Abstract
Growing evidence indicates that neuroinflammation can alter adult neurogenesis by mechanisms as yet unclear. We have previously demonstrated that the neuroinflammatory response and neuronal damage after lipopolysaccharide (LPS) injection is reduced in cyclooxygenase-1 deficient (COX-1-/-) mice. In this study, we investigated the role of CoX-1 on hippocampal neurogenesis during LPS-induced neuroinflammation, using COX-1-/- and wild-type (WT) mice. We found that LPS-induced neuroinflammation resulted in the decrease of proliferation, survival and differentiation of hippocampal progenitor cells in WT but not in COX-1-/- mice. Thus, we demonstrate for the first time that COX-1 is involved in the inhibition of BrdU progenitor cells in proliferation and hippocampal neurogenesis after LPS. These results suggest that COX-1 may represent a viable therapeutic target to reduce neuroinflammation and promote neurogenesis in neurodegenerative diseases with a strong inflammatory component.
Key words: neurogenesis, cyclooxygenase-1, lipopolysaccharide, inflammation, brain
Introduction
Adult neurogenesis occurs in the dentate gyrus (DG) of the hippocampus1,2 and in the subventricular zone (SVZ) of the lateral ventricle.3 Hippocampal neurogenesis plays a role in the maintenance of normal hippocampal function, including learning and memory.4,5 Hippocampal stem cells (HSCs) lie in the subgranular zone (SGZ), along the border between the hilus and the granule cell layer (GCL).6 Many of the HSCs die shortly after their birth,7 whereas the surviving cells migrate into the dentate GCL, differentiate into granule cells8 and project their axons to the CA3 region resulting in their morphological and functional integration into the existing hippocampal circuitry.9 The formation of new granule cells in the DG can be affected by a large number of physiological and pathological stimuli, such as aging and neurodegenerative diseases.10–13
Neuroinflammation is thought to be involved in the pathophysiology of several neurodegenerative diseases, including Alzheimer disease.14 Inflammation can affect the microenvironment of the neurogenic niche15 and influence proliferation of progenitor cells, survival and migration of new neuroblasts, differentiation of new neuroblasts to specific neuronal phenotypes and development of functional synaptic connectivity.16,17
Neuroinflammation is mediated by microglial activation and subsequent release of pro-inflammatory mediators such as prostaglandins, cytokines, chemokines and reactive oxygen or nitrogen species.18 Prostaglandin H synthases or cyclooxygenases (COX)-1 or -2 catalyze the conversion of arachidonic acid to prostaglandins.18 Because of its localization in microglia, and thus, being able to immediately synthesize prostaglandins in response to microglial activation, COX-1 recently emerged as a major player in the acute inflammatory response.19–21 A recent study also suggested a role for COX-1 expressed in brain perivascular cells in mediating the immune-to-brain signaling in response to a systemic lipopolysaccharide (LPS) challenge.22
In this study, we used mice with a null mutation of the COX-1 gene to investigate the role of COX-1 on hippocampal neurogenesis during acute neuroinflammation induced by intracerebroventricular injection of LPS.18 LPS binds to a CD14 receptor and together with the extracellular adaptor protein MD-2 binds to the toll-like receptor 4 (TLR4) expressed by microglia and astrocytes,23 causing a massive glial activation accompanied by a robust and transient transcriptional activation of genes encoding for pro-inflammatory mediators.18
We have previously shown that the genetic deletion or pharmacological inhibition of COX-1 significantly attenuated glial activation, release of pro-inflammatory markers, as well as blood-brain barrier disruption and recruitment of peripheral leukocytes to the inflamed brain in response to LPS.18,20,24 Since LPS has been shown to decrease hippocampal neurogenesis,12,25,26 in this study we investigated whether COX-1 gene deletion positively modulates hippocampal neurogenesis during LPS-induced neuroinflammation. We examined the proliferation, survival and differentiation of neuronal progenitor cells in the DG after LPS injection and demonstrated for the first time that COX-1 gene deletion resulted in an increased proliferation and differentiation of hippocampal progenitor cells in the adult mouse brain during neuroinflammation.
