Abstract
Monoclonal antibodies (mAbs) represent the fastest growing class of therapeutic proteins. The increasing demand for mAb manufacturing and the associated high production costs call for the pharmaceutical industry to improve its current production processes or develop more efficient alternative production platforms. The experimental control of IgG fucosylation to enhance antibody dependent cell cytotoxicity (ADCC) activity constitutes one of the promising strategies to improve the efficacy of monoclonal antibodies and to potentially reduce the therapeutic cost. We report here that the EB66 cell line derived from duck embryonic stem cells can be efficiently genetically engineered to produce mAbs at yields beyond a 1 g/L, as suspension cells grown in serum-free culture media. EB66 cells display additional attractive growth characteristics such as a very short population doubling time of 12–14 h, a capacity to reach very high cell density (>30 million cells/mL) and a unique metabolic profile resulting in low ammonium and lactate accumulation and low glutamine consumption, even at high cell densities. Furthermore, mAbs produced on EB66 cells display a naturally reduced fucose content resulting in strongly enhanced ADCC activity. The EB66 cells have therefore the potential to evolve as a novel cellular platform for the production of high potency therapeutic antibodies.
Key words: duck, embryonic stem cells, monoclonal antibody, fucose, ADCC
Introduction
Monoclonal antibodies (mAbs) constitute a highly successful class of therapeutic proteins, with applications in various fields such as inflammatory diseases, oncology or infectious diseases. Currently, 24 therapeutic mAbs are marketed in the US and more than 200 mAbs are in clinical development, with this number predicted to increase rapidly. Mammalian cell culture, in particular the Chinese hamster ovary (CHO) cell line, emerged as the expression host of choice for the industrial production of therapeutic glycoproteins because of their capacity for proper protein folding, assembly and post-translational modification.1 However, a rapid increase in the number of approved mAbs may cause issues of limited production capacity, high production charges and prohibitive therapeutic costs.2 As a consequence, access of the general population to such innovative therapies may be delayed. Increasing bioreactors capacity would not reduce sufficiently the cost of production, mainly due to downstream processing becoming a bottleneck. Thus, permanent efforts have been dedicated to further improvements of the production yields achieved by current mammalian systems.3 Besides such developments, alternative production systems have been recently explored that could produce proteins with enhanced therapeutic indexes and hence with the potential for lower production costs.4
Antibodies are glycoproteins composed of two heavy and two light chains with a high specificity against defined antigen.5 The Fc portion of immunoglobulin G (IgG) possesses one conserved glycosylation site at Asn-297 in each of the CH2 domains, where complex biantennary type oligosaccharides are attached.6 These carbohydrate moieties are essential to the therapeutic efficacy by providing the antibodies with the ability to activate complement and to bind to Fcγ receptors (FcγRs) of effector cells.7 In cancer treatment, the antibody ADCC function is thought to play a critical role in clinical efficacy in humans.8,9 Lack of fucose on human IgG1 oligosaccharide was demonstrated to improve the binding to Fcγ receptor IIIa (FcγRIIIa) present on effector cells, e.g., natural killer (NK) cells, and thereby enhance ADCC.10 The control of IgG fucosylation is thus one of the promising approaches currently under active investigation to improve the potency of therapeutic antibodies and possibly reduce costs. Production of non-fucosylated therapeutic antibodies has been mostly achieved through glycoengineering strategies in CHO cells,11 but some species naturally have the capability to produce glycoproteins with low fucose. Raju et al.12 reported that chicken IgG1 contain complex glycans with naturally reduced fucose content compared to other species. This peculiarity of avian glycosylation was confirmed by the production of a human IgG1 in eggs of transgenic chicken.13 Such transgenic IgGs were found to harbor N-glycans with low fucose content and exhibit an enhanced ADCC.13 Therefore, a continuous avian cell line that displays “industrial friendly” characteristics, i.e., genetic stability, proliferation in stirred-tank bioreactors to high cell densities as suspension cells, growth in serum-free media, short population doubling time, and is amenable to genetic engineering to produce IgG could be a valuable alternative for the production of therapeutic mAb with an enhanced biological activity.
Over the past ten years, we established and developed a proprietary avian embryonic stem (ES) cell based platform, which involved the isolation of chicken or duck embryonic stem cells and derivation thereof of stable cell lines without genetic, viral or chemical modifications.14 The duck EB66 cell line was thus derived following a multi step process permitting the selection of stable cell lines that maintain some of the unique biological properties of ES cells, such as the expression of ES cells specific markers, e.g., telomerase, SSEA-1, EMA-1, the ability to indefinitely self-renew in vitro and a long-term genetic stability.15 Moreover, EB66 cells have the capacity to proliferate in stirred-tank bioreactors to high cell densities as suspension cells in serum-free or chemically defined culture media. These cells have been demonstrated to be highly susceptible to a very broad range of human and animal viral vaccines and are considered as an alternative for the cost-effective manufacturing of vaccines currently produced in chicken eggs or in primary chicken embryonic fibroblasts.16 Such attractive cell growth properties, coupled with their avian origin and their ability to be efficiently genetically engineered, could also make such cells an attractive platform for the production of mAbs with reduced fucose content and enhanced ADCC activity.
In this report, we demonstrate that EB66 cells can indeed be efficiently genetically engineered and that stable mAbs expressing clones can be isolated, expanded and grown in stirred-tank bioreactors for the production of mAbs at yields beyond 1 g/L. We furthermore show that mAbs purified from EB66 cells display a glycosylation profile similar to the one of mAbs produced on CHO cells, although with a strongly reduced fucose content that significantly enhanced ADCC activity.
Results
DNA transfection of EB66 cells.
