Abstract
Gap junctions and hemichannels are formed by a family of proteins called connexins. Till date up to twenty one different connexins have been characterized and their expression was observed to be spatio-temporally regulated. Gap junctions and hemichannels are involved in transfer of a variety of less than 1 kDa small molecules such as, ions, small metabolites, cAMP, ATP, IP3, prostaglandins, etc. Post-translational modifications of connexins and their interaction with other proteins are reported to be the key regulators of channel functions. Studies during the past decade or so, suggest the physiological important of connexin hemichannels mediating the communication between the cell and its environment. Molecules conveyed through the hemichannels elicit a variety of signaling pathways and influence cellular functions such as, cell cycle, tissue homeostasis, migration, mechanotransduction, oxidative stress. The purpose of the current review is to compile the reported studies so far and provide a general overview in our understanding how the molecular transfer through hemichannels regulates cellular signaling and functions.
Keywords: Case-control study, Connexin, gap junction, molecular epidemiology, ovarian cancer, hemichannel, cell cycle, cellular function and signaling, mechanotransduction
Introduction
Gap junctions (GJs) and hemichannels (HCs) are composed of proteins known as connexins which form a principal means of cell-cell communication in animal tissues, allowing the diffusion of ions, metabolites and secondary messengers [1, 2]. Up to twenty one different connexins have been identified till now [3] and their nomenclature is based on the molecular weight; for example, connexin (Cx) 43 is a 43 kDa protein. Connexins oligomerize into hexameric connexons and docking of two such connexon channels expressed on adjacent cells lead to the construction of a GJ channel. Connexons formed by a single type of connexin are termed homomeric and channels with a mixture of two types of connexins are known as heteromeric channels [1, 4, 5]. Heterotypic channels are formed when two homomeric connexons formed by different connexins dock onto each other [6–8]. Formation of heteromeric-heterotypic GJ channels is also reported [4, 9, 10]. Intermixing of connexins depends on their compatibility, for example, Cx43 cannot form heteromeric channels with Cx26 [11]. Such intermixing could confer special functions to GJ channels as they exhibit different permeability properties in comparison to homomeric-homotypic channels alone [12].
GJs and HCs allow passage of the molecules with a cut off weight of 1 kDa and the specificity depends on the type of connexin. The shape of the pore and the pore-lining residues of connexons contribute to the specificity of molecules that are exchanged [13]. Post-translational modifications, such as phosphorylation and S-nitrosylation, also affect the molecular transfer through HCs [14, 15]. Therefore, intermixing of connexins and post-translational modifications could be critical in regulation of cell communication. Molecular exchange via connexin channels is thought to be essential for signal propagation in cells, and to be involved in growth control, differentiation, embryonic development and reproduction [16, 17]. It has been technically challenging to identify specific molecules that are communicated through GJs formed by different connexins. However, identification of molecules passing through HCs is known to be less challenging as factors released by HCs expressed in cell culture systems can be collected from culture media and analyzed.
Signaling molecules, such as PGE2, ATP, and NAD+, are released through HCs [18–21]. These signaling molecules act in both paracrine and autocrine manner to elicit intracellular signaling responses [22]. HCs mediated exchange of secondary messengers is required for maintenance of cellular physiology. Therefore, studying the molecular exchange via HCs is a critical step to understand how HC mediated communication regulates cellular function. Recently, several specific tools have been developed for study of HCs, including Cx43 HC blocking antibody, blocking peptides towards HCs, and lanthanum ion [23–25]. These reagents have been proven to be invaluable to identify the specific function of connexin-forming HCs and specific molecules released by these channels [20, 23]. Determination of the specificity of connexin forming HCs is of practical importance since HCs can be formed by either connexins or another protein called pannxins. Pannexins albeit sharing no sequence homology with connexins are found to co-exist in certain cells and form HCs in the cell [26]. Similar to connexin forming ones, pannexin-HCs open to extracellular spaces and permit molecular weight less than 1 kDa to pass through. However, unlike connexin, thus far there is no solid evidence suggesting the formation of gap junctions by pannexin in mammalian cells [27]. There is increasing evidence that pannexins are involved in vasodilatation, inflammatory responses, ischemic death of neurons, epilepsy and tumor suppression [28]. Another important task is to identify the signaling pathways associated with the molecules released from HCs and how they modulate the cellular fate. This review summarizes the regulation and function of connexin HCs in several cellular events and the associated signaling pathways.
Cell cycle and development
Though the role of connexins in regulation of cell cycle, cell progression and development has been elaborately studied, the importance of connexin HCs in this regard is still unclear. Recently, it is observed that GJB2 (Cx26) is a susceptible locus in patients with psoriasis, a skin disease that occurs due to increased skin cell division [29]. Cx26 is known to form HCs in skin and inner ear, and mutations of Cx26 genes are associated with skin diseases and deafness [30]. However, it is not clear if abnormally elevated cell division in psoriasis is related to the function of Cx26 HCs. In C6 glioma cell, Cx43 is shown to reduce cell proliferation by impeding the cell cycle progression from G0/G1 to S phase [31]. Mutation of Cx43 at Tyr247 and Tyr265 is observed to reverse the negative proliferative effect of Cx43. Connexins are differentially expressed during cell division and development. Cx26 expression increases between S phase and early G1 phase in actively dividing neocortex cells, whereas Cx43 expression is at its peak between G2 and S phase and decreases during G1 phase [32]. Cx43 phosphorylation is also reported to be associated with the progression of cell cycle. Cx43 phosphorylation is increased during S and G2/M phases of the cell cycle, which may be related to the assembly of fewer gap junction channels [33].