Results
LPS injection reduces proliferation of hippocampal progenitor cells in WT but not in COX-1-/- mice.
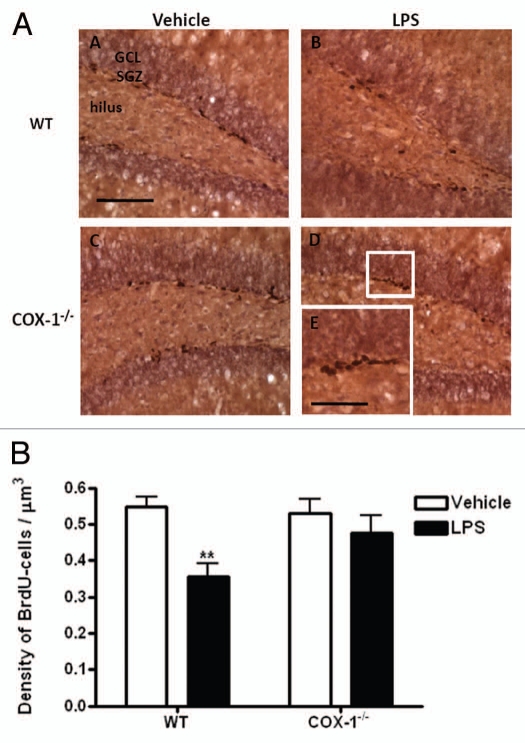
To evaluate progenitor cells proliferation in the DG, BrdU-labeled cells were counted 24 h after LPS injection. The distribution of the BrdU-labeled proliferative cells was found mainly in clusters at the border between the granule cell layer and the hilus, regardless of treatment or genotype (Fig. 1A). Within the area encompassing the granule cell layer and subgranular zone (Fig. 1A), LPS treatment decreased by 35% the mean density of BrdU-positive cells in WT mice (0.36 ± 0.16 vs. 0.55 ± 0.027 in vehicle-treated WT mice, p < 0.01, Fig. 1B). In contrast, the number of BrdU-labeled proliferative cells was not significantly different in LPS-injected COX-1-/- compared with vehicle-injected COX-1-/- mice (0.47 ± 0.05 vs. 0.53 ± 0.04) (Fig. 1B).
Figure 1.
(A) Hippocampal BrdU proliferative cells after LPS-induced neuroinflammation in COX-1-/- and Wt mice. Representative photomicrographs of BrdU immunohistochemistry in the DG for WT (A and B) and COX-1-/- mice (C and D) euthanized 24 h after i.c.v. injection of LPS or vehicle. High-magnification images of BrdU immunostaining is shown in (E). Scale bars = 100 µm (A–D); 50 µm (E). (B) Hippocampal BrdU-proliferative cells after LPS-induced neuroinflammation in COX-1-/- and WT mice. Quantification of BrdU-labeled cells per µm3 of DG, in WT and COX-1-/- mice euthanized 24 h after i.c.v. injection of LPS or vehicle. Data are means ± SEM (n = 5, vehicle-injected; n = 6, LPS-injected), **p < 0.01.
LPS injection reduces hippocampal neurogenesis in WT but not in COX-1-/- mice.
