Abstract
Incubation of the drug-sensitive H69, a small cell lung cancer cell line, with increased concentrations of adriamycin yielded multidrug resistant (MDR) H69AR cells that over-express multidrug resistance-associated protein (MRP1). MRP1 co-transports its substrate with glutathione (GSH), leading to lower intracellular GSH. In this report we tested whether depleting intracellular GSH in MRP1-expressing cells could hyper-sensitize them to anticancer drugs or not. We have found that the GSH contents in MRP1-expressing cells are significantly lower than their corresponding control cells. The treatment with MRP1 substrate verapamil or the GSH synthetase inhibitor buthionine sulfoxi-mine significantly reduced the intracellular GSH contents in MRP1-expressing cells. Interestingly, depleting intracellular GSH contents can hyper-sensitize the MRP1-cDNA transfected BHK cells to daunomycin, but not the adriamycin-selected H69AR cells. Further analyses indicated that anti-apoptotic factor Bcl2 might be a factor responsible for the fact that depleting intracellular GSH could not hyper-sensitize H69AR cells to daunomycin. We hypothesized that knocking down the expression of Bcl2 could hyper-sensitize H69AR cells to daunomycin. Interestingly, infection of H69AR cells with retroviral particles harboring Bcl2 interfering RNAi not only reduced the expression of Bcl2, but also many factors that contribute to MDR, such as Bcl-xl, MRP1 and ABCC3, etc., leading to the MDR H69AR cells more sensitive to daunomycin than the parental H69 cell. Thus, although the mechanisms of the down-regulation of the genes contributing to MDR remain to be elucidated, retroviral particles harboring Bcl2 interfering RNAi could be used as an alternative way to sensitize the MDR cancer cells to anticancer drugs.
Keywords: Small cell lung cancer (SCLC),; Multidrug resistance (MDR),; MRP1; Glutathione (GSH),; Bcl2,; Small interfering RNAi,; PCR array
Introduction
Lung cancer, with ~ 1.3 million annual deaths worldwide, is the leading cause of cancer-related death. Small cell lung cancer (SCLC), which accounts for ~ 15% of all lung cancer cases, is the most aggressive metastatic form of lung cancer. Since SCLC does not respond well to surgery or radiotherapy [1], chemotherapy remains the primary mode to treat SCLC patients. The standard treatment of SCLC is the administration of chemotherapy, such as cisplatin and etoposide, along with thoracic radiotherapy [2]. Although there is a good initial response to chemotherapeutic treatment, most SCLC patients develop MDR. MDR is responsible for relapse in SCLC patients. Thus, virtually no curative treatment for SCLC exists to date,leading to 5-year survival less than 5% [3].
H69 is a drug sensitive SCLC cell line derived from the pleural fluid of a 55-year-old Caucasian male [4]. Upon treatment with increasing concentration of adriamycin the surviving cells, named as H69AR, become MDR [1]. Thus, this phenotype is an acquired MDR [5]. Over-expression of ATP-binding cassette (ABC) transporters, such as ABCB1 [6], ABCG2 [7, 8], MRP1 (ABCC1) [1, 9] and/or other factors, such as anti-apoptotic factor Bcl2 [10], confers acquired MDR. Although how many factors involved in MDR in H69AR cell is still not clear, over-expression of MRP1 definitely contributes to the MDR phenotype in this cell line [9]. In this report, the drug sensitive H69 cells and the drug resistant H69AR cells were used as model system to reverse the MDR phenotype in SCLC cells.
Materials and methods
Materials
Verapamil, daunomycin, buthionine sulfoximine (BSO), GSH, glutathione reductase, α-nicotinamide adenine dinucleotide phosphate (NADPH), 5,5’-dithiobis-2-nitrobenzoic acid (DTNB), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) and ethylenediaminetetraacetic acid (EDTA) were from Sigma-Aldrich. DMEM/F-12 and RPMI-1640 medium were from GIBCO. Fetal bovine serum (FBS) was from Gemini Bio-Products. ABT-737 and its enantiomer were gifts from Abbott Pharmaceuticals.
Cell lines and cell culture
Human CFTR-cDNA transfected baby hamster kidney (BHK/CFTR) cell [11] and MRP1-cDNA transfected BHK (BHK/MRP1) cell [12] were grown in DMEM/F-12 medium containing 5% FBS and 100 µM methotrexate. H69 and H69AR cells (from American Type Culture Collection) were grown in RPMI-1640 medium containing 10% FBS.
RNAi construction, retroviral particle preparation, viral infection and green fluorescent protein (GFP) positive cell sorting
Small interfering RNAi molecules (shown in S1) were designed by using the siRNA design software (http://www.oligoengine.com), synthesized by Mayo Molecular Biology Core, annealed in vitro, digested with BamHI and HindIII (shown in S1) and used to replace the BamHI-HindIII fragment encoding the human Pyk2/RNAi in pGFP.U6.Pyki [13] to produce plasmid DNA containing pGFP.U6.Con, pGFP.U6.Bcl2/630 and pGFP.U6.Bcl2/846, respectively. Retroviral stocks were prepared by co-transfecting the GP2-293 packaging cell with the plasmid DNA mentioned above and the helper pVSV-G. Viral particles were collected according to the method described [13] and used to infect H69 or H69AR cells. The GFP-positive cells were collected by fluorescence-activated cell sorting (FACS) on a BD FACSAria flow cytometer.
Sample preparation and western blot analysis
Cell lysates were prepared by treatment with 2% SDS. Genomic DNA in the cell lysates was sheared by sonication. Protein concentrations were determined by using the “Coomassie-Plus-Protein-Assay-Reagent” from Pierce. Western blot analysis was performed according to the method described previously [14].
Chemosensitivity assay
IC50 value of daunomycin was determined by employing the MTT assay [15]. Briefly, 2,000 cells (for BHK/CFTR and BHK/MRP1) or 20,000 cells (for H69 and H69AR) were plated in a volume of 100 µl per well in 96-well plates and processed with varying concentrations of daunomycin.
Determination of glutathione content
Determination of total intracellular GSH was performed according to the method described [16]. Briefly, GSH was determined by using a reaction mixture containing 50 µl of cell lysates (or controls to set up standard curve), 50 µl of 2.4 mM DTNB, and 50 µl of 10.64 mU/µl glutathione reductase in the assay buffer (pH7.5) containing 153 mM sodium phosphate and 8.4 mM EDTA. After 5 min incubation at 23 °C, the reaction was started by addition of 50 µl NADPH solution (0.6944 mg/ml) in assay buffer. Absorbance at 412 nm was monitored for 4 minutes by the µQuant Universal Microplate Spectrophotometer (BIO-TEK).
PCR array
Total RNA was isolated from the drug-sensitive H69, the MDR H69AR cells and the RNAi treated H69AR cells, including H69AR/con, H69AR/630 and H69AR/846, by using the RNeasy Mini kit (Qiagen) and treated with RNase-free DNase (Turbo DNA-free kit from Ambion). The integrity of the RNA was determined by the denaturing formaldehyde agarose gel electrophoresis and microanalysis (Agilent Bioanalyzer). The isolated total RNA was used to determine the expression profiles of the genes associated with MDR by employing the “Cancer Drug Resistance & Metabolism PCR Array” kit, according to the procedure recommended by the supplier (Super Array Biosciences).
Statistical analysis
The results in Tables were presented as means ± S.D. The two-tailed P values were calculated based on the unpaired t test from GraphPad Software Quick Calcs. By conventional criteria, if P value is less than 0.05, the difference between the two samples is considered to be statistically significant.
Results
MRP1-expressing cells are more resistant to daunomycin than their corresponding control cells
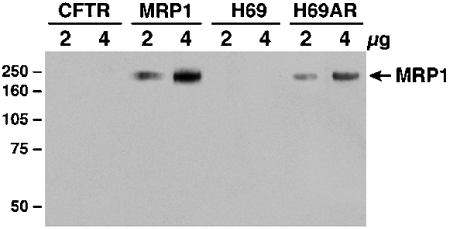
The results in Figure 1 showed that neither the CFTR-cDNA transfected BHK cells nor H69 cells have detectable amounts of MRP1, whereas the MRP1-cDNA transfected BHK cells or the adriamycin-selected H69AR cells expressed high levels of MRP1. The results in Table 1 indicate that the IC50 values of daunomycin for MRP1-expressing BHK/MRP1 or H69AR cells are ~ 7 or 16 fold higher than their corresponding control cells, suggesting that high levels of MRP1 in these cells are responsible for drug resistance.