Extracellular Ca2+ levels and pH, which regulate HC opening, are also known to regulate cell growth and proliferation. Cx43 HCs in 3T3 fibroblasts are observed to release NAD+ [19], which is converted to cyclic ADP-ribose (cADPR) by an ectoenzyme, CD38. Uptake of cADPR by the cell increases intracellular calcium levels, consequently increasing cell proliferation rate by shortening S phase [34]. Neural precursor cells release ATP [35] and released ATP controls intracellular calcium levels via Cx43 HCs [36]. Calcium waves propagating through radial glial cells, a transient embryonic cell type important for neuronal migration, requires connexin HCs and disruption of the wave decreases the proliferation of cortical ventricular zone (VZ) [36]. Blocking of intracellular Ca2+ levels inhibits DNA synthesis in these cells [36]. Another study shows that inhibition of Cx43 HCs reduces neointima formation after vascular injury due to inhibition of smooth muscle cell proliferation of smooth muscle cells [37]. Mutations of Cx30 gene are responsible for ectodermal dysplasia associated with increased proliferation and differentiation of keratinocytes. “gain of function” mutants, G11R and A88V, have enhanced HC function and generate a leakage of ATP [38], which may lead to the enhancement of cell proliferation. Also, recently it is observed that blocking of HCs induces phosphorylation of small GTPase cdc42, which is important in orchestrating cytoskeletal organization during cell division [35]. Additionally, knockdown of Cx43 expression by siRNA in neural precursor cells leads to slower interkinetic nuclear migration and also affects the nuclear length/width ratio [35]. Gap27 peptide, which is designed to block Cx43 HCs, is shown to hinder T cell proliferation by increasing the G0/G1 cell numbers, suggesting the role of HCs in sustaining T cell clonal expansion [39].
During chick retinal pigment epithelium (RPE) development, robust Ca2+ waves are generated and gap junction coupling is involved in this process [40]. Specific peptide blocker, Gap26, of Cx43 HC inhibited release of both ATP and Ca2+ waves in RPE, suggesting the Cx43 HC [41]. The ATP released through Cx43 HCs expressed in RPE influences the mitotic division in retinal ventricular zone, which is important in generation of neurons and glia [41]. Extracellular ATP is a signal for various events during embryonic development. ATP through purinergic receptors evokes Ca2+ waves and promotes retinal progenitor cell proliferation [42]. ATP signaling also initiates the proliferation of rat cerebral cortical astrocytes [43], human neural stem cell [44] and Müller glial cell [45]. Hence, the release of ATP through HCs activates purinergic receptors and triggers gene expression necessary for retinal development, ionic homeostasis cochlea (for details, see “Cochlear homeostasis”) and proliferation of neural precursor cells during neural development.
Ischemic preconditioning
A subtle or sub-lethal insult to a tissue causes protection of the tissue towards a severe insult, and the process is known as preconditioning [46]. There is a general belief that release of protective agents during the initial insult leads to resistance towards permanent tissue damage. Ischemia could be caused due to deprivation of oxygen and glucose or ATP efflux. Cardiac and the neural tissues are most extensively studied to understand the preconditioning phenomenon and various connexin HCs are implicated in release of tissue protective agents. Cx43 HCs are abundantly expressed in cardiac tissue [47]. Under ischemic stress, cardiac myocytes and C6 glioma cells release ATP [48, 49]. ATP efflux leads to increase in intracellular calcium levels; consequently HC open further causing an increase in cellular volume, which ultimately results in apoptotic cell death [50]. Cx43 HC blocking peptide, Gap26 is demonstrated to protect rat neonatal cardiomyocytes and intact rat heart from ischemic injury [50, 51]. However, Cx43 deficient heterozygous (+/−) mice lacked cardio protection during ischemic preconditioning [52]. Also, in isolated cardiac myocytes, where the GJ communication is minimal, Cx43 was observed to be required for ischemic preconditioning [53], suggesting that ischemic preconditioning could be gap junction-independent. These studies indicate that though HC communication is required for cardiac protection, sustained opening results in cardiac injury. Cardiac myocytes subjected to ischemia showed an increase in release of ATP [48], which can be blocked by Gap26 peptide [48]. Another important observation made in this study is that the ATP release through Cx43 HCs is reduced due to prolonged ischemia. Transient opening of HCs is observed in cardio myocytes subjected to ischemia [54]. Regulation of HC opening during ischemia could be important in preventing necrosis due to sustained ATP release. Extracellular ATP is known to communicate through G-protein coupled P2Y2 and P2Y4 purinergic receptors expressed on the cell surface and invoke intracellular calcium levels [55, 56]. Other factors released by HCs and the signaling pathways triggered during preconditioning response are yet to be determined. Apart from the connexin at the cell surface, recent studies have demonstrated that Cx43 is also expressed in mitochondria and plays an important role during ischemic preconditioning [57, 58]. Mitochondria are known to be important for mediating precondition response. Treatment with GJ and HC blockers, such as carbanoxelone and heptanol, results in a decrease of mitochondrial dye uptake [57], implying the existence of functional HCs in mitochondria. During prolonged ischemia, ATP levels decrease leading to lowering of pH and accumulation of reactive oxygen species (ROS), which ultimately results in cell death. These studies suggest that connexin HCs play an important role in release of ATP during the early stages of cardiac ischemia, thereby creating a protective preconditioning effect.