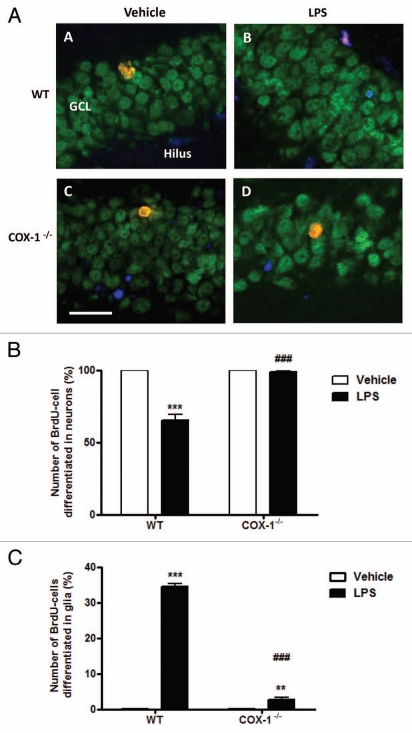
To determine the fate of new hippocampal cells, we examined the phenotype of newly BrdU-positive cells generated 4 weeks after LPS injection by concomitant immunolabeling of BrdU with neuronal (NeuN) and glial (S100β) markers (Fig. 2A). S100β, a marker for astrocytes and oligodendrocytes, labels cell bodies with a better definition than GFAP. The percentage of BrdU-positive cells that co-localized with NeuN immunolabeling was significantly reduced by 35% in LPS-injected compared with vehicle-injected WT mice (p < 0.001). In contrast, the number of neurons did not differ significantly in LPS-injected COX-1-/- mice as compared with vehicle-injected COX-1-/- mice (97.3% ± 0.6% vs. 100% ± 0%; Fig. 2B). Furthermore, LPS-injected WT mice showed a 35% increase (p < 0.001) in the number of BrdU-positive cells that differentiated into glial cells compared with vehicle-injected mice (p < 0.001; Fig. 2C). Only a small percentage of BrdU-positive cells differentiated into glia in LPS-injected COX-1-/- mice compared with their vehicle-injected controls (2.7% ± 0.7% vs. 0%, p < 0.01; Fig. 2C).
Figure 2.
(A) Hippocampal BrdU-cells differentiation after LPS-induced neuroinflammation in COX-1-/- and WT mice. Confocal images of DG for WT (A and B) and COX-1-/- mice (C and D) euthanized 4 weeks after i.c.v. injection of LPS or vehicle. Sections were triple-labeled for BrdU (red), NeuN (green) and S100β (blue). Scale bars = 20 µm. (B) Differentiation of new hippocampal cells 4 weeks after LPS-induced neuroinflammation in COX-1-/- and WT mice. Quantification of percentage of BrdU-cells differentiated into neurons in WT and COX-1-/- mice after 4 weeks of i.c.v. injection of LPS or vehicle. Data are means ± SEM (n = 4), ***p < 0.001, LPS-injected WT mice vs. vehicle-injected WT mice; ###p < 0.001, LPS-injected COX-1-/- mice vs. LPS-injected WT mice. (C) Differentiation of new hippocampal cells 4 weeks after LPS-induced neuroinflammation in COX-1-/- and WT mice. Quantification of percentage of BrdU-cells differentiated into glia in WT and COX-1-/- mice 4 weeks after i.c.v. injection of LPS or vehicle. Data are means ± SEM (n = 4), ***p < 0.001, LPS-injected WT mice vs. vehicle-injected WT mice; **p < 0.01, LPS-injected COX-1-/- mice vs. vehicle-injected COX-1-/- mice; ###p < 0.001, LPS-injected WT mice vs. LPS-injected COX-1-/- mice.
COX-1 is involved in the LPS-induced survival effect of new hippocampal cells.
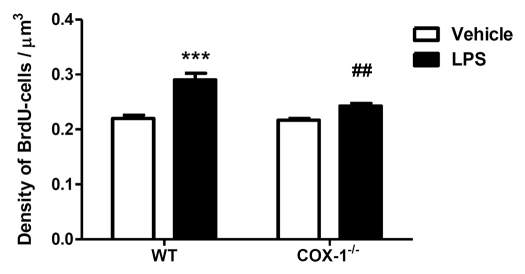
To test whether COX-1 is involved in the post-mitotic BrdU-cell survival, we analyzed the density of BrdU-cells in the granule cell layer and subgranular zone of DG 4 weeks after LPS injection in COX-1-/- and WT mice. LPS significantly increased the density of BrdU-survival cells compared with vehicle-injection in WT mice (0.29 ± 0.01 vs. 0.22 ± 0.006, 24%, p < 0.001), but did not affect survival of new cells in COX-1-/- mice compared with their respective vehicle-injected controls (0.24 ± 0.005 vs. 0.22 ± 0.003) (Fig. 3).
Figure 3.
Survival of new hippocampal cells 4 weeks after LPS-induced neuroinflammation in COX-1-/- and WT mice. Quantification of BrdU-labeled cells per µm3 of DG in WT and COX-1-/- mice 4 weeks after i.c.v. injection of LPS or vehicle. Data are means ± SEM (n = 4), ***p < 0.001, LPS-injected WT mice vs. vehicle-injected WT mice; ##p < 0.01, LPS-injected COX-1-/- vs. LPS-injected WT mice.