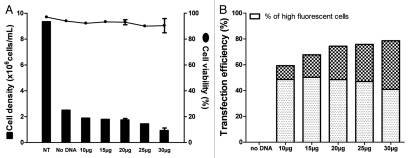
EB66 cells have the attractive capability of growing as adherent or suspension cells and can be transfected under both states by various common transfection procedures, e.g., lipofection, polyfection, electroporation, nucleo-fection. Nucleofection was found to be the most efficient system to transfer a plasmid expression vector into EB66 cells (data not shown) and was selected for further optimization. Optimization of nucleofection efficacy was performed by assessing various factors, e.g., nucleofection programs according to the manufacturer, cell concentration, total cell number, DNA concentration. Using an expression vector for the dsRED reporter gene, good cell viability was conserved at 48 h post DNA nucleofection, although the overall cell density is reduced when compared to non-transfected cells (Fig. 1A). Furthermore, DNA was not toxic to the cells since stable viability and cell density were found in the absence of DNA or presence of increasing concentration of transfected DNA from 10–30 µg. Interestingly, increasing the concentration of transfected DNA correlated with an increase in both the percentage of transfected cells from 60 to 80%, and the frequency of cells transiently expressing elevated levels of dsRED from 10–40% (Fig. 1B). This observation was confirmed by establishing stable EB66 clones expressing a mAb, where an increase in transfected DNA concentration also correlated with an increase in the frequency of stable cell clones expressing higher levels of antibody (data not shown).
Figure 1.
DNA transfection of duck EB66 cells. An expression vector encoding the red fluorescent protein (DsRed) was transfected into EB66 cells at increasing amounts. (A) 48 h post transfection, viable cells were counted and viability was assessed by a trypan blue exclusion. (B) Efficiency of DNA transfection was determined by flow cytometry analysis of the cells expressing DSRed. High fluorescent cells were gated to specifically determine the percentage of EB66 cells expressing dsRed at high levels. NT: non-transfected cells.
Production of monoclonal antibodies in stably transfected EB66 cells.
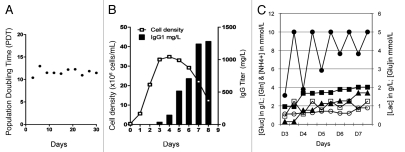
A series of stable EB66 cell lines producing a chimeric IgG1 anti-cancer mAb targeting an undisclosed antigen, referred to here as anti-X mAb, was generated by nucleofection and selection for resistance to Geneticin. A stable EB66 clone selected among the best antibody producers was further studied for its antibody production yield and glycosylation profile. The routine culture of this clone in Erlenmeyer confirmed some of the unique attractive biological properties observed with the parental EB66 cell line, such as a very short population doubling time of 12 h (Fig. 2A), the ability to reach very high cell densities (above 30 millions cells/mL) (Fig. 2B) and an unexpected negligible accumulation of ammonium and lactate and a very low consumption of glutamine (Fig. 2C). The latter data suggest that duck EB66 cells hold a cellular metabolism potentially advantageous for industrial upscaling, and different from that of mammalian cells. In addition, amplification of the selected EB66 producer clone in Erlenmeyer flasks using a standard fed-batch culture strategy led to the production of 1.28 g/L of IgG1 after 8 d in culture with a maximal cell density of approximately 36 million cells/mL at day 4 (Fig. 2B). Production yields of 0.9 g/L were also obtained using the same clone in a 2L stirred-tank bioreactor under basic non-optimized fedbatch conditions (data not shown). The progressive cell death from day 4 to day 8 in culture, concomitant to the accumulation of the mAbs in the cell culture supernatant, did not result in any significant degradation of the secreted antibody, as assessed by size exclusion high performance liquid chromatography (HPLC) of crude cell supernatant and by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), western blot and size exclusion HPLC analysis of the protein-A purified mAbs (data not shown). In addition, analysis of the protein A-purified mAbs by size exclusion HPLC also showed no or minimal aggregation of the purified protein, with more than 98% of the mAbs identified as monomers (data not shown).
Figure 2.
Cell culture growth characteristics and monoclonal antibody production. A stable EB66 clone producing a monoclonal antibody targeting an undisclosed antigen “X” was selected for analysis of its growth properties in 100 mL Erlenmeyer flasks. The population doubling time (PDT) was determined by routine culture of the EB66 producer clone during 30 d (A) and the cell density and antibody production yield was assessed in a fedbatch experiment in which glucose and glutamine concentrations were maintained by addition of concentrated media formulation at 10 g/L and 2 mM, respectively (B). A metabolic analysis (C) was performed by daily analysis of cell culture supernatant samples for the concentration of glucose (dark circles), glutamine (open squares), lactate (dark squares), ammonium (dark triangles) and glutamate (open circles).
Glycosylation profile of monoclonal antibodies produced on EB66 cells.
In order to determine the glycosylation profile of mAbs produced by EB66 cells, the anti-X mAb was purified, treated by N-glycosidase, permethylated and analyzed by matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). In parallel, the coding sequences of the heavy and light chains of a chimeric anti-CD20 IgG1 antibody were synthesized to be the same as that of rituximab, cloned in the expression vector and expressed in stably transfected EB66 clones. The anti-CD20 antibody was purified, similarly analyzed by MALDI-TOF-MS, and compared to the commercial chimeric anti-CD20 rituximab antibody produced on CHO cells.
