found in a multistep process where genetic alterations and signaling pathways deregulation are combined. The insulin-like growth factor system is one of the best-investigated molecular pathways involved in adrenal growth. Several genetic alterations such as loss of imprinting or loss of heterozygosity of the 11p15 gene locus causing a strong IGF2 overexpression have been demonstrated in the majority of adult and childhood ACCs [4-7]. IGF2 binds two distinct receptors, type I (IGF-1R) and type II (IGF-IIR). Similar to the insulin receptor, IGF-1R is a receptor tyrosine kinase composed of two heterodimeric subunits that possesses an intrinsic tyrosine kinase activity, and activates a variety of downstream effectors associated with this receptor family. Since overexpression of IGF-1R has been found in a substantial proportion of ACCs, it is likely that locally produced IGF2 acts as an autocrine or paracrine growth factor in adrenocortical tumorigenesis [8-10]. On the basis of the pivotal role of IGF-1R in IGF2 signaling, it becomes evident that this receptor represents a promising target for adrenocortical tumors therapy. It has been recently reported that suppression of IGF2 /IGF-1R signaling, through the use of the IGF-1R inhibitor NVP-AEW541 or by using blocking antibodies, inhibits ACC cell line proliferation in vitro and in vivo in a human ACC xenograft model [9, 10].
Picropodophyllin (PPP), a member of the cyclolignan family, has recently been described as an inhibitor of IGF-1R. PPP inhibits phosphorylation of IGF-1R without interfering with the highly homologous insulin receptor or tyrosine kinases of other relevant growth factor receptors relevant for cancer cells [11]. PPP induces tumor regression and inhibition of metastasis in several models of human cancer and its administration is well tolerated in vivo [12]. These data prompted us to investigate the effect of PPP on the growth of two established human ACC cell lines (H295R and SW-13).
Materials and Methods
Chemicals
PPP was synthetized in an ultrapure form as described [11]. NVP-AEW541 [13] was provided by Novartis. Stock solutions of both compounds were prepared in DMSO (50 mM and 10 mM, respectively).
Cell culture and proliferation assays
H295R cells were cultured in DMEM/F-12 supplemented with 2% NuSerum, 1% ITS Plus and antibiotics, as described [14]. SW-13 cells were cultured in DMEM/F12 supplemented with 10% FCS and antibiotics. To measure proliferation, cells were seeded in duplicate in 24-well plates at the density of 3×104 cells/well and cultured in complete medium in the presence of the indicated concentration of the different compounds or DMSO added to the culture medium. Cells were counted after 3 days of culture using the COUNTESS automate instrument (Invitrogen).
Immunoblots
H295R and SW-13 cells were treated with the indicated concentrations of different compounds or with DMSO vehicle. Protein extracts were prepared by harvesting cells in RIPA buffer [(50mM Tris-HCl pH 7.4, 150mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 1 mM EDTA, 50mM NaF, Protein Inhibitor Cocktails 1 and 2 (Sigma)]. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Immunoblot was performed using a chemilumi-nescence system for protein detection (ECL Plus, GE Healthcare). Antibodies used were as follows: anti-IGF1Rβ; anti-Akt (total) and phospho-Akt(Ser473); anti-p44/p42 mitogen-activated protein kinase and anti-phospho-p44/p42 mitogen-activated protein kinase (all from Cell Signaling Technology); anti-phosphotyrosine PY20 (Sigma).
Flow cytometry
H295R and SW-13 cells were fixed in 70% ethanol and then treated with RNAse A (50 µg/mL) for 30 min at 37°C. DNA was stained with propidium iodide (50 µg/mL) and cells were analyzed for cell-cycle distribution with a FAC-Scan instrument (Becton Dickinson).
Results
In this study, we used two established human ACC cell lines: the well-differentiated H295R cells, that retain the ability to synthetize steroid hormones, and SW-13 derived from a stage IV tumor that are not steroidogenic. Both cell lines represent suitable models to study the effects of IGF-1R inhibitors since they express high levels of IGF-1R. However, H295R cells, but not SW -13, produce high levels of IGF2, which acts in an autocrine manner to trigger their proliferation [8, 9].
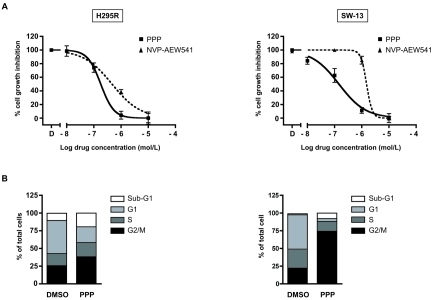
We first evaluated the effect of different doses of PPP on H295R and SW-13 cell proliferation and compared it with the NVP-AEW541 IGF-1R inhibitor. After 3 days of treatment, PPP inhibited cell growth of H295R (IC50 1.8 × 10−7M) and SW-13 (IC50 1.4 × 10−7M) cells in a dose-dependent manner, having a more potent effect than NVP-AEW541 on both cell lines (IC50 4.6 × 10−7M for H295R cells and IC50 1.6 × 10−6M for SW-13 cells; Figure 1A).
