Abstract
OBJECTIVE:
To perform a comparative analysis of the effects of platelet-rich plasma and centrifuged bone marrow aspirate on the induction of bone healing in rabbits.
METHOD:
Twenty adult, male New Zealand rabbits were randomly separated into two equal groups, and surgery was performed to create a bone defect (a cortical orifice 3.3 mm in diameter) in the proximal metaphysis of each rabbit's right tibia. In the first group, platelet-rich plasma was implanted in combination with β-tricalcium phosphate (platelet-rich plasma group), and in the second group, centrifuged bone marrow in combination with β-tricalcium phosphate (centrifuged bone marrow group) was implanted. After a period of four weeks, the animals were euthanized, and the tibias were evaluated using digital radiography, computed tomography, and histomorphometry.
RESULTS:
Seven samples from each group were evaluated. The radiographic evaluation confirmed the absence of fractures in the postoperative limb and identified whether bone consolidation had occurred. The tomographic evaluation revealed a greater amount of consolidation and the formation of a greater cortical bone thickness in the platelet-rich plasma group. The histomorphometry revealed a greater bone density in the platelet-rich plasma group compared with the centrifuged bone marrow group.
CONCLUSION:
After four weeks, the platelet-rich plasma promoted a greater amount of bone consolidation than the bone marrow aspirate concentrate.
Keywords: Bone substitutes, Fracture healing, Radiology, Tomography, Histology
INTRODUCTION
There is a need for the development of safer, more efficient, and more effective surgical procedures for the consolidation of fractures,1 for reconstruction (bone loss) and replacement of bone segments. There is a large demand due to the increasing number of victims of accidents and injuries (falls, traffic accidents, accidents at work, aggressions, and other external causes of morbidity), and of patients suffering from orthopedic diseases (idiopathic, degenerative, metabolic, infectious, auto-immune, congenital, and neoplastic pathologies).
In Brazil, it is expected a significantly increase in life expectancy at birth and rapid growth of the elderly population.2 With this proportional increase in elderly population and the relative aging of country, it is expected an elevation in the incidence of fractures due to osteoporosis. This expectation is aggravated by the prospect of worsening conditions resulting from uncontrolled urban concentration (an increased occurrence of traffic accidents and violence). This scenario makes urgent the search for innovative procedures, practical, and possible to be implemented in the public hospitals in the unified health care system of Brazil (equity).
Researchers are attempting to gain a better understanding of the physiology of bone consolidation3 for the development of surgical techniques for internal and external fracture fixation1. In addition, researchers are searching for the best solutions for possible sequelae and complications, such as vicious consolidation, delayed consolidation, or the absence of consolidation (pseudo-arthrosis).4
New resources and technologies are continually being added to the therapeutic arsenal of orthopedic surgeons. Systemically administered drugs are currently being evaluated, especially medication that is used for the treatment and prevention of osteoporosis, such as bisphosphonates,5,6 osteoconductive agents, synthetic and composite (organic/synthetic) bone substitutes,7 and osteoinductive agents, including platelet-rich plasma (PRP),8-11 bone marrow concentrate (BMC),12-15 bone morphogenetic proteins (BMPs),16,17 and gene therapy.18 Devices that provide osteostimulation using pulsed electromagnetic fields,19 electric microcurrents,20 and extracorporeal shockwaves21 are also being evaluated.
The clinical results of synthetic osteoconductive and osteoinductive materials are still controversial. Autologous bone (an autograft) that is removed from the ilium is still the most widely used type of graft and is considered to be the gold standard of osteoconductive, osteogenic, and osteoinductive agents.22
The use of autografted bone that has been taken from the ilium or another donor region of the patient is not always possible or indicated because of the need for a second surgical procedure, the limitation of the bone stock, and the possible complications (unaesthetic scarring, pain, risk of alterations in local sensitivity, and infection).23 In these cases, synthetic osteoconductive biomaterials [hydroxyapatite, β tricalcium phosphate (βTCP), other phosphates and carbonates, collagen, composites, and other biomaterials used as scaffolds or carriers] are viable options, especially when these materials are used in combination with a biological autologous osteoinductor, such as platelet-rich plasma,24 concentrated bone marrow aspirate,25 or tissue growth factors.17 PRP and centrifuged BMC are the most commonly used osteoinductors, in combination with synthetic osteoconductors, due to their convenience, the technical ease with which they are obtained, intraoperative processing, lack of immunogenicity, non-transmissibility of diseases, and low cost.
