Abstract
OBJECTIVE:
To determine whether preoperative inspiratory muscle training is able to attenuate the impact of surgical trauma on the respiratory muscle strength, in the lung volumes, and diaphragmatic excursion in obese women undergoing open bariatric surgery.
DESIGN:
Randomized controlled trial.
SETTING:
Meridional Hospital, Cariacica/ES, Brazil.
SUBJECTS:
Thirty-two obese women undergoing elective open bariatric surgery were randomly assigned to receive preoperative inspiratory muscle training (inspiratory muscle training group) or usual care (control group).
MAIN MEASURES:
Respiratory muscle strength (maximal static respiratory pressure – maximal inspiratory pressure and maximal expiratory pressure), lung volumes, and diaphragmatic excursion.
RESULTS:
After training, there was a significant increase only in the maximal inspiratory pressure in the inspiratory muscle training group. The maximal expiratory pressure, the lung volumes and the diaphragmatic excursion did not show any significant change with training. In the postoperative period there was a significant decrease in maximal inspiratory pressure in both the groups. However, there was a decrease of 28% in the inspiratory muscle training group, whereas it was 47% in the control group. The decrease in maximal expiratory pressure and in lung volumes in the postoperative period was similar between the groups. There was a significant reduction in the measures of diaphragmatic excursion in both the groups.
CONCLUSION:
The preoperative inspiratory muscle training increased the inspiratory muscle strength (maximal inspiratory pressure) and attenuated the negative postoperative effects of open bariatric surgery in obese women for this variable, though not influencing the lung volumes and the diaphragmatic excursion.
Keywords: Respiratory Muscle Training, Spirometry, Diaphragm, Respiratory Muscles, Bariatric Surgery, Obesity
INTRODUCTION
Patients undergoing bariatric surgery have an impaired lung function in the immediate postoperative period.1 As it is an upper abdominal surgery, changes are inherent to this procedure, such as reduced lung volume, increased respiratory rate, dysfunction of the respiratory muscle, loss of control of breathing, and oxygenation and an increase in pulmonary secretion.1-4 Factors such as open surgery and obesity appear to emphasize the abovementioned changes.1,
It is widely acknowledged that the dysfunction of the respiratory muscles, especially the diaphragm, caused by the upper abdominal surgery is a major cause of postoperative pulmonary complications (PPC), such as atelectasis and pneumonia.8 According to the literature data, the diaphragm muscle dysfunction after an abdominal surgery is mainly due to the reflex inhibition of the phrenic nerve caused by visceral manipulation and postoperative pain.8
Aiming to attenuate the negative effects of surgery in the postoperative period, especially with regard to the respiratory muscle dysfunction, several authors have recommended preoperative inspiratory muscle training (IMT).2,4,9,10 According to Smetana, preoperative IMT appears to be an important strategy in the prevention of PPC and has been used by some authors in thoracic, cardiac and abdominal surgeries.2,4,9,10 However, there are no studies that evaluate the effect of preoperative IMT in obese patients undergoing open bariatric surgery, and only one study has reported the effects of postoperative IMT.11
Therefore, the hypothesis of this study was that preoperative IMT is able to attenuate the negative effects of surgical trauma on respiratory muscle strength, lung volume, and diaphragmatic excursion, thus reducing the risk of PPC in obese patients undergoing open bariatric surgery.
PATIENTS AND METHODS
Patients
The trial was performed with obese patients who were candidates for elective open Roux-en-Y gastric bypass surgery from the Meridional Hospital (Cariacica, ES, Brazil). The inclusion criteria for the present study allowed only females over 18 years of age that did not smoke and did not have respiratory disease. The trial excluded patients who refused to participate in the steps of the research protocol, those with a history of prior abdominal surgery, those who were unable to understand and perform the tests properly as well as those who refused to sign the Informed Consent Form. The present study was approved by the Meridional Hospital Ethics Committee (protocol 02-28/2009).
Measurements
RESPIRATORY MUSCLE STRENGTH: The respiratory muscle strength was determined by the maximal static respiratory pressure measured during the forced inspiration and expiration: maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP). The measurement was carried out using an aneroid manometer (Wika®, Brazil), calibrated in centimeters H2O (±300 cm H2O) and equipped with a 2-mm hole to relieve the oral pressure. The procedure was carried out as described by the ATS (American Thoracic Society) and the ERS (European Respiratory Society).12 MIP and MEP were determined using the residual volume and the total lung capacity, respectively, with the subjects in the sitting position. The inspiratory and expiratory efforts were held for at least 1 s. Patients performed at least three acceptable inspirations/expirations, while wearing a nose clip, to determine the two reproducible inspirations/expirations. The largest values were used in the analysis.
