Abstract
Human monoclonal antibodies (mAbs) have become drugs of choice for the management of an increasing number of human diseases. Human antibody repertoires provide a rich source for human mAbs. Here we review the characteristics of natural and non-natural human antibody repertoires and their mining with non-combinatorial and combinatorial strategies. In particular, we discuss the selection of human mAbs from naïve, immune, transgenic and synthetic human antibody repertoires using methods based on hybridoma technology, clonal expansion of peripheral B cells, single-cell PCR, phage display, yeast display and mammalian cell display. Our reliance on different strategies is shifting as we gain experience and refine methods to the efficient generation of human mAbs with superior pharmacokinetic and pharmacodynamic properties.
Key words: human monoclonal antibodies, B cells, hybridoma technology, display technologies, antibody libraries, antibody engineering
Introduction
Human antibody repertoires are collections of human immunoglobulin (Ig) genes that encode human heavy and light chains. These include VH, D, JH and CH gene segments of the heavy chain locus on chromosome 14; Vκ, Jκ and Cκ gene segments of the kappa light chain locus on chromosome 2; and Vλ, Jλ and Cλ gene segments of the lambda light chain locus on chromosome 22 in the human genome. In addition, transgenic animals with complete or partial human antibody repertoires have been generated. Human antibody repertoires can be mined in vivo and in vitro for human monoclonal antibodies (mAbs).
Chimeric, humanized and human mAbs, which collectively are the prevailing formats of therapeutic mAbs, share human constant domains but are discerned by the origin of their variable domains. Human mAbs (also referred to as fully human mAbs) are defined as having variable domains that are entirely derived from human antibody repertoires, whereas chimeric and humanized mAbs feature variable domains that originate from non-human antibody repertoires. A key feature of human mAbs is their ability to fully blend in with the human immune system. Being indistinguishable from endogenous human antibodies, human mAbs are generally anticipated to have superior pharmacokinetic and pharmacodynamic properties compared to mAbs from nonhuman antibody repertoires. Ideally, human mAbs are not recognized as foreign by the human immune system, i.e., are not immunogenic. Human and humanized mAbs have been found to be less immunogenic than nonhuman and chimeric mAbs, the latter of which trigger human anti-mouse antibody (HAMA), human anti-rat antibody (HARA) and human anti-chimeric antibody (HACA) responses.1 It is expected, but remains to be substantiated, that human mAbs are generally less immunogenic than humanized mAbs.2,3 Human anti-human antibody (HAHA) responses are mainly due to idiotypic, allotypic and glycosylation differences between human mAbs and endogenous human antibodies. Although potentially beneficial for certain therapeutic applications,4 it is likely that fine tuning of human mAb engineering and manufacturing will reduce the remaining immunogenicity of human mAbs.
Hybridoma technology5 has been extremely successful in the generation of mouse and rat mAbs; however, the initial failure to robustly apply this method to the generation of human mAbs paired with the prospect of lucrative intellectual property accelerated the development of a variety of alternative methods over the past three decades.2,6–8 The medical and commercial success of mAbs in the management of human disease drove this growth and diversification.9,10 Although only six out of 28 mAbs that were approved by the US Food and Drug Administration (FDA) and/or the European Medicines Agency (EMA) are human mAbs (Table 1), four of these antibodies were approved as recently as 2009, and their proportion is likely to increase, owing to an unremitting pipeline of human mAbs in Phase 3 clinical trials.11
Table 1.
Human mAbs approved in the United States and the European Union
| Generic name (trade name; company) | Format (origin) | Antigen | Indications | Approval |
| Adalimumab (Humira®; Abbott) | IgG1κ (phage display) | TNFα | Rheumatoid arthritis; juvenile idiopathic arthritis; psoriatic arthritis; plaque psoriasis; ankylosing spondylitis; Crohn's disease | 2002 (FDA), 2003 (EMA) |
| Panitumumab (Vectibix®; Amgen) | IgG2κ (transgenic mouse) | EGFR | Metastatic colorectal cancer | 2006 (FDA), 2007 (EMA) |
| Golimumab (Simponi®; Johnson & Johnson) | IgG1κ (transgenic mouse) | TNFα | Rheumatoid arthritis; psoriatic arthritis; ankylosing spondylitis | 2009 (FDA), 2009 (EMA) |
| Canakinumab (Ilaris®; Novartis) | IgG1κ (transgenic mouse) | IL-1β | Cryopyrin-associated periodic syndrome (CAPS) | 2009 (FDA), 2009 (EMA) |
| Ustekinumab (Stelara®; Johnson & Johnson) | IgG1κ (transgenic mouse) | IL-12 and IL-23 (common p40 subunit) | Plaque psoriasis | 2009 (FDA), 2009 (EMA) |
| Ofatumumab (Arzerra®; Genmab) | IgG1κ (transgenic mouse) | CD20 | Chronic lymphocytic leukemia | 2009 (FDA), 2010 (EMA) |
Here we review the generation of human mAbs from naïve, immune, transgenic and synthetic human antibody repertoires. Strictly speaking, only human mAbs from naïve and immune repertoires, which we summarize as natural repertoires in the next section, as well as human mAbs from transgenic repertoires, which nonetheless are products of artificial immune systems, meet the above stated criterion of having variable domains that are entirely derived from human antibody repertoires and are therefore indistinguishable from endogenous human antibodies. By contrast, human mAbs from synthetic repertoires generally contain artificial sequences that are not encoded in the human genome and cannot be generated by natural processes such as VHDJH, VκJκ and VλJλ rearrangements and somatic hypermutation. Transgenic and synthetic repertoires are summarized as non-natural repertoires in a subsequent section. Furthermore, we distinguish between non-combinatorial and combinatorial strategies for mining human antibody repertoires. Non-combinatorial strategies retrieve human mAbs from single B-cells with the original heavy and light chain pairs (Fig. 1). Combinatorial strategies involve human antibody libraries with randomly combined heavy and light chains (Fig. 2). Hybridoma technology and phage display technology exemplify non-combinatorial and combinatorial mining tools, respectively. Both approaches have led to FDA- and EMA-approved human mAbs (Table 1).
Figure 1.
Non-combinatorial mining of human antibody repertoires. (A) Human antibody repertoires consist of polyclonal mixtures of a variety of B cells that express functional human antibodies, including naive and post-GC B cells that represent naïve and immune repertoires, respectively. Post-GC B cells, which differentiate into antibody secreting cells and memory B cells, provide a human antibody repertoire that has been extensively mined with non-combinatorial strategies. (B) Hybridoma technology (top), EBV immortalization (center), and single-cell sorting (bottom) allow the isolation and expansion of monoclonal B cells which are then screened with an antigen of interest. Cell surface Ig can be utilized to first enrich B cells that express human antibodies to the antigen of interest. (C) Heavy and light chain cDNAs are amplified from mRNA of monoclonal B cells and B-cell lines by RT-PCR. (D) After cloning heavy and light chain cDNAs into ectopic expression vectors, human mAbs are purified from the supernatant of transfected mammalian cells (typically Chinese hamster ovary CHO or mouse myeloma NS0 cells) in high yields.
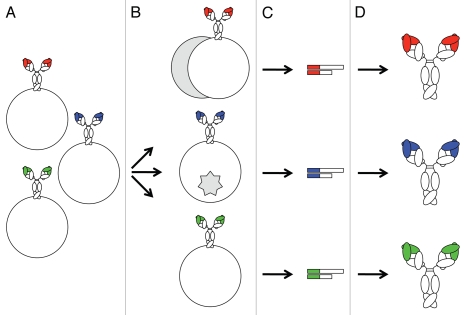
Figure 2.
Combinatorial mining of human antibody repertoires. Polyclonal or oligoclonal mixtures of human B cells (A) are used in bulk to isolate mRNA and amplify cDNA of heavy and light chains (B). (C) Heavy and light chain cDNAs are cloned into display vectors that afford antibody libraries with a physical linkage of genotype (cDNA) and phenotype (protein). Shown as examples are filamentous phage that encode and display Fab (top) and virus-infected mammalian cells that express and display scFv (bottom). Note that heavy and light chains are randomly combined. (D) Antibody libraries are then selected by several rounds of panning on immobilized antigen (top) or screened by a single round of FACS using fluorescently labeled antigen (bottom). (See text for details). The physical linkage of genotype and phenotype allows the enrichment and amplification of displayed antibodies. (E) Human mAbs from antibody libraries are manufactured the same way as human mAbs from monoclonal B cells and B-cell lines.
Natural Repertoires
Each B cell encodes a single antibody with defined specificity. The clonal diversity of the natural human antibody repertoire is defined by the number of B cells that encode antibodies with unique specificities, i.e., the number of independent clones. In an individual, this number is estimated to be at least 108. B cells from primary and secondary lymphoid tissues along with B cells in circulation, i.e., in peripheral blood, constitute the natural human antibody repertoire. The natural repertoire can be divided into naïve and immune repertoires depending on whether or not it has been shaped by antigen encounter. The immune repertoire has been extensively mined for human mAbs, first with non-combinatorial and later with combinatorial strategies. By contrast, combinatorial strategies have dominated access to human mAbs from the naïve repertoire (Table 2).
Table 2.
Principal mining tools for human antibody repertoires
 |
The bone marrow is a primary lymphoid tissue of interest for mining natural human antibody repertoires. The principle site of differentiation of hematopoietic stem cells into immature B cells,12 the bone marrow generates pro-B cells and pre-B cells with partially rearranged heavy and light chain genes. While these partially developed human antibody repertoires are generally not suitable for mining human mAbs, surrobody libraries that pair rearranged heavy chains with surrogate light chains of pre-B cells have been described recently.13 The ultimate products of B-cell development in the bone marrow are immature B cells that express cell surface IgM. Upon exiting the bone marrow and entering the circulation, immature B cells mature to naïve B cells, the latter of which express cell surface IgD in addition to IgM. Immature and naïve B cells collectively provide a fully developed human antibody repertoire that has not been shaped, at least not extensively, by antigen encounter and is therefore termed primary repertoire or naïve repertoire.
When naïve B cells encounter new antigens that bind to their surface Ig they become activated B cells, proliferate and go through differentiation processes that typically involve somatic hypermutation and class switch recombination from the earliest expressed IgM isotype to IgG, IgE and IgA isotypes. These processes are T-cell-dependent and take place in germinal centers (GC) of secondary lymphoid tissues such as lymph nodes, spleen and tonsils. Post-GC B cells with affinity and isotype matured antibodies differentiate further into antibody secreting cells and memory B cells.14 Antibody secreting cells can be divided into proliferating plasma blasts and fully differentiated plasma cells that no longer proliferate. Plasma cells exit secondary lymphoid tissues, enter the circulation, and migrate to the bone marrow where they may survive for weeks, months, or even years in specialized niches. Antibody titers in circulation are maintained by plasma cells residing in the bone marrow.15 In contrast to antibody secreting cells, memory B cells do not secrete antibodies but express cell surface Ig. Memory B cells are found in secondary lymphoid tissues, in circulation and in the bone marrow. Upon recall antigen encounters, memory B cells rapidly differentiate into antibody secreting cells. Both memory B cells and plasma cells can be long-lived, maintaining immunological memory for a lifetime.16
The secondary repertoire, or immune repertoire, is largely defined by the pool of post-GC B cells, plasma blasts, plasma cells and memory B cells. Expression of CD27 distinguishes this B-cell pool from other B cells. Antibody-secreting cells and memory B cells can also arise from T-cell-independent processes that play a role in both adaptive and innate immunity. Adaptive immunity to T-cell-independent antigens such as carbohydrates and lipids contributes to the immune repertoire. By contrast, cells expressing natural antibodies, which play a key role in innate immunity,17 can be considered to belong to the naïve repertoire. These two types are difficult to distinguish and typically share IgM isotype, low affinity and polyreactivity. They illustrate that immune and naïve repertoires often overlap without clear anatomical localizations or functional distinctions.