Cx36 and Cx43 are the major connexin isoforms expressed in brain tissue. In general, Cx36 is abundantly expressed in neuronal tissue and Cx43 is localized to glial tissue. Cx43 and Cx36 HCs show increased opening during ischemia leading to the release of ATP [59] Stimulation by acute ischemic conditions enhances neuronal Cx36 and glial Cx43 HC activity, which favors ATP release and generates preconditioning. Besides ATP, Cx43 HCs also mediate the release of glutamate from astrocytes, and glutathione and other amino acid derivates from hippocampal slices [60;61]. The efflux of glutathione is postulated to have cell protective functions in situations when glutamate reuptake is impaired, such as after stroke [61]. Similar to the observations made in the cardiac tissue, acute ischemia opens HCs leading to ATP release and depletion of intracellular ATP, which finally results in cell death [62]. Metabolic inhibition is also reported to increase ATP release via HCs and the depletion of intracellular ATP reserves could promote cell death [63]. Metabolic inhibition also increases Cx43 expression on the cell surface causing further imbalance in the cellular ionic concentrations, thereby, making the cells vulnerable to cell death [64]. However, Cx36 HCs expressed in the neurons are observed to mediate ischemic preconditioning [65]. Cx36 HCs in neurons mediate the ATP release and cause depolarization of the neurons [65]. Depolarization of neurons ultimately increases neuronal tolerance towards ischemia. Role of HCs in causing glutamate toxicity due to the release of excessive glutamate during ischemic conditions is yet to be studied. Overall, HCs formed by connexins promote cell death in both neurons and cardio myocytes during acute ischemia, but they also can promote tolerance towards ischemia after an encounter with sublethal ischemia.
Cochlear homeostasis
Autosomal Cx26 mutations expressed in inner ear are associated with hearing loss [66]. Interestingly, these mutations are characterized to be both dominant and recessive, which makes the study of Cx26 in cochlear homeostasis very critical. Autosomal mutations of Cx26 display impaired IP3 mediated Ca2+ wave propagation between cells [67]. Cx26 and Cx30 are predominantly expressed in the inner ear and are required for normal hearing [68, 69]. Impaired expression of these two connexins affects propagation of Ca2+ wave [20]. These two connexins also form heteromeric and heterotypic HCs, displaying a range of gating properties [9]. Cx26 is observed to be selectively permeable to anionic molecules [70]. A dominant deafness mutant of Cx26, R75W causes loss in gap junction communication but exhibits normal HC permeability [71, 72]. Cochlear organ cultures lacking pannexin-1 or purinergic receptor P2X7 did not inhibit the propagation of Ca2+ waves [20]. Cx26 HCs expressed in the inner ear release ATP that causes an increase in outer hair motility (OHC), which is important for hearing [70]. Lower extracellular Ca2+ causes ATP release via Cx26 HCs, which affects OHC through purinergic receptor signaling. Apart from ATP, Cx26 HCs also release IP3 and the release is observed to increase with lower extracellular Ca2+ and mechanical stress [73]. Both IP3 and ATP elicit intracellular Ca2+ waves and also increase spreading of the waves between the cells [74]. Lower extracellular calcium levels and mechanical stress experienced by the cochlear membrane can cause release of ATP and IP3. These secondary messengers re-enter into the cells or act through membrane receptors to activate intracellular calcium reserves, thereby, acting in both paracrine and autocrine fashion. Along with propagation of calcium waves secondary messengers such as IP3 could be communicated between cells via GJ channels. Hence, the overall signaling pathway mediated by connexins could be very important in auditory signal propagation. A clear picture of the signaling pathways associated with connexin HC function in the inner ear is yet to be obtained.
Mechanotransduction
Expression of connexins is observed to increase after mechanical stimulation of cardiac and bone cells [75–77]. GJs present in these cells are shown to be important conduits of signaling molecules that are generated due to mechanical stimulation indicating that connexins act as mechanosensory channels [18, 78, 79]. In osteoblasts, both Cx43 and Cx45 are expressed [80], but when transient expression of Cx45 is increased it results in reduction of GJ mediated intercellular communication and downregulation of osteoblastic differentiation markers at the transcriptional level [81, 82]. Osteocytes, similar to their precursor osteoblasts, are observed to express both Cx43 and Cx45, but Cx43 is observed to be in abundance, and mechanical stimulation regulates its expression and function [83;84]. Cx43 knockout mice displayed defective skeletal development and delayed ossification [85]. Osteoblast-specific Cx43 knockout mice showed a decrease in bone formation in response to mechanical loading, suggesting that Cx43 is important in adaptation to mechanical stimulation [86]. These observations indicate that Cx43 is regulated by mechanical stimulation and is the major GJ forming protein in osteoblasts and osteocytes.