LUNG VOLUMES: The evaluation of the pulmonary function was conducted by conventional spirometry using a personal computer version of the NDD EasyOne™ Spirometer Model 2001 (Medizintechnik AG, Zurich, Switzerland). The parameters evaluated directly were as follows: volume, capacity, and flow of the lungs using the slow vital capacity (SVC), the forced vital capacity (FVC) and the maximum voluntary ventilation (MVV) tests, with volunteers in the sitting position and a minimum of three repetitions as recommended by the ATS and the ERS.13 The obtained results were expressed in absolute values and as percentages of the predicted reference values for the Brazilian population.14 The SVC test yielded the following variables: vital capacity (VC), tidal volume (VT), inspiratory reserve volume (IRV), and expiratory reserve volume (ERV). The FVC test allowed the determination of the forced expiratory volume in 1 s (FEV1) and the FEV1-to-FVC ratio.
DIAPHRAGMATIC EXCURSION: Diaphragmatic mobility was evaluated by chest X-ray, in the posteroanterior view, with the patients in the orthostatic position.
Two radiograph exposures of the same film under full inspiration and expiration were performed. Using the digitalized image of the radiograph, the axis (centimeters) and the area (square centimeters) of the right and the left dome of the diaphragm, between full inspiration and expiration, were calculated with software (UTHSCSA, Image Tool for Windows, version 1.28).15 The images were analyzed by the same radiologist, who was blinded to the information regarding to which group each patient belonged.
Protocol
Preoperative assessment (T1)
In the first evaluation, the patients were informed about the research protocol, requested to sign the Informed Consent Term, and then randomly assigned to the IMT group or the control group by opening a sealed envelope. In addition, baseline characteristics, such as name, age, sex, weight, height, waist-hip ratio, smoking history, presence of chronic lung disease or respiratory symptoms (cough, secretion, dyspnea, and chest pain), and other comorbidities were evaluated. Additionally, tests were also conducted to assess the respiratory muscle strength, spirometry, and diaphragmatic excursion.
Intervention (T2)
In the IMT group, training was performed 2-4 weeks before the surgery using the Threshold® IMT (Respironics, Pittsburgh, PA, USA). The program consisted of one daily session that lasted 15 minutes, six times per week, two times supervised by the physiotherapist and unsupervised the other four times. The initial load was calculated at 30% MIP, measured in the preoperative evaluation and re-calculated after a new measure of this variable at each visit to the physiotherapist.4,10 Patients in the control group (CG) received no intervention in the preoperative period.
Patients in the IMT and control groups were assessed two to three days before the surgery with the same preoperative testing. In addition, the patients also received instructions about the care to be taken after the surgery, the importance of cough and early ambulation.
Postoperative Assessment (T3)
Patients were evaluated on the first postoperative day (D1) with the same preoperative testing, and they were followed until discharge from the hospital.
Evaluations were performed only in the afternoon and after the administration of analgesics. Postoperative pain was subjectively rated before conducting the tests using the visual analogue scale (VAS), which ranged from 0 (no pain) to 10 (intense pain).
Patients were submitted to daily chest physiotherapy that was standardized for both groups on the day of the surgery and during the entire stay in the hospital. Each physiotherapy session consisted of diaphragmatic breathing, incentive spirometry, assisted cough, circulatory exercises, and early ambulation. The postoperative pulmonary complications considered were as follows: pneumonia (body temperature≥38°C, productive cough with purulent sputum, presence of pulmonary infiltration on chest X-ray examination, and increased leukocyte count), atelectasis with clinical implications (evidence of pulmonary atelectasis in the chest X-ray associated with respiratory discomfort) and acute respiratory failure (acute inability of the lungs to promote gas exchange, demanding the used of mechanical ventilation).16
Statistical Analysis
To calculate the sample size, the percentage difference of MIP between evaluation T1 and T3 (T1-T3/T1 * 100) between the IMT and control groups was considered as a variable, and the t-test for independent samples was used. Considering the power of 80% and a significance level of 5%, a value of 16 volunteers for each group was determined.