Human immune repertoires are highly attractive because they contain antibodies of high affinity with minimal immunogenicity and off-target reactivity. Unlike nonhuman immune repertoires that have been mined extensively for mAbs, the human immune repertoire lacks both availability and accessibility. First of all, humans cannot be exposed to antigens at will, limiting available immune repertoires to those induced by infections, vaccinations, autoimmunity and alloimmunity. Furthermore, secondary lymphoid tissues such as the spleen, which is a preferred source of mouse, rat and rabbit immune repertoires for mAb mining, are difficult to access in humans. Although tonsillectomy provides access to tonsils, it generally does not coincide with an immune response of interest. Access to the human immune repertoire is therefore generally limited to peripheral blood and, to a lesser extent, bone marrow. As discussed above, these compartments also contain the naïve repertoire. Combinatorial strategies allow the retrieval of human mAbs from the naïve repertoire. Typically, these mAbs need to be improved by affinity maturation in vitro to compensate for their lack of efficient selection by antigens in vivo. The next two sections discuss various features of naïve and immune repertoires that are exploited in non-combinatorial and combinatorial strategies for mining human mAbs.
Non-Combinatorial Mining
Hybridoma technology.
The advent of hybridoma technology had a tremendous impact on biomedical research, yielding mAbs that have become an indispensable part of everyday laboratory work. Today rodent mAbs against a plethora of antigens are widely available and can be produced in almost unlimited quantities due to this robust underlying technology.5 Unfortunately, the therapeutic use of rodent mAbs in humans is limited by their propensity to induce strong HAMA and HARA responses that quickly neutralize the rodent mAb. Given this, the mining of human antibody repertoires using hybridoma technology was investigated extensively. Despite significant efforts, the adaptation of hybridoma technology to the generation of human mAbs has been a slow and difficult process due to the limited number of B cells stimulated with an antigen of interest and a lack of suitable drug-resistant myeloma cell fusion partners for the immortalization of human B cells. Thus, mouse myeloma cells were initially used for the generation of mouse-human heterohybridomas.18–20 Such hybrid cells were shown to have a strong tendency to lose human chromosomes, including chromosome 2 carrying the kappa light chain locus.21 While this precluded an extensive use of mouse myeloma cells for human mAb production, protocols have been established that may ameliorate the undesirable behavior of heterohybridomas and allow long-term production of human mAbs.22
Given the genetic instability of heterohybridomas, the use of alternative fusion partners, particularly human lymphoblastoid cell lines and human myeloma cells, has been explored extensively.23–28 While in principle successful, the yield of viable hybridomas is typically too poor for practical applications. In addition, such hybridomas often secrete permutated Igs derived from both fusion partners. The use of certain mouse-human hybrid cells including heteromyelomas and heterohybridomas as fusion partner appears to resolve these problems, while at the same time generating stable antibody producing hybridomas.29–32 In addition, a promising human myeloma cell line allowing efficient generation of stable and highly productive human hybridomas was described, even though a more widespread use of this cell line awaits the production of a subline lacking expression of an endogenous λ chain.33 Finally, the use of genetically engineered mouse myeloma cells allows for highly efficient formation of stable heterohybridomas,34 with a propensity to produce affinity-matured IgG antibodies when appropriately pre-treated CD27-positive B cells are used for fusion.35
As mentioned above, human hybridoma technology not only suffered from the lack of suitable fusion partners, but also from a shortage of antigen-stimulated B cells. Because ethical considerations typically prevent antigen exposure at will in humans, alternative methods to improve access to the human immune repertoire were developed. Human peripheral blood lymphocytes transplanted into immune-compromised mice were shown to give rise to a secondary humoral immune response upon antigen stimulation,36,37 and provide access to the human memory compartment through hybridoma technology.38,39
In summary, each of the methods described is either labor-intensive or suffers from drawbacks when compared to the classical hybridoma technology. Thus, hybridoma technology has not become the method of choice for mining human antibody repertoires, leading to tremendous efforts to develop the alternative methods discussed in the following sections.
Clonal expansion of peripheral B cells.
One of the most popular methods for harnessing the human immune response involves immortalization of peripheral B cells with Epstein-Barr virus (EBV). The first demonstration of EBV immortalization of human B cells into lymphoblastoid cell lines in vitro, was described almost 40 years ago.40 There have been numerous reports since then, although initial success was limited due to low efficiency of B-cell immortalization and cloning and the small quantities of antibody secreted.41,42 Better cloning efficiency, stability and productivity can be achieved when EBV-immortalized antibody secreting cells are fused with mouse myeloma or mouse-human heteromyeloma cells.43,44 Thus, multi-step procedures involving EBV-immortalization of peripheral B cells followed by fusion with myeloma cells and cloning of hybridomas were established.45–48 This method has yielded several human mAbs with therapeutic potential, including neutralizing antibodies against the hemagglutinin protein derived from the 1918 H1N1 pandemic influenza virus.49
As an alternative to the formation of hybridomas, EBV-transformed cells have been infected with a retrovirus encoding the ras oncogene, yielding cells with similar cloning efficiencies and levels of antibody secretion.50 The most significant improvement of EBV immortalization came from the observation that addition of the TLR9 ligand CpG to human B cells dramatically increases the efficiency of EBV immortalization and facilitates the establishment of clonal lines stably secreting specific antibody. In this manner, mAbs potently neutralizing the SARS corona virus or the H5N1 Influenza A virus were isolated.51,52
Strategies allowing the long-term growth and cloning of human B cells, without immortalization by transformation with a virus or an oncogene, have also been explored. In initial experiments, short-term B-cell clones were formed when cultured in the presence of EL-4B5 thymoma cells in conjunction with human T-cell plus macrophage supernatant.53,54 Improved proliferation and antibody production were found under more defined conditions, when B cells were activated by IL-4 treatment and CD40 triggering.55,56 A combination of both methods together with further refinements proved extremely efficient, allowing direct establishment of viable B-cell cultures from single, antigen-specific B cells.57,58 Similar to EBV immortalization, these B-cell clones secrete sufficient amounts of antibody into the supernatant, and production of specific antibody can be validated prior to cloning of the variable domains. Further, high throughput screens based on short term clonal expansion of activated peripheral B cells are possible and have led to the identification of HIV-1 neutralizing antibodies.59
Recently, a promising method involving genetic reprogramming of human memory B cells was described.60 Retrovirus-mediated expression of Bcl-6 and Bcl-xL in peripheral memory B cells, combined with culturing on CD40L-expressing L cells in the presence of IL-21, converts them to highly proliferating, cell surface Ig-positive, antibody secreting B cells that can be cloned efficiently. The proteins Bcl-6 and Bcl-xL are typically expressed in GC B cells, and the resulting cells have features of GC B cells, including expression of activation-induced deaminase (AID). It is of interest whether this method will find broader application in the generation of human mAbs.
Single-cell PCR.
The most straightforward, though technically challenging, non-combinatorial strategy for mining human antibody repertoires is single-cell polymerase chain reaction (PCR). If both heavy and light chain variable domains can be amplified in an efficient and reliable manner from single cells, then antibodies with original heavy and light chain pairings can be cloned on a cell-by-cell basis without the need to grow individual B-cell clones. In principle, either memory B cells or plasma cells can serve as a source of rearranged antigen-binding sites. As discussed, peripheral memory B cells are known to persist for many years after antigen exposure, thereby offering a diverse and attractive repertoire of antigen specificities readily available in the blood. As memory B cells contain only relatively small quantities of specific RNA, the successful cloning of both heavy and light chain variable domains from a single B cell poses a significant technical challenge. In spite of this, antibodies have successfully been cloned from single peripheral B cells, including mAbs against self-antigens,61,62 tetanus toxoid57 and HIV-1 gp140.63 The efficiency of cloning relevant antibodies has been improved, e.g., by enriching specific B cells using antigen-coated magnetic beads,57 or by directly isolating antigen-specific B cells by fluorescence-activated cell sorting (FACS).63
Compared to memory B cells, plasma cells have the disadvantage that they are present in the blood only for short periods of time after antigen exposure, after which they are only accessible in the bone marrow. Nonetheless, a known antigen exposure or challenge, e.g., a booster immunization, induces circulating plasma cells, of which a majority is specific for the antigen after approximately one week.64 Although no highly plasma cell-specific marker is known to date, they are readily isolated by FACS, e.g., based on their high expression of CD19 and CD38, and intermediate expression of CD45.65 These cells represent dedicated antibody factories and contain huge amounts of specific heavy and light chain mRNAs, thereby facilitating antibody cloning by reverse transcriptase (RT)-PCR. Numerous human mAbs have been cloned from plasma cells to date, including mAbs against tetanus toxoid and influenza virus.64–66 Recently, using a method related to microengraving,67 microwell array chips were successfully used for high-throughput screening of human antibodies secreted by single CD138+ plasma cells followed by RT-PCR cloning.68
Combinatorial Mining
Combinatorial mining of human antibody repertoires is intertwined with the concept of antibody libraries.8,69 Antibody libraries randomly combine heavy and light chain cDNAs obtained through RT-PCR from mRNA of B-cell pools into expression vectors that afford a compartmental or physical linkage of phenotype (protein) and genotype (cDNA). In contrast to non-combinatorial mining, the source of human antibody repertoires subjected to combinatorial mining is B-cell mRNA rather than B cells. Thus, antibody secreting cells, due to their higher heavy and light chain gene transcript levels compared to other B cells, can have much greater representation in antibody libraries. This bias can be highly desirable for the combinatorial mining of immune, but not naïve, repertoires. To increase the diversity of independent clones in antibody libraries from naïve repertoires, RT-PCR of the heavy chain mRNA can be restricted to the IgM isotype. However, combinatorial strategies have inherent biases due to the RT-PCR cloning of heavy and light chain cDNAs and due to the expression and folding of non-natural antibody fragments in non-B-cell environments. Nonetheless, high-throughput DNA sequencing recently confirmed that large antibody libraries can accurately mimic natural human antibody repertoires in terms of molecular diversity.70,71
Like other cDNA libraries, the first antibody libraries were based on lambda phage vectors that carried heavy and light chain cDNAs and facilitated their expression upon infection of Escherichia coli bacteria.72 Lysis plaques of lambda phage on bacterial lawns were then screened with antigens of interest. This method was used to clone a diverse panel of human mAbs in Fab format to tetanus toxoid after generating an antibody library from peripheral blood lymphocytes isolated from human donors who had received a booster vaccination.73,74 A major drawback of this method is its limitation to screening as opposed to selecting antibody libraries. The number of independent clones, i.e., lysis plaques, that can be screened, even if the antigen of interest is in unlimited supply, is practically confined to 106.
Subsequent combinatorial strategies, e.g., phage, yeast and mammalian cell display, replaced screening with selection, allowing the mining of much larger antibody libraries approaching 1011 independent clones. Selection is facilitated by display technologies that physically link a displayed antibody fragment to its cDNA in a defined particle such as a filamentous phage, a ribosome, or a cell. Millions to billions to, theoretically, trillions of independent particles constitute an antibody library that can be mined with antigens of interest. Particles that display antibody fragments with high affinity to the antigen are isolated. Because of the simultaneous isolation of their cDNA, the displayed antibody fragments can be copied, enabling multiple rounds of selection. In other words, the physical linkage of phenotype and genotype effectively links recognition and replication. In addition, DNA sequencing may readily identify phenotype and genotype.