We sought to evaluate the efficacy of two osteoinductive agents - platelet-rich plasma and centrifuged bone marrow aspirate - in conjunction with β tricalcium phosphate, a commercial synthetic osteoconductive agent. The present study is a controlled prospective study that used rabbits as an experimental model to compare the quality of the bone that was formed in tibial defects. The findings of the present study allow for the selection of the most effective osteoinductive agent.
METHODS
The research protocol of the present study was prepared in accordance with Law n° 11,794 from October 8, 2008, which establishes procedures for the scientific use of animals. The present study was approved by the Ethics Committee of Londrina State University (UEL) and the Ethics Commission for the Analysis of Research Projects at the Hospital das Clinicas, University of São Paulo School of Medicine (FMUSP). This study also followed the procedures established in the guide of the Canadian Council on Animal Care26 for the maintenance, management, sedation, analgesia, anesthesia, and euthanasia of small animals.
Twenty New Zealand albino, adult male rabbits (Oryctolagus cuniculus) were used, with a mean weight of 2700 g. The animals, which were clinically healthy and were obtained from a commercial source, were housed at the Vivarium of the University Hospital (UH) in Londrina, Paraná.
To evaluate the autologous osteoinductive agents, two experimental groups were formed, with ten rabbits randomly distributed in each group. The first group received platelet-rich plasma (PRP group), and the second group received centrifuged bone marrow aspirate (BMC group). The two types of osteoinductive agents were associated with a commercial absorbable osteoconductive agent; β tricalcium phosphate (Cerasorb ® M - Curasan AG International - Kleinostheim, Germany), with a grain size of 500 to 1000 µm, a porosity of 65%, and a pore size of 5 to 500 µm.
All of the animals were transferred from the rabbit vivarium to the Surgical Technique Laboratory at the Center for Health Sciences, UEL. After weighing, the animals were anesthetized according to the anesthesia protocol for small animals of the Canadian Council of Animal Care26 (an intramuscular injection in the proximal region of the left pelvic limb of a solution containing 40 mg/kg body weight of 10% dextro-ketamine hydrochloride and 2% 5 mg/kg body weight of xylazine hydrochloride) and were then tattooed on the right ear with numbers from 1 to 20.
For the preparation of the platelet-rich plasma, a 9-ml sample of blood was collected using a cardiac puncture from the rabbits in the PRP group. The blood sample from each animal was sent to the Postgraduate Laboratory of Internal Medicine at the Center for Health Sciences, UEL and was centrifuged for ten minutes at a speed of 1200 rpm at room temperature. The fraction that separated from the PRP was transferred to another sterile tube and was stored. Next, 0.2 ml of 10% calcium gluconate was added to the tube with the PRP, and after a few minutes, a clot was formed (activation of PRP). After these processing steps, the tube with the activated PRP was returned to the Surgical Technique Laboratory for use in the surgical procedure.27
Trichotomies were produced in the region of the anterior tibial tuberosity up to the middle of the leg in both groups of rabbits and in the right iliac crest region in the animals of the BMC group. Antisepsis and asepsis were performed with 2% chlorhexidine gluconate antiseptic and a 0.5% alcoholic solution of chlorhexidine digluconate, respectively.
