Abstract
More than fifteen years after the first identification of a class II isoform of phosphoinositide 3-kinase (PI3K) in Drosophila melanoǵaster this subfamily remains the most enigmatic among all PI3Ks. What are the functions of these enzymes? What are their mechanisms of activation? Which downstream effectors are specifically regulated by these isoforms? Are class I and class II PI3Ks redundant or do they control different intracellular processes? And, more important, do class II PI3Ks have a role in human diseases? The recent increased interest on class II PI3Ks has started providing some answers to these questions but still a lot needs to be done to completely uncover the contribution of these enzymes to physiological processes and possibly to pathological conditions. Here we will summarise the recent findings on the alpha isoform of mammalian class II PI3Ks (PI3K-C2α ) and we will discuss the potential involvement of this enzyme in human diseases.
Keywords: Phosphoinositide 3-kinase, phosphoinositides, glucose transport, insulin secretion, neurosecretion, exocy-tosis
Introduction
Phosphoinositide 3-kinases (PI3Ks) catalyse the phosphorylation of the 3-position of the inositol ring of phosphoinositides. The resulting 3-phosphorylated phosphoinositides can activate several proteins by modulating their intracellular localisation or by inducing conformational changes. Once activated, these proteins can in turn regulate a plethora of intracellular functions, such as cell proliferation, survival, migration, glucose homeostasis and membrane trafficking [1–3]. It is well established that deregulation of PI3Ks-dependent cellular pathways is associated with several diseases, including cancer and diabetes [1,2,4–6].
Three classes of PI3Ks exist, based on their substrate specificity and structure [2,7]. Class I PI3Ks are dimers comprising one catalytic and one regulatory subunit. The catalytic subunits define two subgroups within the class I, with the p110α, β and δ grouped into the class IA subgroup and p110γ classified as IB. It was originally thought that class IA PI3Ks were specifically activated by tyrosine kinase receptors (RTKs) and class IB by G-protein coupled receptors (GPCR). However recent evidence indicates that such a net distinction may not be completely correct: for instance data have clearly shown that p110β can be activated by GPCR as well as RTKs [8]. The catalytic subunits of class IA possess a binding domain for the regulatory subunits, a Ras-binding domain and the “PI3K core”, consisting of C2, helical and catalytic domains. The main in vivo lipid product of class I PI3Ks is phosphatidylinositol 3,4,5-trisphosphate [Ptdlns(3,4,5)P3], a well established second messenger, extensively studied for its role in activation of protein kinase B/Akt, a master regulator of cell survival, migration, proliferation as well as glucose transport and many more cellular functions. Class III PI3K, hVps34, is a monomer possessing the same PI3K core as class I PI3Ks but lacking the Ras binding and the regulatory subunit-binding domains [2,9]. hVps34 is only able to catalyse the synthesis of the monophosphate phosphatidylinositol 3-phosphate (Ptdlns3P), probably because its substrate recognition loop can only allocate the uncharged Ptdlns [9]. hVps34 is closely associated with the protein kinase hVpsl5 which has been described as a hVps34 regulatory protein although its precise role in hVps34 regulation is still not completely defined [9].
The first isoform of class II PI3Ks was identified in Drosophila melanogaster [10]. Class II PI3Ks are monomers of high molecular weight. Mammals possess three class II isoforms: PI3K-C2α, PI3K-C2β and PI3K-C2g which mostly differ from the class I PI3Ks because of extensions at the N-terminus and C-terminus [7], as described in more details below. Data suggest that these isoforms can be activated downstream of RTKs [11–14] and GPCR [15] as well as through distinct mechanisms compared to class I PI3Ks (discussed below). Class II PI3Ks preferentially phosphorylate Ptdlns in vitro, with some activity towards Ptdlns4P also reported. On the contrary the in vitro activity towards phosphatidylinositol 4,5-bisphosphate [Ptdlns(4,5)P2] was reported to be only 1% of the total [16] and it was detected only in the presence of phosphatidylserine for PI3K-C2α [17].
Among all PI3Ks, class II isoforms are still the least investigated and characterised although accumulating data are now revealing the role of these enzymes in several cellular functions. Here we will discuss the recent advances in our understanding of the physiological roles of the mammalian class II isoform PI3K-C2α , with some thoughts on the difficulties and challenges encountered in studying the signalling pathways activated by this specific enzyme. Finally, we will discuss evidence of its role in pathological conditions.
PI3K-C2α
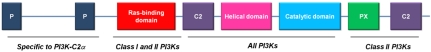
Human PI3K-C2α was cloned from the cell line U937 and it was shown to be ubiquitously expressed, with the highest levels in heart, placenta and ovary [7,17]. Similar to class I PI3Ks, PI3K-C2α possesses a Ras-binding domain and the PI3K core but it lacks the regulatory subunit-binding domain. As the other class II isoforms, PI3K-C2α possesses extensions at the N-terminus and C-terminus regions compared to class I PI3Ks. The C-terminus extensions are conserved between class II isoforms and consist of a phox homology (PX) domain and a second C2 domain. The N-terminus extensions are distinct between the class II isoforms: for instance PI3K-C2α specifically possesses a clathrin-binding region but it lacks the proline rich sequences detected in the corresponding region of PI3K-C2β. Figure 1 shows a schematic representation of the structure of PI3K-C2α , focussing on the similarities and differences of this enzyme compared to other isoforms.
Figure 1.
PI3K-C2α structure. Schematic representation of the protein domains present on PI3K-C2α and descriptive comparison with the other PI3K isoforms.
One of the most peculiar characteristics of PI3K-C2α is its low sensitivity to the classical PI3K inhibitors wortmannin and LY294002 [7,17]. Indeed, in the absence of a selective PI3K-C2α inhibitor, resistance to high concentrations of these inhibitors has often been used as indicator of the involvement of this specific PI3K isoform.
PI3K-C2α: just another PI3K?
Why should the cell need three additional PI3Ks when it has got already one class III and four class I PI3K isoforms? Are class I and class II PI3Ks redundant or do they control different intracellular processes or different steps within the same process? To answer these questions it is necessary to determine whether these enzymes activate the same downstream effectors or they rather activate distinct proteins. The PI3Ks-dependent activation of target proteins requires the interaction of the PI3K lipid product(s) with specific protein domains within the downstream effector. Distinct domains can bind different phosphoinositides. Therefore the first question that needs to be answered is whether class I and class II PI3Ks can generate the same lipid produce(s) in vivo.
In vivo lipid product of PI3K-C2α
The issue of the lipid product of PI3K-C2α (and possibly of class II PI3Ks in general), is often matter of some debate. Original studies showed an activity of class II PI3Ks against both Ptdlns and Ptdlns4P in vitro, although it must be stressed that Ptdlns was the preferential substrate [10,16,18,19]. Even upon addition of clathrin, which is able to increase the in vitro activity of PI3K-C2α in particular towards Ptdlns4P and Ptdlns(4,5)P2, Ptdlns3P remains the main product in vitro [20]. The first suggestion that Ptdlns3P could indeed be the in vivo product of PI3K-C2α upon cellular stimulation came from our study that identified an insulin-dependent pool of Ptdlns3P, specifically generated at the plasma membrane of muscle cells and adipo-cytes [21,22]. The observation that the insulin-mediated synthesis of Ptdlns3P was resistant to treatment with high concentrations of wortman-nin and LY294002 [21] first suggested that the highly resistant PI3K-C2α could have a role in catalysing the synthesis of the insulin-dependent pool of Ptdlns3P in vivo. The conclusive demonstration that this was indeed the case came from a detailed HPLC analysis of phosphoinositides extracted from unstimulated and insulin-stimulated L6 cells upon labelling with [3H]-myo-inositol. Comparison of the phosphoinositides profile of stable cell lines expressing a scrambled control shRNA or two distinct shRNAs specifically targeting PI3K-C2α showed that the insulin-induced synthesis of Ptdlns3P was completely blunted in cells lacking PI3K-C2α [14]. Consistent with these data, the insulin-induced translocation of the Ptdlns3P-binding probe GFP-2XFYVEHrs to the plasma membrane was inhibited upon PI3K-C2α down-regulation. Importantly, parallel HPLC analysis of the levels of Ptdlns(3,4)P2 and Ptdlns(3,4,5) P3 showed that down regulation of PI3K-C2α did not affect the synthesis of these phosphoinositides upon insulin stimulation. This was the first, clear demonstration that Ptdlns3P is the sole in vivo product of PI3K-C2α [14].