Phage display.
The generation and selection of peptide libraries displayed on filamentous phage marked the advent of display technologies.75 The use of phage display for the generation and selection of antibody libraries, displayed in either scFv76–78 or Fab79,80 format, revolutionized the field of mAbs and antibody engineering in the early 1990s, arguably accelerated by an intense competition between researchers at the Medical Research Council (Cambridge, UK) and The Scripps Research Institute (La Jolla, California, USA). Over the past 20 years, phage display has proven to be the most robust and versatile mining tool for human antibody repertoires, yielding human mAbs to virtually any antigen of interest.8 The process of multiple rounds of selection on a purified antigen or on antigen-expressing cells, referred to as panning, can be adapted to positively or negatively select a range of desired antibody properties, such as affinity, specificity, manufacturability and catalytic activity. While scFv (∼25 kDa) and Fab (∼50 kDa) still dominate the phage display format, human antibody repertoires have also been mined through the display and selection of single variable domains (∼12.5 kDa) of heavy and light chain.81,82
Typically, recombinant fusion to the N-terminus of phage protein pIII or a fragment of pIII provides the physical linkage of antibody fragment and phage, facilitating monovalent display in phagemid systems and multivalent display in phage systems.83 Other multivalent display systems use phage proteins pVIII and pIX as fusion partners.79,84 For the generation of human mAbs with high affinity, monovalent Fab display through phage protein pIII is generally preferred.85 Phage display relies on the expression and folding of antibody fragments in the periplasm of gram-negative bacteria, typically Escherichia coli. Although this compartment provides an oxidizing milieu suitable for disulfide bridge formation and mimics the endoplasmic reticulum of eukaryotic cells surprisingly well, prokaryotic factors that skew the selectable diversity of antibody libraries are inevitable. For example, the lack of chaperones in bacteria can lead to inadequate folding of scFv and Fab and result in toxicity.
Human antibody libraries displayed on phage were generated from immune and naïve repertoires at about the same time. The first human immune repertoires mined by phage display were based on peripheral B cells and bone marrow of vaccinated or infected human donors and yielded human mAbs to tetanus toxoid, HIV-1 gp120, and other viral antigens.85–89 Notably, antibody libraries derived from bone marrow of HIV-1-positive human donors with serum antibodies against a variety of other viruses yielded human mAbs to each of these viruses following selection by phage display.90 The bone marrow was conceptualized as the central bank for antigen-experienced B cells that carried a record of antibody responses to past viral exposures. Autoimmune repertoires from human donors who have serum antibodies to DNA and other autoantigens have also been accessed by phage display.91–93 Tumor-infiltrating B cells provide a unique immune repertoire in cancer patients that has been mined with phage display for potentially therapeutic human mAbs to tumor antigens.94–96 Alternatively, peripheral B cells from cancer patients vaccinated with autologous tumor cells have been used to generate and select antibody libraries by phage display.97 Peripheral B cells and phage display were also employed to mine an alloimmune repertoire of a cancer patient who had been treated with allogeneic hematopoietic stem cell transplantation, revealing serum antibodies to tumor antigens that might have potential as therapeutic human mAbs.98
The first human naïve repertoire mined by phage display was based on peripheral B cells and yielded human mAbs to a variety of different antigens that had not been encountered in vivo.99 Importantly, this method allowed the generation of human mAbs to human antigens.100 These studies demonstrated that the random combination of heavy and light chain can yield human mAbs with new specificities. Due to the overlap of naïve and immune repertoires, as well as the bias of mRNA from antibody secreting cells, antibody libraries from naïve repertoires typically contain a mixture of heavy and light chain cDNAs with and without somatic hypermutations. Human mAbs derived from naïve repertoires generally have lower affinities compared to human mAbs from immune repertoires, but this shortcoming can be overcome by increasing the number of independent clones, thereby generating a larger antibody library.101–104 However, it is now common to mature the affinity of human mAbs derived from naïve repertoires with subsequent antibody engineering methods that are also based on display technologies. Methods that involve the randomization of certain sequences within heavy or light chain are discussed in a subsequent subsection on synthetic repertoires.
A method that preserves natural VHDJH, VκJκ and VλJλ sequences and yields human mAbs indistinguishable from endogenous human antibodies involves the shuffling of heavy and light chains.105 This method, known as chain shuffling, can yield excellent human mAbs from moderately diverse antibody libraries with 109 independent clones.106 Chain shuffling can also be used to convert mouse mAbs to human mAbs via sequential replacement of mouse heavy and light chains with human heavy and light chain libraries, respectively, followed by selection through phage display.107 Adalimumab, the first human mAb approved by FDA and EMA (Table 1), was derived from a mouse mAb to human TNFα via this method.108 A similar method, also based on phage display, preserves only the complementarity determining region (CDR) 3 sequences of mouse heavy and light chains, i.e., HCDR3 and LCDR3, while converting everything else to human sequences.109
A general concern about the de novo generation of human mAbs from naïve and synthetic repertoires in vitro is their lack of exposure to negative selection processes in vivo which are integral to immune tolerance mechanisms and efficiently remove undesirable off-target reactivities. Addressing this concern, phage display has also been used to mine non-combinatorial antibody libraries, i.e., antibody libraries that retain original heavy and light chain pairings of human antibody repertoires through in-cell RT-PCR.110 This method is of particular interest for the identification of human antibodies in autoimmune repertoires.
Yeast display.
The expression, folding and display of antibody fragments on the surface of cells from the yeast Saccharomyces cerevisiae involve eukaryotic processes that are similar to those encountered in the natural B-cell environment. Antibody fragments are channeled through endoplasmic reticulum and Golgi apparatus, ensuring proper disulfide bridge formation and N-linked glycosylation.111,112 Thus, yeast display may address some of the above mentioned biases prokaryotic systems impose on antibody libraries. In fact, in a direct comparison based on the same human antibody repertoire, yeast display yielded a more diverse set of human mAbs than phage display.113 The physical linkage of antibody fragment and cell is provided through recombinant fusion to the C-terminus of the secreted protein Aga2, which covalently associates with the cell surface protein Aga1.114 Formats of antibody fragments that have been used for yeast display include scFv,111 Fab115 and scFab.116
Initially limited by the number of independent clones that could be transformed, yeast display has become a powerful mining tool for large antibody libraries from human naïve repertoires.117–119 In addition, yeast display has been successfully employed for the mining of human immune repertoires.113,120 Interestingly, the haploid/diploid lifecycle of yeast can be exploited in recombinatorial strategies that generate large antibody libraries in diploid cells by mating a haploid cell pool harboring a heavy chain library with a haploid cell pool harboring a light chain library.118,120 Such recombinatorial strategies based on yeast display have also been used for chain shuffling.121
In contrast to phage display, antibody libraries displayed on yeast cells are simultaneously screened and selected with an antigen of interest. Screening is carried out by flow cytometry and selection by FACS. The ability to detect and sort individual binding events during the selection process has made yeast display an exceptional method for the affinity maturation of mAbs,122 including human mAbs derived from naïve repertoires by phage display.115
Mammalian cell display.
A screening/selection system based entirely on expression of human antibodies in their natural environment, i.e., the mammalian cell, may be best suited for the mining of human antibody repertoires. Within this system, all necessary components for human antibody synthesis and processing are available at physiological levels. Compared to yeast cells, antibody repertoires expressed in mammalian cells are less biased by properties other than antigen binding. Several laboratories have therefore developed strategies for human mAb mining by mammalian cell display. The most straightforward approach is to transfect an antibody library with a vector directing antibody expression to the cell surface by means of a C-terminal transmembrane region, thereby allowing for selection of cells expressing specific antibody based on antigen binding, e.g., by FACS.
In a proof-of-concept study involving the expression of a mixture of two different CD22-specific scFv on the surface of human embryonic kidney 293T cells, it was estimated that an up to 240-fold enrichment can be achieved with only 2-fold affinity differences.123 It remains to be shown whether this approach will allow for isolation of antibodies from more complex mixtures, such as a library. A general limitation of screening by plasmid transfection is the potential to deliver multiple plasmids per cell, leading to the expression of an ill-defined number of different antibodies on each cell. It would then require multiple rounds of selection in order to enrich for specific antibodies, even if the library diversity is small. This problem can be circumvented with viral expression vectors. As infection rates can be dosed precisely, conditions can be used where one cell expresses exactly one antibody species.
One viral system suitable for screening of human antibody libraries on the surface of mammalian cells is based on vaccinia virus.124 Using separate heavy and light chain libraries, African green monkey kidney BSC-1 cells are co-infected, human IgG antibodies expressed on the cell surface and cells encoding specific antibody isolated by FACS. Because heavy and light chains are expressed from separate vectors, infection rates are kept high to ascertain that a substantial fraction of cells expresses functional antibody. As a consequence, some of the cells isolated will express more than one heavy and light chain pair, and several rounds of selection are beneficial. After screening, specific recombinants can be recovered from small numbers of selected cells, perhaps even single cells.124
A two-step method for the isolation of human antibodies by mammalian cell display has been described recently.125 Using virus like particles with highly repetitive arrays of antigen,126 pools of B cells specific for an antigen of interest are first isolated from peripheral blood lymphocytes of immunized or naturally immune human donors by FACS. Recombinant, antigen-focused scFv libraries are then generated from these B cells and screened by mammalian cell surface display using a Sindbis virus expression system. Infection of baby hamster kidney cells at a low multiplicity of infection ensures that one cell expresses only one antibody species, thus allowing identification of antigen-specific antibodies in a single round of FACS. Due to the highly replication-competent nature of the Sindbis vector, viruses encoding a specific antibody can readily be amplified from single-sorted cells. Thus, the binding properties of antibodies can be characterized prior to cloning, sequencing and subsequent production as whole IgG. Towards a clinical application of the technology, therapeutic mAbs against nicotine and influenza A M2 protein have been isolated, characterized and validated preclinically to date.125,127
A drawback of mammalian cell display is that the number of cells that can be handled at the same time is limited. This is particularly true if the screening is done under conditions where one (or close to one) antibody species is expressed per cell, limiting the diversity of libraries that can be conveniently handled to about 107 independent clones. This is suboptimal for combinatorial libraries and pre-selection of B cells for antigen specificity is advisable. It is therefore no coincidence that the human mAbs isolated by mammalian cell display to date are derived from pools of B cells enriched for antigen specificity.125,127 Because these enriched combinatorial libraries are of low diversity, there is a high likelihood that the resulting human mAbs contain natural pairs of heavy and light chains, i.e., are derived from clonally related B cells. An advantage common to all screening systems based entirely on mammalian cell expression is that they have a built-in selection for efficient expression in mammalian cells. As antibody good manufacturing practice (GMP) processes typically involve expression in mammalian cells, this is expected to have a significant impact on the cost of goods.
Non-Natural Repertoires
Transgenic repertoires.
More than 20 years ago, a proof of concept study first demonstrated that human Ig gene segments introduced into a mouse can be accessed by the mouse immune system and used to express functional antibodies.128 In this approach, a human heavy chain mini locus containing VH, D and JH gene segments linked to a Cµ gene was introduced into an embryonic stem cell that was used to develop a transgenic mouse. In this mouse, a large fraction of lymphoid cells expressed human antibody genes and this led to measurable levels of hybrid IgM with human heavy chains and mouse light chains in the serum. Further, it was shown that conventional hybridoma technology could be used to recover recombinant monoclonal IgM, thus laying the ground for the production of human antibodies in so called humanized mice.