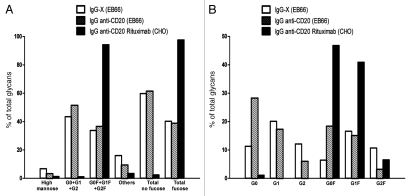
As expected, commercial rituximab was characterized by the predominant presence of fucosylated biantennary G0F, G1F and G2F glycoforms that together represented 95% of the total glycans while the non-fucosylated G0, G1 and G2 glycoforms account for only 1.1% together (Fig. 3A). Exposure of the EB66-produced mAbs to fucosidase provided indirect evidence that duck EB66 cells, similar to mammalian cells, add fucose in an alpha 1–6 linkage (data not shown). The high mannose glycoforms account for 1% of the total glycans (Fig. 3A). In contrast, both the anti-CD20 and the anti-X antibodies produced on EB66 cells display strongly reduced fucose content. Both IgGs are characterized by the predominant presence of non-fucosylated biantenary oligosaccharides that accounts for 60% of the total oligocaccharides (Fig. 3A), while the fucosylated oligosaccharides represent only 40% of the total population (Fig. 3A). High mannose structures account for around 3% of the total oligosaccharides on antibodies produced in EB66 cells (Fig. 3A). No significant differences in the percentage of Neuraminic-N-acetyl sialic acid were detected between the CHO- and EB66-derived antibodies, with a presence accounting for 2% of the total oligosaccharides (data not shown). Interestingly, analysis of the individual fucosylated and non-fucosylated G0, G1 and G2 glycoforms for the two EB66-produced mAbs reveals a grossly similar pattern of relative distribution between the fucosylated and non-fucosylated individual glycoforms (Fig. 3B). The anti-X mAb harbors a higher proportion of G1 and G1F while the EB66 anti-CD20 mAbs harbors a higher proportion of G0 and G0F, similar to the anti-CD20 rituximab, which also displays a higher proportion of G0 and G0F glycoforms (Fig. 3B). Whether such distributions of individual glycoforms are clone-specific or antibody specific remain to be further investigated.
Figure 3.
Comparative N-linked oligosaccharide analysis of CHO- and EB66-produced antibodies. The N-glycan analysis was performed by time-of-flight mass spectrometry (MALDI-TOF-MS) on the commercial anti-CD20 rituximab antibody produced on CHO cells, as well as on an EB66-produced anti-CD20 antibody with the same sequence than rituximab. A second EB66-produced antibody targeting an undisclosed target “X” was also included in the study. Results are presented for each group of oligosaccharide as the percentage of total glycans. G0, G1 and G2 are non-fucosylated nongalactosylated, non-fucosylated monogalatosylated, non-fucosylated digalactosylated oligosaccharides, respectively. G0F, G1F and G2F are fucosylated nongalactosylated, fucosylated monogalatosylated, fucosylated digalactosylated oligosaccharides, respectively. “Others” represent the hybrid glycans and tri-antennary glycans, with or without fucose.
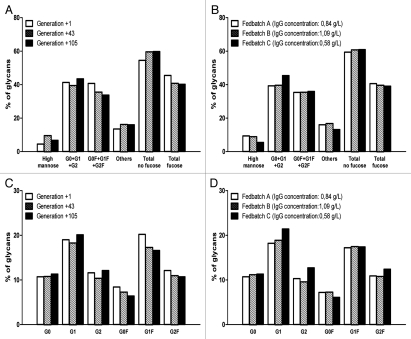
Importantly, this peculiar glycosylation profile of EB66-derived IgGs is stable for a given EB66 producer clone as demonstrated by the analysis of the anti-X antibody produced and purified at successive cell culture generation 1, 43 and 105 (Fig. 4A) or using different culture fedbatch strategies leading to different antibody production yields (Fig. 4B). In all cases, non-fucosylated glycoforms account for around 60% of total glycans, irrespective of the generation number or fedbatch strategy selected. Furthermore, the relative distribution of the individual fucosylated and non-fucosylated G0, G1 and G2 glycoforms was also found to be stable in culture (Fig. 4C and D).
Figure 4.
Stability of the glycosylation profile of EB66-produced monoclonal antibodies. An individual EB66 clone producing the anti-X antibody was selected for its production yields and expanded under fixed culture conditions for 105 generations. Monoclonal antibody batches were purified with EB66 cultures arrested at generation 1, 43 and 105 and were analyzed for their N-linked oligosaccharides composition (A and C). In parallel, an analysis of glycosylation was performed on purified antibody batches prepared from the same EB66 producer clone grown under three fedbatch conditions differing in the feeding strategies and leading to different production yields (B and D).
EB66-produced monoclonal antibodies display an enhanced activation of natural killer cells.
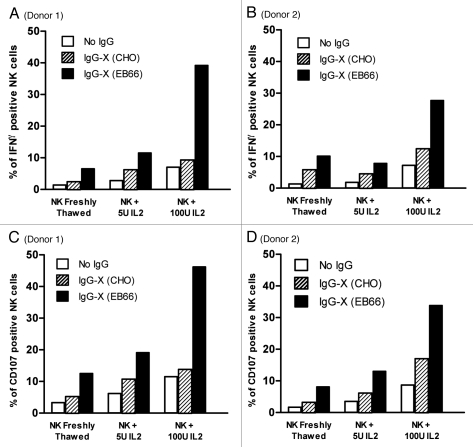
Activation of NK cells by the chimeric anti-X antibody produced on EB66 or CHO cells was assessed by flow cytometry analysis of the percentage of NK cells expressing INFγ (Fig. 5A and B) or the CD107 marker (Fig. 5C and D), when incubated with tumor cells expressing the target antigen “X” and effector cells from two healthy human donors. NK cells were cultured either without Interleukin 2 (IL-2) or with 5 or 100 units of IL-2. In all culture conditions, IgG1 from EB66 clone displayed higher NK activation compared with the same IgG1 produced in CHO cells (Fig. 5).
Figure 5.
Comparative analysis of the activation of Natural Killer cells by CHO- and EB66-produced antibodies. Efficiency of activation of NK cells purified from two independent healthy human donors was assessed in parallel for the monoclonal antibody targeting the undisclosed target “X” and produced either on CHO or on EB66 cells. Induction of expression of INFγ (A and B) and of the membrane antigen CD107a (C and D) was determined by flow cytometry with NK cells cultured without Interleukin 2 (IL-2) or with 5 or 100 units of IL-2.
EB66-produced monoclonal antibodies display an enhanced ADCC activity.
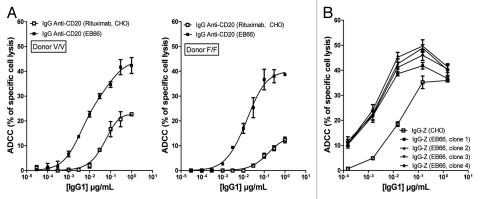
A comparative study was performed in parallel on two independent mAbs produced on EB66 and CHO cells to assess the influence of the cell production substrate and glycosylation profile of the producer mAbs on ADCC activity. The selected antibodies were the anti-CD20 IgG1 (Fig. 6A) and anti-Z IgG1 mAb (Fig. 6B) directed against an undisclosed cancer target that was different from the one recognized by anti-X mAb previously discussed.