The Shapiro-Wilk test was used to calculate the normality of data. Subsequently, for the analysis of the baseline characteristics (Table 1) and surgery data (Table 2), the t-test was used for the independent samples for variables with normal distribution, the Mann-Whitney test for variables not normally distributed and the Chi-square test for nominal variables.
Table 1.
Baseline characteristics (values expressed as mean and SD).
| Variable | IMT Group(n = 15) | Control Group(n = 17) | |
| Age (years) | 36.13±8.12 | 34.8±9.47 | p = 0.679 |
| BMI (kg/m2) | 41.55±4.74 | 42.10±2.98 | p = 0.745 |
| W/H ratio | 0.96±0.09 | 0.89±0.07 | p = 0.025 |
| Hypertension | 9 | 7 | p = 0.287 |
| Diabetes mellitus | 3 | 3 | p = 0.864 |
| Dyslipidemia | 2 | 2 | p = 0.893 |
BMI: body mass index; W/H ratio: waist/hip ratio.
Table 2.
Surgery Data (values expressed as mean and SD).
| Variable | IMT Group(n = 15) | Control Group(n = 17) | |
| Duration of anesthesia (min) | 185.33±28.06 | 176.47±23.89 | p = 0.342 |
| Hospital stay (days) | 2±0.0 | 2.11±0.33 | p = 0.571 |
| VAS | 4.46±1.30 | 4.35±1.62 | p = 0.829 |
| PPC | 0 | 0 |
VAS: visual analogue scale of pain; PPC: postoperative pulmonary complications.
For the analysis of the variables, ANOVA-repeated measures followed by Bonferroni correction were used to compare the three assessments (T1, T2, and T3) in both groups (Table 3).
Table 3.
Respiratory muscle strength, lung volumes and diaphragmatic excursion in the IMT and Control groups (values expressed as mean and SD).
| IMT Group(n = 15) | Control Group(n = 17) | |||||||||
| Variable | Preoperative (T1) | After intervention(T2) | % Dif T1 vs. T2 | Postoperative(T3) | % Dif T1 vs. T3 | Preoperative(T1) | After intervention(T2) | % Dif T1 vs. T2 | Postoperative(T3) | % Dif T1 vs. T3 |
| MIP (cmH2O) | 93.33±23.80 | 120.00±20.35* | ↑ 33% | 63.34±21.60§ | ↓ 28% | 92.94±18.63 | 91.76±20.38 | ↓ 1% | 48.82±19.32§ | ↓ 47%# |
| MEP (cmH2O) | 117.33±34.53 | 142.66±28.90 | ↑ 26% | 49.66±22.71§ | ↓ 56% | 116.47±32.39 | 135.29±34.11 | ↑ 19% | 49.70±22.39§ | ↓ 55% |
| VC (L) | 3.22±0.27 | 3.17±0.31 | ↓ 1.5% | 2.07±0.52§ | ↓ 35% | 3.22±0.54 | 3.29±0.48 | ↑ 1.5% | 1.95±0.46§ | ↓ 39% |
| %VC | 90.66±7.84 | 89.53±8.47 | ↓ 1% | 58.66±15.55§ | ↓ 35% | 88.52±8.66 | 90.94±7.28 | ↑ 1.5% | 54.17±12.43§ | ↓ 39% |
| VT (L) | 0.93±0.34 | 0.90±0.29 | ↓ 3% | 0.65±0.21 | ↓ 23% | 0.79±0.27 | 0.80±0.32 | ↑ 1% | 0.58±0.19 | ↓ 23% |
| IRV (L) | 1.78±0.47 | 1.74±0.43 | ↓ 2% | 1.11±0.27§ | ↓ 37% | 1.83±0.52 | 1.77±0.58 | ↓ 4% | 1.08±0.36§ | ↓ 41% |
| ERV(L) | 0.50±0.20 | 0.53±0.19 | ↑ 6% | 0.33±0.19 | ↓ 30% | 0.57±0.28 | 0.68±0.24 | ↑ 19% | 0.28±0.15§ | ↓ 50% |