Figure 1.
The cyclolignan PPP inhibits cell growth and induces G2/M accumulation and apoptosis in the H295R and SW13 ACC cell lines. A, H295R and SW-13 cells were cultured in 24 well plates in the presence of DMSO or of increasing doses of PPP (10−8 to 10−5 M; black squares) or NVP-AEW541 (10−7 to 10−5 M; black triangles) and counted after 3 days of culture. The experiments were performed in duplicate and represent the mean ± SEM of at least five experiments for PPP and two for NVP-AEW541. B, H295R and SW-13 cells were treated with DMSO as a control or 1 µM PPP for 24h before analysis of cell cycle distribution.
We next evaluated the effect of PPP on cell cycle distribution after a 24h treatment. Exposure of H295R cells to 1 mM PPP increased the fraction of cells in G2/M-phase (from 25% of DMSO control to 38%) and sub-G1 (hypodiploid apoptotic cells; from 10% of DMSO control to 19%) with a corresponding decrease of the fraction of cells in the G1-phase (from 47% of DMSO control to 22%). The PPP-induced G2/M accumulation (from 22% of DMSO control to 74%) and apoptosis (from 2% of DMSO control to 8%) were also observed in SW-13 cells (Figure 1B).
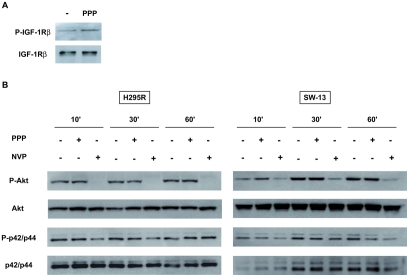
Surprisingly, PPP did not significantly modulate phosphorylation of IGF-1Rβ in H295R cells (Figure 2A). The apoptotic effect of PPP has been shown to be associated with an important inhibition of PI3K/Akt pathway and a moderate effect on the ERK pathway in other cell types [11, 15]. After a 48h serum starvation, H295R and SW13 cells were treated with or without 10−5M PPP for 2h and finally stimulated with 10% serum. We observed that PPP exerts no significant effect on Akt and ERK1/2 phosphorylations whereas NVP-AEW541 exerts a substantial inhibition of Akt and a moderate effect on ERK1/2 phosphorylations. These results suggest that PPP effect does not involve inhibition of the Akt and ERK1/2 effect in adrenocortical cell lines (Figure 2B).
Figure 2.
Effect of PPP on IGF-1Rβ phosphorylation and downstream signaling pathways. A, Tyrosine-phosphorylated proteins were immunoprecipitated using the PY20 antibody from H295R cells and IGF-1Rβ revealed by immunoblotting (top panel), in basal conditions or after pre-incubation with 1 µM PPP for 6 hours. Total IGF-1Rβ expression is also shown (bottom panel). B, Expression of phospho-Akt, total Akt, phospho-ERK1/2, total ERK1/2 in H295R and SW-13 cells was analysed by immunoblot of serum-starved cells incubated with or without 10 µM PPP or NVP-AEW541 during 2h and stimulated with serum for 10, 30 or 60 min.
Discussion
The factors responsible for the incidence of benign adrenocortical tumors and its malignant transformation are not well understood. In addition to IGF2 overexpression, increased levels of the IGF-1R have been found in adrenocortical carcinomas, suggesting an important role for the IGF-system in adrenocortical carcinogenesis [9, 10]. These results indicated that IGF-1R may represent an important target for cancer therapy. Recently some studies using IGF-1R inhibitors such as NVP-AEW541 or an anti-IGF-1R monoclonal antibody produced inhibition of ACC cell growth in vitro and in vivo [9, 10]. The results of a phase I clinical study of an anti-IGF-1R monoclonal antibody in patients with advanced ACC have been reported [16].
In this report we have studied the effect of PPP, a member of the cyclolignan family described as a specific inhibitor of the IGF-1R, on ACC cells proliferation [11]. PPP has been shown to block the phosphorylation of the IGF-1R without affecting the homologous insulin receptor [11]. This represents an obvious advantage over some other IGF-1R inhibitors. Moreover, PPP has been shown to be well tolerated in vivo after oral administration [11, 12]. For these reasons, PPP may represent a compound potentially interesting for drug development for ACC.