Consistent with this, it was also reported that overexpression of a catalitically inactive PI3K-C2α in PC12 cells reduced the steady state levels of Ptdlns3P [23]. Although a reduction in the steady state levels of Ptdlns(3,4,5)P3 was also initially reported, subsequent studies from these authors demonstrated that PI3K-C2α regulates the synthesis of a pool of Ptdlns3P in large dense core vesicles (LCDVs) of PC12 upon stimulation of exocytosis [24] and that this specific phosphoinositide is critical for PI3K-C2α -dependentfunctions [24,25].
Recently, an HPLC analysis performed in insulin-stimulated pancreatic β cells MIN6 upon transient downregulation of PI3K-C2α reported a specific inhibition on the insulin-induced levels of phosphatidylinositol 3,4-bisphosphate [Ptdlns (3,4)P2] but not of Ptdlns3P or Ptdlns(3,4,5)P3 [26]. It must be specified that these experiments were performed in media supplemented with 10% serum in the absence or presence of insulin whereas the HPLC analysis of phosphoinositides extracted from L6 cells was performed in cells labelled for 24h in serum free medium and then stimulated with insulin [14]. Total internal reflection fluorescence microscopy analysis showed a co-distribution of a GFP-tagged PH domain from Aktl [which bind Ptdlns (3,4)P2 and Ptdlns(3,4,5)P3] but not of GFP-2XFYVEHrs with the B isoform of the insulin receptor (IR-B) [26]. The lipid generated by PI3K-C2α activation upon stimulation of insulin secretion by glucose or by membrane depolarisation still needs to be identified.
Further analysis of the in vivo product of PI3K-C2α in different cellular systems and upon distinct stimulation would shed more light into this debated issue.
Downstream effectors of PI3K-C2α: the “usual” Akt?
The best characterised downstream effector of class I PI3Ks is Akt, a master regulator of a plethora of intracellular functions and one of the key players in tumourigenesis. It is therefore not surprising that one of the most asked questions is whether PI3K-C2α is able to activate Akt. Contrasting evidence is present in the literature on this issue. We reported that phosphorylation of Akt (Ser473) and of its downstream target glycogen synthase kinase (GSK) 3(3 upon short stimulation with insulin or platelet derived growth factor was not affected by downregulation of PI3K-C2α in L6 cells, consistent with the fact that the insulin-induced synthesis of Ptdlns(3,4)P2 and Ptdlns(3,4,5)P3 was not inhibited [14]. Consistent with these data, it has been recently reported that extracellular signal-regulated kinase but not Akt Ser473 phosphorylation was inhibited in CHO-IR and HepG2 expressing antisense sequences targeting PI3K-C2α and stimulated with insulin for 10 min [27]. Furthermore downregulation of PI3K-C2α did not affect Akt (Ser473) and GSK3P phosphorylation in HeLa cells in serum [28]. On the other hand, an increase in Akt1 activity was detected in MIN6 cells overexpressing wild type but not catalytically inactive PI3K-C2α and inhibition of the insulin-induced Akt1 activation was reported in these cells upon downregulation of PI3K-C2α [26]. Clearly more work is necessary to understand if there is a link between PI3K-C2α and Akt and the precise mechanisms of this regulation.
The main downstream effectors of PI3K-C2α, namely the proteins activated by the PI3K-C2α-dependent pool of Ptdlns3P, are still unknown. Proteins possessing Ptdlns3P-binding domains such as PX, FYVE and PH domains, are the most likely candidates but they are still undefined. As a result of this, not only it is still unclear how PI3K-C2α can regulate some of the intracellular functions described below, but also detecting the activation of this enzyme is still technically challenging and very time consuming. Currently, the direct activation of PI3K-C2α can only be monitored by performing in vitro kinase assays on the immunoprecipitated enzyme or by analysing its lipid product by HPLC or using fluorescent probes. The latter strategy requires confirmation that the analysed phosphoinositide is indeed dependent on PI3K-C2α activation and this can be done by investigating whether the levels of the specific lipid are reduced by PI3K-C2α inhibition. This is complicated by the fact that there is no specific PI3K-C2α inhibitor and therefore it can only be achieved by downregulating the protein levels using specific siRNA/shRNA or by using a catalytically inactive mutant. In comparison, for instance activation of a class I PI3K can easily be detected by Western blotting analysis of Akt phosphorylation, in particular at its residue Thr308, an event dependent on class I PI3K activation. Of course the identification of the specific class I isoform involved would require in vitro kinase assay and siRNA/shRNA experiments as well but at least the involvement of this class of enzymes can be easily and rapidly tracked down. It is safe to say that the lack of a quick readout for PI3K-C2α activation and of a specific inhibitor for this isoform has limited our understanding of the involvement of this enzyme in physiological and pathological conditions.
Mechanisms of activation of PI3K-C2α
The mechanisms of activation of PI3K-C2α are still not completely defined but evidence accumulated so far strongly suggests that they differ from the mechanisms regulating class I activation. As described above, PI3K-C2α is a monomer and does not possess a regulatory subunit that can modulate its activation. On the other hand, the enzyme specifically possesses several protein domains and it is tempting to speculate that interaction of these domains to regulatory/adaptor proteins or to membrane lipids is involved in its activation. For instance, it was reported that addition of clathrin in an in vitro kinase assay or removal of an N-terminal region of the enzyme which includes the clathrin-binding sites increases PI3K-C2α activity [20]. It has been reported that the full-length PI3K-C2α and the isolated PX domain have the same affinity for Ptdlns(4,5)P2-containing membranes and the same monolayer penetration [29]. Whether this interaction is important for PI3K-C2α activation needs to be investigated indepth. Interestingly, a co-operative role of C2 domain in binding to Ptdlns(4,5)P2-containing membranes was also suggested [29].
We have demonstrated that PI3K-C2α translocates to the plasma membrane upon insulin stimulation of L6 cells [14], which is consistent with the insulin-dependent, PI3K-C2α-mediated synthesis of Ptdlns3P at this cellular compartment. Interestingly, activation of PI3K-C2α in this context appears to be mediated by the insulin-induced activation of the small GTP-binding protein TC10 [14]. Whether other GTPase can have a role in PI3K-C2α activation remains to be determined. It should be noted that in the original paper reporting the insulin-induced activation of PI3K-C2α, association of the enzyme with a 160 kDa protein was detected upon insulin stimulation [11]. The identity of this protein is still unknown and the possibility that this association is involved in the insulin-induced regulation of PI3K-C2α has not been further investigated yet. Similarly, it was suggested that PI3K-C2α may undergo phosphorylation upon insulin stimulation [11] but this hypothesis still needs to be confirmed.