Production of human antibodies in genetically modified mice was immediately recognized as a highly attractive approach for the generation of human mAbs against human antigens, as mice can readily be immunized with any antigen of interest and are not tolerant to most human proteins.129 While the mice described by Brüggemann et al.128 only expressed a human heavy chain mini locus in the presence of the endogenous mouse heavy and light chain loci, the production of fully human antibodies became feasible with the development of mice with additional genetic modifications. In two different laboratories, mice were generated that combined disruptions of the endogenous mouse heavy and kappa light chain loci and transgenes encoding human heavy and kappa light chain loci.130,131 The endogenous mouse lambda light chain locus was left unmodified by both groups because it only contributes to 5–15% of the antibody repertoire.132 In the report by Lonberg et al.131 the human heavy chain locus comprised 3 VH, 16 D, all 6 JH and the Cµ and Cγ1 constant region gene segments, whereas the light chain locus comprised 4 Vκ, all 5 Jκ and the Cκ gene segments. The mice described by Green et al.130 carried a repertoire of similar diversity, with the heavy chain locus including 5 VH, all 25 D, all 6 JH and the Cµ and Cδ constant region gene segments, and the light chain locus including 2 Vκ, all 5 Jκ and the Cκ gene segments. The human transgenes in such mice had undergone VHDJH and VκJκ rearrangements with N region diversification, i.e., additions or deletions of nucleotides at the VH-D-JH and Vκ-Jκ junctions, class switch recombination and somatic hypermutation.131 Despite the limited repertoire present in both transgenic mouse strains, immunization led to the generation of specific human antibodies against several antigens that were accessible by conventional hybridoma technology.130,131 In this respect, transgenic mice have led to a revival of hybridoma technology for the mining of human antibody repertoires.
The finding that transgenic mice containing human heavy and light chain mini loci are capable of mounting a specific humoral immune response comprising human antibodies is surprising in at least two respects. First, it demonstrates that hybrid B-cell receptor complexes consisting of human surface Ig and mouse Igα/Igβ (CD79a/CD79b) chains are functional in vivo and permit largely normal development and function of B cells. Second, only a fraction of the natural heavy and light chain V gene repertoire appears to be sufficient for the generation of antigen-specific antibodies, putting the relative importance of combinatorial diversity into perspective. For the production of antibodies against protein antigens using hyperimmunization protocols, the two non-germline encoded sources of clonal diversity, i.e., junctional diversity and somatic hypermutation, appear to be sufficient. Indeed, it was shown that the HCDR3 repertoire is in large part created by junctional diversity, and sufficient for most antigen specificities.133
Despite the initial success of the transgenic mice carrying mini loci, human mAbs against few antigens were isolated from these mice. The immune responses in these mice were inferior to those of wild type mice, with reduced numbers of mature B cells and lower levels of circulating Igs; however, incorporation of a significantly larger repertoire of Vκ gene segments led to increased levels of pre-B and B cells.134 Mice that incorporated the majority of the human heavy chain and kappa light chain loci were found to recapitulate the human antibody response even better by restoring B-cell compartments to near normal levels.135 Compared to the transgenic mice carrying mini loci,130,131 the animals with near complete loci displayed higher levels of human serum Ig, better class switch recombination efficiency and increased clonal diversity and magnitude of the human antibody response. Accordingly, a panel of human mAbs with high affinity against three human antigens was isolated.135 In contrast to mice, approximately 40% of serum Igs in humans contains a lambda light chain. To further enhance the diversity of humanized B-cell responses, transgenic mice were generated that carried, for the first time, large portions of all three human Ig loci, including the human lambda light chain locus, in a background in which the endogenous mouse heavy and kappa light chain loci had been inactivated. Upon immunization, these mice generated antibodies containing both human kappa and lambda light chains.136
To generate more complete humanized mice, the to date largest fraction of the human germline repertoire was introduced by microcell-mediated chromosome transfer. In this manner, transchromosomal mice were generated that express the complete heavy chain and kappa light chain repertoire in absence of mouse heavy and kappa light chains.137 However, instability of the chromosome fragment containing the kappa light chain locus led to a substantial reduction in the generation of hybridomas, a problem that was solved by crossing IgH transchromosomal mice with Igκ transgenic mice.138 It is interesting to note that of the six human mAbs on the market today, five are derived from humanized mice (Table 1). Of these, panitumumab is derived from Abgenix' XenoMouse,135 whereas the other four are derived from Medarex' UltiMab platform, which is based on the HuMab mouse,134 the Kirin TC mouse137 and a cross of the two, the KM mouse.138
Natural antibody responses are polyclonal. There are cases in which immunotherapy using polyclonal antibodies (pAbs) instead of mAbs is expected to be more efficient. This includes, for example, the treatment or prevention of diseases caused by different viral or bacterial strains or combating viruses prone to formation of escape mutants. In principle, human pAbs could be produced in humanized mice.138 While mice are well suited for the generation of mAbs, their small body size makes them unsuitable for the production of useful amounts of therapeutic or preventive pAbs. One method of accessing the potential of human pAbs is to use recombinant pAbs, i.e., defined cocktails of mAbs.139 In addition, due to advances into the understanding of humoral immune systems of large farm animals combined with significant technological advances, it may soon be possible to generate human pAbs by hyperimmunization of transchromosomal cattle.140
Synthetic repertoires.
Antibody libraries from synthetic repertoires are based on synthetic DNA sequences designed to diversify the antibody sequence.69,141 Depending on the design, human mAbs from synthetic repertoires may or may not be distinguishable from endogenous human mAbs. The first human synthetic repertoire was generated by randomizing all of the 16 amino acids that comprised the HCDR3 sequence of a human anti-tetanus toxoid mAb.142 Subsequent selection by phage display against fluorescein yielded a panel of human mAbs that revealed a shift in specificity from tetanus toxoid to fluorescein. In addition, synthetic repertoires allow the CDR grafting of a peptide motif with known specificity followed by randomization of the flanking sequences and selection by phage display. This method yielded human mAbs that bind human integrins αVβ3 and αIIbβ3 with high affinity.143,144 The randomization of single or multiple CDR sequences followed by selection with phage display has also been utilized for the affinity maturation of human mAbs that were derived from immune or naïve repertoires. This process can yield human mAbs with picomolar affinity.145,146
Antibody libraries that are based on human naïve repertoires but introduce additional sequence diversification through randomizing single or multiple CDR sequences represent, by definition, human synthetic repertoires;147–150 however, not all synthetic repertoires are based on randomized CDR sequences. Using master framework sequences derived from a single human VH and Vλ gene segment, Söderlind et al.151 introduced and recombined a complete set of CDR sequences derived by RT-PCR from human naïve repertoires. Yet another approach of sequence diversification in vitro is based on dispersing mutations throughout heavy and light chain variable domain sequences, as opposed to focusing mutations on defined segments such as CDR sequences. This can be achieved through error-prone PCR or mutagenic Escherichia coli strains. Like focused mutagenesis, dispersed mutagenesis has been utilized for the affinity (or catalytic activity) maturation of human mAbs, usually by phage display152,153 and, more recently, by yeast display.115,154
Owing to the fact that the discussed antibody libraries blend in vivo and in vitro diversity, they are sometimes referred to as semi-synthetic. By contrast, fully synthetic antibody libraries are entirely designed and engineered in vitro. A fully synthetic antibody library that combined a single human VH and Vκ gene segment with randomized HCDR3 and LCDR3 sequences was mined successfully for human mAbs to human antigens.155 Further restrictions that mimic natural CDR sequence diversity in the heavy chain variable domain and use a fixed light chain variable domain yielded human mAbs that closely resemble endogenous human antibodies. These were improved further by introducing natural CDR sequence diversity in the light chain variable domain.156,157 CDR sequence diversity can be further restricted to a four-amino-acid code (Ala, Asp, Ser and Tyr)158 or even a two-amino-acid code (Ser and Tyr),159 demonstrating the versatility of synthetic repertoires and providing molecular insights into specificity and affinity of antibody/antigen interactions. Restricting sequence diversity in fully synthetic and semi-synthetic repertoires has also been used for tailoring antibody libraries toward the selection of human mAbs to a particular class of antigens, such as peptides and carbohydrates.160,161 Fully synthetic antibody libraries that are more complex and based on >1010 independent clones (in scFv or Fab format) reassemble a variety of natural CDR and framework sequences and combine these with randomized CDR sequences.162,163 A fully synthetic antibody library with diversification in all six CDR sequences was reported more recently.164
In addition to phage display, fully synthetic antibody libraries have been mined successfully with ribosome display.165 Ribosome display is an in vitro display technology166 that does not require transformation of prokaryotic or eukaryotic cells.167–169 Ribosome display has been employed to both accomplish and guide the affinity maturation of human mAbs.170 Other less common display technologies, such as retroviral display,171 have also been used for mining synthetic repertoires. In addition to the conventional scFv and Fab formats, synthetic repertoires have facilitated the display and selection of single human variable domains81,172 and, recently, human constant domains with diversified sequences in non-CDR loops of the Ig fold.173,174
Another exciting advance in the area of human synthetic repertoires has been the generation and selection of antibody libraries that feature an expanded genetic code for the incorporation of unnatural amino acids into CDR sequences.175 Collectively, synthetic repertoires have begun to outshine all other human antibody repertoires with respect to diversity and versatility. Human mAbs from synthetic repertoires are poised to become preferred reagents for diagnostic and basic applications; however, their utility for preventive and therapeutic interventions remains to be established in clinical trials.
Outlook
Increased availability and improved accessibility of human antibody repertoires have made human mAbs the entity of choice for therapeutic mAbs. Today, human mAbs to virtually any antigen of interest can be generated. The clinical performance and the commercial viability of currently approved human mAbs provide a robust platform for the development of future generations of human mAbs. Not only will these broaden the scope of targeted antigens and indications, but also improve the activity of intervention through better pharmacodynamic and pharmacokinetic properties. Although an initial goal in the development of human mAbs was to render them indistinguishable from endogenous human antibodies, subtle antibody engineering and expression strategies that retain low immunogenicity, but tune affinity, effector functions and circulatory half-life, as well as lower the cost of goods, are being employed in the development of the next generation of human mAbs.176
Of the currently approved human mAbs (Table 1), one was derived from a naïve repertoire and five were derived from transgenic repertoires. It will be interesting to compare human mAbs from different sources with respect to their pharmacokinetic and pharmacodynamic performance. For example, the human anti-human TNFα mAb, adalimumab, which was derived indirectly from a naïve repertoire as described above, induces a HAHA response in a significant percentage of patients, adversely affecting serum concentrations and clinical responses.177 The recent approval of human anti-human TNFα mAb, golimumab,178 which was derived from a transgenic repertoire, provides a first opportunity to clinically investigate whether human mAbs derived from different human antibody repertoires against the same antigen differ in terms of immunogenicity.
The immune repertoire is a natural repertoire that has not been fully exploited for the mining of human mAbs. Sophisticated in vivo processes that shape the immune repertoire afford endogenous human antibodies with high affinity, minimal immunogenicity and minimal off-target reactivity. The immune repertoire has become increasingly accessible through new methods that facilitate the enrichment, isolation or expansion of antigen-specific post-GC B cells, plasma blasts, plasma cells or memory B cells from peripheral blood. The development of these methods has been spurred by the discovery of endogenous human antibodies of potential therapeutic utility in normal human donors, as well as in those with inflammatory diseases, infectious diseases, and cancer. Endogenous human antibodies that are triggered by preventive or therapeutic interventions, such as vaccination or allogeneic hematopoietic stem cell transplantation, are particularly attractive. An ideal mining strategy for this exceptional source of human mAbs is mammalian cell display as it provides an environment that closely resembles B cells. Consequently, this platform is anticipated to contribute to the development of future generations of human mAbs.