To prepare the centrifuged bone marrow aspirate, a 5-ml sample was collected. A marrow puncture was made in the right iliac bone of the rabbits in the BMC group, and 5 ml of bone marrow was aspirated using strong suction with a heparinized syringe (1∶1000 heparin solution). This material was immediately sent for processing, in a chilled container, to the Postgraduate Internal Medicine Laboratory. The bone marrow sample was transferred to a sterile tube and was centrifuged at 400 rpm for ten minutes. In the intermediate fraction that was formed after centrifugation (“buffy-coat”), stromal bone marrow cells and hematopoietic precursors (mesenchymal stem cells and progenitor cells) were observed. Next, the fraction that was obtained was homogenized in a vortex agitator. Trypan blue dye was then applied to a 50-μl sample in separate Eppendorf tubes to test the cell viability. The viable cells with intact cytoplasmic membranes exhibited no basophilia. A sample was considered viable when the number of stained cells that were observed using optical microscopy was insignificant.25 After the verification of the cell viability, a volume of approximately 0.5 ml of the remaining centrifuged bone marrow aspirate (BMC) was sent to the Surgical Technique Laboratory for use in the operation.
Infiltration of a local anesthetic (0.5% bupivacaine hydrochloride) without a vasoconstrictor was completed before the surgical procedures to improve the postoperative analgesia. For the surgical procedure, each animal was positioned in the supine position while under general anesthesia. A cutaneous access route of 1.5 cm was made on the anteromedial side of the right leg, over the proximal tibial metaphyseal region. Next, the soft tissues were moved to the side, and the periosteum was opened. The exposed medial metaphyseal-diaphyseal transition region of the tibia was perforated with a dental trephine that was 3.3 mm in diameter. A dental drill with controlled rotation (800 rpm) was used to avoid local thermal damage to the bone tissue.
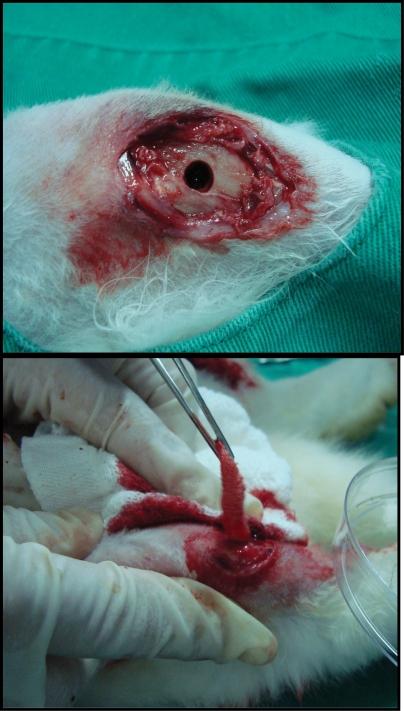
In the PRP group, the platelet-rich plasma (PRP) coagulum was deposited over the ß tricalcium phosphate in a petri dish, forming a cylinder that was inserted into the bone defect (Figures 1A and 1B).
Figure 1.
Bone defect obtained after drilling in the right tibia (a) and after implantation of the βTCP and PRP cylinder (b).
In the BMC group, the centrifuged bone marrow aspirate and beta tricalcium phosphate were homogenized. The resulting paste was introduced into the tibial bone defect.
After completion of the procedures for filling the bone defects, each animal's skin was sutured with polyamide (mononylon) surgical thread, size 3-0. Occlusive dressing was not used and the limb was not immobilized. Next, the animals were intramuscularly administered antibiotic medications, penicillin G benzathine, and procaine (Pencivet ® PPU), and the anti-inflammatory drug piroxicam. After recovery from the anesthesia in their individual transport cages, each rabbit was taken to the vivarium. Normal weight bearing on the limbs was allowed in the immediate postoperative period.
The animals were kept alive for a four-week follow-up period after the operation, which is a sufficient length of time for bone healing in rabbits.5,25
The animals were euthanized using an intracardiac lethal injection with 20% potassium chloride26 under anesthesia by intramuscular injection of a solution of dextro-ketamine hydrochloride and xylazine hydrochloride.26 The same anesthesia was used during the operations and was subject to resolution No. 714 (2002) of the Federal Council of Veterinary Medicine and to Law No. 11794 from October 8, 2008.