We have shown that PPP inhibits growth of two different human ACC cell lines (H295R and SW13) in vitro. Cell cycle analysis revealed that a 24h treatment with PPP drastically increased the fraction on G2/M and sub-G1 phases. These effects on cell cycle have been also observed in multiple myeloma cells after PPP treatment [17]. PPP exerts an important effect on proliferation at lower concentrations than NVP-AEW541. Nevertheless, we could not detect any effect of PPP on rapid phosphorylation of Akt and ERK1/2, whereas it has been largely associated with an inhibition of PI3K/Akt pathway in other cell lines [15]. These results suggest that in ACC cell lines, the inhibitory effect of PPP does not primarily involve these signaling pathways. Recently, other reports revealed that PPP may act with mechanisms different from Akt/Erk inhibition. PPP is a stereoisomere of podo-phyllotoxin (PPT), an established inhibitor of microtubule assembly leading to mitotic arrest presenting a general toxicity [18, 19]. However, PPP used here is an ultrapure compound. It was shown that PPP does not bind to tubulin at concentrations up to 50 µM while PPT does. The effect of PPP observed in our cellular model is observed at a concentration of 0.1 µM.
Further studies are necessary to further characterize the molecular mechanism of the inhibitory action of PPP on adrenocortical cell lines proliferation. This compound may represent an interesting therapeutic tool to be associated to newer drugs in ACT chemotherapy [14, 20-22], in order to develop more selective and specific treatments for clinical use.
Acknowledgments
We thank J. Cazareth for help with cell cycle analysis and Novartis for NVP-AEW541. Research in E.L. laboratory is funded by Institut National du Cancer, CNRS (LIA NEOGENEX) and FP7 ENS@T-CANCER.
Abbreviations
- ACC
adrenocortical carcinoma
- PPP
picropodophyllin
- IGF-1R
type 1 IGF receptor
References
- 1.Libe R, Fratticci A, Bertherat J. Adrenocortical cancer: pathophysiology and clinical management. Endocr Relat Cancer. 2007;14:13–28. doi: 10.1677/erc.1.01130. [DOI] [PubMed] [Google Scholar]
- 2.Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2009;23:273–289. doi: 10.1016/j.beem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Berruti A, Ferrero A, Sperone P, Daffara F, Reimondo G, Papotti M, Dogliotti L, Angeli A, Terzolo M. 2008 Emerging drugs for adrenocortical carcinoma. Expert Opin Emerg Dr. 2008;13:497–509. doi: 10.1517/14728214.13.3.497. [DOI] [PubMed] [Google Scholar]
- 4.Gicquel C, Bertagna X, Schneid H, Francillard-Leblond M, Luton JP, Girard F, Le Bouc Y. Rearrangements at the 11p15 locus and overex-pression of insulin-like growth factor-II gene in sporadic adrenocortical tumors. J Clin Endocrinol Metab. 1994;78:1444–1453. doi: 10.1210/jcem.78.6.7911125. [DOI] [PubMed] [Google Scholar]
- 5.Rosati R, Cerrato F, Doghman M, Pianovski MA, Parise GA, Custodio G, Zambetti GP, Ribeiro RC, Riccio A, Figueiredo BC, Lalli E. High frequency of loss of heterozygosity at 11p15 and IGF2 overexpression are not related to clinical outcome in childhood adrenocortical tumors positive for the R337H TP53 mutation. Cancer Genet Cytogenet. 2008;186:19–24. doi: 10.1016/j.cancergencyto.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West AN, Neale GA, Pounds S, Figueredo BC, Rodriguez Galindo C, Pianovski MA, Oliveira Filho AG, Malkin D, Lalli E, Ribeiro R, Zambetti GP. Gene expression profiling of childhood adrenocortical tumors. Cancer Res. 2007;67:600–608. doi: 10.1158/0008-5472.CAN-06-3767. [DOI] [PubMed] [Google Scholar]
- 7.Giordano TJ, Thomas DG, Kuick R, Lizyness M, Misek DE, Smith AL, Sanders D, Aljundi RT, Gauger PG, Thompson NW, Taylor JM, Hanash SM. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am J Pathol. 2003;162:521–531. doi: 10.1016/S0002-9440(10)63846-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logie A, Boulle N, Gaston V, Perin L, Boudou P, Le Bouc Y, Gicquel C. Autocrine role of IGF-II in proliferation of human adrenocortical carcinoma NCI H295R cell line. J Mol Endocrinol. 1999;23:23–32. doi: 10.1677/jme.0.0230023. [DOI] [PubMed] [Google Scholar]