| FVC (L) | 3.20±0.26 | 3.16±0.29 | ↓ 1% | 2.14 ±0.52§ | ↓ 33% | 3.32±0.50 | 3.35±0.49 | ↑ 1% | 2.02±0.49§ | ↓ 39% |
| %FVC | 90.26±7.29 | 89.01±7.06 | ↓ 1% | 60.20±14.43§ | ↓ 33% | 90.17±8.20 | 91.01±8.52 | ↑ 1% | 55.58±12.52§ | ↓ 39% |
| FEV1 (L) | 2.71± 0.21 | 2.64±0.24 | ↓ 3% | 1.77±0.47§ | ↓ 35% | 2.80±0.43 | 2.81±0.40 | ↑ 0.4% | 1.71±0.42§ | ↓ 39% |
| %FEV1 | 91.93±7.41 | 89.53±6.78 | ↓ 3% | 60.06±15.69§ | ↓ 35% | 91.52±7.18 | 92.23±7.98 | ↑ 0.4% | 56.41±13.42§ | ↓ 39% |
| MVV (L/min) | 108.55±19.90 | 102.32±20.65 | ↓ 6% | 73.71±21.16§ | ↓ 32% | 107.89±16.13 | 107.61±17.91 | ↓ 0.3% | 71.69±19.32§ | ↓ 33% |
| %MVV | 76.33±11.38 | 72.13±13.03 | ↓ 6% | 52.73±18.96§ | ↓ 32% | 76.41±11.43 | 76.41±11.95 | ↓ 0.3% | 51.47±14.03§ | ↓ 33% |
| Axis hemidiaphragmatic R (cm) | 4.80±1.34 | 4.51±1.47 | ↓ 6% | 2.69±1.09§ | ↓ 44% | 4.66±1.36 | 4.48±1.59 | ↓ 4% | 2.28±1.01§ | ↓ 51% |
| Axis hemidiaphragmatic L (cm) | 4.77±1.40 | 4.42±1.44 | ↓ 7% | 2.70±1.20§ | ↓ 43% | 4.77±1.18 | 4.80±1.38 | ↑ 0.6% | 2.43±1.20§ | ↓ 49% |
| Area hemidiaphragmatic R (cm2) | 51.46±19.62 | 49.82±20.89 | ↓ 1% | 28.74±13.33§ | ↓ 44% | 47.51±15.42 | 48.16±17.77 | ↑ 3% | 24.48±12.37§ | ↓ 47% |
| Area hemidiaphragmatic L (cm2) | 49.66±21.14 | 47.31±19.45 | ↓ 4% | 28.28±13.32§ | ↓ 43% | 47.38±14.89 | 50.43±16.21 | ↑ 6% | 24.10±12.66§ | ↓ 49% |
MIP: maximal inspiratory pressure; MEP: maximal expiratory pressure; VC: vital capacity; %VC: percentage of predicted vital capacity; VT: tidal volume; IRV: inspiratory reserve volume; ERV: expiratory reserve volume; FVC: forced vital capacity; %FVC: percentage of predicted forced vital capacity; FEV1: forced expiratory volume in one second; %FEV1: percentage of predicted forced expiratory volume in one second; MVV: maximum voluntary ventilation; %MVV: percentage of predicted maximum voluntary ventilation;
Difference between evaluation T1 vs. T2, p<0.05;
Difference between evaluation T1 vs. T3, p<0.05;
# Difference between percentage difference T1 vs. T3 (%Dif T1 X T3) of evaluation in IMT Group and Control Group, p<0.05.
In addition, the percentage difference of T1 vs. T2 and T1 vs. T3 evaluations between the IMT and control groups was compared using the t-test for independent samples (Table 3).
RESULTS
Baseline characteristics
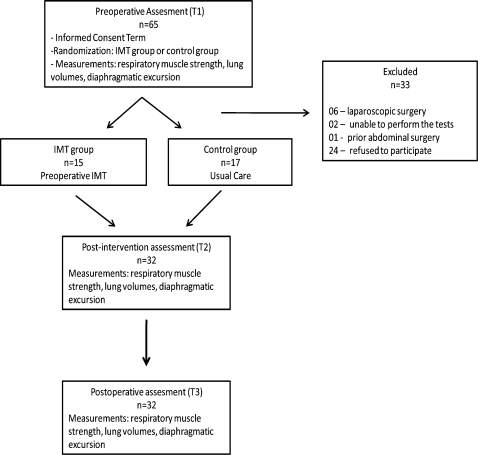
Sixty-five patients who were candidates for elective open bariatric surgery, aged 21-52 years and with BMI 35-53 kg/m2, were evaluated. Of these, 33 were excluded: six by opting to perform surgery by laparoscopy, two for not performing the tests properly, one for already having a previous abdominal surgery, and 24 who did not participate in all the stages of the research protocol (Figure 1).