The rabbit tibias were removed and were dissected from the soft tissue. These tibias were then immediately packed in appropriate containers with a 10% buffered formalin solution and were identified by the animal's number. The respective contra-lateral (left) tibias were removed in the same way and were submitted to bone densitometry to test for equivalence between the groups.
The results of the bone defect consolidation obtained in the PRP and BMC groups were compared using digital radiography, computed tomography, and histology by histomorphometry.
Digital radiography was used to assess whether consolidation had occurred. This technique also verified the incidence of fractures in each tibia (an exclusion criterion).
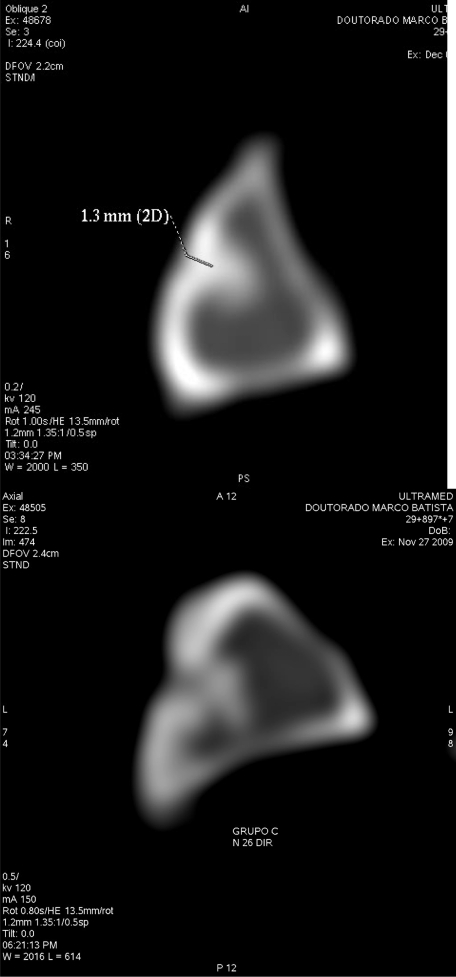
The thickness of the newly formed cortical wall was measured from the volumetric axial acquisition using a tomographic device with multiple rows of detectors followed by reconstruction in the planes of the cortical bone defects (Figures 2A and 2B).
Figure 2.
Axial CT image of the tibia of an animal in the PRP group showing the consolidation of newly formed bone defect and a cortical thickness of 1.3 mm (a) and the BMC group showing no consolidation of the cortical bone defect (b).
Imaging test evaluations were performed by experienced radiologists, and in the case of the radiological evaluations, the evaluations were performed jointly by two radiologists (by consensus).
After the imaging tests, the right tibias were sent for bone histomorphometry, were decalcified, washed, and sliced, and the regions of the bone defects were submitted to routine histology. Slides with 4-µm-thick histological sections were stained using hematoxylin-eosin (HE). The relative formation (%) of bone tissue in each sample was measured using a Zeiss® optical microscope (40X magnification) with the aid of an image analyzer.
The frequencies of consolidation of the defects were compared between the PRP and BMC groups, as measured by digital radiography, according to Fisher's exact test (nominal samples). The normality of the distributions of the parameters of the thickness of the newly formed cortical wall (mm), as measured by CT scan, and of the bone formation (%), as determined by histomorphometry, were confirmed by Pearson's coefficient of variation (PCV<30%) and the Kolmogorov-Smirnov test (KS: p>0.05). Student's t-test for two independent parametric samples was used to compare the results for cortical wall thickness (mm) and bone formation (%) of the BMC and PRP groups. A confidence level of 5% was adopted (α = 0.05), and these statistical tests were two-dimensional.