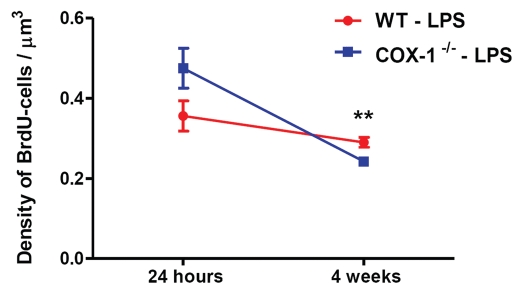
When we compared the survival BrdU-cells at 4 weeks with the BrdU-cells present at 24 h in the DG, after LPS or vehicle injection in WT and COX-1-/- mice, the density of surviving BrdU-cells was drastically decreased. In particularly, the surviving BrdU-cells in vehicle-injected WT and COX-1-/- mice was drastically reduced by 60% after 4 weeks (p < 0.001). LPS-injected COX-1-/- mice showed a minor reduction of surviving BrdU-cells after 4 weeks (49%), whereas the number of BrdU-cells was not significantly affected in LPS-injected WT mice (Fig. 4).
Figure 4.
Comparison between survival of BrdU-labeled cells at 24 h and 4 weeks after LPS injection in WT and COX-1-/- mice. The surviving BrdU-cells in LPS-injected COX-1-/- mice was severely reduced by 49% after 4 weeks, whereas the number of BrdU-cells was not significantly affected in LPS-injected WT mice. Data are means ± SEM (n = 5, 24 h after LPS; n = 4, 4 weeks after LPS); **p < 0.01, LPS-injected COX-1-/- vs. LPS-injected WT mice, 4 weeks after injection.
Discussion
In this study we examined the role of COX-1 in the proliferation, survival and differentiation of new hippocampal progenitor cells after LPS-induced neuroinflammation. We found that LPS-induced neuroinflammation altered the proliferation of progenitor cells, the production rate of new neurons, and the survival of new hippocampal progenitor cells in WT but not in COX-1-/- mice.
Previous data from our lab showed that COX-1 plays a critical role in the neuroinflammatory response to LPS18 or β-amyloid injection.27 Specifically, COX-1 gene deletion or treatment with the COX-1 specific inhibitor SC-560 resulted in decreased glial activation, reduced expression of pro-inflammatory cytokines and chemokines, and limited neuronal damage after central injection of LPS.18 Taken together these data suggest that during neuroinflammation, COX-1 plays a critical role in the propagation of the inflammatory response and in the modulation of the regenerative potency of hippocampal progenitor cells.
COX-1 is predominantly expressed by microglia and perivascular cells, and thus, can be activated, within seconds to minutes, following an acute challenge, and produce prostaglandins that contribute to the inflammatory response.28,29 Activation of COX-1-expressing microglia and subsequent release of pro-inflammatory mediators such as IL-1β, IL-6, TNFα, NO and prostanoids, which have antineurogenic effects, could be responsible for the adverse effects of inflammation on neurogenesis.12,30
TLR4 is abundantly expressed by neural stem/progenitor cells and the absence of TLR4 results in enhanced proliferation and neuronal differentiation,31 suggesting that TLR4 directly modulates self-renewal and the cell-fate decision of neuronal progenitor cells.31 Rolls et al. (2007) reported that LPS treatment decreases the proliferation and differentiation of cultured neural stem/progenitor cells via a nuclear factor kappaB (NFκB) signaling TLR-4 dependent.31
Our results show that inhibition of the proliferation and differentiation of progenitor cells after LPS injection can be caused by the inflammatory environment, and particularly, through an indirect effect on hippocampal progenitor cells. Indeed, neuronal progenitor cells do not express COX-1 (data not shown) either under physiological conditions or after LPS. Thus, the positive modulation of neurogenesis by COX-1 deletion could be an indirect effect attributable to the attenuation of the neuroinflammatory response.18 In this regard, the transcription factors STAT3 and NFκB, which are upregulated after LPS-induced inflammation,18,31 induce the transcription of many pro-inflammatory genes18 and play a critical role in controlling the proliferation of neuronal stem cells32 and neuronal differentiation.18 Supporting a role for these transcription factors, our group has previously demonstrated that, in response to LPS, COX-1-/- mice or WT treated with SC-560 have decreased activation and translocation of NFκB and STAT-3, and reduced levels of the pro-inflammatory cytokines IL-1β, IL-6 and TNFα.18