Mechanical stimulation leads to opening of HCs in many cell types. In human osteoblast cells and osteoblast like MC3T3-E1 cells, mechanical stimulation leads to the release of IP3, Ca2+ and ATP through Cx43 HCs [87, 88]. Cx43 HCs expressed in osteocytes are involved in release of bone regulators, such as PGE2 and ATP in response to fluid flow shear stress [18, 89]. Recent studies indicate that other bone cells, such as chondrocytes also express Cx43 HCs which are involved in release of ATP in response to cyclic compressive strains [90]. In osteocytes, the opening of Cx43 HCs are adaptively regulated in response to the magnitudes and durations of mechanical stimulation [23]. Cx43 HCs are observed to be important for cell survival, which is critical in maintaining the integrity of bone [91]. Osteosarcoma cells when treated with drugs that induce cell cycle arrest resulted in expression of functional HCs and formation of dendritic processes similar to osteocytes [92], implying that HCs may be involved in osteoblast differentiation and osteocyte survival. In our recent studies we have shown that the osteocytic dendritic processes function as mechanosensors [93]. Mechanical stimulation of osteocytes is observed to release PGE2 via Cx43 HCs and this release was observed to be adaptive [23]. The released PGE2 is observed to activate both PI3K/Akt and cAMP-PKA signaling pathways leading to an increase in Cx43 expression [22, 94]. Among four isoforms of PGE2 receptors, E2 and E4 are activated in response to fluid flow shear stress, which leads to the activation of downstream PI3K/Akt and cAMP-PKA signaling events [94, 95]. Cx26 HCs expressed in Cochlea were observed to release IP3 in the presence of mechanical stimulation and lower extracellular Ca2+ [73]. Mechanical stimulation along with lower extracellular Ca2+ potentiated Cx43 HC opening and subsequent release of ATP in astrocytes and endothelial cells [96, 97]. The molecules released via HCs could activate different signaling pathways through interacting with specific receptors, thereby, exerting the biological functions in response to mechanical stimulation.
Conclusion
Apart from GJ communication, connexin-forming HCs play critical roles in modulating cellular functions. As summarized in Figure 1, the molecular transfer through HCs regulates cellular functions. This is likely to be regulated by the factors released through the open HCs. Binding to cell surface receptors by the released factors leads to the activation of intracellular signaling pathways and consequently, the regulation of cellular function and physiology through gene transcription, translational or post-translational events including intracellular trafficking, protein turnover, phosphorylation and other modifications. HC formed by different connexins may regulate distinctive cellular events via the selective message of specific molecules. This raises the possibility that HCs have certain substrate selectivity in exchanging molecules. Recent studies on understanding the pore structure also support this possibility. It is imperative to develop HC-specific tools, such as blocking antibodies, peptides or dominant negative mutants. With these specific reagents, the molecules exchanged via HCs and the activation of specific signaling pathways can be investigated. Moreover, with the development of dominant negative mutants specific for HCs, but not gap junctions, the physiological roles of HCs in specific tissues or cells in vivo will be illustrated using transgenic mouse models.
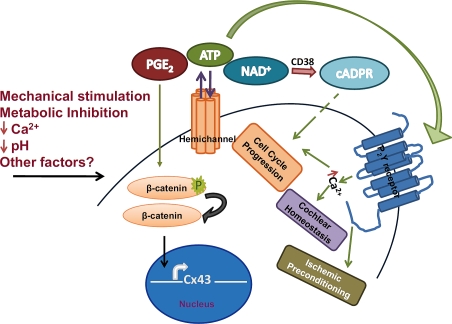
Figure 1.
Connexin hemichannels (HCs) in response to stimuli such as mechanical stimulation, lower extracellular calcium, lower pH or metabolic inhibition mediate release of molecules like PGE2, ATP and NAD+. These metabolites influence cellular signaling through various receptors. ATP is known to influence cellular functions such as cochlear homeostasis, ischemic preconditioning and cell cycle through G-protein coupled purinergic receptor signaling. PGE2 release by HCs due to mechanical stimulation causes activation of β-catenin, which is shown to induce Cx43 transcription. Detailed signaling pathways elicited by HC communication are under studied.
Acknowledgements
This work was supported by National Institutes of Health Grant PO1 AR46798 (to J.X.J) and Welch Foundation grant: AQ-1507 (to J.X.J).
References
- [1].Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- [2].Bennett MVL, Goodenough DA. Gap junctions, electronic coupling, and intercellular communication. Neurosciences Res Prog Bull. 1978;16:373–486. [PubMed] [Google Scholar]
- [3].Söhl G, Willecke K. Gap junction and the connexin family. Cardiovasc Res. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- [4].Jiang JX, Goodenough DA. Heteromeric connexons in lens gap junction channels. Proc Natl Acad Sci U S A. 1996;93:1287–1291. doi: 10.1073/pnas.93.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].He DS, Jiang JX, Taffet SM, Burt JM. Formation of heteromeric gap junction channels by connexin 40 and 43 in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1999;96:6495–6500. doi: 10.1073/pnas.96.11.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].White TW, Bruzzone R. Multiple connexin proteins in single intercellular channels: connexin compatibility and functional consequences. J Bioenerg Biomembranes. 1996;28:339–350. doi: 10.1007/BF02110110. [DOI] [PubMed] [Google Scholar]