Figure 1.
Flowchart of the study participants.
Thus, the data from the remaining 32 patients were evaluated. The IMT group comprised 15 patients, while the control group comprised 17 patients. There was no statistical difference between the groups regarding age, BMI, and comorbidities. However, there was statistical significance only for the variable waist-hip ratio (Table 1).
Surgery Data
The patients underwent open Roux-en-Y gastric bypass surgery, which was performed by the same surgeon. The duration of anesthesia ranged from 120 to 240 minutes, and there was no statistically significant difference between the groups (Table 2).
The majority of the patients remained in the hospital for two days. However, two patients in the control group needed to stay one day longer; one was hospitalized for having difficulty accepting the diet, while the other was hospitalized for abdominal pain and nausea (Table 2).
There was no statistical difference in the subjective sensation of pain assessed by VAS between the groups. None of the patients studied had any postoperative pulmonary complications (Table 2).
Respiratory muscle strength
Based on the data shown in Table 3, the groups were similar in the variables MIP and MEP in the preoperative assessment (T1) before the training, which demonstrated the homogeneity of the sample population.
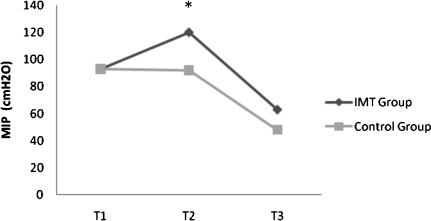
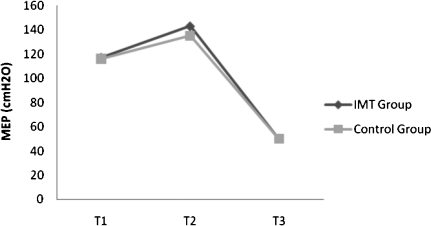
There was an increase in the MIP only in the IMT group. Training did not influence the MEP because there was no significant change in both groups (Table 3; Figure 2 and 3).
Figure 2.
MIP evaluations of T1, T2, and T3 in IMT ((n = 15) and control (n = 17) groups. *Difference between IMT and Control groups (T2), p<0.05. (values expressed as mean)”.
Figure 3.
MEP evaluations of T1, T2, and T3 in IMT (n = 15) and Control (n = 17) groups. (values expressed as mean).
In the postoperative period, there was a significant decrease in MIP and MEP in both groups. However, there was a decrease of 28% in the IMT group compared to 47% in the control group (p<0.05). The decrease in MEP in the postoperative period was similar between the groups (Table 3; Figure 2 and 3).
Lung Volumes
In the preoperative assessment (T1), the lung volumes (VC, VT, IRV, ERV, FVC, FEV1, and MVV) were similar in both groups, which demonstrated the homogeneity of the sample population.
Also, there was no change in the lung volume after the training period (T2) (Table 3).
Although there was a significant decrease in the VC, IRV, FVC, FEV1, and MVV in both groups in the postoperative period, the ERV remained statistically unchanged in the IMT group only. Furthermore, the VT did not decrease in the postoperative period in both groups (Table 3).
Diaphragmatic excursion
In the preoperative assessment (T1), there was no significant difference between the IMT and control groups for the measures of diaphragmatic excursion, which demonstrated the homogeneity of the sample population.
Moreover, measures of diaphragmatic excursion did not show any significant change with training (Table 3).
In the postoperative period, there was a significant reduction in the measures of diaphragmatic excursion in both groups, although the reduction was not statistically different (Table 3).
DISCUSSION
Based on the results of this study, preoperative IMT attenuates the negative effects of open bariatric surgery in the inspiratory muscle strength (MIP). However, IMT did not appear to influence the lung volume and diaphragmatic excursion.
Other studies concerning the preoperative IMT, conducted at least two weeks before the upper abdominal surgery, also showed attenuation of the reduction in MIP.2,4 Kulkarni et al.2 observed that MIP did not reduce the postoperative period in the IMT group. The present results showed a significant postoperative reduction in MIP in both groups. However, this reduction was lower in the IMT group because the protocol of the present study was training for 15 minutes once a day, while in the above mentioned study,2 the training was twice daily. Additionally, the Kulkarni et al.2 study included open and laparoscopic surgeries, and there was no mention of any obese patients. Thus, the above mentioned facts could have contributed to reducing the impact of surgery in the MIP.