Figure 6.
Comparative analysis of the ADCC activity of CHO- and EB66-produced antibodies. The ADCC activity was measured for two independent monoclonal antibodies: the commercial anti-CD20 rituximab and its counterpart anti-CD20 antibody produced on EB66 cells (A), as well as a monoclonal antibody targeting an undisclosed target “Z” produced in parallel on CHO cells and on four individual EB66 clones (B). The anti-hCD20 antibodies were tested using the human Raji cell line as target cells and human peripheral blood mononuclear cells (PBMC) from either a donor homozygous for FcγRIIIa-158V (VV; A, left) or a donor homozygous for FcγRIIIa-158F (FF; A, right). The mean values ± S.D. of triplicates are shown.
The anti-hCD20 antibodies were tested using the human Raji cell line as target cells and human peripheral blood mononuclear cells (PBMC) from either a donor homozygous for FcγRIIIa-158V (VV) or a donor homozygous for FcγRIIIa-158F (FF) (Fig. 6A). This genetic polymorphism at position 158 of the FcγRIIIa receptor was reported to modulate the efficiency of binding of human IgG1 to the receptor, and hence the ADCC activity, with the FcγRIIIa-158V (VV) genotype resulting in a higher affinity to the antibody Fc moiety and a stronger ADCC activity.8 The FcγRIIIa receptor polymorphism was also reported to influence the clinical therapeutic activity of the commercial CHO-produced anti-CD20 rituximab antibody.17–20 Irrespective of the FcγRIIIa receptor genotype of the effector cells, a significantly stronger specific lysis of target Raji cells was obtained with the EB66-produced anti-CD20 IgG (Fig. 6A), confirming a previous report by Niwa et al.21 At a concentration of 1 µg/mL of IgG, maximum specific target lysis was increased 2.5-fold (donor VV) and 4-fold (donor FF) with EB66-produced IgG compared to the CHO-produced commercial rituximab. Furthermore, the concentration of antibodies required to achieve a similar specific target cells lysis was 40-fold (donor VV) and 100-fold (donor FF) lower for EB66-produced IgG compared to the CHO-produced rituximab (Fig. 6A).
The enhanced ADCC activity of EB66-produced antibodies was further confirmed using a second antibody, anti-Z mAb (Fig. 6B). Antibody batches purified from four independent randomly selected EB66 producer clones were tested for their ADCC activity towards a human tumor cell line expressing the target antigen, using non-genotyped human PBMCs as effector cells. As expected, all four antibody batches displayed an enhanced ADCC activity when compared to the same antibody produced from CHO cells (Fig. 6B), confirming and extending previous results obtained with the anti-CD20 antibody.
Influence of the percentage of non-fucosylated IgGs glycoforms on the ADCC activity.
Analysis of a large series of independent stable EB66 clones producing different IgGs has revealed that the glycosylation profile, and especially the lower fucose content, is a well-conserved characteristic of EB66-produced antibodies. However, a variation was observed between the individual EB66 producer clones in the percentage of the total non-fucosylated glycoforms, which can range from 40–80% of the total glycans. While this glycosylation profile was found to be stable within each clone, as illustrated in Figure 4, the question was raised on the influence of this variable fucose content on the ADCC activity of the antibodies produced by the selected stable EB66 producer clones.
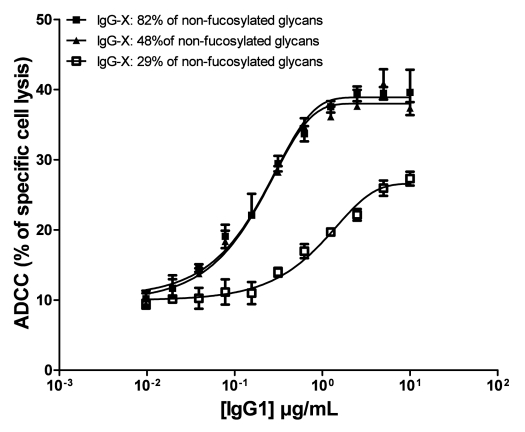
In order to more specifically assess the correlation between the ADCC activity and the percentage of fucose in the tested antibodies, anti-X mAb was produced and purified from three individual EB66 clones previously identified and specifically selected for their ability to produce IgGs with increasing proportions of total non-fucosylated glycoforms (Fig. 7). Analysis of the ADCC activity of these three batches of anti-X antibody, which comprise 29, 48 and 82% of total non-fucosylated oligosaccharides, respectively, shows that a percentage of only 49% of non-fucosylated oligosaccharides is sufficient to provide a maximum ADCC activity, similar to the ADCC activity observed with the IgG batch containing 82% of non-fucosylated oligosaccharides. In contrast, a proportion of 29% of non-fucosylated oligosaccharides leads to much weaker ADCC activity (Fig. 7).
Figure 7.
Influence of the percentage of fucosylation on the ADCC activity. The monoclonal antibody targeting the undisclosed antigen “X” was produced and purified from three individual EB66 clones specifically selected for the production of IgGs with increasing proportions of total non-fucosylated glycoforms of 29, 48 and 82%, respectively. The three batches of purified antibodies were tested for their ADCC activity as indicated in Materials & Methods, using effector cells from a single human donor. The mean values of specific target cell lysis ±S.D. of triplicates are shown.
In vivo clearance of EB66-produced monoclonal antibodies in mice.
Glycosylation plays a major role in controlling the in vivo half-life of antibodies. Given the lower fucose content of EB66-produced antibodies compared to CHO-produced antibodies, a comparative assessment of the in vivo half-life of CHO-and EB66-produced antibodies was performed in mice. Although this animal model is poorly relevant to humans in fully predicting the pharmacokinetics of glycoproteins, it can was used as a preliminary model to evaluate the in vivo clearance of the CHO- versus the EB66-produced IgGs.