Figure 1.
High level of MRP1 protein in H69AR cell. Cells were lysed with 2% SDS and 2 or 4 µg of these cell lysates were resolved on a 7% SDS-PAGE, electro -blotted to a nitrocellulose membrane and probed with MRP1-specific antibody 42.4 [14]. Molecular weight markers (kDa) are indicated on the left.
Table 1.
IC50 of daunomycin (DNM), buthionine sulfoximine (BSO) and verapamil (VRP)
| BHK/CFTR | BHK/MRP1 | H69 | H69AR | |
|---|---|---|---|---|
| DNM (nM) | 1.9 ± 0.1* | 13.9 ± 0.8 | 85.3 ± 7.0 | 1362.2 ±262.1 |
| BSO (µM) | 21.5 ± 3.5 | 20.8 ±9.6 | 290.8 ± 30.8 | 11.3 ± 1.4 |
| VRP (µM) | 35.4 ± 6.6 | 3.1 ±0.8 | 55.5 ± 6.6 | 42.4 ±3.6 |
The expeiriments were performed in triplicate and repeated twice.
MRP1-expression has significant effect on the intracellular GSH content
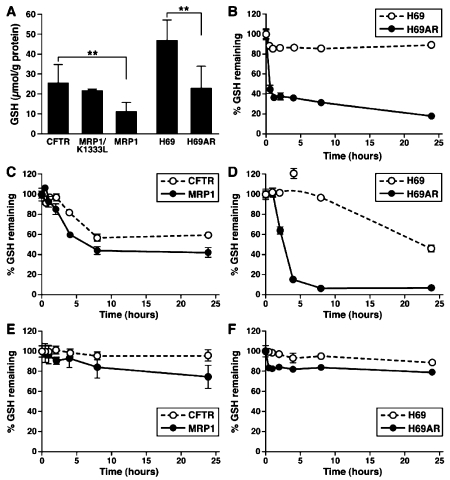
It has been reported that the GSH contents in MRP1-expressing cells are significantly lower than that in their control cells [17-19]. In order to test whether this is the consequence of MRP1-mediated substrate-GSH co-transport, the GSH contents in BHK cells expressing human CFTR, human MRP1 and K1333L-mutated MRP1 were determined. The results in Figure 2A clearly indicate that the GSH content in BHK/K1333L cell is similar to that of BHK/CFTR, but significantly higher than that in BHK/MRP1 cell, suggesting that wt MRP1 constantly pumps the GSH out of the cell whereas the functionally inactive K1333L mutant [14] cannot. The above conclusion is further confirmed by the results showing that the GSH content in MRP1-expressing H69AR cell is significantly lower than that in its parental H69 cell (Figure 2A). It has been shown that MRP1 cannot efficiently transport the reduced GSH out of cell [20], but can efficiently transport the reduced GSH out of cell in the presence of verapamil [21, 22]. Thus, the endogenous GSH must be co -transported out of the cell with MRP1 substrates, such as GSH-dependent flopping of phosphatidylcholine [23].
Figure 2.
GSH contents in MRP1-expressing cells. GSH contents in MRP1-expressing cells. A. GSH contents in MRP1-expressing cells are significantly lower than in their corresponding control cells. Total intracellular GSH was determined in triplicate [n = 55 (CFTR), 4 (K1333L), 65 (MRP1), 50 (H69) and 50 (H69AR)] according to the method described in Materials and Methods. ** indicates that the P value is less than 0.0001. B. Effects of 10 μM verapamil on the intracellular GSH contents. C and D. Effects of 10 μM BSO on the intracellular GSH contents. E and F. Effects of 2 μM daunomycin on the intracellular GSH contents. 106 cells were plated out in each 35 mm dish and incubated overnight. The agents mentioned above were added to the media and incubated at 37 ° C for varying durations.
We have found that exogenous MRP1 substrate verapamil further reduced the GSH content in MRP1-expressing BHK/MRP1 cell, but not in parental BHK cell [24]. We decided to test whether verapamil would also significantly reduce the GSH content in MRP1-expressing H69AR cell. The results shown in Figure 2B clearly indicate that 10 µM verapamil, which is ~ 1/5 of their IC50 values (Table 1), had minimal effect on the GSH content in H69 cell, but significantly further decreased the GSH content in MRP1-expressing H69AR cell.
To test whether the GSH synthetase inhibitor should also have differential effects on the MRP1-expressing cells and their corresponding control cells, the γ-glutamylcysteine synthetase inhibitor BSO was used to treat the two pairs of cells, i.e., BHK/CFTR versus BHK/MRP1 and H69 versus H69AR. The IC50 value of BSO for MRP1-expressing BHK/MRP1 cell is slightly lower than that of BHK/CFTR cell (Table 1) whereas the effects of BSO on the intracellular GSH content in BHK/MRP1 cell is higher than that in BHK/CFTR cell (Figure 2C). For un-knownreason, the IC50 value of BSO for H69 cell is ~ 26 fold higher than for H69AR (Table 1). Interestingly, the effect of BSO on the intracellular GSH content in H69AR cells is significantly higher than in their parental H69 cells (Figure 2D), suggesting that the synergetic effects of BSO on GSH synthetase and the MRP1-mediated GSH transport are significantly higher than that the effect of BSO on GSH synthetase. This result also explains why the IC50 value of BSO for H69 cell is significantly higher than for H69AR cell.
Since MRP1-expressing cells, such as BHK/MRP1 or H69AR, are more resistant to daunomycin than their corresponding control cells (Table 1), daunomycin must be the substrate of MRP1. Thus, treatment with daunomycin might also significantly reduce the GSH content in MRP1-expressing cells. To test this hypothesis, GSH contents were determined in the presence or absence of daunomycin. As shown in Figure 2E and 2F, the GSH contents, upon treatment with daunomycin, in control cells, such as BHK/CFTR or H69, were not significantly reduced, whereas ~ 25% or ~ 20% of GSH was depleted in MRP1-expressing BHK/MRP1 or H69AR cells, indicating that verapamil is a more potent MRP1 substrate than daunomycin in depleting the intracellular GSH.
Treatments with the combination of verapamil and BSO hyper-sensitized the MRP1-expressing BHK/MRP1 cells to daunomycin
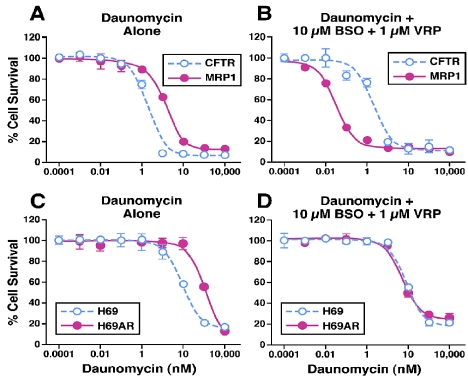
Since either verapamil or BSO can efficiently decrease the intracellular GSH contents in MRP1-expressing cells, these treatments might significantly sensitize the MRP1-expressing cells to anticancer drugs. Indeed, in the absence of verapamil and BSO, the MRP1-expressing BHK/MRP1 cells are more resistant to daunomycin than the control BHK/CFTR cells (Figure 3A and Table 2). Upon treatment with 0.1 µM verapamil, the IC50 value of daunomycin for either BHK/CFTR or BHK/MRP1 was not significantly affected (Table 2). However, upon treatment with 10 µM BSO, 10 µM BSO + 0.1 µM verapamil, or 1 µM verapamil, the IC50 values of daunomycin for BHK/CFTR cells were not significantly affected (Table 2), whereas these values decreased from 14 nM to 4.4, 3.7 or 4.8 nM (Table 2). Of note, the IC50 value for BHK/CFTR cell, upon treatment with 10 µM BSO + 1µM verapamil, was not significantly affected, whereas this value for BHK/MRP1 cells was decreased from 13.92 to 0.03 nM (Table 2 and Figure 3B), suggesting that depleting intracellular GSH content by BSO and verapamil significantly inhibits the MRP1-mediated daunomycin transport. These results also indicate that depleting intracellular GSH content can hypersensitize the MRP1-expressing BHK/MRP1 cells to anticancer drug daunomycin.