- [7].Barrio LC, Suchyna T, Bargiello T, Xu LX, Roginski RS, Bennett MVL, Nicholson BJ. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by voltage. Proc Natl Acad Sci USA. 1991;88:8410–8414. doi: 10.1073/pnas.88.19.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bouvier D, Spagnol G, Chenavas S, Kieken F, Vitrac H, Brownell S, Kellezi A, Forge V. Sorgen PL. Characterization of the structure and intermolecular interactions between the connexin40 and connexin43 carboxyl-terminal and cytoplasmic loop domains. J Biol Chem. 2009;284:34257–34271. doi: 10.1074/jbc.M109.039594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yum SW, Zhang J, Vallunas V, Kanaporis G, Brink PR, White TW. Scherer SS. Human connexin26 and connexin30 form functional heteromeric and heterotypic channels. Am J Physiol Cell Physiol. 2007;293:C1032–C1048. doi: 10.1152/ajpcell.00011.2007. [DOI] [PubMed] [Google Scholar]
- [10].Cottrell GT, Wu Y, Burt JM. Cx40 and Cx43 expression ratio influences heteromeric/heterotypic gap junction channel properties. Am J Physiol Cell Physiol. 2002;282:C1469–C1482. doi: 10.1152/ajpcell.00484.2001. [DOI] [PubMed] [Google Scholar]
- [11].Gemel J, Valiunas V, Brink PR. Beyer EC. Connexin43 and connexin26 form gap junctions, but not heteromeric channels in co-expressing cells. J Cell Sci. 2004;117:2469–2480. doi: 10.1242/jcs.01084. [DOI] [PubMed] [Google Scholar]
- [12].Ayad WA, Locke D, Koreen IV. Harris AL. Heteromeric, but not homomeric, connexin channels are selectively permeable to inositol phosphates. J Biol Chem. 2006;281:16727–16739. doi: 10.1074/jbc.M600136200. [DOI] [PubMed] [Google Scholar]
- [13].Weber PA, Chang HC, Spaeth KE, Nitsche JM. Nicholson BJ. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys J. 2004;87:958–973. doi: 10.1529/biophysj.103.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bao X, Lee SC, Reuss L. Altenberg GA. Change in permeant size selectivity by phosphorylation of connexin 43 gap-junction hemichannels by PKC. Proc Natl Acad Sci. 2007;104:4919–4924. doi: 10.1073/pnas.0603154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Retamal MA, Cortés CJ, Reuss L, Bennett MV. Sáez J. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Nat Acad Sci (USA) 2006;103:4475–4480. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Reaume AG, De Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM. Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- [17].Simon AM, Goodenough DA, Li E. Paul DL Female infertility in mice lacking connexin37. Nature (Lond) 1997;385:525–529. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- [18].Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E. Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–3106. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bruzzone S, Guida L, Zocchi E, Franco L. De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- [20].Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H. Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Garré JM, Retamal MA, Cassina P, Barbeito L, Bukauskas FF, Sáez JC, Bennett MV. Abudara V. FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc Nat Acad Sci (USA) 2010;107:22659–22664. doi: 10.1073/pnas.1013793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xia X, Batra N, Shi Q, Bonewald LF, Sprague E. Jiang JX. Prosglandin promotion of osteocyte gap junction function through transcriptional regulation of connexin 43 by glycogen synthase kinase 3/□-catenin signaling. Mole Cell Biol. 2010;30:206–219. doi: 10.1128/MCB.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E. Jiang JX. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem. 2008;283:26374–26382. doi: 10.1074/jbc.M803136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Evans WH. Leybaert L. Mimetic peptides as blockers of connexin channel-facilitated intercellular communication. Cell Commun Adhes. 2007;14:265–273. doi: 10.1080/15419060801891034. [DOI] [PubMed] [Google Scholar]
- [25].Kondo RP, Wang S-Y, John SA, Weiss JN. Goldhaber JI. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- [26].Shestopalov VI. Panchin Y. Pannexins and gap junction protein diversity. Cell Mol Life Sci. 2008;65:376–394. doi: 10.1007/s00018-007-7200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dahl G. Locovei S. Pannexin: to gap or not to gap, is that a question? IUBMB Life. 2006;58:409–419. doi: 10.1080/15216540600794526. [DOI] [PubMed] [Google Scholar]
- [28].D'hondt C, Ponsaerts R, De Smedt H, Vinken M, de Vuyst E, De Bock M, Wang N, Rogiers V, Leybaert L, Himpens B. Bultynck G. Pannexin channels in ATP release and beyond: an unexpected rendezvous at the endoplasmic reticulum. Cell Signal. 2011;23:305–316. doi: 10.1016/j.cellsig.2010.07.018. [DOI] [PubMed] [Google Scholar]
- [29].Sun LD, Cheng H, Wang ZX, Ahang AP, Wang PG, Xu JH, Zhu QX, Zhou HS, Ellinghaus E, Zhang FR, Pu XM, Yang XQ, Zhang JZ, Xu AE, Wu RN, Xu LM, Peng L, Helms CA, Ren YQ, Zhang C, Zhang SM, Nair RP, Wang HY, Lin GS, Stuart PE, Lin Y. Y Nat Genet. 2010;42:1005–1009. doi: 10.1038/ng.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gerido DA, DeRosa AM, Richard G. White TW. Aberrant hemichannels properties of Cx26 mutations causing skin disease and deafness. Am J Physiol Cell Physiol. 2007;293:C337–C345. doi: 10.1152/ajpcell.00626.2006. [DOI] [PubMed] [Google Scholar]