Association of PI3K-C2α to IR-B has also been reported in pancreatic β cells MIN6, as assessed by fluorescence resonance energy transfer (FRET) analysis and co-immunoprecipitation studies of overexpressed constructs [26]. Efficiency of FRET increases in conditions that stimulate insulin secretion. Specifically, the NPEY motif in the juxta-membrane region of IR-B appears to be involved in this association. On the other hand, no phosphorylated insulin receptor was recovered in PI3K-C2α immunopre-cipitates from insulin-stimulated CHO-IR cells [11] and from neurotrophin-3-stimulated 3T3-L1 adipocytes [30]. Nevertheless a very important distinction between class I and class II PI3K activation emerges from this study [26], with class I PI3K being specifically activated downstream of the A isoform of IR and PI3K-C2α specifically activated by IR-B.
Taken together these data suggest that, even when stimulated by the same growth factor, class I PI3Ks and PI3K-C2α are activated in distinct manner and result in distinct, complementary effects, as described in more details later.
Several lines of evidence indicate that calcium can activate PI3K-C2α in many cellular systems. Stimulation of de-endotheliased rabbit aortic vascular smooth muscle (VSM) with KCI. noradrenaline or ionomycin is able to increase PI3K-C2α activity assessed by in vitro kinase assay on the immunoprecipitated endogenous protein [31]. Incubation of VSM in Ca2+-free EGTA-containing media completely prevents the noradrenaline-induced activation of PI3K-C2α demonstrating the critical role of calcium in this process. Similarly, it has also been reported that increasing calcium concentration in the lipid kinase assay in vitro performed using immunoprecipitated recombinant PI3K-C2α enhances the activity of the enzyme in a dose-dependent manner [24]. Consistent with this, a calcium-dependent recruitment of the GFP-2XFYVE domain to the LDVCs was detected in PC12 cells using digitonin permeabilisation in the presence of calcium-containing buffers and it was reported that increasing calcium concentration enhanced the synthesis of Ptdlns3P in vitro using purified chromaffin cells [24]. Taken together these data indicate that the activity of PI3K-C2α can be enhanced by increasing concentrations of calcium and that stimulus able to trigger intracellular calcium increase can modulate the PI3K-C2α-dependent synthesis of Ptdlns3P in vitro and in vivo. We have recently reported that PI3K-C2α regulates insulin granule exocytosis induced by membrane depolarisation [32], a process dependent on increase of intracellular calcium and similar to what observed in VSM contraction (discussed below). This strongly suggests that the enzyme may be activated by calcium in different cellular contexts.
How calcium activates PI3K-C2α is still not defined but it is noteworthy that PI3K-C2α but not p110α, has been shown to be able to utilise calcium as cofactor for phosphate transfer in in vitro kinase assays [12]. Interestingly, in these conditions, the enzyme appears to lose even its low activity towards Ptdlns4P being able to solely phosphorylate Ptdlns to generate Ptdlns3P in vitro [12]. The main candidates for mediating the calcium-dependent activation of PI3K-C2α are the two C2 domains within the enzyme although this needs to be better investigated. Activation of PI3K-C2α by calcium may represent a unique mechanism and a specific and selective way to active this PI3K isoform.
Functions of PI3K-C2α
In the last few years, studies investigating the involvement of PI3K-C2α in cellular functions have exponentially grown. Although the processes in which the enzyme has been involved are different and apparently distinct, many similarities exist which possibly suggest some common mechanisms of action and activation of PI3K-C2α in different cellular contexts.
PI3K-C2α and exocytosis
PI3K-C2α is emerging as a key regulator of exocytosis in distinct cellular contexts, suggesting a very critical requirement for this enzyme in this process.
PI3K-C2α in insulin secretion
The first suggestion that PI3K-C2α could have a role in insulin secretion came from a study reporting that the insulin-induced transcription of the gene encoding β-cell glucokinase (βGK) was not inhibited by high concentrations of PI3K inhibitors or by dominant negative mutant of p85 [33]. More recently, the direct involvement of PI3K-C2α in this process has been demonstrated by downregulation of PI3K-C2α and overexpression of wild type and catalytically inactive PI3K-C2α in MIN6 [26]. More important, this study showed that downregulation of PI3K-C2α reduces the glucose-induced stimulation of insulin secretion in these cells [26]. In this model, PI3K-C2α is proposed to be part of an insulin-dependent feedback loop involving activation of Akt1 which in turn controls the glucose-stimulated insulin secretion, partially through upregulation of β-GK [26]. This may not be the only mechanism by which PI3K-C2α can control insulin secretion: indeed for instance overexpression of PI3K-C2α does not affect insulin secretion, although it is able to increase the basal β-GK promoter activity.
More recently, we have demonstrated that PI3K-C2α has an additional role in insulin secretion, being also involved in the final steps of insulin granules exocytosis [32]. We showed that downregulation of PI3K-C2α in rat insulinoma cells INS1 strongly inhibited secretion of insulin induced by membrane depolarisation, therefore in the absence of the metabolic contribution derived from the glucose consumption. Downregulation of the enzyme did not affect the total insulin content or the increase in intracellular calcium upon KCI stimulation, indicating a defect in the exocytosis of the insulin granules. No effect on the number of insulin granules proximal to the plasma membrane in resting cells, or on the expression levels of proteins important for exocytosis was detected in cells lacking PI3K-C2α. Data suggested a specific role for the enzyme at the level of the fusion of the insulin granules to the plasma membrane.
PI3K-C2α in neurosecretory granules release
PI3K-C2α has also a role in neurosecretory granules exocytosis [23]. Specifically, it was reported that an anti-PI3K-C2α was able to inhibit the carbachol-induced catecholamine release in adrenal chromaffin cells. Data suggested a specific role for PI3K-C2α in the ATP-dependent priming of neurosecretory granules. Similarly, release of human growth hormone (hGH) by PC12 upon treatment with high K+ was enhanced by overexpression of wild type PI3K-C2α and inhibited by overexpression of a kinase dead PI3K-C2α mutant. The observation that overexpression of the binding probe GFP-2XFYVE (but not the mutant GFP-2XFYVEC215S, which is unable to bind Ptdlns3P) was also able to inhibit the release of hGH in PC12 and of catecholamine in chromaffin cells supported the hypothesis of a specific role for Ptdlns3P in this process, which was confirmed by subsequent studies [24,25].
Data obtained in neurosecretion and insulin secretion suggest the interesting hypothesis that PI3K-C2α may be involved in control of exocytosis in different cellular contexts, possibly regulating final steps, common to the process and irrespective of the cellular systems investigated. In this respect, although it is not a classical exocytotic process, it is noteworthy that PI3K-C2α is also involved in translocation of the glucose transporter protein (GLUT)4 to the plasma membrane upon insulin stimulation [14], as discussed below.
PI3K-C2α and glucose transport
We have demonstrated that downregulation of PI3K-C2α in muscle cells reduces glucose transport by partially inhibiting GLUT4 translocation to the plasma membrane [14]. This is consistent with the role of Ptdlns3P in regulation of GLUT4 translocation [21,22,34–38]. Initially, our observation that exogenous Ptdlns3P was able to induce translocation of the GLUT4 to the plasma membrane but it was not sufficient to induce glucose uptake [21] led us to hypothesise that the enzyme might be involved in movement of the GLUT4-containing vesicles to the plasma membrane [14]. The demonstration that PI3K-C2α has a role in insulin secretion and neurosecretion as well as GLUT4 translocation calls for some thinking about other potential additional mechanisms of PI3K-C2α action.