RESULTS
The results for the defect consolidation, newly formed cortical wall thickness (mm), and bone formation (%) are shown in Tables 1, 2, and 3, respectively.
Table 1.
Measurements of the consolidation of defects filled with PRP+βTCP and BMC+βTCP using radiographic evaluation. Comparison by Fisher's exact test (α = 0.05 bilateral).
| CONSOLIDATION-RADIOGRAPHICEVALUATION | GRAFT MATERIAL | |||
| PRP+βTCP | BMC+βTCP | |||
| n | % | n | % | |
| Consolidation | 7 | 43.8 | 7 | 43.8 |
| No consolidation | 0 | 0.0 | 1 | 6.2 |
| Fracture1 | 1 | 6.2 | 0 | 0.0 |
| TOTAL | 8 | 50.0 | 8 | 50.0 |
| Fisher | p = 1.00 | |||
Tibial fracture, case not included in the statistical analysis.
Table 2.
Cortical thickness (mm) formed after bone consolidation in defects filled with PRP+βTCP and BMC+βTCP, measured using computed axial tomography. Normality assessment using Pearson's Coefficient of Variation (PCV) and the Kolmogorov-Smirnov test (KS). Comparison between grafts using Student's t-test (α = 0.05 bilateral).
| CORTICALTHICKNESS (mm) | GRAFT MATERIAL | |
| PRP+βTCP | BMC+βTCP | |
| M | 1.96 | 1.00 |
| SD | 0.36 | 0.26 |
| SEM | 0.13 | 0.15 |
| MAX | 2.3 | 1.2 |
| MIN | 1.3 | 0.7 |
| N | 7 | 3 |
| PCV (%) | 18.39 | 26.46 |
| KS (p) | >0.10 | - |
| Student's t-test | t = 4.10 | p = 0.004* |
Table 3.
Bone tissue formed by area (%) after consolidation of defects filled with PRP+βTCP or BMC+βTCP, as measured using histomorphometry. Normality assessment using Pearson's Coefficient of Variation (PCV) and the Kolmogorov-Smirnov test (KS). Comparison using Student's t-test (α = 0.05 bilateral).
| BONE TISSUE FORMED (%) | GRAFT MATERIAL | |
| PRP+βTCP | BMC+βTCP | |
| M | 65.02 | 36.88 |
| SD | 5.71 | 9.05 |
| SEM | 2.16 | 3.42 |
| MAX | 72.13 | 48.17 |
| MIN | 59.07 | 26.39 |
| N | 7 | 7 |
| PCV (%) | 8.78 | 24.55 |
| KS (p) | >0.10 | >0.10 |
| Student's t-test | t = 6.96 | p≅0.00* |
DISCUSSION
Platelet-rich plasma8-11,23, and centrifuged bone marrow aspirate12-15, are agents with proven osteoinductive activities and are often used in orthopedic bone reconstruction surgery. We compared the effects of platelet-rich plasma and centrifuged bone marrow aspirate combined with a commercial synthetic osteoconductive material (ß tricalcium phosphate) on the consolidation of bone defects. Autogenous bone grafts that use ground cortical-spongy bone are considered to be the gold standard. These grafts exhibit proven osteoinductive and osteoconductive properties that overlap with those of the agents studied22,23 and that would hinder the assessment of differences between PRP and BMC.
Unlike other reports,28 no blood samples were collected for the platelet counts in the present study because the amount of blood available (9 ml) was insufficient. We opted to carry out only one centrifugation according to the Arenas protocol.27 One-step centrifugation was sufficient to obtain a platelet concentration that was approximately six times that of plasma, which is 320,000 platelets/ml according to the work of Wilson.28 The platelet concentration obtained with one centrifugation is approximately 2,000,000 platelets/ml, which is sufficient for the effects of PRP.27
The tibias of seven rabbits from each group were evaluated using computed tomography and histomorphometry. In the PRP group, two rabbits died due to complications during surgery, and another rabbit presented with a fracture of the tibia. In the BMC group, one animal was excluded because of a technical problem with the cell viability test for the sample of centrifuged bone marrow aspirate, and another was excluded due to self-mutilation of the operated limb. One case did not present with consolidation.