Figure 3.
Effects of verapamil and BSO on the sensitivity of MRP1-expressing cells and their corresponding control cells to daunomycin. Effects of verapamil and BSO on the sensitivity of MRP1-expressing cells and their corresponding control cells to daunomycin. A and C. Cells were incubated with varying concentrations of daunomycin. B and D. Cells were incubated with varying concentrations of daunomycin in the presence of 10 µM buthionine sulfoximine (BSO) and 1 µM verapamil (VRP). Cell survival was determined by MTT assay according to the method described in Materials and Methods.
Table 2.
IC50 of Daunomycin (DNM) in the presence of buthionine sulfoximine (BSO) and/or verapamil (VRP)
| Drug combination | BHK/CFTR (nM)b | BHK/MRP1 (nM) | RFa | H69 (nM) | H69AR (nM) | RFa |
|---|---|---|---|---|---|---|
| DNM | 1.92 ± 0.08 | 13.92 ±0.76 | 7.25 | 85.32 ± 6.95 | 1362.23 ± 262.13 | 15.97 |
| DNM + 10 µM BSO | 2.12 ±0.29 | 4.42 ±0.15 | 2.08 | 92.26 ± 1.52 | 133.82 ± 3.53 | 1.45 |
| DNM + 0.1 µM VRP | 2.37 ±0.57 | 16.90 ± 4.69 | 7.19 | 94.83 ± 4.78 | 1216.15 ± 63.85 | 12.82 |
| DNM + 1µM VRP | 2.50 ± 0.07 | 4.81 ±0.10 | 1.92 | 82.85 ±12.22 | 477.35 ± 11.21 | 5.76 |
| DNM + 10 µM BSO + 0.1 µM VRP | 2.12 ±0.32 | 3.69 ±0.31 | 1.74 | 92.84 ± 2.43 | 143.51 ± 15.86 | 1.55 |
| DNM + 10 µM BSO + 1 µM VRP | 2.05 ± 0.08 | 0.03 ± 0.00 | 0.01 | 87.82 ± 2.52 | 147.11 ± 28.53 | 1.68 |
| DNM + 10 µM VRP | 81.83 ± 4.83 | 182.70 ± 4.70 | 2.23 | |||
| DNM + 10 µM VRP | 76.73 ± 5.11 | 34.36 ± 5.61 | 0.45 |
RF = Resistance Factor
The experiments were performed in triplicate and repeated twice.
Treatments with verapamil + BSO can reverse the drug resistance of the MRP1-expressing H69AR cell, but cannot hyper-sensitize them to anti-cancer drug
The above results implied that MRP1-expressing cancer cells could, upon treatment with 10 µM BSO + 1µM verapamil, be selectively killed by anticancer drug daunomycin. To test this possibility, the MRP1-expressing H69AR cells and their control H69 cells were treated with either BSO, verapamil or the combination of BSO and verapamil (in the presence of varying concentration of daunomycin). As expected, the MRP1-expressing H69AR cells are more resistant to daunomycin than their control H69 cells (Figure 3C and Table 2). Similar to BHK/CFTR control cells, the treatments with BSO, verapamil or BSO + verapamil did not have a significant effect on the IC50 value of daunomycin for H69 control cells (Table 2 and Figure 3D). In contrast, the treatments with either 10 µM BSO or 1 µM verapamil decreased the IC50 value of daunomycin for H69AR cells from 1362 nM to 134 nM or 477 nM (Table 2). Of note, upon treatment with 10 µM BSO + 1 µM verapamil, the IC50 value of daunomycin for H69AR cells was similar to that the treatment with 10 µM BSO alone (Table 2 and Figure 3D), indicating that depleting intracellular GSH content by BSO + verapamil can reverse the MDR in H69AR cells, but cannot hyper-sensitize them to daunomycin. We then tested whether higher concentration of verapamil would hyper-sensitize them to daunomycin or not. Interestingly, upon treatment with 10 µM verapamil or 10 µM verapamil + 10 µM BSO, the IC50 values of daunomycin for MRP1-expressing H69AR cell decreased from 1362 nM to 183 nM or 34 nM (Table 2), indicating that depleting intracellular GSH content by BSO + verapamil cannot hyper-sensitize them to daunomycin. The different response (to these treatments) between the MRP1-expressing BHK/MRP1 cells and H69AR cells implies that some other factors might also contribute to MDR phenotype in H69AR cells.
Anti-apoptotic factor Bcl2 may be another factor that contributes to MDR phenotype in MRP1-expressing H69AR cells
In order to determine which factor(s) may also contribute to MDR phenotype in MRP1-expressing H69AR cells, total RNA was isolated from the parental H69 and the MDR H69AR cells and used to determine the gene expression profiles by employing the “Cancer Drug Resistance and Metabolism PCR Array” kit. As shown in Table 3, ABCC1 (MRP1), ABCC2, ABCC3, ABCC6, GSTP1 and many other genes were highly over-expressed in H69AR cell in comparing to H69 cell, whereas the expression of TOP1, TOP2A, TOP2B, SULT1E1, APC, BRCA1, BRCA2, XPA, ERBB4, IGF1R, IGF2R, NFKB1 or TNFRSF11A in H69AR cell was significantly lower than in H69 cell. Since anti-apoptotic factors, such as Bcl2 or Bcl-xL, play an important role in MDR, we paid particular attention to these factors. However, Bcl-xL was not included in this kit and the expression of Bcl2 was not accurately determined. In order to determine the expression levels of these factors, whole cell lysates were used to do western blot analysis. The results in Figure 4A indicated that the expression level of Bcl-xL in H69AR is slightly lower than in H69 cell, whereas the expression level of Bcl2 in H69AR is ~ 3 fold higher than in H69 (Figure 4B).
Table 3.
Comparison of gene expression in H69 and H69AR cells
| Genes | H69 | H69AR | Genes | H69 | H69AR | Genes | H69 | H69AR | Genes | H69 | H69AR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ABCC1 | 1.00 | 15.95 | CYP1A2 | 1.00 | 5.62 | APC | 1.00 | 0.17 | IGF2R | 1.00 | 0.25 |
| ABCC2 | 1.00 | 5.62 | CYP2B6 | 1.00 | 5.62 | BRCA1 | 1.00 | 0.25 | AR | 1.00 | 5.62 |
| ABCC3 | 1.00 | 5.43 | CYP2C8 | 1.00 | 5.62 | BRCA2 | 1.00 | 0.09 | ESR1 | 1.00 | 5.62 |
| ABCC5 | 1.00 | 0.18 | CYP2C9 | 1.200 | 5.62 | XPA | 1.00 | 0.06 | PPARD | 1.00 | 5.59 |
| ABCC6 | 1.00 | 33.89 | CYP2C19 | 1.00 | 5.62 | CCND1 | 1.00 | 7.94 | PPARG | 1.00 | 5.62 |
| ABCG2 | 1.00 | 5.62 | CYP2E1 | 1.00 | 5.62 | CDKN2A | 1.00 | 5.44 | RARG | 1.00 | 3.94 |
| RBI | 1.00 | 5.62 | CYP3A4 | 1.00 | 5.62 | EGFR | 1.00 | 8.34 | AHR | 1.00 | 3.98 |
| TOPI | 1.00 | 0.12 | GSTP1 | 1.00 | 15.97 | ERBB2 | 1.00 | 5.62 | NFKB1 | 1.00 | 0.25 |
| TOP2A | 1.00 | 0.12 | NAT2 | 1.00 | 5.62 | ERBB4 | 1.00 | 0.09 | NFKB1B | 1.00 | 5.29 |
| TOP2B | 1.00 | 0.17 | SULT1E1 | 1.00 | 0.17 | IGF1R | 1.00 | 0.25 | TNFRSF11A | 1.00 | 0.12 |
Figure 4.