The differentiation of neuronal progenitor cells toward neuronal or glial lineage is determined by specific signaling cascades regulating the activation of different transcription factors. Multiple processes directing cell fate determination can be modulated by different cytokines and transcription factors. IL-6, TNFα and IL-132,33 promote astrocytic differentiation by activating the transcription factors STAT3 and NFκB.34,35 Furthermore, activation of cytokine receptors on neuronal progenitor cells stimulates the Notch1 pathway signaling, which inhibits neuronal differentiation through a hairy-enhancer-of split 1 (Hes-1)-mediated mechanism.15,36 Notch signaling increases the expression of Hes-1,36 which antagonizes pro-neural basic helix-loop-helix transcription factors and represses the commitment of neuronal progenitor cells to a neuronal fate.37 The cross-talk of all these transcription factors and their co-activators could modulate astrocytic fate specification in hippocampal progenitor cells after LPS-induced neuroinflammation. Furthermore, LPS injection downregulates the expression of NeuroD2, a marker of neuronal progenitor cells, which is thought to play a role in the determination and maintenance of the neuronal cells fate.38
Functional stimuli, including learning, memory and environmental enrichment can increase the number of surviving BrdU-cells by a “survival promoting effect.”39,40 In our study, we showed that neuroinflammation also has a survival promoting effect on new hippocampal progenitor cells. After 4 weeks of LPS injection, WT mice showed a 24% increase in surviving BrdU-cells compared with vehicle-injected WT mice. This LPS-induced survival promoting effect was not observed in COX-1-/- mice. This could be due to the reduced neuroinflammatory response observed in COX-1-/- mice, as neuroinflammation could trigger a survival effect in the new hippocampal progenitor cells to replace damaged neurons in the inflamed brain, although many new progenitor cells will differentiate into glial cells.
When we compare the survival of BrdU positive cells 24 h and 4 weeks after LPS injection, COX-1-/- mice showed a strong decrease in surviving cells that was not observed in WT mice (49% vs. 17%, respectively). This difference could be linked to the reduction of inflammatory response and neuronal damage in COX-1-/- mice after LPS, which would make unnecessary for new hippocampal cells to survive as in vehicle-injected WT and COX-1-/- mice.
New hippocampal neurons that survive chronic inflammatory stress differentiate and integrate as granule neurons, although with a heightened degree of synaptic plasticity.41,42 Recent work showed that new neurons exposed to the chronic pathological environment are severely controlled,41,43,44 and can exhibit subtle changes in development of dendritic arborizations and spine density,45 which are dependent on the characteristics of the pathological environment.45 Apical dendrites reached the molecular layer during the first 2 weeks after their birth, and the dendritic arborizations and the development of spines occurs during the first 3–4 weeks.41 Since pilot studies in our lab showed that LPS-induced neuroinflammation peaks at 24 h and then resolves in few days (Choi et al. 2008), one could speculate that new hippocampal cells develop normally and integrate into the hippocampal network, without undergoing morphological or functional changes. However, only electrophysiological studies can confirm a functional integration of new hippocampal cells into the hippocampal functional circuitry.