The radiographs did not provide quantitative data on bone consolidation. However, these radiographs provided a qualitative assessment of the occurrence or the lack of consolidation. These radiographs also allowed for the confirmation of the presence of fractures during the postoperative period (an exclusion criterion). In the PRP group, all of the cases presented consolidation. One case was excluded from the analysis due to the presence of a fracture of the tibia in the region of the defect. In the BMC group, one case did not present with consolidation. However, no statistically significant difference in occurrence of bone healing was found between the two groups (p = 1.00) (Table 1).
The CT scan proved to be more reliable for confirming the consolidation of the cortical defects of the tibia, and this scan also enabled a quantitative assessment of the bone formation. The thickness of the cortical region of the defect that formed in the PRP group was found to be greater (p = 0.004) than that of the BMC group (Table 2).
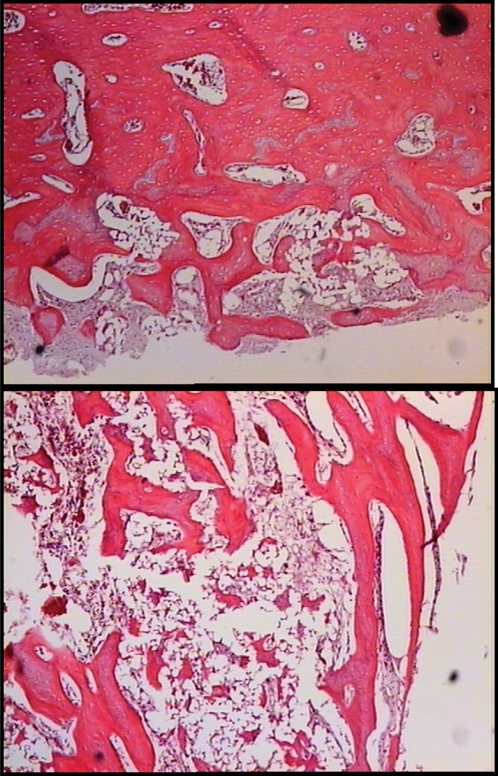
Histomorphometry of the samples from the PRP group demonstrated greater relative bone formation per area (p≅0.00) than the samples from the BMC group (Table 3). In the photographic images that were obtained from the slides of the BMC group, a lower bone tissue density and a low regenerated trabecular density were observed (Figure 3).
Figure 3.
Photomicrographs of bone defect consolidation in an animal in the PRP group (a) and the BMC group (b).
During the four-week period, the larger amount of consolidation that was observed in the PRP group than in the BMC group may be explained by the relative differences in the factors that were observed in the blood compositions of these groups. The PRP group presented with significantly greater amounts of blood platelets and the fundamental proteins secreted by these platelets, such as platelet-derived growth factor (PDGF), transforming growth factor β (TGF β1 and TGF β2), vascular endothelial growth factor (VEGF), and epithelial growth factor (EGF),28 than the BMC group. This finding is important because these proteins are needed to begin the healing process in all tissue types.
PDGF is the first and principal growth factor that is secreted during any tissue damage, i.e., its action initiates the process of induction and differentiation of connective tissue, which culminates in healing of the injury.24
The mesenchymal stem cells of the centrifuged bone marrow may require a longer period of time for consolidation, similar to that obtained by the platelet-rich plasma (wall thickness). However, based on the results of the histomorphometry, it is unlikely that BMC produces the same quality of bone as PRP because we observed a significantly greater amount of fibrosis formation in the interstices of the newly formed bone in the region of the consolidated defect when BMC was used, and these tissues would be difficult to replace later.