Finally, it is important to keep in mind that endogenous human antibodies are polyclonal whereas human mAbs, by definition, are monoclonal. In general, polyclonal or even oligoclonal responses are thought to be more proficient in mounting effector functions compared to monoclonal responses.179 While non-recombinant human pAbs purified from human donors have long been used in preventive and therapeutic interventions, recombinant human pAbs would afford much broader applicability.139 Thus, despite current practical and regulatory challenges in manufacturing and formulation, human pAbs will likely be a factor in future antibody therapy. Mining strategies that yield a panel of human mAbs to a variety of different epitopes of an antigen of interest are suitable for the development of human pAbs. Nonetheless, it remains possible that cocktails of human mAbs that are individual products of conventional mining strategies are still inferior to truly polyclonal human antibodies that emerged as complex mixtures in vivo. Thus, evolving mining strategies from monoclonal to polyclonal screening and selection offers a worthy challenge for antibody engineers.
Acknowledgements
We thank Drs. Gregg J. Silverman (University of California, San Diego) and Brian C. Shaffer (National Institutes of Health) for critically reading the manuscript. This work was supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Abbreviations
- Ig
immunoglobulin
- mAbs
monoclonal antibodies
- HAMA
human anti-mouse antibody
- HARA
human anti-rat antibody
- HACA
human anti-chimeric antibody
- HAHA
human anti-human antibody
- FDA
Food and Drug Administration
- EMA
European Medicines Agency
- GC
germinal centers
- EBV
Epstein-Barr virus
- AID
activation-induced deaminase;
- PCR
polymerase chain reaction
- RT
reverse transcriptase
- CDR
complementarity-determining region
- FACS
fluorescence-activated cell sorting
- GMP
good manufacturing practice
- pAbs
polyclonal antibodies
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/12187
Conflict-of-interest and financial disclosure statements
R.R.B. is an employee of Cytos Biotechnology AG and holds stock options in the company.
References
- 1.Hwang WY, Foote J. Immunogenicity of engineered antibodies. Methods. 2005;36:3–10. doi: 10.1016/j.ymeth.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Weiner LM. Fully human therapeutic monoclonal antibodies. J Immunother. 2006;29:1–9. doi: 10.1097/01.cji.0000192105.24583.83. [DOI] [PubMed] [Google Scholar]
- 3.Swann PG, Tolnay M, Muthukkumar S, Shapiro MA, Rellahan BL, Clouse KA. Considerations for the development of therapeutic monoclonal antibodies. Curr Opin Immunol. 2008;20:493–499. doi: 10.1016/j.coi.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033–1040. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 6.Steinitz M. Three decades of human monoclonal antibodies: past, present and future developments. Hum Antibodies. 2009;18:1–10. doi: 10.3233/HAB-2009-0196. [DOI] [PubMed] [Google Scholar]
- 7.Lonberg N. Fully human antibodies from transgenic mouse and phage display platforms. Curr Opin Immunol. 2008;20:450–459. doi: 10.1016/j.coi.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Hoogenboom HR. Selecting and screening recombinant antibody libraries. Nat Biotechnol. 2005;23:1105–1116. doi: 10.1038/nbt1126. [DOI] [PubMed] [Google Scholar]
- 9.Reichert JM. Monoclonal antibodies in the clinic. Nat Biotechnol. 2001;19:819–822. doi: 10.1038/nbt0901-819. [DOI] [PubMed] [Google Scholar]
- 10.Dimitrov DS, Marks JD. Therapeutic antibodies: current state and future trends—is a paradigm change coming soon? Methods Mol Biol. 2009;525:1–27. doi: 10.1007/978-1-59745-554-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichert JM. Antibodies to watch in 2010. MAbs. 2010;2:84–100. doi: 10.4161/mabs.2.1.10677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu L, Yee H, Chan C, Kashyap AK, Horowitz L, Horowitz M, et al. Combinatorial surrobody libraries. Proc Natl Acad Sci USA. 2008;105:10756–10761. doi: 10.1073/pnas.0805293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 15.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 16.Lanzavecchia A, Sallusto F. Human B cell memory. Curr Opin Immunol. 2009;21:298–304. doi: 10.1016/j.coi.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vollmers HP, Brandlein S. Natural IgM antibodies: the orphaned molecules in immune surveillance. Adv Drug Deliv Rev. 2006;58:755–765. doi: 10.1016/j.addr.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Lane HC, Shelhamer JH, Mostowski HS, Fauci AS. Human monoclonal anti-keyhole limpet hemocyanin antibody-secreting hybridoma produced from peripheral blood B lymphocytes of a keyhole limpet hemocyanin-immune individual. J Exp Med. 1982;155:333–338. doi: 10.1084/jem.155.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nowinski R, Berglund C, Lane J, Lostrom M, Bernstein I, Young W, et al. Human monoclonal antibody against Forssman antigen. Science. 1980;210:537–539. doi: 10.1126/science.7423202. [DOI] [PubMed] [Google Scholar]
- 20.Schlom J, Wunderlich D, Teramoto YA. Generation of human monoclonal antibodies reactive with human mammary carcinoma cells. Proc Natl Acad Sci USA. 1980;77:6841–6845. doi: 10.1073/pnas.77.11.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croce CM, Shander M, Martinis J, Cicurel L, D'Ancona GG, Koprowski H. Preferential retention of human chromosome 14 in mouse X human B cell hybrids. Eur J Immunol. 1980;10:486–488. [Google Scholar]
- 22.Yoshinari K, Arai K, Kimura H, Matsumoto K, Yamaguchi Y. Long-term production of human monoclonal antibodies by human-mouse heterohybridomas. J Immunol Methods. 1995;186:17–25. doi: 10.1016/0022-1759(95)00125-t. [DOI] [PubMed] [Google Scholar]
- 23.Chiorazzi N, Wasserman RL, Kunkel HG. Use of Epstein-Barr virus-transformed B cell lines for the generation of immunoglobulin-producing human B cell hybridomas. J Exp Med. 1982;156:930–935. doi: 10.1084/jem.156.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cote RJ, Morrissey DM, Houghton AN, Beattie E, Jr, Oettgen HF, Old LJ. Generation of human monoclonal antibodies reactive with cellular antigens. Proc Natl Acad Sci USA. 1983;80:2026–2030. doi: 10.1073/pnas.80.7.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Croce CM, Linnenbach A, Hall W, Steplewski Z, Koprowski H. Production of human hybridomas secreting antibodies to measles virus. Nature. 1980;288:488–489. doi: 10.1038/288488a0. [DOI] [PubMed] [Google Scholar]
- 26.Houghton AN, Brooks H, Cote RJ, Taormina MC, Oettgen HF, Old LJ. Detection of cell surface and intracellular antigens by human monoclonal antibodies. Hybrid cell lines derived from lymphocytes of patients with malignant melanoma. J Exp Med. 1983;158:53–65. doi: 10.1084/jem.158.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larrick JW, Truitt KE, Raubitschek AA, Senyk G, Wang JC. Characterization of human hybridomas secreting antibody to tetanus toxoid. Proc Natl Acad Sci USA. 1983;80:6376–6380. doi: 10.1073/pnas.80.20.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsson L, Kaplan HS. Human-human hybridomas producing monoclonal antibodies of predefined antigenic specificity. Proc Natl Acad Sci USA. 1980;77:5429–5431. doi: 10.1073/pnas.77.9.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper MD, Kirkpatrick R. Production of stable heterohybridomas producing human monoclonal antibodies. Methods Mol Biol. 1995;45:29–39. doi: 10.1385/0-89603-308-2:29. [DOI] [PubMed] [Google Scholar]
- 30.Ostberg L, Pursch E. Human X (mouse X human) hybridomas stably producing human antibodies. Hybridoma. 1983;2:361–367. doi: 10.1089/hyb.1983.2.361. [DOI] [PubMed] [Google Scholar]
- 31.Posner MR, Elboim H, Santos D. The construction and use of a human-mouse myeloma analogue suitable for the routine production of hybridomas secreting human monoclonal antibodies. Hybridoma. 1987;6:611–625. doi: 10.1089/hyb.1987.6.611. [DOI] [PubMed] [Google Scholar]
- 32.Teng NN, Lam KS, Calvo Riera F, Kaplan HS. Construction and testing of mouse—human heteromyelomas for human monoclonal antibody production. Proc Natl Acad Sci USA. 1983;80:7308–7312. doi: 10.1073/pnas.80.23.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karpas A, Dremucheva A, Czepulkowski BH. A human myeloma cell line suitable for the generation of human monoclonal antibodies. Proc Natl Acad Sci USA. 2001;98:1799–1804. doi: 10.1073/pnas.98.4.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dessain SK, Adekar SP, Stevens JB, Carpenter KA, Skorski ML, Barnoski BL, et al. High efficiency creation of human monoclonal antibody-producing hybridomas. J Immunol Methods. 2004;291:109–122. doi: 10.1016/j.jim.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Adekar SP, Jones RM, Elias MD, Al-Saleem FH, Root MJ, Simpson LL, et al. Hybridoma populations enriched for affinity-matured human IgGs yield high-affinity antibodies specific for botulinum neurotoxins. J Immunol Methods. 2008;333:156–166. doi: 10.1016/j.jim.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Duchosal MA, Eming SA, Fischer P, Leturcq D, Barbas C, 3rd, McConahey PJ, et al. Immunization of hu-PBL-SCID mice and the rescue of human monoclonal Fab fragments through combinatorial libraries. Nature. 1992;355:258–262. doi: 10.1038/355258a0. [DOI] [PubMed] [Google Scholar]
- 37.Carlsson R, Martensson C, Kalliomaki S, Ohlin M, Borrebaeck CA. Human peripheral blood lymphocytes transplanted into SCID mice constitute an in vivo culture system exhibiting several parameters found in a normal humoral immune response and are a source of immunocytes for the production of human monoclonal antibodies. J Immunol. 1992;148:1065–1071. [PubMed] [Google Scholar]
- 38.Eren R, Lubin I, Terkieltaub D, Ben-Moshe O, Zauberman A, Uhlmann R, et al. Human monoclonal antibodies specific to hepatitis B virus generated in a human/mouse radiation chimera: the Trimera system. Immunology. 1998;93:154–161. doi: 10.1046/j.1365-2567.1998.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawada-Hirai R, Jiang I, Wang F, Sun SM, Nedellec R, Ruther P, et al. Human anti-anthrax protective antigen neutralizing monoclonal antibodies derived from donors vaccinated with anthrax vaccine adsorbed. J Immune Based Ther Vaccines. 2004;2:5. doi: 10.1186/1476-8518-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nilsson K, Klein G, Henle W, Henle G. The establishment of lymphoblastoid lines from adult and fetal human lymphoid tissue and its dependence on EBV. Int J Cancer. 1971;8:443–450. doi: 10.1002/ijc.2910080312. [DOI] [PubMed] [Google Scholar]
- 41.Kozbor D, Roder JC. Requirements for the establishment of high-titered human monoclonal antibodies against tetanus toxoid using the Epstein-Barr virus technique. J Immunol. 1981;127:1275–1280. [PubMed] [Google Scholar]
- 42.Steinitz M, Klein G, Koskimies S, Makel O. EB virus-induced B lymphocyte cell lines producing specific antibody. Nature. 1977;269:420–422. doi: 10.1038/269420a0. [DOI] [PubMed] [Google Scholar]