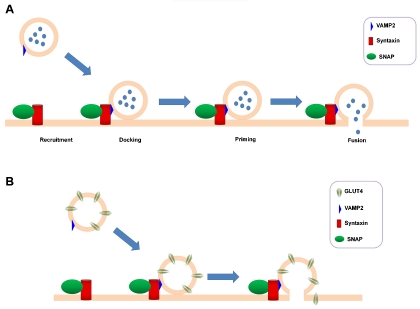
In a very simplistic and schematic representation, neurosecretion and insulin secretion can be represented as processes requiring the recruitment of the granules to the plasma membrane, their docking, priming (when the granules become competent for fusion) and finally fusion (Figure 2A). An important role in these processes is played by the soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) complex. Although GLUT4 translocation is not a classical exocytotic process and it is regulated by completely different mechanisms, it also relies on SNAREs assembly and on a final fusion event (Figure 2B).
Figure 2.
Schematic representation of exocytosis and GLUT4 translocation. (A) In a very simplistic model, exocytosis involves the initial recruitment of the granules to the plasma membrane, followed by their docking, priming and finally fusion. Different syntaxins and SNAPs can be involved in this process, according to the specific cellular system. (B) GLUT4 translocation also involves recruitment of GLUT4-containing vesicles to the plasma membrane, docking and fusion.
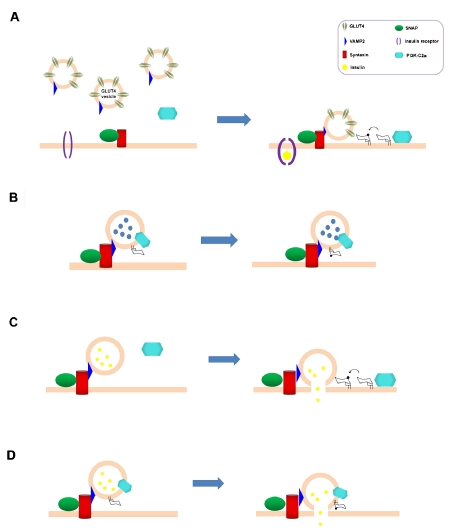
It is therefore tempting to speculate that PI3K-C2α may control some steps which are common between all these processes. For instance, PI3K-C2α may control exocytosis by facilitating the interaction between granules/vesicles and plasma membrane. In this respect an interesting observation came from a recent proteomics analysis showing that the protein VAMP8 associated with the insulin secretory granules in INS-1E cells [39]. Interestingly, VAMP8 has been shown to bind Ptdlns3P [40]. Whether a potential PI3K-C2α-dependent pool of Ptdlns3Patthe plasma membrane can bind VAMP8 in order to facilitate recruitment of the insulin granules is a fascinating hypothesis that needs to be tested. This would first require to determine where PI3K-C2α is activated during the process of insulin secretion, if at the plasma membrane (as for GLUT4 translocation, Figure 3A, C) or on the granules (as for neurosecretory granules, Figure 3B, D). As discussed above, association of PI3K-C2α to the IR-B has been reported [26], suggesting a potential role for the enzyme at the plasma membrane.
Figure 3.
Involvement of PI3K-C2α in exocytosis and GLUT4 translocation. (A) Upon insulin stimulation, PI3K-C2α is recruited to the plasma membrane where it generates a pool of Ptdlns3P which, in a mechanism still not defined, participates to GLUT4 translocation. (B) During neurosecretion, PI3K-C2α (which is associated to the granules) generates Ptdlns3P on the granules. (CD) PI3K-C2α is also required for insulin granules exocytosis. Whether the enzyme translocates from the cytoplasm to the plasma membrane upon cellular stimulation (C) or it is associated to the granules (D) remains to be defined.
Another fascinating hypothesis is that Ptdlns3P itself may be directly involved in the fusion event during the exocytosis process. Recent studies using reconstituted proteoliposomes have shown that Ptdlns3P can directly support fusion in particular experimental conditions [41], and that this phosphoinositide is part of a minimal set of lipids required for fusion [42]. Based on these data it is tempting to speculate that PI3K-C2α may regulate insulin granule fusion directly by maintaining a pool of Ptdlns3P necessary for this event.
We have also suggested the possibility of a role for PI3K-C2α in regulation of calpain 10, a protease which has been involved both in GLUT4 translocation [43–45] and in insulin secretion [46], processes also requiring activation of PI3K-C2α [14,32].
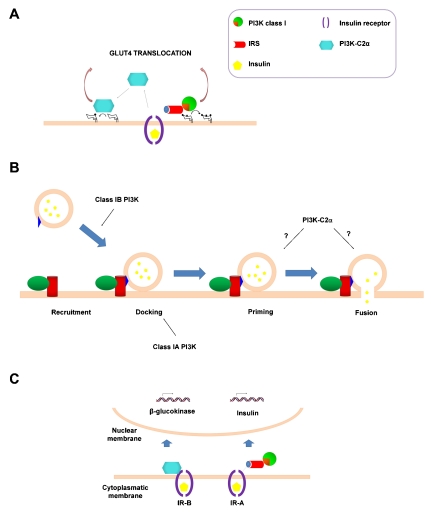
A final thought about the similarities between these processes concerns the involvement of different PI3K isoforms. It is well established that class I PI3K activation is critical for GLUT4 translocation. Our data suggest that a cooperative action of PI3K-C2Cα and class I isoforms is necessary to fully activate this process and glucose transport [14], as schematically represented in Figure 4A. Similarly, together with data discussed above on the role of PI3K-C2α in insulin secretion, it has been reported that knockout mice for the class I p110γ lack the first phase of insulin secretion [47] and that specific blockade of p110γ impairs insulin secretion [48]. More recently, the involvement of class IA isoforms has also been suggested by data obtained using a mouse model lacking PIK3R1 specifically in the β cells and PIK3R2 systemically (these are two of the three genes encoding for the class IA regulatory subunits). These mice show reduced glucose tolerance and insulin secretion in response to glucose because of defects in the exocytosis machinery [49].
Figure 4.
Co-operative roles of class I PI3Ks and PI3K-C2α. (A) Class I PI3Ks and PI3K-C2α are both required for full GLUT4 translocation and glucose transport in muscle cells. (B) Members of the class I subfamily and PI3K-C2α have been demonstrated to be involved in the process of insulin secretion. (C) Activation of class I PI3K downstream of the A isoform of the insulin receptor (IR-A) is required for transcription of the insulin gene whereas activation of PI3K-C2α downstream of IR-B controls transcription of β-GK [26,33].
Taken together these data suggest that, as for the GLUT4 translocation, insulin secretion requires a co-operative action of class I PI3Ks and PI3K-C2α (Figure 4B). In this respect, it is noteworthy that activation of class I PI3K downstream of IR-A has been shown to be important for the insulin-induced transcription of the insulin gene whereas PI3K-C2α regulates the IR-B-mediated transcription of β-GK [26,33], again indicative of a co-operative activity of the two classes (Figure 4C).