CONCLUSION
Better consolidation, as indicated by the formation of thicker cortical walls and a higher relative quantity of bone tissue per area, was observed in the group that was treated with platelet-rich plasma and ß tricalcium than in the animals that were treated with centrifuged bone marrow aspirate and ß tricalcium phosphate.
The platelet-rich plasma promoted a more effective consolidation than the centrifugation of the bone marrow aspirate.
ACKNOWLEDGEMENTS
We thank Mr. Eduardo Arnhold, Director of Welfare Odontological Surgical Product Imports, for the donation of the osteoconductive agent.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Alonso JE, Lee J, Burgess AR, Browner BD. The management of complex orthopedic injuries. Surg Clin North Am. 1996;76:879–903. doi: 10.1016/s0039-6109(05)70486-2. 10.1016/S0039-6109(05)70486-2 [DOI] [PubMed] [Google Scholar]
- 2.Instituto Brasileiro de Geografia e Estatistica – IBGE(BR) Revisao 2008. Informacao demografica e socioeconomica numero 24. Rio de Janeiro: IBGE; 2008. Projecao da populacao do brasil por sexo e idade – 1980-2050. [Google Scholar]
- 3.Einhorn T. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998:S7–21. doi: 10.1097/00003086-199810001-00003. 10.1097/00003086-199810001-00003 [DOI] [PubMed] [Google Scholar]
- 4.Croci AT. Retarde de consolidacao e pseudoartrose. Acta Ortop Bras. 1997;5:26–34. [Google Scholar]
- 5.Guarniero R, Vaz CES, Santana PJ, Molin ED, Braun J, Harada MS. Avaliacao do efeito do ibandronato na consolidacao de fratura: Estudo experimental em coelhos. Rev Bras Ortop. 2007;42:254–60. [Google Scholar]
- 6.Santana PJ. Sao Paulo: Faculdade de Medicina da Universidade de Sao Paulo; 1999. Estudo da consolidacao de fraturas na desnutricao proteica: trabalho experimental com o uso de alendronato em ratos [tese] [Google Scholar]
- 7.De Long WG, Jr, Einhorn TA, Koval K, Mckee M, Smith W, Sanders, et al. Bone grafts and bone grafts substitutes in orthopaedic trauma surgery. J Bone Joint Surg Am. 2007;89:649–58. doi: 10.2106/JBJS.F.00465. 10.2106/JBJS.F.00465 [DOI] [PubMed] [Google Scholar]
- 8.Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxilofac Implants. 1999;14:529–35. [PubMed] [Google Scholar]
- 9.Whitman DH, Berry RL. A technique for improving the handling of particulate cancellous bone and marrow grafts using platelet gel. J Oral Maxilofac Surg. 1998;56:1217–8. doi: 10.1016/s0278-2391(98)90776-5. 10.1016/S0278-2391(98)90776-5 [DOI] [PubMed] [Google Scholar]
- 10.Andrew JG, Hoyland JA, Fremont AJ, Marsh DR. Platelet-derived growth factor expression in normally healing human fractures. Bone. 1995;16:455–60. doi: 10.1016/8756-3282(95)90191-4. [DOI] [PubMed] [Google Scholar]
- 11.Lind M, Schumaker B, Soballe K, Keller J, Melsen F, Bunger C. Transforming growth factor-beta enhances fracture healing in rabbit tibiae. Acta Orthop Scand. 1993;64:553–6. doi: 10.3109/17453679308993691. 10.3109/17453679308993691 [DOI] [PubMed] [Google Scholar]
- 12.Connoly JF. Injectable bone marrow preparations to stimulate osteogenic repair. Clin Orthop Relat Res. 1995;313:8–18. [PubMed] [Google Scholar]
- 13.Connoly J. Clinical use of marrow osteoprogenitor cells to stimulate osteogenesis. Clin Orthop Relat Res. 1998;355:257–66. doi: 10.1097/00003086-199810001-00026. 10.1097/00003086-199810001-00026 [DOI] [PubMed] [Google Scholar]