- 43.Bron D, Feinberg MB, Teng NN, Kaplan HS. Production of human monoclonal IgG antibodies against Rhesus (D) antigen. Proc Natl Acad Sci USA. 1984;81:3214–3217. doi: 10.1073/pnas.81.10.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozbor D, Roder JC, Chang TH, Steplewski Z, Koprowski H. Human anti-tetanus toxoid monoclonal antibody secreted by EBV-transformed human B cells fused with murine myeloma. Hybridoma. 1982;1:323–328. doi: 10.1089/hyb.1.1982.1.323. [DOI] [PubMed] [Google Scholar]
- 45.Geylis V, Kourilov V, Meiner Z, Nennesmo I, Bogdanovic N, Steinitz M. Human monoclonal antibodies against amyloid-beta from healthy adults. Neurobiol Aging. 2005;26:597–606. doi: 10.1016/j.neurobiolaging.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Lang AB, Furer E, Senyk G, Larrick JW, Cryz S., Jr Systematic generation of antigen specific human monoclonal antibodies with therapeutical activities using active immunization. Hum Antibodies Hybridomas. 1990;1:96–103. [PubMed] [Google Scholar]
- 47.Ohlin M, Broliden PA, Danielsson L, Wahren B, Rosen J, Jondal M, et al. Human monoclonal antibodies against a recombinant HIV envelope antigen produced by primary in vitro immunization. Characterization and epitope mapping. Immunology. 1989;68:325–331. [PMC free article] [PubMed] [Google Scholar]
- 48.Yu X, McGraw PA, House FS, Crowe J., Jr An optimized electrofusion-based protocol for generating virus-specific human monoclonal antibodies. J Immunol Methods. 2008;336:142–151. doi: 10.1016/j.jim.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu X, Tsibane T, McGraw PA, House FS, Keefer CJ, Hicar MD, et al. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature. 2008;455:532–536. doi: 10.1038/nature07231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shammah S, Mantovani TL, Dalla-Favera R, Casali P. Generation of human monoclonal antibodies by transformation of lymphoblastoid B cells with ras oncogene. J Immunol Methods. 1993;160:19–25. doi: 10.1016/0022-1759(93)90004-q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simmons CP, Bernasconi NL, Suguitan AL, Mills K, Ward JM, Chau NV, et al. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 2007;4:178. doi: 10.1371/journal.pmed.0040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Traggiai E, Becker S, Subbarao K, Kolesnikova L, Uematsu Y, Gismondo MR, et al. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wen L, Hanvanich M, Werner-Favre C, Brouwers N, Perrin LH, Zubler RH. Limiting dilution assay for human B cells based on their activation by mutant EL4 thymoma cells: total and antimalaria responder B cell frequencies. Eur J Immunol. 1987;17:887–892. doi: 10.1002/eji.1830170624. [DOI] [PubMed] [Google Scholar]
- 54.Zubler RH, Erard F, Lees RK, Van Laer M, Mingari C, Moretta L, et al. Mutant EL-4 thymoma cells polyclonally activate murine and human B cells via direct cell interaction. J Immunol. 1985;134:3662–3668. [PubMed] [Google Scholar]
- 55.Banchereau J, de Paoli P, Valle A, Garcia E, Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991;251:70–72. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- 56.Rousset F, Garcia E, Banchereau J. Cytokine-induced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J Exp Med. 1991;173:705–710. doi: 10.1084/jem.173.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lagerkvist AC, Furebring C, Borrebaeck CA. Single, antigen-specific B cells used to generate Fab fragments using CD40-mediated amplification or direct PCR cloning. Biotechniques. 1995;18:862–869. [PubMed] [Google Scholar]
- 58.Weitkamp JH, Kallewaard N, Kusuhara K, Feigelstock D, Feng N, Greenberg HB, et al. Generation of recombinant human monoclonal antibodies to rotavirus from single antigen-specific B cells selected with fluorescent virus-like particles. J Immunol Methods. 2003;275:223–237. doi: 10.1016/s0022-1759(03)00013-9. [DOI] [PubMed] [Google Scholar]
- 59.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwakkenbos MJ, Diehl SA, Yasuda E, Bakker AQ, van Geelen CM, Lukens MV, et al. Generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells by genetic programming. Nat Med. 2010;16:123–128. doi: 10.1038/nm.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci USA. 2008;105:9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 64.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meijer PJ, Andersen PS, Haahr Hansen M, Steinaa L, Jensen A, Lantto J, et al. Isolation of human antibody repertoires with preservation of the natural heavy and light chain pairing. J Mol Biol. 2006;358:764–772. doi: 10.1016/j.jmb.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 66.Poulsen TR, Meijer PJ, Jensen A, Nielsen LS, Andersen PS. Kinetic, affinity and diversity limits of human polyclonal antibody responses against tetanus toxoid. J Immunol. 2007;179:3841–3850. doi: 10.4049/jimmunol.179.6.3841. [DOI] [PubMed] [Google Scholar]
- 67.Love JC, Ronan JL, Grotenbreg GM, van der Veen AG, Ploegh HL. A microengraving method for rapid selection of single cells producing antigen-specific antibodies. Nat Biotechnol. 2006;24:703–707. doi: 10.1038/nbt1210. [DOI] [PubMed] [Google Scholar]
- 68.Jin A, Ozawa T, Tajiri K, Obata T, Kondo S, Kinoshita K, et al. A rapid and efficient single-cell manipulation method for screening antigen-specific antibody-secreting cells from human peripheral blood. Nat Med. 2009;15:1088–1092. doi: 10.1038/nm.1966. [DOI] [PubMed] [Google Scholar]
- 69.Rader C. Antibody libraries in drug and target discovery. Drug Discov Today. 2001;6:36–43. doi: 10.1016/s1359-6446(00)01595-6. [DOI] [PubMed] [Google Scholar]
- 70.Glanville J, Zhai W, Berka J, Telman D, Huerta G, Mehta GR, et al. Precise determination of the diversity of a combinatorial antibody library gives insight into the human immunoglobulin repertoire. Proc Natl Acad Sci USA. 2009;106:20216–20221. doi: 10.1073/pnas.0909775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Persson MA. Twenty years of combinatorial antibody libraries, but how well do they mimic the immunoglobulin repertoire? Proc Natl Acad Sci USA. 2009;106:20137–20138. doi: 10.1073/pnas.0912118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huse WD, Sastry L, Iverson SA, Kang AS, Alting-Mees M, Burton DR, et al. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science. 1989;246:1275–1281. doi: 10.1126/science.2531466. [DOI] [PubMed] [Google Scholar]
- 73.Mullinax RL, Gross EA, Amberg JR, Hay BN, Hogrefe HH, Kubitz MM, et al. Identification of human antibody fragment clones specific for tetanus toxoid in a bacteriophage lambda immunoexpression library. Proc Natl Acad Sci USA. 1990;87:8095–8099. doi: 10.1073/pnas.87.20.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Persson MA, Caothien RH, Burton DR. Generation of diverse high-affinity human monoclonal antibodies by repertoire cloning. Proc Natl Acad Sci USA. 1991;88:2432–2436. doi: 10.1073/pnas.88.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 76.McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348:552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- 77.Clackson T, Hoogenboom HR, Griffiths AD, Winter G. Making antibody fragments using phage display libraries. Nature. 1991;352:624–628. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- 78.Breitling F, Dubel S, Seehaus T, Klewinghaus I, Little M. A surface expression vector for antibody screening. Gene. 1991;104:147–153. doi: 10.1016/0378-1119(91)90244-6. [DOI] [PubMed] [Google Scholar]
- 79.Kang AS, Barbas CF, Janda KD, Benkovic SJ, Lerner RA. Linkage of recognition and replication functions by assembling combinatorial antibody Fab libraries along phage surfaces. Proc Natl Acad Sci USA. 1991;88:4363–4366. doi: 10.1073/pnas.88.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoogenboom HR, Griffiths AD, Johnson KS, Chiswell DJ, Hudson P, Winter G. Multi-subunit proteins on the surface of filamentous phage: methodologies for displaying antibody (Fab) heavy and light chains. Nucleic Acids Res. 1991;19:4133–4137. doi: 10.1093/nar/19.15.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holt LJ, Herring C, Jespers LS, Woolven BP, Tomlinson IM. Domain antibodies: proteins for therapy. Trends Biotechnol. 2003;21:484–490. doi: 10.1016/j.tibtech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 82.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 83.O'Connell D, Becerril B, Roy-Burman A, Daws M, Marks JD. Phage versus phagemid libraries for generation of human monoclonal antibodies. J Mol Biol. 2002;321:49–56. doi: 10.1016/s0022-2836(02)00561-2. [DOI] [PubMed] [Google Scholar]
- 84.Shi L, Wheeler JC, Sweet RW, Lu J, Luo J, Tornetta M, et al. De novo selection of high-affinity antibodies from synthetic fab libraries displayed on phage as pIX fusion proteins. J Mol Biol. 2010 doi: 10.1016/j.jmb.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 85.Barbas C, 3rd, Kang AS, Lerner RA, Benkovic SJ. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci USA. 1991;88:7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burton DR, Barbas C, 3rd, Persson MA, Koenig S, Chanock RM, Lerner RA. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci USA. 1991;88:10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zebedee SL, Barbas C, 3rd, Hom YL, Caothien RH, Graff R, DeGraw J, et al. Human combinatorial antibody libraries to hepatitis B surface antigen. Proc Natl Acad Sci USA. 1992;89:3175–3179. doi: 10.1073/pnas.89.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barbas C, 3rd, Crowe J, Jr, Cababa D, Jones TM, Zebedee SL, Murphy BR, et al. Human monoclonal Fab fragments derived from a combinatorial library bind to respiratory syncytial virus F glycoprotein and neutralize infectivity. Proc Natl Acad Sci USA. 1992;89:10164–10168. doi: 10.1073/pnas.89.21.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barbas C, 3rd, Collet TA, Amberg W, Roben P, Binley JM, Hoekstra D, et al. Molecular profile of an antibody response to HIV-1 as probed by combinatorial libraries. J Mol Biol. 1993;230:812–823. doi: 10.1006/jmbi.1993.1203. [DOI] [PubMed] [Google Scholar]
- 90.Williamson RA, Burioni R, Sanna PP, Partridge LJ, Barbas C, 3rd, Burton DR. Human monoclonal antibodies against a plethora of viral pathogens from single combinatorial libraries. Proc Natl Acad Sci USA. 1993;90:4141–4145. doi: 10.1073/pnas.90.9.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barbas SM, Ditzel HJ, Salonen EM, Yang WP, Silverman GJ, Burton DR. Human autoantibody recognition of DNA. Proc Natl Acad Sci USA. 1995;92:2529–2533. doi: 10.1073/pnas.92.7.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roben P, Barbas SM, Sandoval L, Lecerf JM, Stollar BD, Solomon A, et al. Repertoire cloning of lupus anti-DNA autoantibodies. J Clin Invest. 1996;98:2827–2837. doi: 10.1172/JCI119111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Siegel DL. Translational applications of antibody phage display. Immunol Res. 2008;42:118–131. doi: 10.1007/s12026-008-8044-y. [DOI] [PubMed] [Google Scholar]