PI3K-C2α and endocytosis
Early studies showed that PI3K-C2α can associate with clathrin and regulate clathrin-dependent endocytosis [20]. Specifically, it was reported that clathrin-coated vesicles accumulated in the cytosol in cells overexpressing PI3K-C2α whereas they were mostly localised at the cell periphery in untransfected cells. Inhibition of endocytosis of transferrin, displacement of mannose 6-phosphate receptors from trans-Golgi network and a reduced localisation of Lamp1 and Lamp2 in lysosomes was detected in cells over-expressing PI3K-C2α, indicative of a defect in clathrin-mediated transport [20]. More recently, a specific role for PI3K-C2α in dynamin-independent endocytosis has also been demonstrated by data showing a reduced internalisation of diphtheria toxin in HeLa cells upon downregulation of PI3K-C2α [50]. In the same experiments, internalisation of transferrin receptor did not appear to be affected, indicating a specific role for the enzyme in dynamin-independent endocytosis. Data also indicated that PI3K-C2α can regulate the dynamin-independent internalisation of endogenous proteins, such as CD59, and fluid-phase uptake. Interestingly, the process does not seem to require other PI3Ks, including the class II PI3K-C2β. In this context relocation of PI3K-C2α seems to be crucial for the enzyme to exert its intracellular functions. Indeed translocation of PI3K-C2α to the plasma membrane and to vesicular compartments at the periphery of the cells occurs upon internalisation and, during internalisation, PI3K-C2α co-localises with the internalised proteins. Consistent with the fact that the enzyme is not involved in this process, transferrin internalisation does not induce any intracellular relocation of PI3K-C2α.
PI3K-C2α and vascular smooth muscle contraction
PI3K-C2α is expressed in aorta and in vascular smooth muscle cells (VSCMs) and it is activated upon treatment of VSM with KCI. noradrenaline or ionomycin [31]. Downregulation of PI3K-C2α reduced the noradrenaline-induced contraction of VSCMs. Specifically, data obtained upon downregulation of PI3K-C2α and using high concentrations of PI3K inhibitors suggested that PI3K-C2α regulates key events crucial for muscle contraction, including Rho GTP-loading, and phosphorylation of 20 kDa myosin light chain (MLC). Importantly, down-regulation of p110α had no effect on contraction [31]. Downregulation of PI3K-C2α also affected the ionomycin-induced contraction of VSMs (without inhibiting the increase in the intracellular calcium), the ionomycin-induced mono and di-phosphorylation of MLC, the phosphorylation/inactivation of the phosphatase MYPT1 and the noradrenaline-induced di-phosphorylation of MLC [51]. Similarly, it was reported that cyclic adenosine 5’-monophosphate inhibited the calcium-induced activation of PI3K-C2α and related signalling pathway [52]. More recently, it was shown that PI3K-C2α is activated in aorta and mesenteric arteries stimulated with KCI. with parallel activation of Rho and phosphorylation of MYPT1 [53]. More important, hyperactivation of PI3K-C2α but not p110α was detected in spontaneously hypertensive rats (SHR) compared to normoten-sive rats with concomitant increase of Rho activity and MYPT1 phosphorylation. Calcium channel blocker nicardipine as well as infusion of high concentrations of wortmannin inhibited all these effects to the values of normotensive rats, ultimately reducing systolic blood pressure.
Taken together these data not only demonstrated a specific role for PI3K-C2α in VSM contraction but they also suggested that this enzyme has a role in pathological conditions.
It is also worth mentioning that our demonstration that PI3K-C2α has a role in insulin secretion induced by depolarisation of pancreatic β cell plasma membrane without affecting the increase in intracellular calcium [32] suggests that there must be a common mechanism of activation of this enzyme, in distinct cellular systems, possibly to regulate similar processes.
PI3K-C2α and cell survival
Another interesting, but still not completely clear aspect of PI3K-C2α signalling is its potential role in promoting cell survival. Downregulation of PI3K-C2α in HeLa cells has been shown to increase apoptosis in a mechanism involving the intrinsic pathway [28]. Indeed downregulation of the enzyme increased the levels of activated caspase 9 and Poly-(ADP-ribose) polymerase (PARP) and downregulation of Bax/Bak was able to rescue the inhibitory effect of PI3K-C2α knock down on cell viability. On the contrary, no effect on Akt phosphorylation at its residue Ser473 and on GSK3β phosphorylation was detected in these cells. When tested in a panel of 23 carcinoma cell lines, PI3K-C2α downregulation reduced viability by more than 50% compared to the corresponding control cell lines in more than half of them [28]. Similarly, reduced cell proliferation and anchorage-independent growth together with increased apoptosis was reported in Mahlavu cells, a hepatoma cell line, upon PI3K-C2α downregulation [54] and increased apoptosis was reported in CHO-IR expressing antisense sequences targeting PI3K-C2α [55]. Consistent with these data, it has been recently reported that overexpression of PI3K-C2α in mesenchymal stem cells (MSCs) is able to increase the survival rate of these cells in hypoxic conditions [56]. Specifically, a decrease in PARP levels and increase in the ratio Bcl2/Bax together with a reduced cell death was detected in MSCs over-expressing PI3K-C2α. In contrast to these data, we did not detect any effect on cell proliferation and growth in L6 muscle cells upon stable downregulation of PI3K-C2α [14], consistent with the lack of effect detected in human bladder smooth muscle cells BdSMC and human lung epithelial fibroblast cells WI-38 [28]. Similarly, no effect on proliferation was detected in rat insulinoma cells INS1, at least when analysed in normal growing conditions [32].
Taken together these data suggest that PI3K-C2α may have a role in regulation of cell proliferation and/or survival, possibly specifically in cancer cells but the precise role of the enzyme in this process and the signalling pathways regulated by PI3K-C2α in this context remain to be defined. How does PI3K-C2α regulate cell survival? The only data available so far suggest that this occur mostly through regulation of the intrinsic apoptotic pathway rather than through Akt activation [54]. Future results will undoubtedly shed new light into the potential contribution of PI3K-C2α to the activation of pro-survival signalling in cancer cells.
First report of a PI3K-C2α knock out mouse model
A knock out model for PI3K-C2α has been recently described [57]. It must be mentioned that a truncated form of PI3K-C2α was still detected in brain and liver of the knock out mice, corresponding to an enzyme lacking the C -terminal PX and C2 domains. Although the PI3K activity in brain and liver was almost blunted in PI3K-C2α-/- mice, the fact that these mice seem to still express a truncated form of PI3K-C2α, albeit with a lower expression compared to the full length protein in wild type mice, must be taken into account. The survival curve of mice indicated that PI3K-C2α was essential for normal postnatal development and at 4 to 6 weeks of age, PI3K-C2α-/- mice presented reduced body fat and lean body mass resulting in reduced body weight compared to wild type mice. Approximately 30% of PI3K-C2α-/- mice died by 6 months of age, compared to only 5% of wild type mice. Specifically, PI3K-C2α-/- mice presented all features of chronic renal failure and histological analysis revealed a severe glomerulonephropathy. More detailed investigation demonstrated that the defects occurred specifically at level of podocyte morphology and function and they are not a consequence of alteration in the immune response.
Whether other phenotypes will be revealed by generation of distinct knock-out and/or knock-in mouse models remain to be defined. Furthermore, it would be particularly interesting to determine the effect of high-fat diet on these models.
PI3K-C2α and human diseases
While it is well established that deregulation of class I PI3K-dependent pathways is associated with several diseases, including diabetes and cancer, there is currently no clear demonstration of a role for PI3K-C2α in these diseases, although data accumulated so far strongly suggest that this may be the case.
Diabetes
The fact that PI3K-C2α contributes to both glucose disposal into muscle cells [14] and insulin secretion [26,32] suggests that deregulation of signalling pathways controlled by this enzyme may ultimately have a role in Type 2 diabetes. Based on the data accumulated so far, it is tempting to speculate that deregulation of PI3K-C2α-dependent signalling may result in inhibition of glucose disposal, a hallmark of insulin resistance, and in reduced insulin secretion, indicative of pancreatic β cell dysfunction. Although at the moment this is only a fascinating hypothesis, it must be noted that we have recently reported a specific downregulation of PI3K-C2α mRNA in islets from Type 2 diabetic compared to non diabetic individuals [32]. Whether this is an early event in the disease progression or it is a secondary effect and whether this contributes to pancreatic β cell loss of function remains to be determined.