Barbalho-Moulim et al.1 compared the effects on the lung function of open and laparoscopic bariatric surgery without any specific preoperative training. A 23% postoperative decrease in MIP was observed in the laparoscopic surgery patients compared to a 37% decrease in the open surgery patients. In the present study, the MIP decreased less than 23% for seven patients in the IMT group and only one in the control group (p<0.05), suggesting that the preoperative IMT in the open bariatric surgery could make an equivalent value of the MIP in the laparoscopic surgery.
IMT did not attenuate the negative effects of surgery in the MEP, which could be due to a surgical incision that caused direct trauma to the abdominal muscles and impaired the functioning of these muscles.1 The MEP decreased 56 and 55% in the IMT and control groups, respectively, for the patients studied. These values are similar to those assessed by Barbalho-Moulim et al.,1 where the MEP fell 61% in open surgery patients while the decrease was 27% in laparoscopic surgery patients; this was because of lower trauma to the abdominal muscles.
In a recently published study,11 the authors evaluated the effect of IMT on bariatric surgery, however, in the postoperative period. Similar to our results, the authors also reported an increase in MIP in the IMT group compared with the control group but without a significant effect on MEP; this may be because the training was directed at the inspiratory muscles.11 In addition, other modalities of training also influenced the respiratory muscle strength in the postoperative period of bariatric surgery.17
According to the literature data, the dysfunction of the respiratory muscles is considered the main cause of PPC,2,8 which can cause alveolar collapse that contributes to the formation of atelectasis leading to pulmonary infections.18 Thus, IMT appears to be an alternative to prevent these complications.2,4,5,10 In the present study, CPP was not observed in either group, which can be attributed to the fact that although these patients manifested risk factors for this kind of a complication, such as obesity, open upper abdominal surgery and anesthesia time of more than 180 minutes,5 they were young, without chronic respiratory diseases and subjected to postoperative chest physiotherapy. To test the above mentioned hypothesis, further studies are required to evaluate patients undergoing open bariatric surgery with other risk factors, such as age over 60 years, smoking or chronic lung disease.5
The lung volumes did not change with training and showed a similar decrease postoperatively in both groups, concluding that IMT does not influence these variables. Similar results were also found in the study by Dronkers et al.,4 which demonstrated that although IMT improved MIP, it did not affect the values of vital capacity.
In situations where the response was related to the type of training offered (specificity of training), the training should be aimed at increasing the lung volumes. Thus, the lung expansion modalities could increase the values of the lung volumes assessed by spirometry. However, Kulkarni et al.2 and Cattano et al.,19 who reported preoperative training with incentive spirometry and deep breathing, observed that this type of intervention had no effect on postoperative lung volumes.
According to the results of the current study, there was no increase in the diaphragmatic motion after the training period. As a result of obesity, the patients had a mechanical restriction of diaphragmatic excursion caused by the deposition of fat in the abdomen that compressed the chest. This compression resulted in excessive strain to the diaphragm, causing mechanical disadvantage to that muscle and thereby reducing its strength and efficiency.20 Thus, as obesity is not attenuated with the training proposed in the present study, the effect of diaphragmatic mobility in the study population is limited.
The impact of surgery on the diaphragmatic excursion was slightly lower in the IMT group, although the difference was not statistical significance. The reduced movement of the diaphragm muscle in the postoperative period of open bariatric surgery has been reported by the abovementioned authors.1 This reduction was observed because the surgery affected the compliance of the abdomen and increased the intra-abdominal pressure,21 thereby inhibiting the action of the muscle by the reflex mechanisms.22,23 When associated with the impaired chest mechanics due to obesity, those changes further decreased the diaphragmatic mobility.20 There are no studies on preoperative IMT in bariatric surgery that evaluated the diaphragmatic excursion to compare to the results of the present study. However, intensive training (longer duration or frequency) and/or with different loads could possibly facilitate IMT to exert some effect on this variable. In addition, it is likely that IMT could demonstrate better results for non-obese patients because they did not present the chest mechanics restriction of diaphragmatic excursion like the obese patients and they have lower respiratory muscle strength.24,25
CONCLUSION
Preoperative IMT increased the inspiratory muscle strength (MIP) and attenuated the negative postoperative effects of open bariatric surgery in obese women for this variable, although it did not influence lung volume. The diaphragmatic excursion appears to have been slightly influenced by IMT. However, more studies with different protocols are required to evaluate the effect of IMT on the prevention of diaphragmatic dysfunction in the postoperative period of open bariatric surgery.