Neuroinflammation is a complex mechanism, in which different pathways, intracellular and extracellular, can interact and both directly and indirectly modulate the neurogenic niche. In addition to a TLR4-dependent mechanism, we suggest that COX-1 plays a role in the inhibition of hippocampal neurogenesis after LPS injection.32,34,46 COX-1-derived prostaglandins, such as PGE2, could directly affect the neurogenic niche, although it remains to be fully elucidated which type(s) of prostaglandins and downstream receptors play a role in the modulation of neurogenesis.42 A recent study by Keen et al. (2009) tested the hypothesis that activation of specific PGE2 receptors is responsible for suppression of hippocampal neurogenesis after LPS-induced neuroinflammation. They demonstrated that EP1, but not EP2, is expressed by progenitor cells. EP1 receptor gene deletion protected hippocampal progenitor cells from PGE2-mediated toxicity directly and also indirectly by partially suppressing microglia activation and the propagation of inflammatory response. In contrast, EP2 was not detectably expressed by progenitor cells, and its ablation did not protect from direct toxic effect of PGE2.
Overall, our past data suggest that the inhibition of hippocampal neurogenesis by LPS in WT, but not COX-1-/- mice, could be linked to the increase in NFκB activation, expression of pro-inflammatory cytokines, propagation of neuroinflammation and PGE2 levels, all of which are attenuated by COX-1 gene deletion.18 We demonstrate for the first time that in LPS-induced neuroinflammation, COX-1 activity is detrimental to the generation of new hippocampal progenitor cells. Thus, COX-1 inhibition may represent a viable therapeutic target to promote neurogenesis in neurodegenerative diseases with a strong inflammatory component, where neuronal loss and memory deficits are known to occur.
Materials and Methods
Animals and procedures.
All animal procedures were performed under an animal study proposal approved by the National Institutes of Health (NIH) Animal Care and Use Committee, in accordance with NIH guidelines on the care and use of laboratory animals. Three-month-old male WT and homozygous (COX-1-/-) mice on a C57BL/6-129/Ola genetic background were used.47 Vehicle (sterile saline, 5 µl) and LPS (Escherichia coli serotype 0127:B8; 5 µg in 5 µl of sterile saline) were administered by intracerebroventricular (i.c.v.) injection into the lateral ventricle as previously described.18
5-bromo-2′-deoxyuridine (BrdU) was used to label the neuronal progenitor cell in proliferation. Mice were given 2 BrdU injections with an interval of 12 h, the first 30 min prior to LPS injection (dissolved in 0.9% NaCl; 50 mg/Kg; 10 mg/ml, i.p.; Sigma Aldrich, St. Louis, MO). To detect the proliferation of hippocampal progenitor cells, mice were euthanized 24 h after LPS injection. To detect the phenotype of new born progenitor cells, mice were euthanized 4 weeks after LPS injection.
DNA denaturation, immunohistochemistry and immunoflourescence.
Immunohistochemistry and immunofluorescent triple labeling for BrdU were performed on free-floating 40 µm sagittal sections that were pretreated by denaturing DNA, as described previously in reference 48. The antibodies were mouse BrdU-antibody (1:100, DAKO, Denmark), rat anti-BrdU for triple labeling (1:200; Accurate Chemical and scientific, Westbury NY), rabbit anti-S-100β (1:200; Abcam, Cambridge, MA), mouse anti-NeuN (1:1,500; Chemicon, Billerica, MA). For immunohistochemistry the peroxidase method (ABC system) with biotinylated donkey anti-mouse IgG antibodies and diaminobenzidine as chromogen was used (Vector laboratories). Immunohistochemistry images were detected with a light microscope Olympus CKX41, using X40 and X60 objective (Olympus). The fluorescent antibodies 488-Alexa Flour anti-mouse IgG (1:500), 594-Alexa Fluor anti-rat IgG (1:500) and 405-Alexa Fluor anti-rabbit IgG (1:500) (Invitrogen, Carlsbad, CA) were used. Fluorescent signals were detected and processed using an inverted confocal microscope (model IX81, Olympus America Inc., Center Valley, PA) with Fluoview 1000 scanning head. Fluorescent images were acquired using a UPlanSApo X10 numerical aperture (NA) 0.4 dry objective and a UPlanSApo X60 NA 1.42 oil immersion objective (Olympus America Inc.), and were processed using Imaris 5.7 (Bitplane) and assembled using Adobe Photoshop CS.
Cell counting and volumetric analysis.