Cancer
In vitro data, in particular on the potential role of PI3K-C2α in regulation of survival, suggest that PI3K-C2α may have a role in cancer. However no study so far has properly investigated a direct involvement of PI3K-C2α in cancer development and progression. Very few studies have investigated the potential association of PI3K-C2α expression level and cancer. For instance a slight decrease in the DNA copy number but a slight increase in mRNA levels was reported in 19 hepatitis B-positive hepatocellular carcinoma compared to matched non-tumour counterparts [54]. We have recently analysed the expression and localisation of all PI3K isoforms in pancreatic ductal adenocarcinoma specimens compared to normal tissues by immunohistochemistry [58]. PI3K-C2α was expressed in a subset of the samples and it was found localised in acini and ducts both in cancer and normal tissue. A higher expression was detected in acini with high cellular atypia and in dysplastic ducts. On the contrary, PI3K-C2β was only visibly stained in few samples. An interesting observation was made in a study which characterised the side-population of the breast cancer cell line MCF7, a rare cell population enriched in cancer stem-like cells with increased tumourigenicity in vivo [59]. The authors showed that PI3KC2A, the gene encoding for PI3K-C2α, was one of the genes expressed at higher levels in the side population compared to the normal population [59].
There is no doubt that more studies will improve our understanding of the potential role of PI3K-C2α in cancer in the near future.
Cardiovascular diseases
As discussed above, PI3K-C2α activity increases in aortae from SHRs, which is paralleled by increased Rho activity, MYPT1 phosphorylation and systolic blood pressure [53]. It is noteworthy that not only treatment with the calcium blocker nicardipine but also infusion with high concentrations of wortmannin (which inhibited PI3K-C2α activity) also strongly reduced systolic blood pressure in aortas and mesenteric arteries of SHRs. These data have suggested a novel role for PI3K-C2α in hypertension.
It is also worth mentioning that transplantation of MSCs overexpressing PI3K-C2α in rats after myocardial infarction resulted in reduced infarct size and area of fibrosis, with improved heart function [56].
Conclusions
In the last years we have learned that an increasing number of intracellular functions are regulated by PI3K-C2α. Although the processes regulated by this enzyme are different, some similarities have emerged, suggesting some common mechanisms of activation and action of PI3K-C2α in different cellular systems. More important, data so far strongly indicate that this enzyme complements the function of class I PI3K isoforms in many processes rather than just having a redundant role. Now evidence suggests that PI3K-C2α may also play a role in pathological conditions, which, together with the fact that this enzyme acts differently from class I enzymes, for sure will fuel a huge interest in precisely defining the contribution of this enzyme to pathological conditions in the near future. Our understanding of PI3K-C2α is still limited by the lack of selective inhibitors and of quick and easy readout of its activation status. The development of specific PI3K-C2α inhibitors and identification of specific downstream effectors would greatly improve our knowledge of the physiological functions of this enzyme and its contribution to pathological conditions.
Acknowledgements
We would like to thank Prof Marco Falasca and Dr Charlotte Edling for critical reading of the manuscript. Simona Mazza is supported by a Diabetes UK PhD studentship (grant BDA:09/0003971 to TM). We thank Diabetes UK and Diabetes Research & Wellness Foundation for their support.
References
- [1].Cantley LC. The phosphoinositide 3-kinasepathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- [2].Vanhaesebroeck B, Guillermet-Guibert J, Grau-pera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–41. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- [3].Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Gen. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- [4].Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev. 2010;20:87–90. doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Falasca M. PI3K/Akt signalling pathway specific inhibitors: a novel strategy to sensitize cancer cells to anti-cancer drugs. Curr Pharm Des. 2010;16:1410–6. doi: 10.2174/138161210791033950. [DOI] [PubMed] [Google Scholar]
- [6].Foukas LC, Withers DJ. Phosphoinositide signalling pathways in metabolic regulation. Curr Top Microbiol Immunol. 2010;346:115–41. doi: 10.1007/82_2010_59. [DOI] [PubMed] [Google Scholar]
- [7].Falasca M, Maffucci T. Role of class II phosphoinositide 3-kinase in cell signalling. Biochem Soc Trans. 2007;35:211–4. doi: 10.1042/BST0350211. [DOI] [PubMed] [Google Scholar]
- [8].Guillermet-Guibert J, Bjorklof K, Salpekar A, Gonella C, Ramadani F, Bilancio A, Meek S, Smith AJ, Okkenhaug K, Vanhaesebroeck B. The pllObeta isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with pllOgamma. Proc Nati Acad Sci U S A. 2008;105:8292–7. doi: 10.1073/pnas.0707761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J. 2008;410:1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- [10].MacDougall LK, Domin J, Waterfield MD. A family of phosphoinositide 3-kinases in Drosophila identifies a new mediator of signal transduction. Curr Biol. 1995;5:1404–15. doi: 10.1016/s0960-9822(95)00278-8. [DOI] [PubMed] [Google Scholar]
- [11].Brown RA, Domin J, Arcaro A, Waterfield MD, Shepherd PR. Insulin activates the alpha isoform of class II phosphoinositide 3-kinase. JBiol Chem. 1999;274:14529–32. doi: 10.1074/jbc.274.21.14529. [DOI] [PubMed] [Google Scholar]
- [12].Arcaro A, Zvelebil MJ, Wallasch C, Ullrich A, Waterfield MD, Domin J. Class II phosphoinositide 3-kinases are downstream targets of activated polypeptide growth factor receptors. Mol Cell Biol. 2000;20:3817–30. doi: 10.1128/mcb.20.11.3817-3830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arcaro A, Khanzada UK, Vanhaesebroeck B, Tetley TD, Waterfield MD, Seckl MJ. Two distinct phosphoinositide 3-kinases mediate polypeptide growth factor-stimulated PKB activation. EMBOJ. 2002;21:5097–108. doi: 10.1093/emboj/cdf512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Falasca M, Hughes WE, Dominguez V, Sala G, Fostira F, Fang MQ, Cazzolli R, Shepherd PR, James DE, Maffucci T. The role of phosphoinositide 3-kinase C2alpha in insulin signaling. J Biol Chem. 2007;282:28226–36. doi: 10.1074/jbc.M704357200. [DOI] [PubMed] [Google Scholar]
