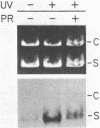
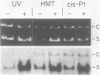
Abstract
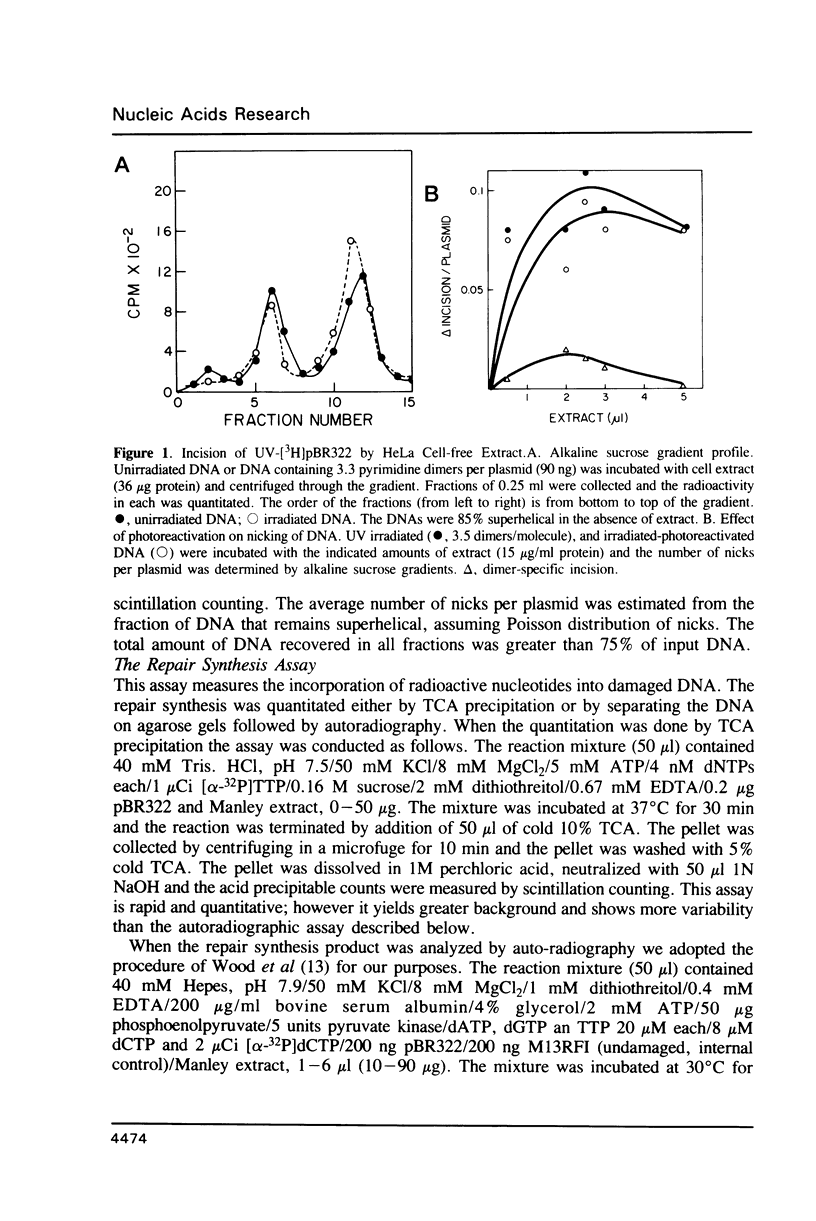
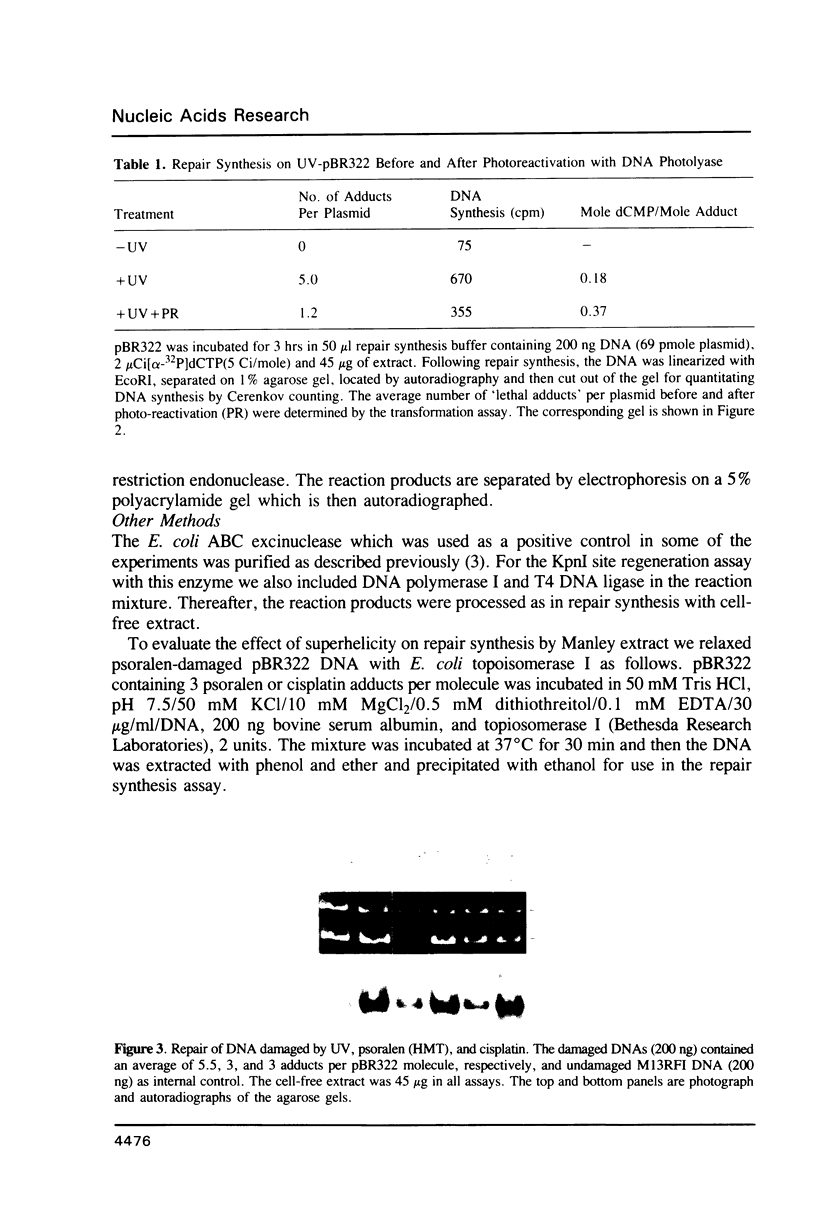
We searched for nucleotide excision repair in human cell-free extracts using two assays: damage-specific incision of DNA (the nicking assay) and damage-stimulated DNA synthesis (the repair synthesis assay). HeLa cell-free extract prepared by the method of Manley et al. (1980) has a weak nicking activity on UV irradiated DNA and the nicking is only slightly reduced when pyrimidine dimers are eliminated from the substrate by DNA photolyase. In contrast to the nicking assay, the extract gives a strong signal with UV irradiated substrate in the repair synthesis assay. The repair synthesis activity is ATP dependent and is reduced by about 50% by prior treatment of the substrate with DNA photolyase indicating that this fraction of repair synthesis is due to removal of pyrimidine dimers by nucleotide excision. Psoralen and cisplatin adducts which are known to be removed by nucleotide excision repair also elicited repair synthesis activity 5-10 fold above the background synthesis. When M13RF DNA containing a uniquely placed psoralen adduct was used in the reaction, complete repair was achieved in a fraction of molecules as evidenced by the restoration of psoralen inactivated KpnI restriction site. This activity is absent in xeroderma pigmentosum group A cells. We conclude that our cell-free extract contains the human nucleotide excision repair enzyme activity.