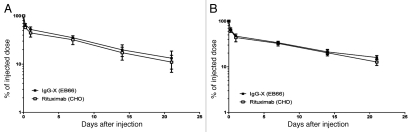
The EB66-produced anti-X mAb and the commercial CHO-produced rituximab were injected via the retro orbital sinus route into BALB/c mice at doses of 10 mg/kg and 20 mg/kg and blood samples were collected regularly and monitored by ELISA for the concentration of the human IgGs. The kinetics of clearance was similar for CHO- and EB66-produced IgGs (Fig. 8), with approximately 50–60% of the administrated IgGs cleared during the α-phase, and the remainder eliminated within the β-phase. An in vivo half-life (t1/2) of approximately 10 d for 10 mg/kg and 12 d for 20 mg/kg was determined for both CHO- and EB66-produced IgGs.
Figure 8.
In vivo clearance of EB66-produced monoclonal antibodies in mice. BALB/c mice were injected via the retro orbital sinus route with the commercial CHO-produced anti-CD20 rituximab antibody and its counterpart anti-CD20 antibody produced on EB66 cells at 10 mg/kg (A) or 20 mg/kg (B). The antibody concentrations in plasma were monitored using a human IgG-specific ELISA. The serum concentration 5 min after injection is considered as 100%. Data are the mean ± S.D. of four animals per group. The serum half-life of the administrated IgG1 was calculated from the slope of the elimination β-phase.
Discussion
The high production and treatment costs associated with the use of therapeutic mAbs is a strong incentive to the development of antibodies with higher potencies, in particular antibodies with enhanced ADCC activities.9,17–21 Since ADCC activity is predominantly controlled by the presence of core fucose in the N-linked oligosaccharides at position Asn-297 of the Fc fragment, glycoengineering is considered by many as a promising approach to improve the specific biological activity of therapeutic mAbs. Most strategies currently under development are thus focused on genetic engineering of the hamster CHO cell line to reduce or eliminate the cell line ability to synthesize fucose. For instance, overexpression of β(1,4)-N-acetyl-glucosaminyltransferase III (GnTIII) in CHO cells, a glycosyltransferase catalyzing the addition of the bisecting GlcNac residue to the N-linked oligosaccharide, and thus restraining fucosylation, was showed to confer to the cell line the ability to produce antibodies with enhanced ADCC activity.22–24 An alternative strategy is the experimental knockdown in CHO cells of two key genes involved in oligosaccharide fucose modification, α1,6-fucosyltransferase (FUT8) and GDP-mannose 4,6-dehydratase (GMD).25–27 Several preclinical and clinical trials using non-fucosylated antibodies generated with such engineered CHO cell lines are currently underway.
Beside CHO cells, alternative production systems such as yeast, Lemna minor algae or Psyscomitrilla patens moss have also been recently considered for glycoengineering with the aim to produce non-fucosylated antibodies with enhanced ADCC activity.28–32 While these systems may offer attractive potential advantages such as cost-effectiveness compared with mammalian cell cultures and a reduced risk of the presence of animal adventitious agents, they also present some natural drawbacks such as the presence of non-human oligosaccharides that may elicit in humans an immune response and alter the pharmacokinetics of the antibodies. Humanization of such yeast or plant production hosts by glycoengineering, i.e., elimination of endogenous genes responsible for undesired glycosylations and introduction of human genes controlling the preferred glycosylations, is therefore warranted and is currently actively pursued by several investigators.28–32
Some cells naturally produce proteins with lower fucose content. The critical role of the FUT-8 gene in fucosylation and the correlation between low FUT-8 expression and higher ADCC activity of the produced antibodies was thus initially reported for the rat hybridoma Y2B/0 cell line.33 Such cells transfected to stably produce an IgG1 were shown to naturally express low levels of FUT-8 mRNA leading to the production of antibodies with a low fucose content and increased ADCC activity, while overexpression of FUT-8 in Y2B/0 cells led to an increase of fucosylated oligosaccharides and a decrease in ADCC activity.33 Although such cells have been considered as a potential production host for antibodies with enhanced ADCC, their poor growth performances, sanitary characterization and traceability hindered their industrial development.
The duck EB66 cell line has been generated and developed as an alternative production system to chicken eggs for the industrial manufacture of human and veterinary vaccines.14,16 EB66 cells were derived from duck embryonic stem cells with no genetic, viral or chemical modifications to gain benefits from some of the unique biological properties of ES cells, in particular the strong expression of telomerase controlling the longer-term genetic stability of the cells. In addition, such cells were adapted to grow in suspension in serum-free or chemically defined culture media and were shown to efficiently propagate both at small scale and in stirred-tank bioreactors up to a 250L scale.14,16 A master cell bank (MCB) and a post-production cell bank (PPCB) were produced in compliance with the Good Manufacturing Practices (GMP) and were fully and extensively characterized for their sanitary status according to international guidelines. A Biological Master File compiling all traceability and characterization data was filed with the US Food and Drug Administration in 2008. While this duck EB66 cell line was originally developed for the production of viral vaccines, the fact that avian species have been described to naturally produce proteins with low fucose content prompted this investigation on the use of the duck EB66 cells for the production of antibodies with reduced fucose content and enhanced ADCC activity.12,13
We report here that EB66 cells can be efficiently DNA transfected and that stable antibody producer clones can be isolated using standard selection procedures. In addition, we demonstrate that the producer clones are characterized by a very short population doubling time of less than 15 h and that these cells can reach very high cell densities (above 30 million cells/mL) in standard Erlenmeyers or stirred-tank bioreactors cultures. Intriguingly, EB66 cells were also found to accumulate only very limited ammonium and lactate and consume little glutamine, even when grown at very high cell densities. Although the metabolism profile of duck EB66 cells remains poorly characterized and must be better defined, such results may imply a potentially advantageous behavior during culture upscaling compared to mammalian cells known to rapidly accumulate toxic metabolites. Finally, with limited optimization in the production process, mAb production yields higher than 1 gram per liter were obtained, with a very short cell culture kinetic of 8 d and a peak of cell viability at 4 d. Such high cell densities (up to 50 million cells/mL) and very short culture kinetic suggest that a lengthening of the cell culture viability by a few days should further significantly improve the production yield. This is currently under investigation through an optimization of the cell culture feeding strategy. Altogether, these results suggest that duck EB66 cells harbor a combination of unique properties very attractive for the industrial manufacture of antibodies: short doubling-time, high cell density, low accumulation of toxic metabolites and high production yield.