- 94.Hansen MH, Nielsen H, Ditzel HJ. The tumor-infiltrating B cell response in medullary breast cancer is oligoclonal and directed against the autoantigen actin exposed on the surface of apoptotic cancer cells. Proc Natl Acad Sci USA. 2001;98:12659–12664. doi: 10.1073/pnas.171460798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coronella JA, Spier C, Welch M, Trevor KT, Stopeck AT, Villar H, et al. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J Immunol. 2002;169:1829–1836. doi: 10.4049/jimmunol.169.4.1829. [DOI] [PubMed] [Google Scholar]
- 96.Pavoni E, Monteriu G, Santapaola D, Petronzelli F, Anastasi AM, Pelliccia A, et al. Tumor-infiltrating B lymphocytes as an efficient source of highly specific immunoglobulins recognizing tumor cells. BMC Biotechnol. 2007;7:70. doi: 10.1186/1472-6750-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cai X, Garen A. Anti-melanoma antibodies from melanoma patients immunized with genetically modified autologous tumor cells: selection of specific antibodies from single-chain Fv fusion phage libraries. Proc Natl Acad Sci USA. 1995;92:6537–6541. doi: 10.1073/pnas.92.14.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baskar S, Suschak JM, Samija I, Srinivasan R, Childs RW, Pavletic SZ, et al. A human monoclonal antibody drug and target discovery platform for B-cell chronic lymphocytic leukemia based on allogeneic hematopoietic stem cell transplantation and phage display. Blood. 2009;114:4494–4502. doi: 10.1182/blood-2009-05-222786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marks JD, Hoogenboom HR, Bonnert TP, McCafferty J, Griffiths AD, Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991;222:581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- 100.Griffiths AD, Malmqvist M, Marks JD, Bye JM, Embleton MJ, McCafferty J, et al. Human anti-self antibodies with high specificity from phage display libraries. EMBO J. 1993;12:725–734. doi: 10.1002/j.1460-2075.1993.tb05706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vaughan TJ, Williams AJ, Pritchard K, Osbourn JK, Pope AR, Earnshaw JC, et al. Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat Biotechnol. 1996;14:309–314. doi: 10.1038/nbt0396-309. [DOI] [PubMed] [Google Scholar]
- 102.Sheets MD, Amersdorfer P, Finnern R, Sargent P, Lindquist E, Schier R, et al. Efficient construction of a large nonimmune phage antibody library: the production of high-affinity human single-chain antibodies to protein antigens. Proc Natl Acad Sci USA. 1998;95:6157–6162. doi: 10.1073/pnas.95.11.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Haard HJ, van Neer N, Reurs A, Hufton SE, Roovers RC, Henderikx P, et al. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J Biol Chem. 1999;274:18218–18230. doi: 10.1074/jbc.274.26.18218. [DOI] [PubMed] [Google Scholar]
- 104.Ling MM. Large antibody display libraries for isolation of high-affinity antibodies. Comb Chem High Throughput Screen. 2003;6:421–432. doi: 10.2174/138620703106298608. [DOI] [PubMed] [Google Scholar]
- 105.Marks JD, Griffiths AD, Malmqvist M, Clackson TP, Bye JM, Winter G. By-passing immunization: building high affinity human antibodies by chain shuffling. Biotechnology. 1992;10:779–783. doi: 10.1038/nbt0792-779. [DOI] [PubMed] [Google Scholar]
- 106.Kwong KY, Baskar S, Zhang H, Mackall CL, Rader C. Generation, affinity maturation and characterization of a human anti-human NKG2D monoclonal antibody with dual antagonistic and agonistic activity. J Mol Biol. 2008;384:1143–1156. doi: 10.1016/j.jmb.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jespers LS, Roberts A, Mahler SM, Winter G, Hoogenboom HR. Guiding the selection of human antibodies from phage display repertoires to a single epitope of an antigen. Biotechnology. 1994;12:899–903. doi: 10.1038/nbt0994-899. [DOI] [PubMed] [Google Scholar]
- 108.Osbourn J, Groves M, Vaughan T. From rodent reagents to human therapeutics using antibody guided selection. Methods. 2005;36:61–68. doi: 10.1016/j.ymeth.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 109.Rader C, Cheresh DA, Barbas C., 3rd A phage display approach for rapid antibody humanization: designed combinatorial V gene libraries. Proc Natl Acad Sci USA. 1998;95:8910–8915. doi: 10.1073/pnas.95.15.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chapal N, Peraldi-Roux S, Bresson D, Pugniere M, Mani JC, Granier C, et al. Human anti-thyroid peroxidase single-chain fragment variable of Ig isolated from a combinatorial library assembled in-cell: insights into the in vivo situation. J Immunol. 2000;164:4162–4169. doi: 10.4049/jimmunol.164.8.4162. [DOI] [PubMed] [Google Scholar]
- 111.Boder ET, Wittrup KD. Yeast surface display for screening combinatorial polypeptide libraries. Nat Biotechnol. 1997;15:553–557. doi: 10.1038/nbt0697-553. [DOI] [PubMed] [Google Scholar]
- 112.Gai SA, Wittrup KD. Yeast surface display for protein engineering and characterization. Curr Opin Struct Biol. 2007;17:467–473. doi: 10.1016/j.sbi.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bowley DR, Labrijn AF, Zwick MB, Burton DR. Antigen selection from an HIV-1 immune antibody library displayed on yeast yields many novel antibodies compared to selection from the same library displayed on phage. Protein Eng Des Sel. 2007;20:81–90. doi: 10.1093/protein/gzl057. [DOI] [PubMed] [Google Scholar]
- 114.Feldhaus MJ, Siegel RW. Yeast display of antibody fragments: a discovery and characterization platform. J Immunol Methods. 2004;290:69–80. doi: 10.1016/j.jim.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 115.van den Beucken T, Pieters H, Steukers M, van der Vaart M, Ladner RC, Hoogenboom HR, et al. Affinity maturation of Fab antibody fragments by fluorescent-activated cell sorting of yeast-displayed libraries. FEBS Lett. 2003;546:288–294. doi: 10.1016/s0014-5793(03)00602-1. [DOI] [PubMed] [Google Scholar]
- 116.Walker LM, Bowley DR, Burton DR. Efficient recovery of high-affinity antibodies from a single-chain Fab yeast display library. J Mol Biol. 2009;389:365–375. doi: 10.1016/j.jmb.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Feldhaus MJ, Siegel RW, Opresko LK, Coleman JR, Feldhaus JM, Yeung YA, et al. Flow-cytometric isolation of human antibodies from a nonimmune Saccharomyces cerevisiae surface display library. Nat Biotechnol. 2003;21:163–170. doi: 10.1038/nbt785. [DOI] [PubMed] [Google Scholar]
- 118.Blaise L, Wehnert A, Steukers MP, van den Beucken T, Hoogenboom HR, Hufton SE. Construction and diversification of yeast cell surface displayed libraries by yeast mating: application to the affinity maturation of Fab antibody fragments. Gene. 2004;342:211–218. doi: 10.1016/j.gene.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 119.Benatuil L, Perez JM, Belk J, Hsieh CM. An improved yeast transformation method for the generation of very large human antibody libraries. Protein Eng Des Sel. 2010;23:155–159. doi: 10.1093/protein/gzq002. [DOI] [PubMed] [Google Scholar]
- 120.Weaver-Feldhaus JM, Lou J, Coleman JR, Siegel RW, Marks JD, Feldhaus MJ. Yeast mating for combinatorial Fab library generation and surface display. FEBS Lett. 2004;564:24–34. doi: 10.1016/S0014-5793(04)00309-6. [DOI] [PubMed] [Google Scholar]
- 121.Lou J, Geren I, Garcia-Rodriguez C, Forsyth CM, Wen W, Knopp K, et al. Affinity maturation of human botulinum neurotoxin antibodies by light chain shuffling via yeast mating. Protein Eng Des Sel. 2010 doi: 10.1093/protein/gzq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boder ET, Midelfort KS, Wittrup KD. Directed evolution of antibody fragments with monovalent femtomolar antigen-binding affinity. Proc Natl Acad Sci USA. 2000;97:10701–10705. doi: 10.1073/pnas.170297297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ho M, Nagata S, Pastan I. Isolation of anti-CD22 Fv with high affinity by Fv display on human cells. Proc Natl Acad Sci USA. 2006;103:9637–9642. doi: 10.1073/pnas.0603653103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smith ES, Shi S, Zauderer M. Construction of cDNA libraries in vaccinia virus. Methods Mol Biol. 2004;269:65–76. doi: 10.1385/1-59259-789-0:065. [DOI] [PubMed] [Google Scholar]
- 125.Beerli RR, Bauer M, Buser RB, Gwerder M, Muntwiler S, Maurer P, et al. Isolation of human monoclonal antibodies by mammalian cell display. Proc Natl Acad Sci USA. 2008;105:14336–14341. doi: 10.1073/pnas.0805942105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jennings GT, Bachmann MF. The coming of age of virus-like particle vaccines. Biol Chem. 2008;389:521–536. doi: 10.1515/bc.2008.064. [DOI] [PubMed] [Google Scholar]
- 127.Beerli RR, Bauer M, Schmitz N, Buser RB, Gwerder M, Muntwiler S, et al. Prophylactic and therapeutic activity of fully human monoclonal antibodies directed against influenza A M2 protein. Virol J. 2009;6:224. doi: 10.1186/1743-422X-6-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bruggemann M, Caskey HM, Teale C, Waldmann H, Williams GT, Surani MA, et al. A repertoire of monoclonal antibodies with human heavy chains from transgenic mice. Proc Natl Acad Sci USA. 1989;86:6709–6713. doi: 10.1073/pnas.86.17.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol. 2005;23:1117–1125. doi: 10.1038/nbt1135. [DOI] [PubMed] [Google Scholar]
- 130.Green LL, Hardy MC, Maynard-Currie CE, Tsuda H, Louie DM, Mendez MJ, et al. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nat Genet. 1994;7:13–21. doi: 10.1038/ng0594-13. [DOI] [PubMed] [Google Scholar]
- 131.Lonberg N, Taylor LD, Harding FA, Trounstine M, Higgins KM, Schramm SR, et al. Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature. 1994;368:856–859. doi: 10.1038/368856a0. [DOI] [PubMed] [Google Scholar]
- 132.Alter-Wolf S, Blomberg BB, Riley RL. Old mice retain bone marrow B1 progenitors, but lose B2 precursors, and exhibit altered immature B cell phenotype and light chain usage. Mech Ageing Dev. 2009;130:401–408. doi: 10.1016/j.mad.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu JL, Davis MM. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity. 2000;13:37–45. doi: 10.1016/s1074-7613(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 134.Fishwild DM, O'Donnell SL, Bengoechea T, Hudson DV, Harding F, Bernhard SL, et al. High-avidity human IgGkappa monoclonal antibodies from a novel strain of minilocus transgenic mice. Nat Biotechnol. 1996;14:845–851. doi: 10.1038/nbt0796-845. [DOI] [PubMed] [Google Scholar]
- 135.Mendez MJ, Green LL, Corvalan JR, Jia XC, Maynard-Currie CE, Yang XD, et al. Functional transplant of megabase human immunoglobulin loci recapitulates human antibody response in mice. Nat Genet. 1997;15:146–156. doi: 10.1038/ng0297-146. [DOI] [PubMed] [Google Scholar]
- 136.Nicholson IC, Zou X, Popov AV, Cook GP, Corps EM, Humphries S, et al. Antibody repertoires of four- and five-feature translocus mice carrying human immunoglobulin heavy chain and kappa and lambda light chain yeast artificial chromosomes. J Immunol. 1999;163:6898–6906. [PubMed] [Google Scholar]