- [15].Maffucci T, Cooke FT, Foster FM, Traer CJ, Fry MJ, Falasca M. Class II phosphoinositide 3-kinase defines a novel signaling pathway in cell migration. J Cell Biol. 2005;169:789–99. doi: 10.1083/jcb.200408005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Arcaro A, Volinia S, Zvelebil MJ, Stein R, Watton SJ, Layton MJ, Gout I, Ahmadi K, Downward J, Waterfield MD. Human phosphoinositide 3-kinase C2beta, the role of calcium and the C2 domain in enzyme activity. J Biol Chem. 1998;273:33082–90. doi: 10.1074/jbc.273.49.33082. [DOI] [PubMed] [Google Scholar]
- [17].Domin J, Pages F, Volinia S, Rittenhouse SE, Zvelebil MJ, Stein RC, Waterfield MD. Cloning ofa human phosphoinositide 3-kinase with a C2domain that displays reduced sensitivity to theinhibitorwortmannin. BiochemJ. 1997;326:139–47. doi: 10.1042/bj3260139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Virbasius JV, Guilherme A, Czech MP. Mouse p170 is a novel phosphatidylinositol 3-kinase containing a C2 domain. J Biol Chem. 1996;271:13304–7. doi: 10.1074/jbc.271.23.13304. [DOI] [PubMed] [Google Scholar]
- [19].Misawa H, Ohtsubo M, Copeland NG, Gilbert DJ, Jenkins NA, Yoshimura A. Cloning and characterization of a novel class II phosphoinositide 3-kinase containing C2 domain. Biochem Biophys Res Commun. 1998;244:531–9. doi: 10.1006/bbrc.1998.8294. [DOI] [PubMed] [Google Scholar]
- [20].Gaidarov I, Smith ME, Domin J, Keen JH. The class II phosphoinositide 3-kinase C2alpha is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol Cell. 2001;7:443–9. doi: 10.1016/s1097-2765(01)00191-5. [DOI] [PubMed] [Google Scholar]
- [21].Maffucci T, Brancaccio A, Piccolo E, Stein RC, Falasca M. Insulin induces phosphatidylinositol-3-phosphate formation through TC10 activation. EMBOJ. 2003;22:4178–89. doi: 10.1093/emboj/cdg402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Falasca M, Maffucci T. Rethinking phosphatidylinositol 3-monophosphate. Biochim Biophys Acta. 2009;1793:1795–803. doi: 10.1016/j.bbamcr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- [23].Meunier FA, Osborne SL, Hammond GR, Cooke FT, Parker PJ, Domin J, Schiavo G. Phosphatidylinositol 3-kinase C2alpha is essential for ATP-dependent priming of neurosecretory granule exocytosis. Mol Biol Cell. 2005;16:4841–51. doi: 10.1091/mbc.E05-02-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wen PJ, Osborne SL, Morrow IC, Parton RG, Domin J, Meunier FA. Ca2+-regulated pool of phosphatidylinositol-3-phosphate produced by phosphatidylinositol 3-kinase C2alpha on neurosecretory vesicles. Mol Biol Cell. 2008;19:5593–603. doi: 10.1091/mbc.E08-06-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Osborne SL, Wen PJ, Boucheron C, Nguyen HN, Hayakawa M, Kaizawa H, Parker PJ, Vitale N, Meunier FA. PIKfyve negatively regulates exocytosis in neurosecretory cells. J Biol Chem. 2008;283:2804–13. doi: 10.1074/jbc.M704856200. [DOI] [PubMed] [Google Scholar]
- [26].Leibiger B, Moede T, Uhles S, Barker CJ, Creveaux M, Domin J, Berggren PO, Leibiger IB. Insulin-feedback via PI3K-C2alpha activated PKBalpha/Akt1 is required for glucosestimulated insulin secretion. FASEB J. 2010;24:1824–37. doi: 10.1096/fj.09-148072. [DOI] [PubMed] [Google Scholar]
- [27].Cui ZG, Hong NY, Kang HK, Lee DH, Lee YK, Park DB. The α-isoform of class II phosphoinositide 3-kinase is necessary for the activation of ERK but not Akt/PKB. Mol Cell Biochem. 2011;346:95–101. doi: 10.1007/s11010-010-0596-1. [DOI] [PubMed] [Google Scholar]
- [28].Elis W, Triantafellow E, Wolters NM, Sian KR, Caponigro G, Borawski J, Gaither LA, Murphy LO, Finan PM, Mackeigan JP. Down-regulation of class II phosphoinositide 3-kinase alpha expression below a critical threshold induces apoptotic cell death. Mol Cancer Res. 2008;6:614–23. doi: 10.1158/1541-7786.MCR-07-0262. [DOI] [PubMed] [Google Scholar]
- [29].Stahelin RV, Karathanassis D, Bruzik KS, Wa-terfield MD, Bravo J, Williams RL, Cho W. Structural and membrane binding analysis of the Phox homology domain of phosphoinositide 3-kinase-C2alpha. J Biol Chem. 2006;281:39396–406. doi: 10.1074/jbc.M607079200. [DOI] [PubMed] [Google Scholar]
- [30].Ursø B, Brown RA, O'Rahilly S, Shepherd PR, Siddle K. The alpha-isoform of class II phosphoinositide 3-kinase is more effectively activated by insulin receptors than IGF receptors, and activation requires receptor NPEY motifs. FEBS Lett. 1999;460:423–6. doi: 10.1016/s0014-5793(99)01388-5. [DOI] [PubMed] [Google Scholar]
- [31].Wang Y, Yoshioka K, Azam MA, Takuwa N, Sa-kurada S, Kayaba Y, Sugimoto N, Inoki I, Ki-mura T, Kuwaki T, Takuwa Y. Class II phosphoinositide 3-kinase alpha-isoform regulates Rho, myosin phosphatase and contraction in vascular smooth muscle. Biochem J. 2006;394:581–92. doi: 10.1042/BJ20051471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dominguez V, Raimondi C, Somanath S, Bugliani M, Loder MK, Edling CE, Divecha N, da Silva-Xavier G, Marselli L, Persaud SJ, Turner MD, Rutter GA, Marchetti P, Falasca M, Maffucci T. Class II phosphoinositide 3-kinase regulates exocytosis of insulin granules in pancreatic beta cells. J Biol Chem. 2011;286:4216–25. doi: 10.1074/jbc.M110.200295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Leibiger B, Leibiger IB, Moede T, Kemper S, Kulkarni RN, Kahn CR, de Vargas LM, Berggren PC. Selective insulin signaling through A and B insulin receptors regulates transcription of insulin and glucokinase genes in pancreatic beta cells. Mol Cell. 2001;7(3):559–70. doi: 10.1016/s1097-2765(01)00203-9. [DOI] [PubMed] [Google Scholar]
- [34].Chaussade C, Pirola L, Bonnafous S, Blondeau F, Brenz-Verca S, Tronchere H, Portis F, Rusconi S, Payrastre B, Laporte J, Van Obberghen E. Expression of myotubularin by an adenoviral vector demonstrates its function as a phosphatidylinositol 3-phosphate [Ptdlns(3)P] phosphatase in muscle cell lines: involvement of Ptdlns(3)P in insulin-stimulated glucose transport. Mol Endocrinol. 2003;17:2448–60. doi: 10.1210/me.2003-0261. [DOI] [PubMed] [Google Scholar]
- [35].Ishiki M, Randhawa VK, Poon V, Jebailey J, Klip A. Insulin regulates the membrane arrival, fusion, and C-terminal unmasking of glucose transporter-4 via distinct phosphoinositides. J Biol Chem. 2005;280:28792–802. doi: 10.1074/jbc.M500501200. [DOI] [PubMed] [Google Scholar]