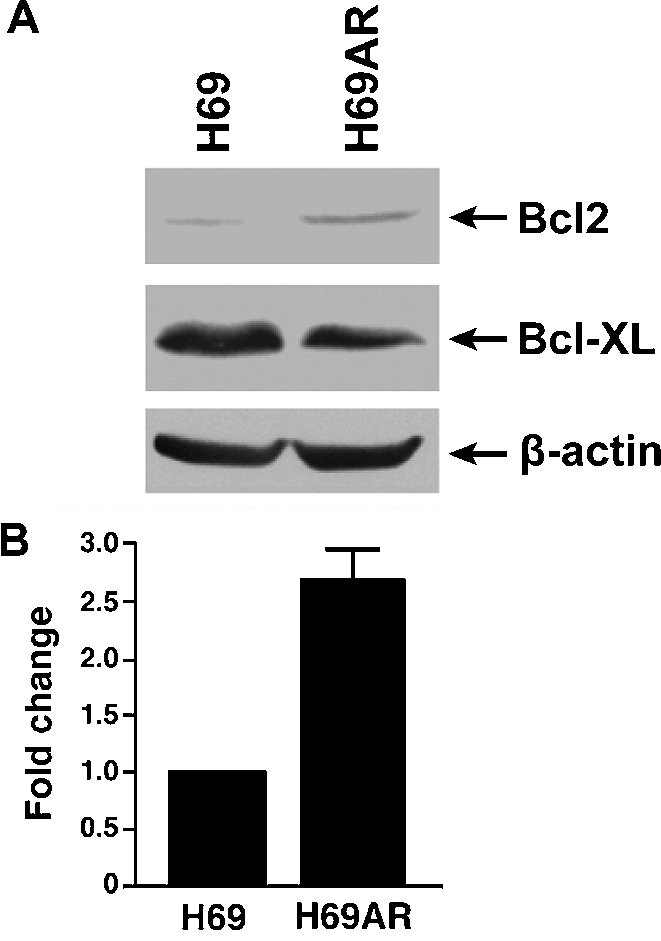
Bcl2 expression is significantly up-regulated in H69AR cell. Bcl2 expression is significantly up-regulated in H69AR cell. 100 ug of whole cell lysates were resolved on a 10% polyacrylamide gel, electro-blotted to a nitrocellulose membrane and probed with either mouse anti-human Bcl2, Bcl-xL or β-actin (used as a loading control) monoclonal antibody (A). The intensities of the Bcl2 bands were measured by a densitometer and the ratio of the corresponding band intensities were calculated based on the intensity of the β-actin band (B).
Bcl2 inhibitor ABT-737 might be the substrate of MRP1
Thus, anti-apoptotic factor Bcl2 was considered as another major factor affecting drug resistance in H69AR cell. If that would be the case, inhibition of Bcl2 function might, upon treatment with verapamil and BSO, hyper-sensitize the MRP1-expressing H69AR cells to daunomycin. To test this possibility, Bcl2 family protein inhibitor ABT-737 [25] and its enantiomer were used to treat the H69 control cells and the MRP1-expressing H69AR cells. The results in Table 4 indicate that ABT-737 enantiomer did not have a significant effect on the IC50 values of daunomycin for either H69 or H69AR, whereas 0.1 µM Bcl2 inhibitor ABT-737 decreased the IC50 values of daunomycin for H69 from 86 nM to 21 nM. Furthermore, 1 µM or 10 µM Bcl2 inhibitor ABT-737 completely killed the H69 control cells (Table 4). In contrast, 10 µM ABT-737 did not have any additional effect on the IC50 value of daunomycin for MRP1-expressing H69AR cell (Table 4), implying that ABT-737 might be the substrate of MRP1. Actually, our preliminary results indicated that ABT-737 did inhibit the MRP1-mediated leukotriene C4 transport (data not shown).
Table 4.
IC50 of Daunomycin (DNM) in the presence of Bcl-2 inhibitor ABT-737
| H69 (nM) | H69AR (nM) | |
|---|---|---|
| DNM alone | 86.64 | 1331.40 |
| DNM + 10 μM BSO + 1 μM VRP | 85.68 | 127.95 |
| DNM + 10 μM BSO + 1 μM VRP + 0.1μM ABT-737 | 21.33 | 131.27 |
| DNM + 10 μM BSO + 1 μM VRP + 1μM ABT-737 | 0.01 | 118.87 |
| DNM + 10 μM BSO + 1 μM VRP + 10μM ABT-737 | 0.00 | 134.90 |
| DNM + 10 μM BSO + 1 μM VRP + 0.1μM ABT-737 enantiomer | 81.72 | 101.95 |
| DNM + 10 μM BSO + 1 μM VRP + 1μM ABT-737 enantiomer | 85.51 | 111.48 |
| DNM + 10 μM BSO + 1 μM VRP + 10μM ABT-737 enantiomer | 53.90 | 77.15 |
Infection of H69AR with retroviral particles harboring RNAi molecule completely reversed the drug resistance
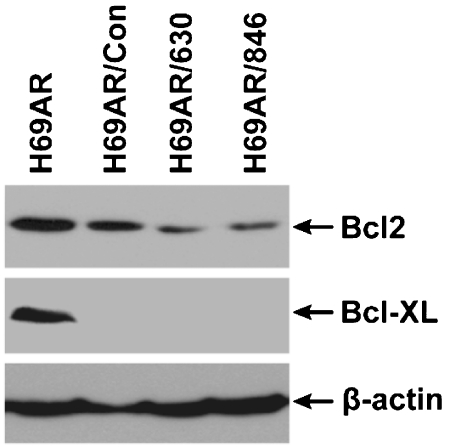
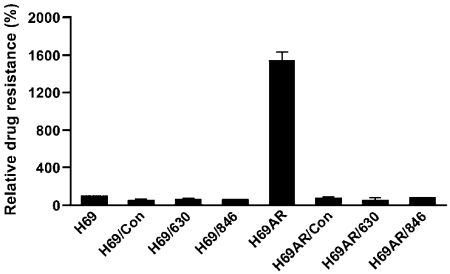
If the above conclusion is correct, we cannot inhibit Bcl2 by ABT-737 and then take the advantage of MRP1-mediated substrate-GSH co-transport to hyper-sensitize the MRP1-expressing H69AR cells to anticancer drugs. Thus, we decided to test whether knocking down the expression of Bcl2 would, upon treatment with verapamil and BSO, hyper-sensitize the MRP1-expressing H69AR cells to anticancer drugs. Infection of H69AR cells with retroviral particles harboring Bcl2-specific RNAi, including Bcl2/630 and Bcl2/846, partially knocked down the expression of Bcl2 [Figure 5, 49 ± 28% (n = 6) and 49 ± 27% (n = 6)]. For unknown reasons, infection of H69AR cells with retroviral particles harboring control RNAi also slightly knocked down the expression of Bcl2 [Figure 5, 89 ± 37% (n = 6)]. Unexpectedly, infections of H69AR cells with retroviral particles harboring either control RNAi or Bcl2-specrific RNAi completely knocked down the expression of Bcl-xl (Figure 5). We then tested whether infection of H69AR cells with retroviral particles harboring RNAi molecules would affect the drug sensitivity or not. As shown in Figure 6, the IC50 values of daunomycin for viral infected H69AR cells, including H69AR/Con, H69AR/630 and H69AR/846, were significantly less than the parental H69AR cell, implying that the genes associated with MDR, such as ABCC1 (MRP1) and ABCC6 shown in Table 3, in H69AR cells might be knocked down by retroviral infection.
Figure 5.
Retroviral partiele harboring Bcl2 small interfering RNAi molecule not only affects the expression of Bcl2 but also Bcl-xL. 80 μg of whole cell lysates were resolved on a 10% polyacrylamide gel, electro-blotted to a nitrocellulose membrane and probed with mouse anti-human Bcl2 mAb, Bcl-xL mAb or β-actin mAb.
Figure 6.
Retroviral particle infection reversed the daunomycin resistance of H69AR cell. IC50 value of daunomycin was determined by MTT assay and the IC50 value for H69 cell was considered as 100%. The samples are: H69 or H69AR (un-infected H69 or H69AR); H69/Con or H69AR/Con (H69 or H69AR cells infected with retroviral particles harboring control interfering RNAi); H69/630 or H69AR/630 (H69 or H69AR cells infected with retroviral particles harboring Bcl2-specific RNAi 630); H69/846 or H69AR/846 (H69 or H69AR cells infected with retroviral particles harboring Bcl2-specific RNAi 846).