CLINICAL MESSAGES
Preoperative IMT attenuated the negative postoperative effects of open bariatric surgery in obese women on inspiratory muscle strength.
Whereas dysfunction of the respiratory muscles is the main cause of postoperative pulmonary complications after abdominal surgery, preoperative IMT may be an alternative to prevent such complications.
ACKNOWLEDGEMENTS
The authors would like to thank the patients, the BIOSCAN Meridional and the National Council for Scientific and Technological Development (CNPq), Proc. N° 579981/2008-8 and N° 504228/2010-2 for their contribution and support.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Barbalho-Moulim MC, Miguel GPS, Forti EMP, Cesar MC, Azevedo JLMC, Costa D. Silicone-Ring Roux-en-Y Gastric Bypass in the Treatment of Obesity: Effects of Laparoscopic Versus Laparotomic Surgery on Respiration. Obes Surg. 2011;21:194–9. doi: 10.1007/s11695-009-9823-9. 10.1007/s11695-009-9823-9 [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni SR, Fletcher E, McConnell AK, Poskitt KR, Whyman MR. Pre-operative inspiratory muscle training preserves postoperative inspiratory muscle strength following major abdominal surgery – a randomised pilot study. Ann R Coll Surg Engl. 2010;92:700–5. doi: 10.1308/003588410X12771863936648. 10.1308/003588410X12771863936648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manzano RM, Carvalho CRF, Saraiva BM, Vieira RJE. Chest physiotherapy during immediate postoperative period among patients undergoing upper abdominal surgery: randomized clinical trial. Sao Paulo Med J. 2008;126:269–73. doi: 10.1590/S1516-31802008000500005. 10.1590/S1516-31802008000500005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dronkers J, Veldman A, Hoberg E, van der Waal C. Prevention of pulmonary complications after upper abdominal surgery by preoperative intensive inspiratory muscle training: a randomized controlled pilot study. Clin Rehab. 2008;22:134–42. doi: 10.1177/0269215507081574. 10.1177/0269215507081574 [DOI] [PubMed] [Google Scholar]
- 5.Smetana GW. Postoperative pulmonary complications: An update on risk assessment and reduction. Cleve Clin J Med. 2009;76(Suppl. 4):S60–5. doi: 10.3949/ccjm.76.s4.10. 10.3949/ccjm.76.s4.10 [DOI] [PubMed] [Google Scholar]
- 6.Eichenberger AS, Proietti S, Wicky S, Frascarolo P, Suter M, Spahn DR, et al. Morbid obesity and postoperative pulmonary atelectasis: an underestimated problem. Anesth Analg. 2002;95:1788–92. doi: 10.1097/00000539-200212000-00060. 10.1097/00000539-200212000-00060 [DOI] [PubMed] [Google Scholar]
- 7.Weller WE, Rosati C. Comparing outcomes of laparoscopic versus open bariatric surgery. Ann Surg. 2008;248:10–5. doi: 10.1097/SLA.0b013e31816d953a. 10.1097/SLA.0b013e31816d953a [DOI] [PubMed] [Google Scholar]
- 8.Laghi F, Tobin MJ. Disorders of the Respiratory Muscles. Am J Respir Crit Care Med. 2003;168:10–48. doi: 10.1164/rccm.2206020. 10.1164/rccm.2206020 [DOI] [PubMed] [Google Scholar]
- 9.Nomori H, Kobayashi R, Fuyuno G, Morinaga S, Yashima H. Preoperative respiratory muscle training. Assessment in thoracic surgery patients with reference to postoperative pulmonary complications. CHEST. 1994;105:1782–8. doi: 10.1378/chest.105.6.1782. 10.1378/chest.105.6.1782 [DOI] [PubMed] [Google Scholar]
- 10.Hulzebos EHJ, Helders PJM, Favie NJ, de Bie RA, de la Riviere AB, van Meeteren NLU. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery. JAMA. 2006;296:1851–7. doi: 10.1001/jama.296.15.1851. 10.1001/jama.296.15.1851 [DOI] [PubMed] [Google Scholar]