A detailed characterization of the glycosylation profile was performed on three independent mAb candidates produced on EB66 cells. This analysis confirmed that EB66-produced antibodies display a low fucose content, with non-fucosylated glycoforms accounting for over 50% of the total oligosaccharides, while CHO-produced antibodies comprise a proportion of less than 5% of non-fucosylated oligosaccharides. Importantly, no abnormal oligosaccharides were detected and the percentage of sialic acids and high mannose glycans were low and very similar to those obtained with CHO-produced antibodies. No conclusion could be drawn on the presence or absence of N-glycolylneuraminic sialic acids (NGNA) and alpha-Gal xenogeneic antigens on the EB66-produced antibodies. Of interest, these oligosaccharides are known to be immunogenic in humans, but have been reported to be absent in avian species.12 Absence of such glycans could provide the EB66 cell line with an additional advantages for the production of antibodies with lower risks of immunogenicity. This assumption remains to be more thoroughly assessed in a future study. The glycosylation profiles of different antibody batches purified from independent EB66 producer clones were also found to be relatively constant, with all batches displaying low fucose content; however, the percentage of non-fucosylated oligosaccharides did vary among the batches from different individual EB66 producer clones, accounting from 30–80% of the total glycoforms depending of the EB66 clone tested. This glycosylation profile was shown to be stable, with no major influence of the cell line passage level or fedbatch process on the percentage of non-fucosylated glycoforms, although the study was performed on one EB66 producer clone only and must be extended to a larger number of independent individual clones to draw a more definitive conclusion. Finally, a comparative study in BALB/c mice confirmed that the in vivo clearance of EB66-produced antibodies is similar to the clearance of CHO-produced antibodies. Although of limited relevance to predict the pharmacokinetic of EB66-produced antibodies in humans, such data indicate that no major differences affecting the antibody clearance are observed when compared to CHO-produced antibodies.
As expected, the low fucose content of EB66-produced antibodies was correlated with an enhanced activation of human NK cells, as measured by the expression of the Interferon-γ and CD107 genes and with a strongly increased ADCC activity for all tested antibodies, irrespective of the genotype of the FcγRIIIa gene in the effector cells.21 In contrast to EB66-produced antibodies, and as previously described,17–21 the CHO-produced antibodies were more biologically active with effector cells with the FcγRIIIa-158V (VV) genotype, but in all cases were much less active than their EB66-produced counterparts. By selecting individual EB66 clones producing the same antibody with increasing amounts of non-fucosylated oligosaccharides, it was furthermore possible to establish that antibodies with a proportion of non-fucosylated oligosaccharides of 48% were as biologically active as antibodies with 82% of non-fucosylated oligosaccharides. In contrast, a ratio of 29% of non-fucosylated oligosaccharides was insufficient to enhance the ADCC activity of the antibody to optimal levels. While the reason for these variations in the percentage of antibody fucosylation among individual EB66 producer clones is unclear and must be further assessed, the observation that reducing fucosylation beyond 50% may not be required to achieve maximal is consistent with previous reports using mAbs produced on glycoengineered CHO cells or on rat Y2B/0 cells.25,34 These results furthermore support predictions from earlier structural models that only one of the two Fc-fucose residues needs to be absent for increased binding affinity towards FcγRIIIa.35
In summary, the unique biological properties of the duck EB66 cell line, together with their extensive sanitary characterization according to current international guidelines, provide these cells with the potential to evolve as a standard cellular platform for the industrial production of therapeutic mAbs with increased ADCC activity, and beyond, for the production of therapeutic proteins.
Materials and Methods
Cell culture.
Suspension EB66 cells are maintained in routine cell culture at 37°C in the EX-CELL EBx-GRO-I medium (SAFC-Biosciences, St. Louis, USA) supplemented with 2.5 mM of L-glutamine. For small scale experiments in Erlenmeyers, cells are maintained in agitation at 7.5% CO2 in a humidified atmosphere. For larger scale experiments (2 L stirred-tank bioreactors), cell culture are performed in a fed-batch process at 37°C, pH 7.5 with an oxygen partial pressure at 50%, a control of glucose at 10 g/L and Glutamine at 2 mM.
Expression vectors.
EB66-specific expression vectors were developed that comprise a single nptII resistance cassette to allow plasmid amplification in Escherichia coli and clone selection after transfection in EB66 cells. The nptII gene encodes the neomycin phosphotransferase protein and provides a resistance to Kanamycin in prokaryotic cells and to Neomycin in eukaryotic cells. Genes encoding a red fluorescent protein (DsRed gene from pDsRed2-nuc, Clonetech USA) or human antibody light chain and human antibody heavy chain were cloned in tandem under the control of regulatory sequences specifically selected for good expression in duck EB66 cells.
DNA transfection.
A red fluorescent protein expression vector was transiently transfected by nucleofection (Amaxa, Germany) in suspension EB66 cells in Ultra-Low Attachment six-well plates. DsRED expression was measured by flow cytometry 48 h after transfection. A control of nucleofection was performed under similar experimental conditions, but without added DNA. Establishment of stable antibody producer clones was achieved by transfection of the IgG1 expression vectors in adherent EB66 cells grown in serum free medium imMEDIAte ADVANTAGE™ Ex66522 (SAFC-Biosciences, St. Louis, MO, USA) supplemented with 2.5 mM of L-glutamine. After Geneticin selection, resistant clones were isolated and grown as suspension cells in culture medium supplemented with 0.15 mg/mL of Geneticin in Ultra-Low Attachment 96-well plates. IgG1 expression was monitored by an anti-human Fast ELISA® detection kit (RD Biotech, France). The best producing clones were amplified successively in 24-well plates, six-well plates, 100 mL Erlenmeyer and 2L stirred-tank bioreactors.