BrdU-labeled cells were counted in the subgranular zone, which was defined as a 2-nucleus-wide band below the apparent border between the GCL and the hilus.8 We detected one every six sections and covered the entire area of dentate gyrus. To measure the DG area, slices were stained with hematoxylin (Vector, Burlingome, CA) and the area was traced using ImageJ Software. The mean density of BrdU-labeled cells in each mouse was calculated as the total number of labeled nuclei divided by the volume of DG (cell density per µm3).49 To determine the percentage of neuronal differentiation of newborn cells, one every six sections was analyzed for the entire area of dentate gyrus. For each animal 50 BrdU-positive cells were randomly selected and analyzed.49
Statistical analysis.
All data are expressed as means ± SEM. Statistical significance was assessed with a two-way ANOVA followed by Bonferroni's post hoc test using GraphPad Prism. Significance was taken at p < 0.05.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIA. We would like to thank Henriette Van Praag for useful discussion and technical advice, Yosuke Mukoyama for kindly providing microscopy and Erik Runko for critically reading and editing this manuscript.
References
- 1.Laplagne DA, Esposito MS, Piatti VC, Morgenstern NA, Zhao C, van Praag H, et al. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS Biol. 2006;4:409. doi: 10.1371/journal.pbio.0040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, et al. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 3.Doetsch F, Scharff C. Challenges for brain repair: insights from adult neurogenesis in birds and mammals. Brain Behav Evol. 2001;58:306–322. doi: 10.1159/000057572. [DOI] [PubMed] [Google Scholar]
- 4.Jessberger S, Clark RE, Broadbent NJ, Clemenson G, Jr, Consiglio A, Lie DC, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 6.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 7.Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. doi: 10.1016/S0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- 8.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 9.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Limke TL, Rao MS. Neural stem cells in aging and disease. J Cell Mol Med. 2002;6:475–496. doi: 10.1111/j.1582-4934.2002.tb00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, et al. Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse. 2010;64:721–728. doi: 10.1002/syn.20800. [DOI] [PubMed] [Google Scholar]
- 12.Russo I, Barlati S, Bosetti F. Effects of Neuroinflammation on the Regenerative Capacity of Brain Stem Cells. J Neurochem. 2010;116:947–956. doi: 10.1111/j.1471-4159.2010.07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 14.Skaper SD. The brain as a target for inflammatory processes and neuroprotective strategies. Ann NY Acad Sci. 2007;1122:23–34. doi: 10.1196/annals.1403.002. [DOI] [PubMed] [Google Scholar]
- 15.Keohane A, Ryan S, Maloney E, Sullivan AM, Nolan YM. Tumour necrosis factor alpha impairs neuronal differentiation but not proliferation of hippocampal neural precursor cells: Role of Hes1. Mol Cell Neurosci. 2010;43:127–135. doi: 10.1016/j.mcn.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 17.Pluchino S, Muzio L, Imitola J, Deleidi M, Alfaro-Cervello C, Salani G, et al. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain. 2008;131:2564–2578. doi: 10.1093/brain/awn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi SH, Langenbach R, Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 2008;22:1491–1501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci. 2009;30:174–181. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aid S, Bosetti F. Targeting cyclooxygenases-1 and -2 in neuroinflammation: Therapeutic implications. Biochimie. 2010;93:46–45. doi: 10.1016/j.biochi.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosetti F, Choi SH. Rethinking the role of cyclooxygenase-1 in neuroinflammation: more than homeostasis. Cell Cycle. 2010;9:2919–2920. doi: 10.4161/cc.9.15.12715. [DOI] [PubMed] [Google Scholar]