Retroviral particle infection significantly knocked down the expression of MRP1
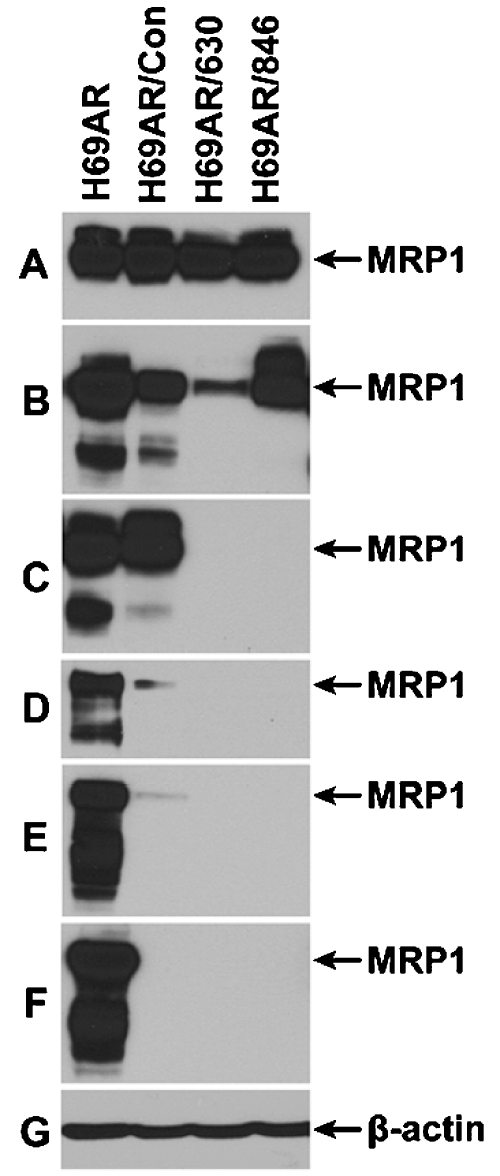
If above conclusion is correct, the amount of MRP1 protein in retroviral particle infected H69AR cells should be significantly less than in their parental H69AR cells. In order to test this possibility, whole cell lysates from H69AR, H69AR/Con, H69AR/630 and H69AR/846 were used to do western blot analyses. As shown in Figure 7F, infection of H69AR cells with viral particles harboring Bcl2/630 or Bcl2/846, after 29th passage in vitro, completely knocked down the expression of MRP1, whereas the cells infected with viral particles harboring control RNAi remained trace amount of MRP1. We wondered whether the freshly harvested samples should also contain much less MRP1 protein than the parental H69AR cells. Interestingly, when the FACS-sorted H69AR/Control, H69AR/630 or H69AR/846 cells were lysed after 2nd passages in vitro, the samples contained similar amount of MRP1 as the parental H69AR cell (Figure 7A). However, the amount of MRP1 in retroviral particle infected cells is gradually decreased along with incubation time in vitro (Figure 7B to 7F).
Figure 7.
Retroviral partiele infection knocked down the expression of ABC transporter MRP1 in a time-dependent manner. 80 µg of whole cell lysates were resolved on a 7% polyacrylamide gel and performed western blot assay as described in Figure 1. A. The FACS-sorted H69AR/Control, H69AR/630 or H69AR/846 cells were lysed after 2nd passages in vitro. B. The FACS-sorted cells were lysed after 4th passages in vitro. C. The FACS-sorted were lysed after 6th passages in vitro. D. The FACS-sorted cells were lysed after 17th passages in vitro. E. The FACS-sorted cells were lysed after 24th passages in vitro. F. The FACS-sorted cells were lysed after 29th passages in vitro.
Retroviral particle infection also affects other gene expression
We wondered how the control RNAi, Bcl2/630 or Bcl2/846 RNAi molecules affect the expression of Bcl-xL or MRP1. Blast search found no identical sequence between the control RNAi and the human Bcl-xL mRNA (S3) or the human MRP1 mRNA (S4). Yet, infection of H69AR cell with viral particles harboring the control RNAi molecule completely knocked down the expression of Bcl-xL (Figure 5) or MRP1 (Figure 7F). Thus, knocking down the expression of Bcl-xL or MRP1 by control RNAi must be directly related to retroviral particle infection, but not to the RNAi sequence. If this will be the case, retroviral particle infection may also affect other gene expression. To test this possibility, total RNA was isolated from H69AR, H69AR/con, H69AR/630 and H69AR/846 cells and used to determine the expression profiles of the genes associated with MDR and drug metabolism. As shown in Table 5, the mRNA levels of BRCA2, CYP2E1, CYP3A5, DHFR, EGFR, ESR2, FOS, GSK3A, IGF1R, NFKB1, RB1, TNFRSF11A, TP53 and XPA in H69AR/con, H69AR/630 and H69AR/846 cells are significantly higher than in their parental H69AR cells, whereas the mRNA levels of ABCC1 (MRP1), ABCC3, ABCC6, CCND1, CDK4, CYP1A2, CYP2D6, ELK, ERBB3, NIF1A, IGF2R, MET, MVP, NFKB1E, PPARD, RARB, RXRA, TOP1 and TPMT in H69AR/con, H69AR/630 and H69AR/846 cells are significantly lower than in their parental H69AR cells. Thus, retroviral particle infection has significant effect on the expression of genes associated with MDR and drug metabolism, suggesting that retroviral particle infection could be used as an alternative approach to overcome the MDR in SCLC or other cancer cells.
Table 5.
Comparison of gene expression for H69AR and RNAi treated H69AR
| Genes | H69AR | H69AR/Con | H69AR/630 | H69AR/846 | Genes | H69AR | H69AR/Con | H69AR/630 | H69AR/846 |
|---|---|---|---|---|---|---|---|---|---|
| ABCC1 | 1.00 | 0.01 | 0.01 | 0.01 | ERCC3 | 1.00 | 0.99 | 0.69 | 0.93 |
| ABCC2 | 1.00 | 1.86 | 1.32 | 1.34 | ESR1 | 1.00 | 1.39 | 0.88 | 0.41 |
| ABCC3 | 1.00 | 0.01 | 0.01 | 0.01 | ESR2 | 1.00 | 7.56 | 2.56 | 3.46 |
| ABCC5 | 1.00 | 0.74 | 0.55 | 0.71 | FGF2 | 1.00 | 1.64 | 0.56 | 0.78 |
| ABCC6 | 1.00 | 0.07 | 0.03 | 0.03 | FOS | 1.00 | 36.75 | 7.19 | 21.73 |
| AHR | 1.00 | 0.95 | 1.19 | 0.72 | GSK3A | 1.00 | 6.76 | 4.67 | 5.97 |
| AP1S1 | 1.00 | 0.68 | 0.33 | 0.44 | GSTP1 | 1.00 | 0.81 | 0.81 | 0.77 |
| APC | 1.00 | 0.82 | 0.93 | 0.87 | NIF1A | 1.00 | 0.33 | 0.24 | 0.31 |
| ARNT | 1.00 | 0.96 | 0.79 | 0.67 | IGF1R | 1.00 | 2.29 | 2.11 | 1.54 |
| ATM | 1.00 | 1.10 | 0.84 | 1.24 | IGF2R | 1.00 | 0.37 | 0.24 | 0.43 |
| BAX | 1.00 | 1.34 | 0.88 | 1.14 | MET | 1.00 | 0.44 | 0.23 | 0.41 |
| BCL2 | 1.00 | 1.03 | 0.42 | 0.59 | MSH2 | 1.00 | 0.97 | 1.31 | 1.14 |
| BCL2L1 | 1.00 | 0.23 | 0.10 | 0.18 | MVP | 1.00 | 0.47 | 0.28 | 0.31 |
| BLMH | 1.00 | 0.90 | 1.03 | 0.82 | NFKB1 | 1.00 | 2.78 | 2.30 | 2.01 |
| BRCA1 | 1.00 | 1.60 | 0.83 | 1.36 | NFKB2 | 1.00 | 1.23 | 0.60 | 0.85 |
| BRCA2 | 1.00 | 9.64 | 3.94 | 8.68 | NFKB1B | 1.00 | 1.62 | 0.59 | 0.96 |
| CCND1 | 1.00 | 0.23 | 0.26 | 0.12 | NFKB1E | 1.00 | 0.49 | 0.52 | 0.49 |
| CCNE1 | 1.00 | 1.44 | 1.25 | 1.41 | PPARA | 1.00 | 2.08 | 0.96 | 1.52 |
| CDK2 | 1.00 | 0.85 | 0.67 | 0.76 | PPARD | 1.00 | 0.22 | 0.23 | 0.32 |
| CDK4 | 1.00 | 0.35 | 0.31 | 0.34 | RARA | 1.00 | 0.95 | 0.63 | 0.85 |
| CDKN1A | 1.00 | 1.15 | 0.40 | 0.67 | RARB | 1.00 | 0.38 | 0.31 | 0.33 |
| CDKN1B | 1.00 | 0.86 | 0.46 | 0.73 | RARG | 1.00 | 0.93 | 0.39 | 0.43 |
| CDKN2A | 1.00 | 0.70 | 0.68 | 0.65 | RBI | 1.00 | 2.15 | 1.60 | 2.19 |
| CDKN2D | 1.00 | 1.25 | 0.93 | 0.92 | RELB | 1.00 | 2.85 | 0.28 | 0.51 |
| CLPTMll | 1.00 | 0.78 | 0.38 | 0.59 | RXRA | 1.00 | 0.54 | 0.60 | 0.54 |
| CYP1A1 | 1.00 | 4.06 | 3.68 | 4.18 | RXRB | 1.00 | 0.78 | 0.79 | 0.70 |
| CYP1A2 | 1.00 | 0.15 | 0.12 | 0.07 | SOD1 | 1.00 | 0.80 | 1.31 | 0.90 |
| CYP2D6 | 1.00 | 0.57 | 0.44 | 0.63 | SULT1E1 | 1.00 | 1.13 | 0.76 | 0.55 |
| CYP2E1 | 1.00 | 6.21 | 3.68 | 2.54 | TNFRSF11A | 1.00 | 19.67 | 9.83 | 17.25 |
| CYP3A5 | 1.00 | 3.34 | 1.85 | 2.30 | TOPI | 1.00 | 0.34 | 0.46 | 0.35 |
| DHFR | 1.00 | 4.54 | 4.60 | 4.85 | TOP2A | 1.00 | 1.03 | 0.61 | 0.84 |
| EGFR | 1.00 | 30.58 | 7.52 | 15.76 | TOP2B | 1.00 | 1.00 | 0.71 | 0.84 |
| ELK | 1.00 | 0.62 | 0.61 | 0.70 | TP53 | 1.00 | 18.33 | 7.32 | 14.12 |
| EPHX1 | 1.00 | 0.71 | 0.78 | 0.89 | TPMT | 1.00 | 0.47 | 0.44 | 0.44 |
| ERBB3 | 1.00 | 0.45 | 0.11 | 0.34 | XPA | 1.00 | 2.87 | 2.74 | 2.78 |
| ERBB4 | 1.00 | 1.03 | 0.37 | 0.82 | XPC | 1.00 | 2.11 | 1.00 | 1.56 |
Discussion