Full text
PDF



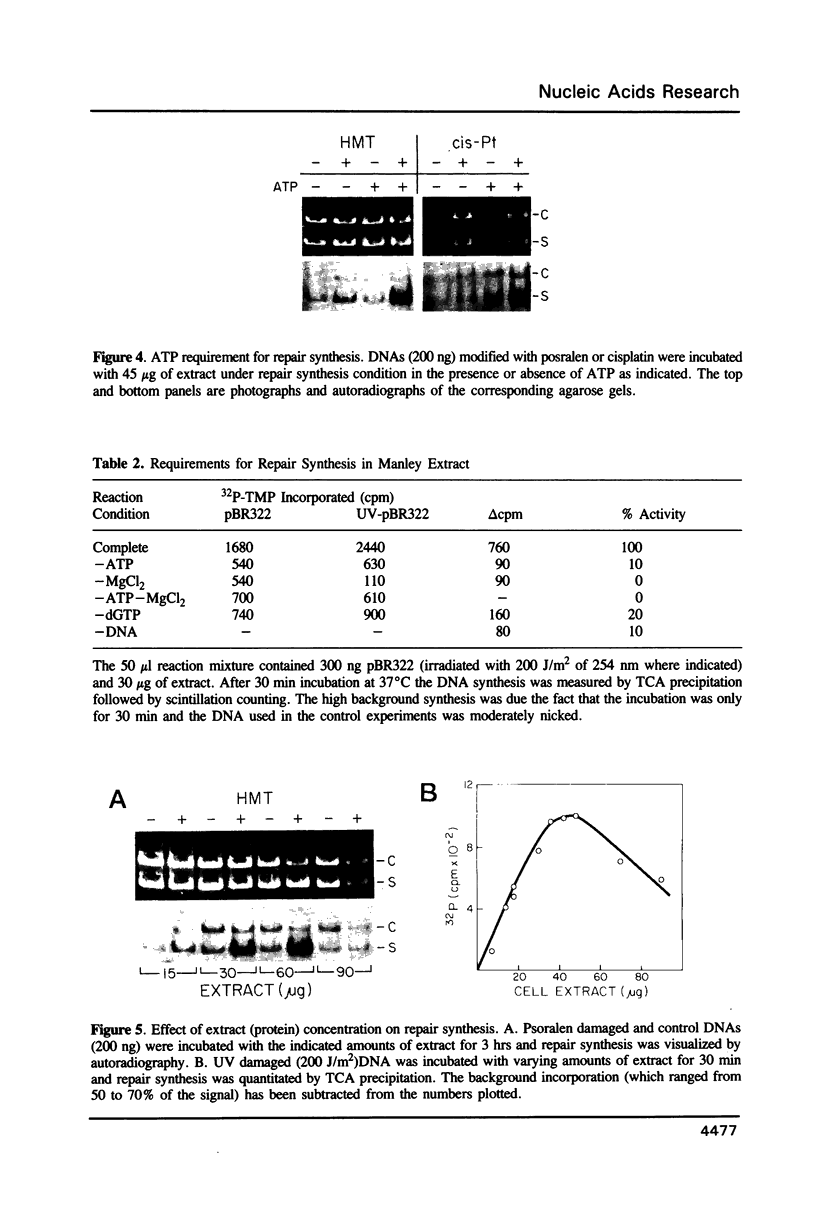
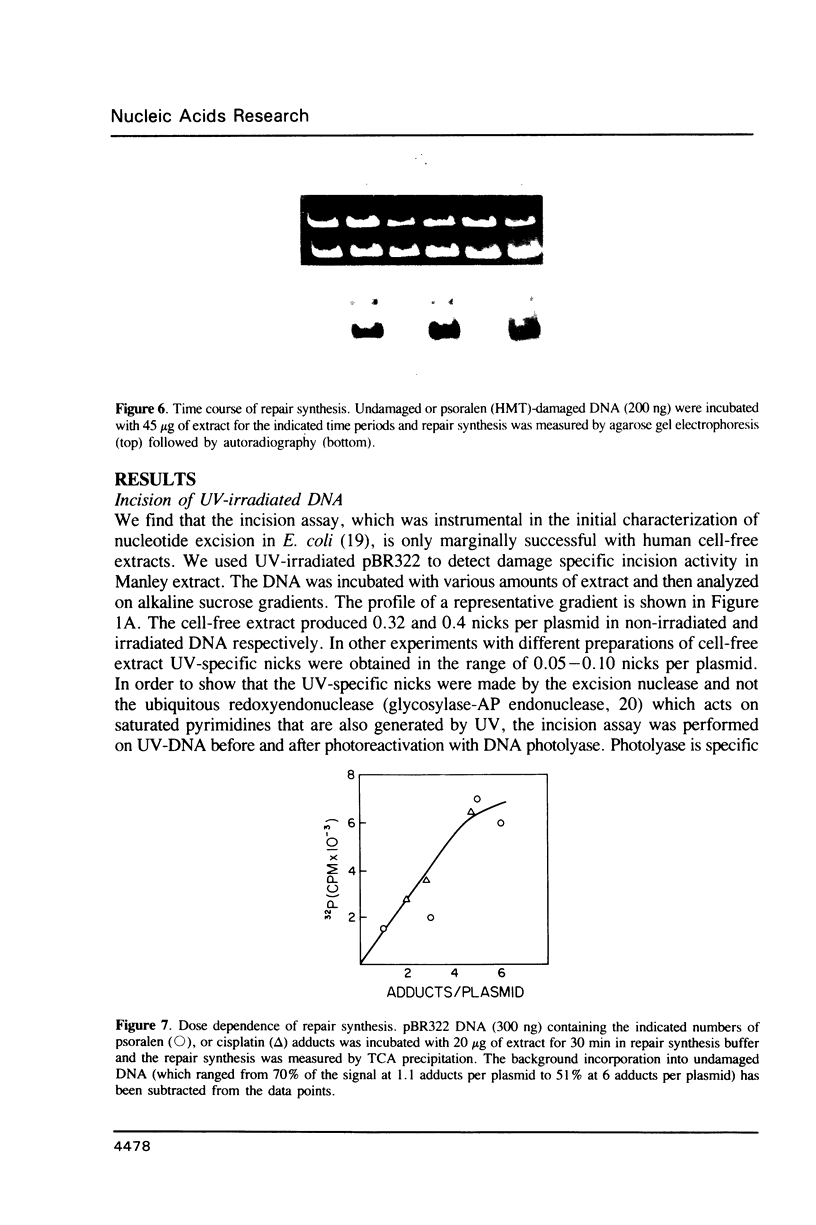
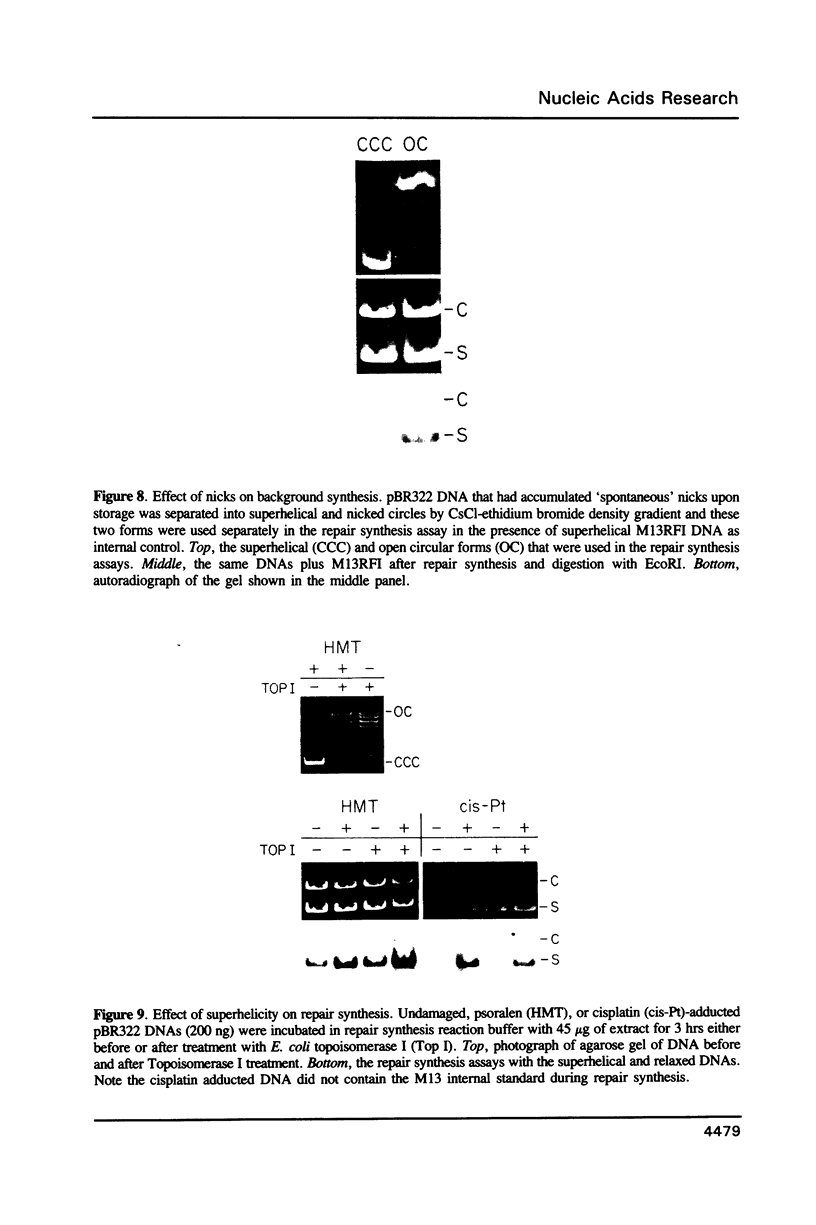





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ciarrocchi G., Linn S. A cell-free assay measuring repair DNA synthesis in human fibroblasts. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1887–1891. doi: 10.1073/pnas.75.4.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch P. W., Helland D. E., Haseltine W. A. Mechanism of action of a mammalian DNA repair endonuclease. Biochemistry. 1986 Apr 22;25(8):2212–2220. doi: 10.1021/bi00356a054. [DOI] [PubMed] [Google Scholar]
- Dresler S. L., Gowans B. J., Robinson-Hill R. M., Hunting D. J. Involvement of DNA polymerase delta in DNA repair synthesis in human fibroblasts at late times after ultraviolet irradiation. Biochemistry. 1988 Aug 23;27(17):6379–6383. doi: 10.1021/bi00417a028. [DOI] [PubMed] [Google Scholar]
- Dresler S. L., Lieberman M. W. Requirement of ATP for specific incision of ultraviolet-damaged DNA during excision repair in permeable human fibroblasts. J Biol Chem. 1983 Oct 25;258(20):12269–12273. [PubMed] [Google Scholar]
- Eastman A., Schulte N. Enhanced DNA repair as a mechanism of resistance to cis-diamminedichloroplatinum(II). Biochemistry. 1988 Jun 28;27(13):4730–4734. doi: 10.1021/bi00413a022. [DOI] [PubMed] [Google Scholar]
- Kano Y., Fujiwara Y. Defective thymine dimer excision from xeroderma pigmentosum chromatin and its characteristic catalysis by cell-free extracts. Carcinogenesis. 1983 Nov;4(11):1419–1424. doi: 10.1093/carcin/4.11.1419. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. K., Briley L. P. Reparative strand incision in saponin-permeabilized human fibroblasts. Mutat Res. 1987 Nov;184(3):237–243. doi: 10.1016/0167-8817(87)90022-8. [DOI] [PubMed] [Google Scholar]