- 14.Paley D, Young MC, Wiley AM, Fornasier VL, Jackson RW. Percutaneus bone marrow grafting of fractures and bone defects.An experimental study in rabbits. Clin Orthop Relat Res. 1986;208:300–12. [PubMed] [Google Scholar]
- 15.Sharma S, Garg NK, Veliath AJ, Subramanian S, Srivastava KK. Percutaneous bone marrow grafting of osteotomies and bony defects in rabbits. Acta Orthop Scand. 1992;63:166–9. doi: 10.3109/17453679209154815. 10.3109/17453679209154815 [DOI] [PubMed] [Google Scholar]
- 16.Urist MR. Bone formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. 10.1126/science.150.3698.893 [DOI] [PubMed] [Google Scholar]
- 17.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. J Bone Joint Surg Am. 2002;84:1032–44. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Hannallah D, Peterson B, Lieberman JR, Fu FH, Huard J. Gene therapy in orthopaedic surgery. J Bone Surg Am. 2002;84:1046–61. 10.1302/0301-620X.84B7.13195 [PubMed] [Google Scholar]
- 19.Donley BG, Ward DM. Implantable electricalstimulation in high-risk hindfoot fusion. Foot Ankle Int. 2002;23:13–8. doi: 10.1177/107110070202300103. [DOI] [PubMed] [Google Scholar]
- 20.Esquisatto MAM, Levada VMO, Mendonca JS, Dalge G, Santos GT, Mendonca FS. Efeitos de diferentes intensidades de microcorrente no reparo osseo em ratos Wistar. Rev Bras Ortop. 2006;41:331–5. [Google Scholar]
- 21.Valchanov VD, Michailov P. High energy shock waves in the treatment of delayed and non-union of fractures. Int Orthop. 1991;15:181–4. doi: 10.1007/BF00192289. [DOI] [PubMed] [Google Scholar]
- 22.Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions. Injury. 2007;38:S75–S80. doi: 10.1016/j.injury.2007.02.012. 10.1016/j.injury.2007.02.012 [DOI] [PubMed] [Google Scholar]
- 23.D'Elia CA. Sao Paulo: Faculdade de Medicina da Universidade de Sao Paulo; 2009. Comparacao entre o uso do plasma rico em plaquetas associado com aspirado de medular ossea ao enxerto autologo de iliaco na consolidacao das osteotomias da tibia proximal: estudo prospectivo randomizado [dissertacao] [Google Scholar]
- 24.Delgado R, Bonatelli APF, Alves MTS. Estudo sobre a associacao de ceramica a plasma rico em plaquetas na coluna vertebral de ratos. Acta Ortop Bras. 2009;17:282–5. 10.1590/S1413-78522009000500006 [Google Scholar]
- 25.Vaz CES. Sao Paulo: Faculdade de Medicina da Universidade de Sao Paulo; 2006. Avaliacao do efeito de centrifugado osteogenico de medulla ossea na consolidacao de fratura: estudo experimental em coelhos [tese] [Google Scholar]
- 26.Canadian Council on Animal Care CCAC. Guide to the care and use of experimental animals; 1998. Available at: http://www.ccac.ca/en/CCAC_Programs/Guidelines_Policies/GUIDES/SPANISH/toc_v1.htm.
- 27.Arenas GCF. Obtencao do plasma rico em plaquetas para infiltracoes e procedimentos cirurgicos. Sao Paulo: 2010. Protocolo Cantadori. (Registrado na Biblioteca Nacional do RJ, N° Registro: 508.102, Livro:962, Folha: 379). [Google Scholar]
- 28.Wilson EMK, Barbieri HB, Mazzer N. Estimulação da cicatrização óssea pelo plasma rico em plaquetas. Estudo experimental em coelhos. Acta Ortop Bras. 2006;14:208–12. 10.1590/S1413-78522006000400006 [Google Scholar]