Chemotherapy remains the primary mode of SCLC treatment. However, occurrence of MDR limits the success of chemotherapeutic treatment of cancers. Over-expression of MRP1 in cancer cells confers them resistant to multiple anticancer drugs, presumably due to MRP1-mediated co-transport of anticancer drugs with GSH. GSH is one of the major reducing agents inside the cancer cells. If the rate of endogenous de novo GSH synthesis could not counterbalance the rate of export catalyzed by MRP1-mediated transport, the GSH concentration inside the MRP1-expressing cell should be decreased. Indeed, exposure of the MRP1-expressing cells to either arsenate [26] or verapamil [24] greatly decreased the intracellular GSH. Thus, it may be possible to selectively kill the MRP1-expressing cancer cells by altering the redox state inside the cancer cells. The results in Figure 2 clearly indicate that cells expressing high levels of MRP1 have significantly less GSH than in their corresponding control cells and treatments with verapamil and/or BSO further decrease the intracellular GSH and result in hyper-sensitizing the MRP1-cDNA transfected BHK cells to daunomycin (Figure 3B and Table 2), implying that the MRP1-over-expressing cancer cells could be selectively killed by depleting the intracellular GSH. Unfortunately, however, the same treatment did decrease the IC50 value of daunomycin for MRP1-expressing H69AR cells, but did not hyper-sensitize these drug-selected H69AR cancer cells to daunomycin (Table 2), presumably due to the long term adriamycin selection [1] enhanced the expression of MRP1 and other factors associated with MDR.
Indeed, many factors associated with MDR, such as anti-apoptotic factor Bcl2 (Figure 4), MRP1, ABCC6, ABCG2, GSTP1 and other factors listed in Table 3, were significantly up-regulated in H69AR cells. Thus, even though the treatments with MRP1 substrate verapamil and/or GSH synthetase inhibitor BSO can deplete the intracellular GSH contents and overcome the drug resistance caused by over-expression of MRP1, these treatments cannot hyper-sensitize the MRP1-over-expressing cancer cells to daunomycin. In other words, in order to hyper-sensitize the MRP1-over-expressing cancer cells to anticancer drugs, one has to knock-down the expression of other factors, such as Bcl2, associated with MDR.
Since Bcl2 is a major factor that contributes to MDR, we hypothesized that knocking down the expression of Bcl2 might hyper-sensitize the MRP1-expressing H69AR cells to daunomycin. Interestingly, infection of H69AR cells with retro-viral particles harboring Bcl2/RNAi not only reduced the expression of Bcl2 (Figure 5), but also knocked down the expression of Bcl-xL (Figure 5) and MRP1 (Figure 7F). Although we cannot take the advantage of depleting the intracellular GSH content to hyper-sensitize them to anti-cancer drugs, retroviral infection did knock down many genes associated with MDR (Table 5) and sensitize them to anti-cancer drugs (Figure 6), suggesting that retroviral infection could be used to treat SCLC and other MDR cancer cells. However, whether this is a common feature in other MDR cancer cells is not clear yet. Further experiments to test this possibility in other MDR cancer cells are required. In addition, understanding the molecular mechanism of the down-regulation of the genes associated with MDR may also facilitate the development of a novel therapeutic approach.
What is the molecular mechanism of the down-regulation of the genes associated with MDR? Blast search found that 630/RNAi or 846/RNAi not only has the identical sequence at the position of 630 or 846 in Bcl2 mRNA, but also several short stretches of identical sequence within the coding region and the non-coding region of Bcl2 mRNA (S2). We speculated that the short stretches of the identical sequences within the coding region might play a role in destabilizing the Bcl2 mRNA. This hypothesis is supported by the fact that: 1) 630/RNAi or 846/RNAi has several short stretches of identical sequences within the coding region of Bcl-xl mRNA (S3); 2) 630/RNAi or 846/RNAi has several short stretches of identaical sequences within the coding region of MRP1 mRNA (S4). However, whether the short stretches of identical sequences within the non-coding region will also play a role in destabilizing the mRNA is not known yet. Blast search found that control RNAi has one short stretch of identical sequence within the non-coding region of Bcl2 mRNA (S2). Infection of H69AR cells with retroviral particles harboring control RNAi moderately reduced the expression of Bcl2 (Figure 5), implying that the short stretches of identical sequences within the non-coding region may also play a role in destabilizing the mRNA. This conclusion may be supported by the fact that 630/RNAi and 846/RNAi have several short stretches of identical sequences within the non-coding region of Bcl-xL mRNA (S3) or MRP1 mRNA (S4). However, although blast search found no identical sequence between the control RNAi and the human Bcl-xL mRNA (S3) or the human MRP1 mRNA (S4), infection of H69AR cells with viral particles harboring the control RNAi completely knocked down the expression of Bcl-xL (Figure 5) and MRP1 (Figure 7F). Thus, knocking down the expression of Bcl-xL or MRP1 by control RNAi must be directly related to retroviral particle infection, but not to the RNAi sequence. In fact, as shown in Table 5, the mRNA levels of BRCA2, CYP2E1, CYP3A5, DHFR, EGFR, ESR2, FOS, GSK3A, IGF1R, NFKB1, RB1, TNFRSF11A, TP53 and XPA in retroviral particle infected cells, including H69AR/con, H69AR/630 and H69AR/846, are significantly higher than in their parental H69AR cells, whereas the mRNA levels of ABCC1 (MRP1), ABCC3, ABCC6, CCND1, CDK4, CYP1A2, CYP2D6, ELK, ERBB3, NIF1A, IGF2R, MET, MVP, NFKB1E, PPARD, RARB, RXRA, TOP1 and TPMT in retroviral particle infected cells, including H69AR/con, H69AR/630 and H69AR/846, are significantly lower than in their parental H69AR cells. Thus, retroviral particle infection not only affects the expression of Bcl-xL and MRP1, but also many other genes associated with MDR and drug metabolism. What is the molecular mechanism of the up- or down-regulation of the genes associated with MDR? One possible mechanism could be that the proviral DNA inserted into chromosome might strongly bind many transcription factors or repressors and compete these factors with the promoter regions of the genes associated with MDR, resulting in up- or down-regulation of these genes. Another possible mechanism could be that retroviral particle infection might generate stress that forces the infected cells responding to this pressure by regulating their gene expression profiles. Regardless of which mechanism is used, understanding the molecular mechanism of the gene regulations by retroviral infection may facilitate the development of a novel therapeutic approach to treat MDR cancers.