Purification of monoclonal antibodies.
Recombinant antibodies were purified from EB66 culture supernatant using standard procedures based on Protein A-affinity chromatography using MabSelect™ (Amersham Biosciences) and were stored in 100 mM glycine HCl/100 mM tris-HCl (pH 8). Antibody integrity was analyzed using a SE-HPLC Superdex™ 200 (Amersham Biosciences).
Analysis of N-linked oligosaccharides in purified monoclonal antibodies.
Glycosylation profiling was carried out by Proteodynamics (Clermont-Ferrand, France). N-linked oligosaccharides were released by digestion of antibodies with N-glycosidase F (Roche, Switzerland). The released carbohydrates were permethylated and analyzed using a matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) with positive ion mode.
Activation of natural killer cells.
NK cells were isolated from two healthy donors and cultured without Interleukin 2 (IL-2) or with 5 or 100 units of IL-2. Tumor cell lines selected for the expression of the undisclosed target “X” were amplified and incubated with anti-X mAb and NK cells. Detection of INFγ was measured by intracellular staining with an anti-INFγ antibody coupled with phycoerythrin (PE). Degranulation of NK cells was assessed with a PCy5-conjugated CD107a mAb (BD Bioscience). Cells were analyzed by flow cytometry (Beckman Coulter).
Antibody-dependent cell cytotoxicity assays.
Cytotoxic activity was assessed using a standard 51Cr-release assay.33 The ADCC activity of purified anti-human CD20 antibodies was measured using human Raji cells as targets and PBMC from two human donors as effector cells, a donor homozygous for FcγRIIIa-158V (VV) and a donor homozygous for FcγRIIIa-158F (FF). Target cells were labeled with 100 µCi 51Cr for 1 h at 37°C, washed four times with culture medium (RPMI supplemented with fetal calf serum), and then plated at the Effector:Target cell (E:T) ratio of 30:1 in a 96-well flat plate. The anti-Her2/neu mAb trastuzumab (Roche, Switzerland) was used as a negative control. For ADCC assays, the indicated mAb was incubated with target cells for 20 min before addition of effector cells. After 4-h incubation at 37°C, 25 µl of supernatant were removed from each well and added on a LumaPlate™ (PerkinElmer). The 51Cr activity was counted in a scintillation counter. Each test was performed in triplicate. The results are expressed as the percentage of lysis, which is calculated according to the following equation: (experimental release − spontaneous release)/(maximal release − spontaneous release) × 100. Experimental release represents the mean count per minute (cpm) for the target cells in the presence of effector cells, spontaneous release represents the mean cpm for target cells incubated without effector cells, and maximal release represents the mean cpm for target cells incubated with 1% Triton X-100. For anti-cancer antibody molecules anti-X or anti-Z, specific tumor cells were used as target cells.
Pharmacokinetic study in BALB/c mice.
Evaluation of IgG half-life in mice was carried out by the Mouse Clinical Institute (Illkirch, France). Antibodies were administered via the retro orbital sinus route at a dose of 10 mg/kg and 20 mg/kg to female BALB/c mice aged 7 wks. Blood samples were collected from four animals per treatment group per time point at 5 min, 6 h, 24 h, 7 days, 14 c and 21 c after administration. The samples were collected by tail clip into tubes containing potassium EDTA as anticoagulant and processed to plasma. Plasmas were stored at −80°C until anti-human Fast ELISA® analysis.
Acknowledgements
The authors would like to thank all members of VIVALIS team for their continuous support and assistance in this project, Drs. C. Merles and M. Theisen for the excellent work in the analysis of the glycosylation profile of the antibodies (Proteodynamics, Clermont-Ferrand, France) and Dr. Tania Sorg for the mouse in vivo clearance study (Mouse Clinical Institute, Illkirch, France).
Abbreviations
- mAb
monoclonal antibody
- ADCC
antibody-dependent cell cytotoxicity
- IgG
immunoglobulin
- ES
embryonic stem
- NK
natural killer cells
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/12350
References
- 1.Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- 2.Butler M. Animal cell cultures: recent achievements and perspectives in the production of biopharmaceuticals. Appl Microbiol Biotechnol. 2005;68:283–291. doi: 10.1007/s00253-005-1980-8. [DOI] [PubMed] [Google Scholar]
- 3.Rita Costa A, Elisa Rodrigues M, Henriques M, Azeredo J, Oliveira R. Guidelines to cell engineering for monoclonal antibody production. Eur J Pharm Biopharm. 2010;74:127–138. doi: 10.1016/j.ejpb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Schirrmann T, Al-Halabi L, Dübel S, Hust M. Production systems for recombinant antibodies. Front Biosci. 2008;13:4576–4594. doi: 10.2741/3024. [DOI] [PubMed] [Google Scholar]
- 5.Janeway CA, Travers P, Walport M, Shlomchik MJ, editors. Immunobiology, The immune system in health and disease. 5th edition. London: Garland Publishing; 2001. Antigen recognition by B cell and T cell receptors; pp. 93–122. [Google Scholar]
- 6.Rademacher TW, Homans SW, Parekh RB, Dwek RA. Immunoglobulin G as a glycoprotein. Biochem Soc Symp. 1986;51:131–148. [PubMed] [Google Scholar]