- [31].Herrero-González S, Gangoso E, Giaume C, Naus CC, Medina JM. Tabemero A. Connexin43 inhibits the oncogenic activity of c-Src in C6 glioma cells. Oncogene. 2010;29:5712–5723. doi: 10.1038/onc.2010.299. [DOI] [PubMed] [Google Scholar]
- [32].Bittman KS. LoTurco JJ. Differential regulation of connexin26 and 43 in murine neocortical precursors. Cereb Cortex. 1999;9:188–195. doi: 10.1093/cercor/9.2.188. [DOI] [PubMed] [Google Scholar]
- [33].Solan JL, Fry MD, Tenbroek EM. Lampe PD. Connexin43 phosphorylation on S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci. 2003;116:2203–2211. doi: 10.1242/jcs.00428. [DOI] [PubMed] [Google Scholar]
- [34].Franco L, Zocchi E, Usai C, Guida L, Bruzzone S, Costa A. De Flora A. Paracrine roles of NAD+ and cyclic ADP-ribose in increasing intracellular calcium and enhancing cell proliferation of 3T3 fibroblasts. J Biol Chem. 2001;276:21642–21648. doi: 10.1074/jbc.M010536200. [DOI] [PubMed] [Google Scholar]
- [35].Liu X, Hashimoto-Torii K, Torii M, Ding C. Rakic P. Gap junctions/hemichannels modulate interkinetic nuclear migration in the forebrain precursors. J Neurosci. 2010;30:4197–4209. doi: 10.1523/JNEUROSCI.4187-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Weissman TA, Requelme PA, Ivic L, Flint AC. Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- [37].Song M, Yu X, Cui X, Zhu G, Zhao G, Chen J. Huang L. Blockade of connexin 43 hemichannels reduces neointima formation after vascular injury by inhibiting proliferation and phenotypic modulation of smooth muscle cells. Exp Biol Med (Maywood) 2009;234:1192–1200. doi: 10.3181/0902-RM-80. [DOI] [PubMed] [Google Scholar]
- [38].Essenfelder GM, Bruzzone R, Lamartine J, Charollais A, Blanchet-Bardon C, Barbe MT, Meda P. Waksman G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum Mo Genet. 2004;13:1703–1714. doi: 10.1093/hmg/ddh191. [DOI] [PubMed] [Google Scholar]
- [39].Oviedo-Orta E, Perreau M, Evans WH. Potolicchio I. Control of the proliferation of activated CD4+ T cells by connexins. J Leukoc Biol. 2010;88:79–86. doi: 10.1189/jlb.0909613. [DOI] [PubMed] [Google Scholar]
- [40].Pearson RA, Catsicas M, Becker DL, Bayley P, Lüneborg NL. Mobbs P. Ca(2+) signalling and gap junction coupling within and between pigment epithelium and neural retina in the developing chick. Eur J Neurosci. 2004;19:2435–2445. doi: 10.1111/j.0953-816X.2004.03338.x. [DOI] [PubMed] [Google Scholar]
- [41].Pearson RA, Dale N, Llaudet E. Mobbs P. ATP release via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- [42].Pearson R, Catsicas M, Becker D. Mobbs P. Purinergic and muscarinic modulation of the cell cycle and calcium signaling in the chick retinal ventricular zone. J Neurosci. 2002;22:7569–7579. doi: 10.1523/JNEUROSCI.22-17-07569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Neary JT. Zhu Q. Signaling by ATP receptors in astrocytes. Neuroreport. 1994;5:1617–1620. doi: 10.1097/00001756-199408150-00019. [DOI] [PubMed] [Google Scholar]
- [44].Ryu JK, Choi HB, Hatori K, Heisel RL, Pelech SL, McLamon JG. Kim SU. Adenosine triphosphate induces proliferation of human neural stem cells: Role of calcium and p70 ribosomal protein S6 kinase. J Neurosci Res. 2003;72:352–362. doi: 10.1002/jnr.10507. [DOI] [PubMed] [Google Scholar]
- [45].Milenkovic I, Weick M, Wiedemann P, Reichenbach A. Bringmann A. P2Y receptormediated stimultion of Müller glial cell DNA synthesis: dependence on EGF and PDGF receptor transactivation. Invest Ophthalmol Vis Sci. 2003;44:1211–1220. doi: 10.1167/iovs.02-0260. [DOI] [PubMed] [Google Scholar]
- [46].Murry CE, Jennings RB. Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- [47].John S, Cesario D. Weiss JN. Gap junctional hemichannels in the heart. Acta Physiol Scand. 2003;179:23–31. doi: 10.1046/j.1365-201X.2003.01197.x. [DOI] [PubMed] [Google Scholar]
- [48].Clarke TC, Williams OJ, Martin PE. Evans WH. ATP release by cardiac myocytes in a simulated ischaemia model: inhibition by a connexin mimetic and enhancement by an antiarrhythmic peptide. Eur J Pharmacol. 2009;605:9–14. doi: 10.1016/j.ejphar.2008.12.005. [DOI] [PubMed] [Google Scholar]
- [49].Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, Yang J, Kang J. Nedergaard M. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Shintani-Ishida K, Uemura K. Yoshida K. Hemichannels in cardiomyocytes open transiently during ischemia and contribute to reperfusion injury following brief ischemia. Am J Physiol Heart Circ Physiol. 2007;293:H1714, H1720. doi: 10.1152/ajpheart.00022.2007. [DOI] [PubMed] [Google Scholar]
- [51].Hawat G, Benderdour M, Rousseau G. Baroudi G. Connexin 43 mimetic peptide Gap26 confers protection to intact heart against myocardial ischemia injury. Pflugers Arch. 2010;2010:583–592. doi: 10.1007/s00424-010-0849-6. [DOI] [PubMed] [Google Scholar]
- [52].Schwanke U, Konietzka I, Duschin A, Li X, Schulz R. Heusch G. No ischemic procondititioning in heterozygous connexin43-deficient mice. Am J Physiol Heart Circ Physiol. 2002;283:H1740–H1742. doi: 10.1152/ajpheart.00442.2002. [DOI] [PubMed] [Google Scholar]