- 137.Tomizuka K, Shinohara T, Yoshida H, Uejima H, Ohguma A, Tanaka S, et al. Double trans-chromosomic mice: maintenance of two individual human chromosome fragments containing Ig heavy and kappa loci and expression of fully human antibodies. Proc Natl Acad Sci USA. 2000;97:722–727. doi: 10.1073/pnas.97.2.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ishida I, Tomizuka K, Yoshida H, Tahara T, Takahashi N, Ohguma A, et al. Production of human monoclonal and polyclonal antibodies in TransChromo animals. Cloning Stem Cells. 2002;4:91–102. doi: 10.1089/153623002753632084. [DOI] [PubMed] [Google Scholar]
- 139.Haurum JS. Recombinant polyclonal antibodies: the next generation of antibody therapeutics? Drug Discov Today. 2006;11:655–660. doi: 10.1016/j.drudis.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 140.Kuroiwa Y, Kasinathan P, Sathiyaseelan T, Jiao JA, Matsushita H, Sathiyaseelan J, et al. Nat Biotechnol. 2009;27:173–181. doi: 10.1038/nbt.1521. [DOI] [PubMed] [Google Scholar]
- 141.Sidhu SS, Fellouse FA. Synthetic therapeutic antibodies. Nat Chem Biol. 2006;2:682–688. doi: 10.1038/nchembio843. [DOI] [PubMed] [Google Scholar]
- 142.Barbas C, 3rd, Bain JD, Hoekstra DM, Lerner RA. Semisynthetic combinatorial antibody libraries: a chemical solution to the diversity problem. Proc Natl Acad Sci USA. 1992;89:4457–4461. doi: 10.1073/pnas.89.10.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barbas C, 3rd, Languino LR, Smith JW. High-affinity self-reactive human antibodies by design and selection: targeting the integrin ligand binding site. Proc Natl Acad Sci USA. 1993;90:10003–10007. doi: 10.1073/pnas.90.21.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chung J, Rader C, Popkov M, Hur YM, Kim HK, Lee YJ, et al. Integrin alphaIIbbeta3-specific synthetic human monoclonal antibodies and HCDR3 peptides that potently inhibit platelet aggregation. FASEB J. 2004;18:361–363. doi: 10.1096/fj.03-0586fje. [DOI] [PubMed] [Google Scholar]
- 145.Yang WP, Green K, Pinz-Sweeney S, Briones AT, Burton DR, Barbas C., 3rd CDR walking mutagenesis for the affinity maturation of a potent human anti-HIV-1 antibody into the picomolar range. J Mol Biol. 1995;254:392–403. doi: 10.1006/jmbi.1995.0626. [DOI] [PubMed] [Google Scholar]
- 146.Schier R, McCall A, Adams GP, Marshall KW, Merritt H, Yim M, et al. Isolation of picomolar affinity anti-c-erbB-2 single-chain Fv by molecular evolution of the complementarity determining regions in the center of the antibody binding site. J Mol Biol. 1996;263:551–567. doi: 10.1006/jmbi.1996.0598. [DOI] [PubMed] [Google Scholar]
- 147.Hoogenboom HR, Winter G. By-passing immunisation. Human antibodies from synthetic repertoires of germline VH gene segments rearranged in vitro. J Mol Biol. 1992;227:381–388. doi: 10.1016/0022-2836(92)90894-p. [DOI] [PubMed] [Google Scholar]
- 148.Griffiths AD, Williams SC, Hartley O, Tomlinson IM, Waterhouse P, Crosby WL, et al. Isolation of high affinity human antibodies directly from large synthetic repertoires. EMBO J. 1994;13:3245–3260. doi: 10.1002/j.1460-2075.1994.tb06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.de Kruif J, Boel E, Logtenberg T. Selection and application of human single chain Fv antibody fragments from a semi-synthetic phage antibody display library with designed CDR3 regions. J Mol Biol. 1995;248:97–105. doi: 10.1006/jmbi.1995.0204. [DOI] [PubMed] [Google Scholar]
- 150.Hoet RM, Cohen EH, Kent RB, Rookey K, Schoonbroodt S, Hogan S, et al. Generation of high-affinity human antibodies by combining donor-derived and synthetic complementarity-determining-region diversity. Nat Biotechnol. 2005;23:344–348. doi: 10.1038/nbt1067. [DOI] [PubMed] [Google Scholar]
- 151.Soderlind E, Strandberg L, Jirholt P, Kobayashi N, Alexeiva V, Aberg AM, et al. Recombining germline-derived CDR sequences for creating diverse single-framework antibody libraries. Nat Biotechnol. 2000;18:852–856. doi: 10.1038/78458. [DOI] [PubMed] [Google Scholar]
- 152.Low NM, Holliger PH, Winter G. Mimicking somatic hypermutation: affinity maturation of antibodies displayed on bacteriophage using a bacterial mutator strain. J Mol Biol. 1996;260:359–368. doi: 10.1006/jmbi.1996.0406. [DOI] [PubMed] [Google Scholar]
- 153.Cesaro-Tadic S, Lagos D, Honegger A, Rickard JH, Partridge LJ, Blackburn GM, et al. Turnover-based in vitro selection and evolution of biocatalysts from a fully synthetic antibody library. Nat Biotechnol. 2003;21:679–685. doi: 10.1038/nbt828. [DOI] [PubMed] [Google Scholar]
- 154.Zhou Y, Drummond DC, Zou H, Hayes ME, Adams GP, Kirpotin DB, et al. Impact of single-chain Fv antibody fragment affinity on nanoparticle targeting of epidermal growth factor receptor-expressing tumor cells. J Mol Biol. 2007;371:934–947. doi: 10.1016/j.jmb.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pini A, Viti F, Santucci A, Carnemolla B, Zardi L, Neri P, et al. Design and use of a phage display library. Human antibodies with subnanomolar affinity against a marker of angiogenesis eluted from a two-dimensional gel. J Biol Chem. 1998;273:21769–21776. doi: 10.1074/jbc.273.34.21769. [DOI] [PubMed] [Google Scholar]
- 156.Sidhu SS, Li B, Chen Y, Fellouse FA, Eigenbrot C, Fuh G. Phage-displayed antibody libraries of synthetic heavy chain complementarity determining regions. J Mol Biol. 2004;338:299–310. doi: 10.1016/j.jmb.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 157.Lee CV, Liang WC, Dennis MS, Eigenbrot C, Sidhu SS, Fuh G. High-affinity human antibodies from phage-displayed synthetic Fab libraries with a single framework scaffold. J Mol Biol. 2004;340:1073–1093. doi: 10.1016/j.jmb.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 158.Fellouse FA, Wiesmann C, Sidhu SS. Synthetic antibodies from a four-amino-acid code: a dominant role for tyrosine in antigen recognition. Proc Natl Acad Sci USA. 2004;101:12467–12472. doi: 10.1073/pnas.0401786101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fellouse FA, Li B, Compaan DM, Peden AA, Hymowitz SG, Sidhu SS. Molecular recognition by a binary code. J Mol Biol. 2005;348:1153–1162. doi: 10.1016/j.jmb.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 160.Cobaugh CW, Almagro JC, Pogson M, Iverson B, Georgiou G. Synthetic antibody libraries focused towards peptide ligands. J Mol Biol. 2008;378:622–633. doi: 10.1016/j.jmb.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schoonbroodt S, Steukers M, Viswanathan M, Frans N, Timmermans M, Wehnert A, et al. Engineering antibody heavy chain CDR3 to create a phage display Fab library rich in antibodies that bind charged carbohydrates. J Immunol. 2008;181:6213–6221. doi: 10.4049/jimmunol.181.9.6213. [DOI] [PubMed] [Google Scholar]
- 162.Knappik A, Ge L, Honegger A, Pack P, Fischer M, Wellnhofer G, et al. Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J Mol Biol. 2000;296:57–86. doi: 10.1006/jmbi.1999.3444. [DOI] [PubMed] [Google Scholar]
- 163.Rauchenberger R, Borges E, Thomassen-Wolf E, Rom E, Adar R, Yaniv Y, et al. Human combinatorial Fab library yielding specific and functional antibodies against the human fibroblast growth factor receptor 3. J Biol Chem. 2003;278:38194–38205. doi: 10.1074/jbc.M303164200. [DOI] [PubMed] [Google Scholar]
- 164.Rothe C, Urlinger S, Lohning C, Prassler J, Stark Y, Jager U, et al. The human combinatorial antibody library HuCAL GOLD combines diversification of all six CDRs according to the natural immune system with a novel display method for efficient selection of high-affinity antibodies. J Mol Biol. 2008;376:1182–1200. doi: 10.1016/j.jmb.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 165.Hanes J, Schaffitzel C, Knappik A, Pluckthun A. Picomolar affinity antibodies from a fully synthetic naive library selected and evolved by ribosome display. Nat Biotechnol. 2000;18:1287–1292. doi: 10.1038/82407. [DOI] [PubMed] [Google Scholar]
- 166.Rothe A, Hosse RJ, Power BE. In vitro display technologies reveal novel biopharmaceutics. FASEB J. 2006;20:1599–610. doi: 10.1096/fj.05-5650rev. [DOI] [PubMed] [Google Scholar]
- 167.Hanes J, Pluckthun A. In vitro selection and evolution of functional proteins by using ribosome display. Proc Natl Acad Sci USA. 1997;94:4937–4942. doi: 10.1073/pnas.94.10.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.He M, Taussig MJ. Antibody-ribosome-mRNA (ARM) complexes as efficient selection particles for in vitro display and evolution of antibody combining sites. Nucleic Acids Res. 1997;25:5132–5134. doi: 10.1093/nar/25.24.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schaffitzel C, Hanes J, Jermutus L, Pluckthun A. Ribosome display: an in vitro method for selection and evolution of antibodies from libraries. J Immunol Methods. 1999;231:119–135. doi: 10.1016/s0022-1759(99)00149-0. [DOI] [PubMed] [Google Scholar]
- 170.Thom G, Cockroft AC, Buchanan AG, Candotti CJ, Cohen ES, Lowne D, et al. Probing a protein-protein interaction by in vitro evolution. Proc Natl Acad Sci USA. 2006;103:7619–7624. doi: 10.1073/pnas.0602341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Urban JH, Schneider RM, Compte M, Finger C, Cichutek K, Alvarez-Vallina L, et al. Selection of functional human antibodies from retroviral display libraries. Nucleic Acids Res. 2005;33:35. doi: 10.1093/nar/gni033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen W, Zhu Z, Feng Y, Xiao X, Dimitrov DS. Construction of a large phage-displayed human antibody domain library with a scaffold based on a newly identified highly soluble, stable heavy chain variable domain. J Mol Biol. 2008;382:779–789. doi: 10.1016/j.jmb.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xiao X, Feng Y, Vu BK, Ishima R, Dimitrov DS. A large library based on a novel (CH2) scaffold: identification of HIV-1 inhibitors. Biochem Biophys Res Commun. 2009;387:387–392. doi: 10.1016/j.bbrc.2009.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wozniak-Knopp G, Bartl S, Bauer A, Mostageer M, Woisetschlager M, Antes B, et al. Introducing antigen-binding sites in structural loops of immunoglobulin constant domains: Fc fragments with engineered HER2/neu-binding sites and antibody properties. Protein Eng Des Sel. 2010 doi: 10.1093/protein/gzq005. [DOI] [PubMed] [Google Scholar]
- 175.Liu CC, Mack AV, Tsao ML, Mills JH, Lee HS, Choe H, et al. Protein evolution with an expanded genetic code. Proc Natl Acad Sci USA. 2008;105:17688–17693. doi: 10.1073/pnas.0809543105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 177.Aarden L, Ruuls SR, Wolbink G. Immunogenicity of anti-tumor necrosis factor antibodies-toward improved methods of anti-antibody measurement. Curr Opin Immunol. 2008;20:431–435. doi: 10.1016/j.coi.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 178.Mazumdar S, Greenwald D. Golimumab. MAbs. 2009;1:422–431. doi: 10.4161/mabs.1.5.9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Logtenberg T. Antibody cocktails: next-generation biopharmaceuticals with improved potency. Trends Biotechnol. 2007;25:390–394. doi: 10.1016/j.tibtech.2007.07.005. [DOI] [PubMed] [Google Scholar]