Acknowledgements
This manuscript was supported in part by a grant from the National Institutes for Health R01 CA89087. We'd like to thank Henry J. Travis (from Dr. Rafael Fonseca's lab) for his help in determination of the integrity of the RNA used in PCR Array and Daniel D. Johnson (from Dr. Aleksander Sekulic's lab) for his help in PCR Array. We thank Irene Beauvais for preparation of the manuscript and Marv Ruona for preparation of the graphics.
Abbreviations
- ABC
ATP-binding cassette
- ABCC1 or MRP1
multidrug resistance-associated protein
- ABCG2
breast cancer resistance protein
- BHK
baby hamster kidney
- BSO
buthionine sulfoximine
- CFTR
cystic fibrosis transmembrane-conductance regulator
- DTNB
5,5’-dithiobis-2-nitrobenzoic acid
- EDTA
ethylenediaminetetraacetic acid
- GFP
green fluorescent protein
- GSH
glutathione
- MDR
multidrug resistance
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide
- NADPH
α-nicotinamide adenine dinucleotide phosphate
- SCLC
small cell lung cancer
- SDS
sodium dodecyl sulfate.
S1. Sequences of small interfering RNAi
1. Oligonucleotide sequences of control RNAi (underlined)
GGA AAG GAC GCG GGA TCC ATA CTC GCT CGC GCT CTT TCA AGA GAA GAG CGC GAG CGA GTA CCT TTC CTG CGC CCT AGG TAT GAG CGA GCG CGA GAA AGT TCT CTT CTC GCG CTC GCT CAT BamH I
TGT TTT TTG GAA AAA GCT TGG 81 bases ACA AAA AAC CTT TTT CGA ACC Hind III
2. Oligonucleotide sequences of Bcl2/630 (underlined)
GGA AAG GAC GCG GGA TCC GGG CAT CTT CTC CTC CCA TTC AAG AGA TGG GAG GAG AAG ATG CCT TTC CTG CGC CCT AGG CCC GTA GAA GAG GAG GGT AAG TTC TCT ACC CTC CTC TTC TAC BamH I
CCC GTT TTT TGG AAA AAG CTT GG 83 bases GGG CAA AAA ACC TTT TTC GAA CC Hind III
3. Oligonucleotide sequences of Bcl2/846 (underlined)
GGA AAG GAC GCG GGA TCC GCT GCA CCT GAC GCC CTT CTT CAA GAG AGA AGG GCG TCA GGT
CCT TTC CTG CGC CCT AGG CGA CGT GGA CTG CGG GAA GAA GTT CTC TCT TCC CGC AGT CCA BamH I
GCA GCT TTT TTG GAA AAA GCT TGG 84 bases CGT CGA AAA AAC CTT TTT CGA ACC Hind III
S2. Identical sequences between small interfering RNAi and human Bcl2 cDNA (Open reading frame 494-1213)
1. Identical sequences between the control RNAi (1 CATACTC GCTCGCGCTCT 18) and human Bcl2 cDNA
Control RNAi: 5 CTCGCTC 11
Bcl2 cDNA: 5902 CTCGCTC 5908
2. Identical sequences between the Bcl2/630 RNAi (1 CGGGCATCTTCTCCTCCCA 19) and human Bcl2 cDNA
630 RNAi: 1 CGGGCATCTTCTCCTCCCA 19
Bcl2 cDNA: 630 CGGGCATCTTCTCCTCCCA 648
630 RNAi: 9 TTCTCCTC 16
Bcl2 cDNA: 4143 TTCTCCTC 4150
630 RNAi: 8 CTTCTCC 14
Bcl2 cDNA: 802 CTTCTCC 808
630 RNAi: 9 TTCTCCT 15
Bcl2 cDNA: 1127 TTCTCCT 1133
630 RNAi: 11 CTCCTCC 17
Bcl2 cDNA: 2254 CTCCTCC 2260
630 RNAi: 5 CATCTTC 11
Bcl2 cDNA: 2844 CATCTTC 2850
630 RNAi: 4 GCATCTT 10
Bcl2 cDNA: 4236 GCATCTT 4242
630 RNAi: 13 CCTCCCA 19
Bcl2 cDNA: 4400 CCTCCCA 4406
3. Identical sequences between the Bcl2/846 RNAi (1 GCTGCA CCTGACGCCCTTC 19) and human Bcl2 cDNA
846 RNAi: 1 GCTGCACCTGACGCCCTTC 19
Bcl2cDNA: 847 GCTGCACCTGACGCCCTTC 865
846 RNAi: 5 CACCTGAC 12
Bcl2cDNA: 773 CACCTGAC 780
846 RNAi: 4 GCACCTG 10
Bcl2 cDNA: 1042 GCACCTG 1048
846 RNAi: 13 GCCCTTC 19
Bcl2 cDNA: 2468 GCCCTTC 2474
846 RNAi: 6 ACCTGAC 12
Bcl2 cDNA: 2732 ACCTGAC 2738
846 RNAi: 1 GCTGCAC 7
Bcl2 cDNA: 4043 GCTGCAC 4049
846 RNAi: 1 GCTGCAC 7
Bcl2 cDNA: 4414 GCTGCAC 4420
846 RNAi: 3 TGCACCT 9
Bcl2 cDNA: 5971 TGCACCT 5977
S3. Identical sequences between small interfering RNAi and human Bcl-xL cDNA (Open reading frame 367-1068)
1. Identical sequences between the control RNAi (1 CATACTC GCTCGCGCTCT 18) and human Bcl-xL cDNA
No short stretch of identical sequence has been found between the control RNAi and Bcl-xL cDNA.
2. Identical sequences between the Bcl2/630 RNAi (1 CGGGCATCTTCTCCTCCCA 19) and human Bcl-xL cDNA
630 RNAi: 2 GGGCATCT 9
Bcl-xL cDNA:2450 GGGCATCT 2457
630 RNAi: 13 CCTCCC 18
Bcl-xL cDNA:2461 CCTCCC 2466
630 RNAi: 3 GGCATCTT 10
Bcl-xL cDNA: 1911 GGCATCTT 1918
630 RNAi: 1 CGGGCAT 7
Bcl-xL cDNA:673 CGGGCAT 679
630 RNAi: 9 TTCTCCT 15
Bcl-xL cDNA: 796 TTCTCCT 802
630 RNAi: 13 CCTCCCA 19
Bcl-xL cDNA: 1093 CCTCCCA 1099
3. Identical sequences between the Bcl2/846 RNAi (1 GCTGCACCTGACGCCCTTC 19) and human Bcl-xL cDNA
846 RNAi: 1 GCTGCACCTG 10
Bcl-xL cDNA: 199 GCTGCACCTG 208
846 RNAi: 4 GCACCTG 10
Bcl-xL cDNA: 537 GCACCTG 543
846 RNAi: 6 ACCTGAC 12
Bcl-xL cDNA:686 ACCTGAC 692
846 RNAi: 7 CCTGACG 13
Bcl-xL cDNA: 1008 CCTGACG 1014
S4. Identical sequences between small interfering RNAi and human MRP1 cDNA (Open reading frame 197-4792)
1. Identical sequences between the control RNAi (1 CATACTC GCTCGCGCTCT 18) and human MRP1 cDNA
No short stretch of identical sequence has been found between the control RNAi and MRP1 cDNA.