- 22.García-Bueno B, Serrats J, Sawchenko PE. Cerebrovascular cyclooxygenase-1 expression, regulation and role in hypothalamic-pituitary-adrenal axis activation by inflammatory stimuli. J Neurosci. 2009;29:12970–12981. doi: 10.1523/JNEUROSCI.2373-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 24.Choi SH, Aid S, Choi U, Bosetti F. Cyclooxygenases-1 and -2 differentially modulate leukocyte recruitment into the inflamed brain. Pharmacogenomics J. 2010;10:448–457. doi: 10.1038/tpj.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 27.Choi SH, Bosetti F. Cyclooxygenase-1 null mice show reduced neuroinflammation in response to beta-amyloid. Aging (Albany NY) 2009;1:234–244. doi: 10.18632/aging.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milatovic D, Zaja-Milatovic S, Montine KS, Shie FS, Montine TJ. Neuronal oxidative damage and dendritic degeneration following activation of CD14-dependent innate immune response in vivo. J Neuroinflammation. 2004;1:20. doi: 10.1186/1742-2094-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aïd S, Bosetti F. Targeting cyclooxygenases-1 and -2 in neuroinflammation: Therapeutic implications. Biochimie. 2011;93:46–51. doi: 10.1016/j.biochi.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das S, Basu A. Inflammation: a new candidate in modulating adult neurogenesis. J Neurosci Res. 2008;86:1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- 31.Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9:1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- 32.Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NFkappaB signaling. BMC Neurosci. 2006;7:64. doi: 10.1186/1471-2202-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourke E, Kennedy EJ, Moynagh PN. Loss of Ikappa B-beta is associated with prolonged NFkappaB activity in human glial cells. J Biol Chem. 2000;275:39996–40002. doi: 10.1074/jbc.M007693200. [DOI] [PubMed] [Google Scholar]
- 34.Mondal D, Pradhan L, LaRussa VF. Signal transduction pathways involved in the lineage-differentiation of NSCs: can the knowledge gained from blood be used in the brain? Cancer Invest. 2004;22:925–943. doi: 10.1081/CNV-200039679. [DOI] [PubMed] [Google Scholar]
- 35.Cimini A, Ceru MP. Emerging roles of peroxisome pro-liferator-activated receptors (PPARs) in the regulation of neural stem cells proliferation and differentiation. Stem Cell Rev. 2008;4:293–303. doi: 10.1007/s12015-008-9024-2. [DOI] [PubMed] [Google Scholar]
- 36.Chojnacki A, Shimazaki T, Gregg C, Weinmaster G, Weiss S. Glycoprotein 130 signaling regulates Notch1 expression and activation in the self-renewal of mammalian forebrain neural stem cells. J Neurosci. 2003;23:1730–1741. doi: 10.1523/JNEUROSCI.23-05-01730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura Y, Sakakibara S, Miyata T, Ogawa M, Shimazaki T, Weiss S, et al. The bHLH gene hes1 as a repressor of the neuronal commitment of CNS stem cells. J Neurosci. 2000;20:283–293. doi: 10.1523/JNEUROSCI.20-01-00283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonow RH, Aid S, Zhang Y, Becker KG, Bosetti F. The brain expression of genes involved in inflammatory response, the ribosome and learning and memory is altered by centrally injected lipopolysaccharide in mice. Pharmacogenomics J. 2009;9:116–126. doi: 10.1038/tpj.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 40.Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakubs K, Bonde S, Iosif RE, Ekdahl CT, Kokaia Z, Kokaia M, et al. Inflammation regulates functional integration of neurons born in adult brain. J Neurosci. 2008;28:12477–12488. doi: 10.1523/JNEUROSCI.3240-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keene CD, Chang R, Stephen C, Nivison M, Nutt SE, Look A, et al. Protection of hippocampal neurogenesis from toll-like receptor 4-dependent innate immune activation by ablation of prostaglandin E2 receptor subtype EP1 or EP2. Am J Pathol. 2009;174:2300–2309. doi: 10.2353/ajpath.2009.081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, et al. Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron. 2006;52:1047–1059. doi: 10.1016/j.neuron.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Wood JC, Jackson JS, Jakubs K, Chapman KZ, Ekdahl CT, Kokaia Z, et al. Functional integration of new hippocampal neurons following insults to the adult brain is determined by characteristics of pathological environment. Exp Neurol. 2011;229:484–493. doi: 10.1016/j.expneurol.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 46.Matousek SB, Hein AM, Shaftel SS, Olschowka JA, Kyrkanides S, O'Banion MK. Cyclooxygenase-1 mediates prostaglandin E(2) elevation and contextual memory impairment in a model of sustained hippocampal interleukin-1beta expression. J Neurochem. 2010;114:247–258. doi: 10.1111/j.1471-4159.2010.06759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 48.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 49.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]