- [36].Kanda H, Tamori Y, Shinoda H, Yoshikawa M, Sakaue M, Udagawa J, Otani H, Tashiro F, Miyazaki J, Kasuga M. Adipocytes from Muncl8cnull mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J Clin Invest. 2005;115:291–301. doi: 10.1172/JCI22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kong AM, Horan KA, Sriratana A, Bailey CG, Collyer LJ, Nandurkar HH, Shisheva A, Layton MJ, Rasko JE, Rowe T, Mitchell CA. Phosphatidylinositol 3-phosphate [Ptdlns3P] is generated at the plasma membrane by an inositol poly-phosphate 5-phosphatase: endogenous Ptdlns3P can promote GLUT4 translocation to the plasma membrane. Mol Cell Biol. 2006;26:6065–81. doi: 10.1128/MCB.00203-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lodhi IJ, Bridges D, Chiang SH, Zhang Y, Cheng A, Geletka LM, Weisman LS, Saltiel AR. Insulin stimulates phosphatidylinositol 3-phosphate production via the activation of Rab5. Mol Biol Cell. 2008;19:2718–28. doi: 10.1091/mbc.E08-01-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brunner Y, Couté Y, lezzi M, Foti M, Fukuda M, Hochstrasser DF, Wollheim CB, Sanchez JC. Proteomics analysis of insulin secretory granules. Mol Cell Proteomics. 2007;6:1007–17. doi: 10.1074/mcp.M600443-MCP200. [DOI] [PubMed] [Google Scholar]
- [40].Dai S, Zhang Y, Weimbs T, Yaffe MB, Zhou D. Bacteria-generated Ptdlns(3)P recruits VAMP8 to facilitate phagocytosis. Traffic. 2007;8:1365–74. doi: 10.1111/j.1600-0854.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- [41].Mima J. Phosphoinositides and SNARE chaperones synergistically assemble and remodel SNARE complexes for membrane fusion. Proc Nati Acad Sci U S A. 2009;106:16191–6. doi: 10.1073/pnas.0908694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mima J, Wickner W. Complex lipid requirements for SNARE- and SNARE chaperone-dependent membrane fusion. J Biol Chem. 2009;284:27114–22. doi: 10.1074/jbc.M109.010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Paul DS, Harmon AW, Winston CP, Patel YM. Calpain facilitates GLUT4 vesicle translocation during insulin-stimulated glucose uptake in adipocytes. Biochem J. 2003;376:625–32. doi: 10.1042/BJ20030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Logie LJ, Brown AE, Yeaman SJ, Walker M. Calpain inhibition and insulin action in cultured human muscle cells. Mol Genet Metab. 2005;85:54–60. doi: 10.1016/j.ymgme.2005.01.003. [DOI] [PubMed] [Google Scholar]
- [45].Brown AE, Yeaman SJ, Walker M. Targeted suppression of calpain-10 expression impairs insulin-stimulated glucose uptake in cultured primary human skeletal muscle cells. Mol Genet Metab. 2007;91:318–24. doi: 10.1016/j.ymgme.2007.05.001. [DOI] [PubMed] [Google Scholar]
- [46].Marshall C, Hitman GA, Partridge CJ, Clark A, Ma H, Shearer TR, Turner MD. Evidence that an isoform of calpain-10 is a regulator of exocytosis in pancreatic beta-cells. Mol Endocrinol. 2005;19:213–24. doi: 10.1210/me.2004-0064. [DOI] [PubMed] [Google Scholar]
- [47].MacDonald PE, Joseph JW, Yau D, Diao J, As-ghar Z, Dai F, Oudit GY, Patel MM, Backx PH, Wheeler MB. Impaired glucose-stimulated insulin secretion, enhanced intraperitoneal insulintolerance, and increased beta-cell mass in micelacking the pllOgamma isoform of phosphoinositide3-kinase. Endocrinology. 2004;145:4078–83. doi: 10.1210/en.2004-0028. [DOI] [PubMed] [Google Scholar]
- [48].Pigeau GM, Kolic J, Ball BJ, Hoppa MB, Wang YW, Rückle T, Woo M, Manning Fox JE, Mac-Donald PE. Insulin granule recruitment and exocytosis is dependent on p110gamma in insulinoma and human beta-cells. Diabetes. 2009;58:2084–92. doi: 10.2337/db08-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kaneko K, Ueki K, Takahashi N, Hashimoto S, Okamoto M, Awazawa M, Okazaki Y, Ohsugi M, Inabe K, Umehara T, Yoshida M, Kakei M, Kita-mura T, Luo J, Kulkarni RN, Kahn CR, Kasai H, Cantley LC, Kadowaki T. Class IA phosphatidy-linositol 3-kinase in pancreatic β cells controls insulin secretion by multiple mechanisms. Cell Metab. 2010;12:619–32. doi: 10.1016/j.cmet.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Krag C, Malmberg EK, Salcini AE. PI3KC2<x, a class II PI3K, is required for dynamin-independent internalization pathways. J Cell Sci. 2010;123:4240–50. doi: 10.1242/jcs.071712. [DOI] [PubMed] [Google Scholar]
- [51].Yoshioka K, Sugimoto N, Takuwa N, Takuwa Y. Essential role for class II phosphoinositide 3-kinase alpha-isoform in Ca2+-induced, Rho-and Rho kinase-dependent regulation of myosin phosphatase and contraction in isolated vascular smooth muscle cells. Mol Pharmacol. 2007;71:912–20. doi: 10.1124/mol.106.032599. [DOI] [PubMed] [Google Scholar]
- [52].Azam MA, Yoshioka K, Ohkura S, Takuwa N, Sugimoto N, Sato K, Takuwa Y. Ca2+-independent, inhibitory effects of cyclic adenosine 5’-monophosphate on Ca2+ regulation of phosphoinositide 3-kinase C2alpha, Rho, and myosin phosphatase in vascular smooth muscle. J Pharmacol Exp Ther. 2007;320:907–16. doi: 10.1124/jpet.106.111443. [DOI] [PubMed] [Google Scholar]
- [53].Seok YM, Azam MA, Okamoto Y, Sato A, Yoshioka K, Maeda M, Kim I, Takuwa Y. Enhanced Ca2+-dependent activation of phosphoinositide 3-kinase class IIα isoform-Rho axis in blood vessels of spontaneously hypertensive rats. Hypertension. 2010;56:934–41. doi: 10.1161/HYPERTENSIONAHA.110.160853. [DOI] [PubMed] [Google Scholar]
- [54].Ng SK, Neo SY, Yap YW, Karuturi RK, Loh ES, Liau KH, Ren EC. Ablation of phosphoinositide-3-kinase class II alpha suppresses hepatoma cell proliferation. Biochem Biophys Res Commun. 2009;387:310–15. doi: 10.1016/j.bbrc.2009.07.013. [DOI] [PubMed] [Google Scholar]
- [55].Kang S, Song J, Kang J, Kang H, Lee D, Lee Y, Park D. Suppression of the alpha-isoform of class II phosphoinositide 3-kinase gene expression leads to apoptotic cell death. Biochem Biophys Res Commun. 2005;329:6–10. doi: 10.1016/j.bbrc.2005.01.091. [DOI] [PubMed] [Google Scholar]
- [56].Eun LY, Song BW, Cha MJ, Song H, Kim IK, Choi E, Chang W, Lim S, Choi EJ, Ham O, Lee SY, Byun KH, Jang Y, Hwang KC. Overexpression of phosphoinositide-3-kinase class II alpha enhances mesenchymal stem cell survival in infarcted myocardium. Biochem Biophys Res Commun. 2010;402:272–9. doi: 10.1016/j.bbrc.2010.10.013. [DOI] [PubMed] [Google Scholar]
- [57].Harris DP, Vogel P, Wims M, Moberg K, Humphries J, Jhaver KG, Dacosta CM, Shadoan MK, MK Xu N, Hansen GM, Balakrishnan S, Domin J, Powell DR, Oravecz T. Requirement for the class II phosphoinositide 3-kinase C2{alpha} in maintenance of glomerular structure and function. Mol Cell Biol. 2011;31:63–80. doi: 10.1128/MCB.00468-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Edling CE, Selvaggi F, Buus R, Maffucci T, Di Sebastiano P, Friess H, Innocenti P, Kocher HM, Falasca M. Key role of phosphoinositide 3-kinase class IB in pancreatic cancer. Clin Cancer Res. 2010;16:4928–37. doi: 10.1158/1078-0432.CCR-10-1210. [DOI] [PubMed] [Google Scholar]
- [59].Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E, 3rd, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Nati Acad Sci U S A. 2007;104:16158–63. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]