2. Identical sequences between the Bcl2/630 RNAi (1 CGGGCATCTTCTCCTCCCA 19) and human MRP1 cDNA
630 RNAi: 11 CTCCTCCCA 19
MRP1 cDNA:2432 CTCCTCCCA 2440
630 RNAi: 4 GCATCTTC 11
MRP1 cDNA:3129 GCATCTTC 3136
630 RNAi: 2 GGGCATC 8
MRP1 cDNA:535 GGGCATC 541
630 RNAi: 11 CTCCTCC 17
MRP1 cDNA:1027 CTCCTCC 1033
630 RNAi: 13 CCTCCCA 19
MRP1 cDNA:2168 CCTCCCA 2174
630 RNAi: 8 CTTCTCC 14
MRP1 cDNA:2191 CTTCTCC 2197
630 RNAi: 11 CTCCTCC 17
MRP1 cDNA:2944 CTCCTCC 2950
630 RNAi: 11 CTCCTCC 17
MRP1 cDNA:2947 CTCCTCC 2953
630 RNAi: 8 CTTCTCC 14
MRP1 cDNA:3421 CTTCTCC 3427
630 RNAi: 12 TCCTCCC 18
MRP1 cDNA:3602 TCCTCCC 3608
630 RNAi: 13 CCTCCCA 19
MRP1 cDNA:4877 CCTCCCA 4883
3. Identical sequences between the Bcl2/846 RNAi (1 GCTGCA CCTGACGCCCTTC 19) and human MRP1 cDNA
846 RNAi: 1 GCTGCACC 8
MRP1 cDNA: 1336 GCTGCACC 1343
846 RNAi: 11 ACGCCCTT 18
MRP1 cDNA: 1862 ACGCCCTT 1869
846 RNAi: 13 GCCCTTC 19
MRP1 cDNA: 1584 GCCCTTC 1590
846 RNAi: 6 ACCTGAC 12
MRP1 cDNA: 2074 ACCTGAC 2080
846 RNAi: 1 GCTGCAC 7
MRP1 cDNA: 3352 GCTGCAC 3358
846 RNAi: 5 CACCTGA 11
MRP1 cDNA: 4415 CACCTGA 4421
846 RNAi: 13 GCCCTTC 19
MRP1 cDNA: 4436 GCCCTTC 4442
846 RNAi: 2 CTGCACC 8
MRP1 cDNA: 4630 CTGCACC 4636
References
- [1].Mirski SE, Gerlach JH, Cole SP. Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res. 1987;47:2594–2598. [PubMed] [Google Scholar]
- [2].Laurie SA, Logan D, Markman BR, Mackay JA, Evans WK. Practice guideline for the role of combination chemotherapy in the initial management of limited-stage small-cell lung cancer. Lung Cancer. 2004;43:223–240. doi: 10.1016/j.lungcan.2003.09.002. [DOI] [PubMed] [Google Scholar]
- [3].Fischer B, Arcaro A. Current status of clinical trials for small cell lung cancer. Rev Recent Clin Trials. 2008;3:40–61. doi: 10.2174/157488708783330503. [DOI] [PubMed] [Google Scholar]
- [4].Gazdar AF, Carney DN, Russell EK, Sims HL, Baylin SB, Bunn PA, Jr, Guccion JG, Minna JD. Establishment of continuous, clonable cultures of small-cell carcinoma of lung which have amine precursor uptake and decarboxyla-tion cell properties. Cancer Res. 1980;40:3502–3507. [PubMed] [Google Scholar]
- [5].Nooter K, Stoter G. Molecular mechanisms of multidrug resistance in cancer chemotherapy. Pathol Res Pract. 1996;192:768–780. doi: 10.1016/S0344-0338(96)80099-9. [DOI] [PubMed] [Google Scholar]
- [6].Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- [7].Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- [8].Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line [see comments] Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- [10].Reed JC. Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresis-tance. Curr Opin Oncol. 1995;7:541–546. doi: 10.1097/00001622-199511000-00012. [DOI] [PubMed] [Google Scholar]
- [11].Chang XB, Tabcharani JA, Hou YX, Jensen TJ, Kartner N, Alon N, Hanrahan JW, Riordan JR. Protein kinase A (PKA) still activates CFTR chloride channel after mutagenesis of all 10 PKA consensus phosphorylation sites. J Biol Chem. 1993;268:11304–11311. [PubMed] [Google Scholar]
- [12].Chang XB, Hou YX, Riordan JR. ATPase activity of purified multidrug resistance-associated protein [published erratum appears in J Biol Chem 1998 Mar 27;273(13):7782] J Biol Chem. 1997;272:30962–30968. doi: 10.1074/jbc.272.49.30962. [DOI] [PubMed] [Google Scholar]
- [13].Lipinski CA, Tran NL, Menashi E, Rohl C, Kloss J, Bay RC, Berens ME, Loftus JC. The tyro-sine kinase pyk2 promotes migration and invasion of glioma cells. Neoplasia. 2005;7:435–445. doi: 10.1593/neo.04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hou Y, Cui L, Riordan JR, Chang XB. Allos-teric interactions between the two non-equivalent nucleotide binding domains of multidrug resistance protein MRP1. J Biol Chem. 2000;275:20280–20287. doi: 10.1074/jbc.M001109200. [DOI] [PubMed] [Google Scholar]
- [15].Cole SP, Downes HF, Slovak ML. Effect of calcium antagonists on the chemosensitivity of two multidrug-resistant human tumour cell lines which do not overexpress P-glycoprotein. Br J Cancer. 1989;59:42–46. doi: 10.1038/bjc.1989.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reduc-tase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- [17].Cole SP, Downes HF, Mirski SE, Clements DJ. Alterations in glutathione and glutathione-related enzymes in a multidrug-resistant small cell lung cancer cell line. Mol Pharmacol. 1990;37:192–197. [PubMed] [Google Scholar]
- [18].Campling BG, Baer K, Baker HM, Lam YM, Cole SP. Do glutathione and related enzymes play a role in drug resistance in small cell lung cancer cell lines? Br J Cancer. 1993;68:327–335. doi: 10.1038/bjc.1993.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rappa G, Gamcsik MP, Mitina RL, Baum C, Fodstad O, Lorico A. Retroviral transfer of MRP1 and gamma-glutamyl cysteine syn-thetase modulates cell sensitivity to L-buthionine-S,R-sulphoximine (BSO): new rationale for the use of BSO in cancer therapy. Eur J Cancer. 2003;39:120–128. doi: 10.1016/s0959-8049(02)00447-1. [DOI] [PubMed] [Google Scholar]
- [20].Leier I, Jedlitschky G, Buchholz U, Center M, Cole SP, Deeley RG, Keppler D. ATP-dependent glutathione disulphide transport mediated by the MRP gene-encoded conjugate export pump. Biochem J. 1996;314:433–437. doi: 10.1042/bj3140433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Loe DW, Deeley RG, Cole SP. Verapamil stimulates glutathione transport by the 190-kDa multidrug resistance protein 1 (MRP1) J Pharmacol Exp Ther. 2000;293:530–538. [PubMed] [Google Scholar]
- [22].Salerno M, Loechariyakul P, Saengkhae C, Garnier-Suillerot A. Relation between the ability of some compounds to modulate the MRP1-mediated efflux of glutathione and to inhibit the MRPI-mediated efflux of daunorubicin. Biochem Pharmacol. 2004;68:2159–2165. doi: 10.1016/j.bcp.2004.08.010. [DOI] [PubMed] [Google Scholar]
- [23].Huang Z, Chang X, Riordan JR, Huang Y. Fluorescent modified phosphatidylcholine flop-pase activity of reconstituted multidrug resistance-associated protein MRP1. Biochim Biophys Acta. 2004;1660:155–163. doi: 10.1016/j.bbamem.2003.11.010. [DOI] [PubMed] [Google Scholar]
- [24].Trompier D, Chang XB, Barattin R, du Moulinet D'Hardemare A, Di Pietro A, Baubichon- Cortay H. Verapamil and its derivative trigger apoptosis through glutathione extrusion by multidrug resistance protein MRP1. Cancer Res. 2004;64:4950–4956. doi: 10.1158/0008-5472.CAN-04-0143. [DOI] [PubMed] [Google Scholar]
- [25].Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O'Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- [26].Zaman GJ, Lankelma J, van Tellingen O, Beijnen J, Dekker H, Paulusma C, Oude Elferink RP, Baas F, Borst P. Role of glutathione in the export of compounds from cells by the ul-tidrug-resistance-associated protein. Proc Natl Acad Sci U S A. 1995;92:7690–7694. doi: 10.1073/pnas.92.17.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]