- Kodadek T., Gamper H. Efficient synthesis of a supercoiled M13 DNA molecule containing a site specifically placed psoralen adduct and its use as a substrate for DNA replication. Biochemistry. 1988 May 3;27(9):3210–3215. doi: 10.1021/bi00409a013. [DOI] [PubMed] [Google Scholar]
- La Belle M., Linn S. In vivo excision of pyrimidine dimers is mediated by a DNA N-glycosylase in Micrococcus luteus but not in human fibroblasts. Photochem Photobiol. 1982 Sep;36(3):319–324. doi: 10.1111/j.1751-1097.1982.tb04381.x. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Ozols R. F., Lai G. M., Fojo A., Rothenberg M., Hamilton T. C. Increased DNA repair as a mechanism of acquired resistance to cis-diamminedichloroplatinum (II) in human ovarian cancer cell lines. Cancer Res. 1988 Oct 15;48(20):5713–5716. [PubMed] [Google Scholar]
- Mortelmans K., Friedberg E. C., Slor H., Thomas G., Cleaver J. E. Defective thymine dimer excision by cell-free extracts of xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2757–2761. doi: 10.1073/pnas.73.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles G. M., Van Houten B., Sancar A. Utilization of DNA photolyase, pyrimidine dimer endonucleases, and alkali hydrolysis in the analysis of aberrant ABC excinuclease incisions adjacent to UV-induced DNA photoproducts. Nucleic Acids Res. 1987 Feb 11;15(3):1227–1243. doi: 10.1093/nar/15.