- 9.Barlaskar FM, Spalding AC, Heaton JH, Kuick R, Kim AC, Thomas DG, Giordano TJ, Ben-Josef E, Hammer GD. Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab. 2009;94:204–212. doi: 10.1210/jc.2008-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almeida MQ, Fragoso MC, Lotfi CF, Santos MG, Nishi MY, Costa MH, Lerario AM, Maciel CC, Mattos GE, Jorge AA, Mendonca BB, Latronico AC. Expression of insulin-like growth factor-II and its receptor in pediatric and adult adrenocortical tumors. J Clin Endocrinol Metab. 2008;93:3524–3531. doi: 10.1210/jc.2008-0065. [DOI] [PubMed] [Google Scholar]
- 11.Girnita A, Girnita L, del Prete F, Bartolazzi A, Larsson O, Axelson M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004;64:236–242. doi: 10.1158/0008-5472.can-03-2522. [DOI] [PubMed] [Google Scholar]
- 12.Menu E, Jernberg-Wiklund H, Stromberg T, De Raeve H, Girnita L, Larsson O, Axelson M, Asosingh K, Nilsson K, Van Camp B, Vanderkerken K. Inhibiting the IGF-1 receptor tyrosine kinase with the cyclolignan PPP: an in vitro and in vivo study in the 5T33MM mouse model. Blood. 2006;107:655–660. doi: 10.1182/blood-2005-01-0293. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Echeverria C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro HG, Cozens R, Evans DB, Fabbro D, Furet P, Porta DG, Liebetanz J, Martiny-Baron G, Ruetz S, Hofmann F. In vivo anti-tumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-1R kinase. Cancer Cell. 2004;5:231–239. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 14.Doghman M, El Wakil A, Cardinaud B, Thomas E, Wang J, Zhao W, Peralta Del Valle MHC, Figueiredo BC, Zambetti GP, Lalli E. Regulation of insulin-like growth factor-mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors. Cancer Res. 2010;70:4666–4675. doi: 10.1158/0008-5472.CAN-09-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasilcanu D, Girnita A, Girnita L, Vasilcanu R, Axelson M, Larsson O. The cyclolignan PPP induces activation loop-specific inhibition of tyrosine phosphorylation of the insulin-like growth factor-1 receptor. Link to the phosphatidyl inositol-3 kinase/Akt apoptotic pathway. Oncogene. 2004;23:7854–7862. doi: 10.1038/sj.onc.1208065. [DOI] [PubMed] [Google Scholar]
- 16.Haluska P, Worden F, Olmos D, Yin D, Schteingart D, Batzel GN, Paccagnella ML, de Bono JS, Gualberto A, Hammer GD. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemoth Pharmacol. 2010;65:765–773. doi: 10.1007/s00280-009-1083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stromberg T, Ekman S, Girnita L, Dimberg LY, Larsson O, Axelson M, Lennartsson J, Hellman U, Carlson K, Osterborg A, Vanderkerken K, Nilsson K, Jernberg-Wiklund H. IGF-1 receptor tyrosine kinase inhibition by the cyclolignan PPP induces G2/M-phase accumulation and apoptosis in multiple myeloma cells. Blood. 2006;107:669–678. doi: 10.1182/blood-2005-01-0306. [DOI] [PubMed] [Google Scholar]
- 18.Linder S, Shoshan MC, Gupta RS. Picropodo-phyllotoxin or podophyllotoxin does not induce cell death via insulin-like growth factor-I receptor. Cancer Res. 2007;67:2899. doi: 10.1158/0008-5472.CAN-06-0635. [DOI] [PubMed] [Google Scholar]
- 19.Larsson O, Axelson M. Cyclolignans as inhibitors of the insulin-like growth factor-I receptor. Cancer Res. 2007;67 doi: 10.1158/0008-5472.can-03-2522. 2899-a. [DOI] [PubMed] [Google Scholar]
- 20.Doghman M, Cazareth J, Lalli E. The T cell factor/beta-catenin antagonist PKF115-584 inhibits proliferation of adrenocortical carcinoma cells. J Clin Endocrinol Metab. 2008;93:3222–3225. doi: 10.1210/jc.2008-0247. [DOI] [PubMed] [Google Scholar]
- 21.Doghman M, Cazareth J, Douguet D, Madoux F, Hodder P, Lalli E. Inhibition of adrenocortical carcinoma cell proliferation by SF-1 inverse agonists. J Clin Endocrinol Metab. 2009;94:2178–2183. doi: 10.1210/jc.2008-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luconi M, Mangoni M, Gelmini S, Poli G, Nesi G, Francalanci M, Pratesi N, Cantini G, Lombardi A, Pepi M, Ercolino T, Serio M, Orlando C, Mannelli M. Rosiglitazone impairs proliferation of human adrenocortical cancer: preclinical study in a xenograft mouse model. Endocr Relat Cancer. 2010;17:169–177. doi: 10.1677/ERC-09-0170. [DOI] [PubMed] [Google Scholar]