- 7.Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 2008;20:471–478. doi: 10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Nimmerjahn F, Ravetch JV. Fcg receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 9.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8:226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 10.Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcgammaRIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 11.Yamane-Ohnuki N, Satoh M. Production of therapeutic antibodies with controlled fucosylation. mAbs. 2009;1:230–236. doi: 10.4161/mabs.1.3.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raju TS, Briggs JB, Borge SM, Jones AJ. Species-specific variation in glycosylation of IgG: evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology. 2000;10:477–486. doi: 10.1093/glycob/10.5.477. [DOI] [PubMed] [Google Scholar]
- 13.Zhu L, van de Lavoir MC, Albanese J, Beenhouwer DO, Cardarelli PM, Cuison S, et al. Production of human monoclonal antibody in eggs of chimeric chickens. Nat Biotechnol. 2005;23:1159–1169. doi: 10.1038/nbt1132. [DOI] [PubMed] [Google Scholar]
- 14.Guehenneux F, Pain B. Avian cell lines for the production of useful substances. 2003 WO/2003/076601. [Google Scholar]
- 15.Biswas A, Hutchins R. Embryonic stem cells. Stem Cells Dev. 2007;16:213–222. doi: 10.1089/scd.2006.0081. [DOI] [PubMed] [Google Scholar]
- 16.Guehenneux F, Moreau K, Esnault M, Mehtali M. Duck embryonic derived stem cell lines for the production of viral vaccines. 2008 WO/2008/129058. [Google Scholar]
- 17.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 18.Anolik JH, Campbell D, Felgar RE, Young F, Sanz I, Rosenblatt J, et al. The relationship of FcgRIIIa genotype to degree of B cell depletion by rituximab in the treatment of systemic lupus erythematosus. Arthritis Rheum. 2003;48:455–459. doi: 10.1002/art.10764. [DOI] [PubMed] [Google Scholar]
- 19.Dall'Ozzo S, Tartas S, Paintaud G, Cartron G, Colombat P, Bardos P, et al. Rituximab-dependent cytotoxicity by natural killer cells: influence of FcgRIIIa polymorphism on the concentration effect relationship. Cancer Res. 2004;64:4664–4669. doi: 10.1158/0008-5472.CAN-03-2862. [DOI] [PubMed] [Google Scholar]
- 20.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphism independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Niwa R, Hatanaka S, Shoji-Hosaka E, Sakurada M, Kobayashi Y, Uehara A, et al. Enhancement of the antibody-dependent cellular cytotoxicity of low-fucose IgG1 Is independent of FcgammaRIIIa functional polymorphism. Clin Cancer Res. 2004;10:6248–6255. doi: 10.1158/1078-0432.CCR-04-0850. [DOI] [PubMed] [Google Scholar]
- 22.Umaña P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol. 1999;17:176–180. doi: 10.1038/6179. [DOI] [PubMed] [Google Scholar]
- 23.Schuster M, Umana P, Ferrara C, Brünjer P, Gerdes C, Waxenecker G, et al. Improved effector functions of a therapeutic monoclonal Lewis Y-specific antibody by glycoform engineering. Cancer Res. 2005;65:7934–7941. doi: 10.1158/0008-5472.CAN-04-4212. [DOI] [PubMed] [Google Scholar]
- 24.Mössner E, Brünker P, Moser S, Püntener U, Schmidt C, Herter S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B cell cytotoxicity. Blood. 2010;115:4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanda Y, Yamane-Ohnuki N, Sakai N, Yamano K, Nakano R, Inoue M, et al. Comparison of cell lines for stable production of fucose-negative antibodies with enhanced ADCC. Biotechnol Bioeng. 2006;94:680–688. doi: 10.1002/bit.20880. [DOI] [PubMed] [Google Scholar]
- 26.Kanda Y, Imai-Nishiya H, Kuni-Kamoshi R, Mori K, Inoue M, Kitajima-Miyama K, et al. Establishment of a GDP-mannose 4,6-dehydratase (GMD) knockout host cell line: a new strategy for generating completely non-fucosylated recombinant therapeutics. J Biotechnol. 2007;130:300–310. doi: 10.1016/j.jbiotec.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 27.Imai-Nishiya H, Mori K, Inoue M, Wakitani M, Iida S, Shitara K, et al. Double knockdown of alpha1,6-fucosyltransferase (FUT8) and GDP-mannose 4,6-dehydratase (GMD) in antibody-producing cells: a new strategy for generating fully non-fucosylated therapeutic antibodies with enhanced ADCC. BMC Biotechnol. 2007;7:84. doi: 10.1186/1472-6750-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potgieter TI, Cukan M, Drummond JE, Houston-Cummings NR, Jiang Y, Li F, et al. Production of monoclonal antibodies by glycoengineered Pichia pastoris J. Biotechnol. 2009;139:318–325. doi: 10.1016/j.jbiotec.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton SR, Gerngross TU. Glycosylation engineering in yeast: the advent of fully humanized yeast. Curr Opin Biotechnol. 2007;18:387–392. doi: 10.1016/j.copbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Sethuraman N, Stadheim TA, Zha D, Prinz B, Ballew N, et al. Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nat Biotechnol. 2006;24:210–215. doi: 10.1038/nbt1178. [DOI] [PubMed] [Google Scholar]
- 31.Cox KM, Sterling JD, Regan JT, Gasdaska JR, Frantz KK, Peele CG, et al. Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat Biotechnol. 2006;24:1591–1597. doi: 10.1038/nbt1260. [DOI] [PubMed] [Google Scholar]
- 32.Schuster M, Jost W, Mudde GC, Wiederkum S, Schwager C, Janzek E, et al. In vivo glyco-engineered antibody with improved lytic potential produced by an innovative non-mammalian expression system. Biotechnol J. 2007;2:700–708. doi: 10.1002/biot.200600255. [DOI] [PubMed] [Google Scholar]
- 33.Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 34.Scallon B, McCarthy S, Radewonuk J, Cai A, Naso M, Raju TS, et al. Quantitative in vivo comparisons of the Fcg receptor-dependent agonist activities of different fucosylation variants of an immunoglobulin G antibody. Int Immunopharmacol. 2007;7:761–772. doi: 10.1016/j.intimp.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Ferrara C, Stuart F, Sondermann P, Brünker P, Umaña P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem. 2006;281:5032–5036. doi: 10.1074/jbc.M510171200. [DOI] [PubMed] [Google Scholar]