- [53].Padilla F, Garcia-Dorado D, Rodriguez-Sinovas A, Ruiz-Meana M, Inserte J. Soler-Soler J. Protection afforded by ischemic preconditioning is not mediated by effects on cell-to-cell electrical coupling during myocardial ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2003;285:H1909–H1916. doi: 10.1152/ajpheart.00438.2003. [DOI] [PubMed] [Google Scholar]
- [54].Shintani-Isida K, Uemura K. Yoshida K. Hemichannels in cardiomyocytes open transiently during ischemia and contribute to reperfusion injury following brief ischemia. Am J Physiol Heart Circ Physiol. 2007;293:H1714–H1720. doi: 10.1152/ajpheart.00022.2007. [DOI] [PubMed] [Google Scholar]
- [55].Bennett MVL, Contreras JE, Bukauskas FF. Saez JC. New roles for astrocytes: Gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Evans WH, de Vuyst E. Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signaling limelight. Biochem J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Miro-Casas E, Ruiz-Meana M, Agullo E, Stahlhofen S, Rodriguez-Sinovas A, Cabestrero A, Jorge I, Torre I, Vazquez J, Boengler K, Schulz R, Heusch G. Garcia-Dorado D. Connexin43 in cardiomyocyte mitochondria contributes to mitochondrial potassium uptake. Cardiovasc Res. 2009;83:747–756. doi: 10.1093/cvr/cvp157. [DOI] [PubMed] [Google Scholar]
- [58].Schulz R, Boengier K, Totzeck A, Luo Y, Garcia-Dorado D. Heusch G. Connexin 43 in ischemic pre- and posconditioning. Heart Fail Rev. 2007;12:261–266. doi: 10.1007/s10741-007-9032-3. [DOI] [PubMed] [Google Scholar]
- [59].Orellana JA, Sáez PJ, Shoji KF, Schalper KA, Plalacios-Prado N, Velarde V, Giaume C, Bennett MV. Sáez JC. Modulation of brain hemichannels and gap junction channels by pro-inflammatory agents and their possible role in neurodegeneration. Antioxid Redox Signal. 2009;11:369–399. doi: 10.1089/ars.2008.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ye ZC, Wyeh MS, Baltan-Tekkok S. Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Stridh MH, Tranberg M, Weber SG, Blomstrand F. Sandberg M. Stimulated efflux of amino acids and glutathione from cultured hippocampal slices by omission of extracellular calcium: likely involvement of connexin hemichannels. J Biol Chem. 2008;283:10347–10356. doi: 10.1074/jbc.M704153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Thompson RJ, Zhou N. macVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- [63].Contreras JE, Sánchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MVL. Sáez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Porc Natl Acad Sci. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez JC. Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Schock SC, Leblanc D, Hakim AM. Thompson CS. ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem Biophys Res Commun. 2008;368:138–144. doi: 10.1016/j.bbrc.2008.01.054. [DOI] [PubMed] [Google Scholar]
- [66].Apps SA, Rankin WA. Kurmis AP. Connexin 26 mutations in autosomal recessive deafness disorders: a review. Int J Audiol. 2007;46:75–81. doi: 10.1080/14992020600582190. [DOI] [PubMed] [Google Scholar]
- [67].Beltramello M, Piazza V, Bukauskas FF, Pozzan T. Mammano F. Impaird permeability to ins (1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nat Cell Biol. 2005;7:63–69. doi: 10.1038/ncb1205. [DOI] [PubMed] [Google Scholar]
- [68].Cohen-Salmon M, Ott T, Michel V, Hardelin J- P, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K. Petit C. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Cur Biol. 2002;12:1106–1111. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Teubner B, Michel V, Pesch J, Lautemann J, Cohen-Salmon M, Söhl G, Jahnke K, Winterhager E, Herberhold C, Hardelin JP, Petit C. Willecke K. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet. 2003;12:13–21. doi: 10.1093/hmg/ddg001. [DOI] [PubMed] [Google Scholar]
- [70].Zhao HB. Connexin26 is responsible for anionic molecule permeability in the cochlea for intercellualr signallling and metabolic communications. Eur J Neurosci. 2005;21:1859–1868. doi: 10.1111/j.1460-9568.2005.04031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chen Y, Deng Y, Bao X, Reuss L. Altenberg GA. Mechanism of the defect in gap-junctional communication by expression of a connexin 26 mutant associated with dominant deafness. FASEB J. 2005;19:1516–1518. doi: 10.1096/fj.04-3491fje. [DOI] [PubMed] [Google Scholar]
- [72].Deng Y, Chen Y, Reuss L. Altenberg GA. Mutations of connexin 26 at position 75 and dominant deafness: essential role of arginine for the generation of functional gap-junctional channels. Hear Res. 2006;220:87–94. doi: 10.1016/j.heares.2006.07.004. [DOI] [PubMed] [Google Scholar]
- [73].Gossman DG. Zhao HB. Hemichannel-mediated inositol 1,4,5-trisphosphate (IP3) release in the cochlea: a novel mechanism of IP3 intercellular signaling. Cell Commun Adhes. 2008;15:305–315. doi: 10.1080/15419060802357217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Majumder P, Crispino G, Rodriguez L, Ciubotaru CD, Anselmi F, Piazza V, Bortoiozzi M. Mammano F. ATP-mediated cell-cell signaling in the organ of Corti: the role of connexin channels. Purinergic Signal. 2010;6:167–187. doi: 10.1007/s11302-010-9192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Cheng B, Zhao S, Luo J, Sprague E, Bonewald LF. Jiang JX. Expression of functional gap junctions and regulation by fluid flow shear stress in osteocyte-like MLO-Y4 cells. J Bone Miner Res. 2001;16:249–259. doi: 10.1359/jbmr.2001.16.2.249. [DOI] [PubMed] [Google Scholar]