- 11.Casali CCC, Pereira APM, Martinez JAB, Souza HCD, Gastaldi AC. Effects of inspiratory muscle training on muscular and pulmonary function after bariatric surgery in obese patients. Obes Surg. 2011 doi: 10.1007/s11695-010-0349-y. doi: 10.1007/s11695-010-0349-y. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society/European Respiratory Society. ATS/ERS Statement on Respiratory Muscle Testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. 10.1164/rccm.166.4.518 [DOI] [PubMed] [Google Scholar]
- 13.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 14.Pereira CAC, Sato T, Rodrigues SC. New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol. 2007;33:397–406. doi: 10.1590/s1806-37132007000400008. 10.1590/S1806-37132007000400008 [DOI] [PubMed] [Google Scholar]
- 15.Fernandes M, Cukier A, Ambrosino N, Leite JJ, Zanetti Feltrim MI. Respiratory pattern, thoracoabdominal motion and ventilation in chronic airway obstruction. Monaldi Arch Chest Dis. 2007;67:209–16. doi: 10.4081/monaldi.2007.477. [DOI] [PubMed] [Google Scholar]
- 16.McAlister FA, Bertsch K, Man J, Bradley J, Jacka M. Incidence of and Risk Factors for Pulmonary Complications after Nonthoracic Surgery. Am J Respir Crit Care Med. 2005;171:514–7. doi: 10.1164/rccm.200408-1069OC. 10.1164/rccm.200408-1069OC [DOI] [PubMed] [Google Scholar]
- 17.Forti E, Ike D, Barbalho-Moulim M, Rasera I, Jr, Costa D. Effects of chest physiotherapy on the respiratory function of postoperative gastroplasty patients. Clinics. 2009;64:683–9. doi: 10.1590/S1807-59322009000700013. 10.1590/S1807-59322009000700013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen NT, Goldeman C, Rosenquist CJ, Arango A, Cole CJ, Lee SJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234:279–89. doi: 10.1097/00000658-200109000-00002. 10.1097/00000658-200109000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cattano D, Altamirano A, Vannucci A, Melnikov V, Cone C, Hagberg CA. Preoperative use of incentive spirometry does not affect postoperative lung function in bariatric surgery. Translational Research. 2010;156:265–72. doi: 10.1016/j.trsl.2010.08.004. 10.1016/j.trsl.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 20.Koenig SM. Pulmonary complications of obesity. Am J Med Sci. 2001;321:249–79. doi: 10.1097/00000441-200104000-00006. 10.1097/00000441-200104000-00006 [DOI] [PubMed] [Google Scholar]
- 21.Nguyen NT, Lee SL, Anderson JT, Palmer LS, Canet F, Wolfe BM, et al. Evaluation of intraabdominal pressure after laparoscopic and open gastric bypass. Obes Surg. 2001;11:40–5. doi: 10.1381/096089201321454097. 10.1381/096089201321454097 [DOI] [PubMed] [Google Scholar]
- 22.Kim Soo Hwan, Na Sungwon, Choi Jin-Sub, Na Se Hee, Shin Seokyung, Koh Shin Ok. An Evaluation of Diaphragmatic Movement by M-Mode Sonography as a Predictor of Pulmonary Dysfunction After Upper Abdominal Surgery. Anesth Analg. 2010;110:1349–54. doi: 10.1213/ANE.0b013e3181d5e4d8. 10.1213/ANE.0b013e3181d5e4d8 [DOI] [PubMed] [Google Scholar]
- 23.Berdah SV, Picaud R, Jammes Y. Surface diaphragmatic electromyogram changes after Laparotomy. Clin Physiol & Func Im. 2002;22:157–60. doi: 10.1046/j.1365-2281.2002.00406.x. 10.1046/j.1365-2281.2002.00406.x [DOI] [PubMed] [Google Scholar]
- 24.Sabino PG, Silva BM, Brunetto AF. Nutritional status is related to fat-free mass, exercise capacity and inspiratory strength in severe chronic obstructive pulmonary disease patients. Clinics. 2010;65:599–605. doi: 10.1590/S1807-59322010000600007. 10.1590/S1807-59322010000600007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa D, Barbalho MC, Miguel GPS, Forti EMP, Azevedo JLMC. The impact of obesity on pulmonary function in adult women. Clinics. 2008;63:719–24. doi: 10.1590/S1807-59322008000600002. 10.1590/S1807-59322008000600002 [DOI] [PMC free article] [PubMed] [Google Scholar]