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida C., Reinhard P., Linn S. DNA repair synthesis in human fibroblasts requires DNA polymerase delta. J Biol Chem. 1988 Jan 5;263(1):501–510. [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Sancar A., Rupert C. S. Determination of plasmid molecular weights from ultraviolet sensitivities. Nature. 1978 Mar 30;272(5652):471–472. doi: 10.1038/272471a0. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. Escherichia coli DNA photolyase is a flavoprotein. J Mol Biol. 1984 Jan 15;172(2):223–227. doi: 10.1016/s0022-2836(84)80040-6. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Nissen-Meyer J., Strike P. Incision of ultraviolet-irradiated DNA by extracts of E. coli requires three different gene products. Nature. 1976 Oct 7;263(5577):524–526. doi: 10.1038/263524a0. [DOI] [PubMed] [Google Scholar]
- Tessman J. W., Isaacs S. T., Hearst J. E. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. Conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985 Mar 26;24(7):1669–1676. doi: 10.1021/bi00328a015. [DOI] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Hearst J. E., Sancar A. Analysis of sequential steps of nucleotide excision repair in Escherichia coli using synthetic substrates containing single psoralen adducts. J Biol Chem. 1988 Nov 15;263(32):16553–16560. [PubMed] [Google Scholar]
- Waldstein E. A., Peller S., Setlow R. B. UV-endonuclease from calf thymus with specificity toward pyrimidine dimers in DNA. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3746–3750. doi: 10.1073/pnas.76.8.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. D., Robins P., Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988 Apr 8;53(1):97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]