- [76].Thi MM, Kojima T, Cowin SC, Weinbaum S. Spray DC. Fluid flow stress remodels expression and function of junctional proteins in cultured bone cells. Am J Physiol (Cell Physiol) 2003;284:C389–C403. doi: 10.1152/ajpcell.00052.2002. [DOI] [PubMed] [Google Scholar]
- [77].Yamada K, Green KG, Samarel AM. Saffitz JE. Distinct pathways regulate expression of cardiac electrical and mechanical junctionproteins in response to stretch. Circ Res. 2005;97:346–353. doi: 10.1161/01.RES.0000178788.76568.8a. [DOI] [PubMed] [Google Scholar]
- [78].Fialová M, Dlugosová K, Okruhicová L, Kristek F, Manoach M. Tribulová N. Adaption o fthe heart to hypertension is associated with maladaptive gap junction connexin-43 remodeling. Physiol Res. 2008;57:7–11. doi: 10.33549/physiolres.931101. [DOI] [PubMed] [Google Scholar]
- [79].Saunders MM, You J, Zhou Z, Li Z, Yellowley CE, Kunze EL, Jacobs CR. Donahue HJ. Fluid flow-induced prostaglandin E2 response of osteoblastic ROS 17/2.8 cells is gap junction-mediated and independent of cytosolic calcium. Bone. 2003;32:350–356. doi: 10.1016/s8756-3282(03)00025-5. [DOI] [PubMed] [Google Scholar]
- [80].Laing JG, Manley-Markowski RN, Koval M, Civitelli R. Steinberg TH. Connexin45 interacts with zonula occludens-1 and connexin43 in osteoblastic cells. J Biol Chem. 2001;276:23051–23055. doi: 10.1074/jbc.M100303200. [DOI] [PubMed] [Google Scholar]
- [81].Lecanda F, Towler DA, Ziambaras K, Cheng S- L, Koval M, Steinberg TH. Civitelli R. Gap junctional communication modulates gene expression in osteoblastic cells. Mole Biol Cell. 1998;9:2249–2258. doi: 10.1091/mbc.9.8.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Koval M, Geist ST, Westphale EM, Kemendy AE, Civitelli R, Beyer EC. Steinberg TH. Transfected connexin45 alters gap junction permeability in cells expressed endogenous connexin43. J Cell Biol. 1995;130:987–1005. doi: 10.1083/jcb.130.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gluhak-Heinrich J, Gu S, Pavlin D. Jiang JX. Mechanical loading stimulates expression of connexin 43 in aveolar bone cells in the tooth movement model. Cell Commun Adhes. 2006;13:115–125. doi: 10.1080/15419060600634619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lohmann CH, Schwartz Z, Liu Y, Li Z, Simon BJ, Sylvia VL, Dean DD, Bonewald LF, Donahue HJ. Boyan BD. Pulsed electromagnetic fileds affect phenotype and connexin 43 protein expression in MLO-Y4 osteocyte-like cells and ROS 17/2.8 osteoblast-like cells. J Orthop, Res. 2003;21:326–334. doi: 10.1016/S0736-0266(02)00137-7. [DOI] [PubMed] [Google Scholar]
- [85].Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH. Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151:931–943. doi: 10.1083/jcb.151.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Grimston SK, Brodt MD, Silva MJ. Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1) J Bone Miner Res. 2008;23:879–886. doi: 10.1359/JBMR.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Romanello M, Pani B, Bicego M. D'Andrea P. Mechanically induced ATP release from human osteoblastic cells. Biochem Biophys Res Commun. 2001;289:1275–1281. doi: 10.1006/bbrc.2001.6124. [DOI] [PubMed] [Google Scholar]
- [88].Romanello M, Veronesi V. D'Andrea P.Mechanosensitivityandintercellular communication in HOBIT osteoblastic cells: A possible role for gap junction hemichannels. Biorheology. 2003;40:119–121. [PubMed] [Google Scholar]
- [89].Genetos DC, Kephart CJ, Zhang Y, Yellowley CE. Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Garcia M. Knight MM. Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. J Orthop Res. 2010;28:510–515. doi: 10.1002/jor.21025. [DOI] [PubMed] [Google Scholar]
- [91].Plotkin LI, Manolagas SC. Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- [92].Brounais B, David E, Chipoy C, Trichet V, Ferré V, Charrier C, Duplomb L, Berreur M, Rédini F, Heymann D. Blanchard F. Long term oncostatin M treatment induces an osteocyte-like differentiation on osteosarcoma and calveria cells. Bone. 2009;44:830–839. doi: 10.1016/j.bone.2008.12.021. [DOI] [PubMed] [Google Scholar]
- [93].Burra S, Nicolella DP, Francis WL, Freitas CJ, Mueschke NJ, Poole C, Jiang JX. Dendritic process of osteocytes is a mechanotransducer that induces the opening of hemichannels. Proc Natl Acad Sci. 2010;107:13648–13653. doi: 10.1073/pnas.1009382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Cherian PP, Cheng B, Gu S, Sprague E, Bonewald LF. Jiang JX. Effects of mechanical strain on the function of gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J Biol Chem. 2003;278:43146–43156. doi: 10.1074/jbc.M302993200. [DOI] [PubMed] [Google Scholar]
- [95].Kitase Y, Barragan L, Qiang H, Kondoh S, Jiang JX, Johnson ML. Bonewald LF. Mechical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the ☐-catenin and PKA pathways. J Bone Miner Res. 2010;25:2381–2392. doi: 10.1002/jbmr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Gomes P, Srinivas SP, Van Driessche W, Vereecke J. Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2005;46:1208–1218. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- [97].Stout CE, Costantin JL, Naus CCG. Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]