Abstract
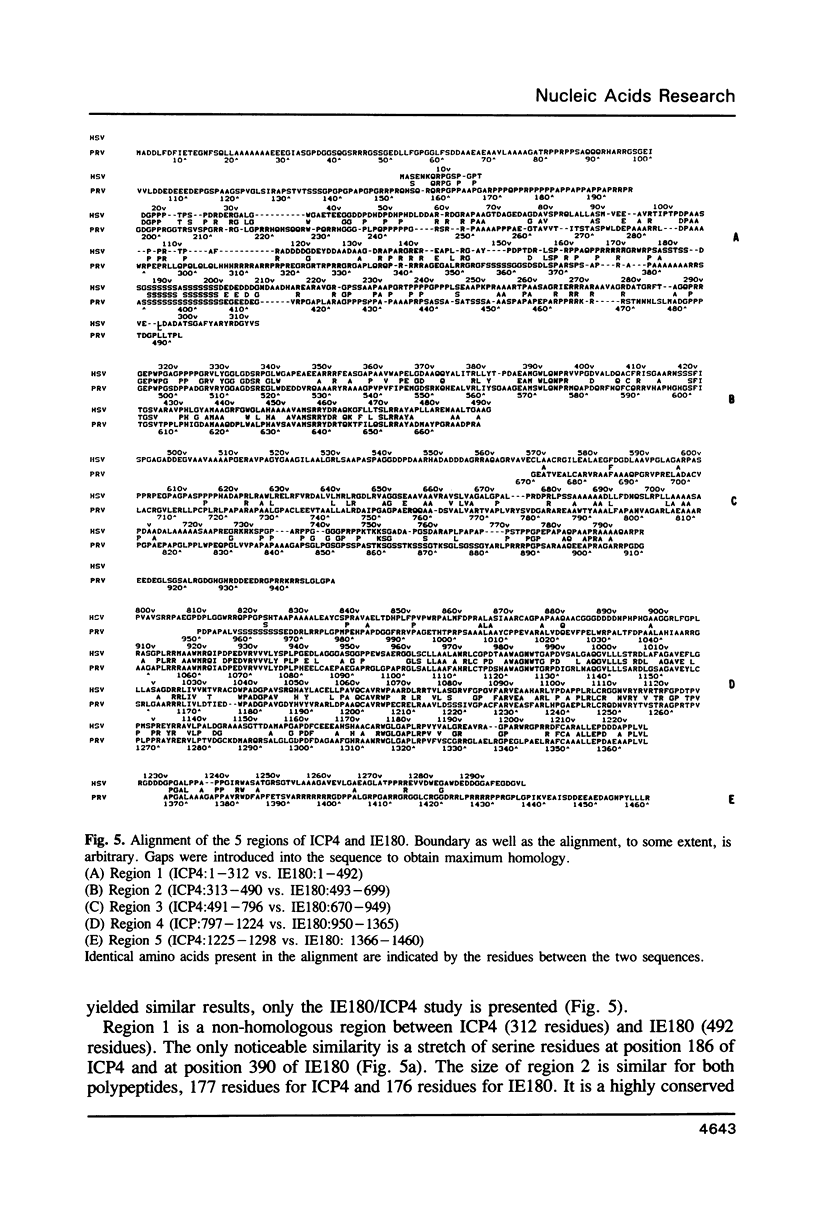
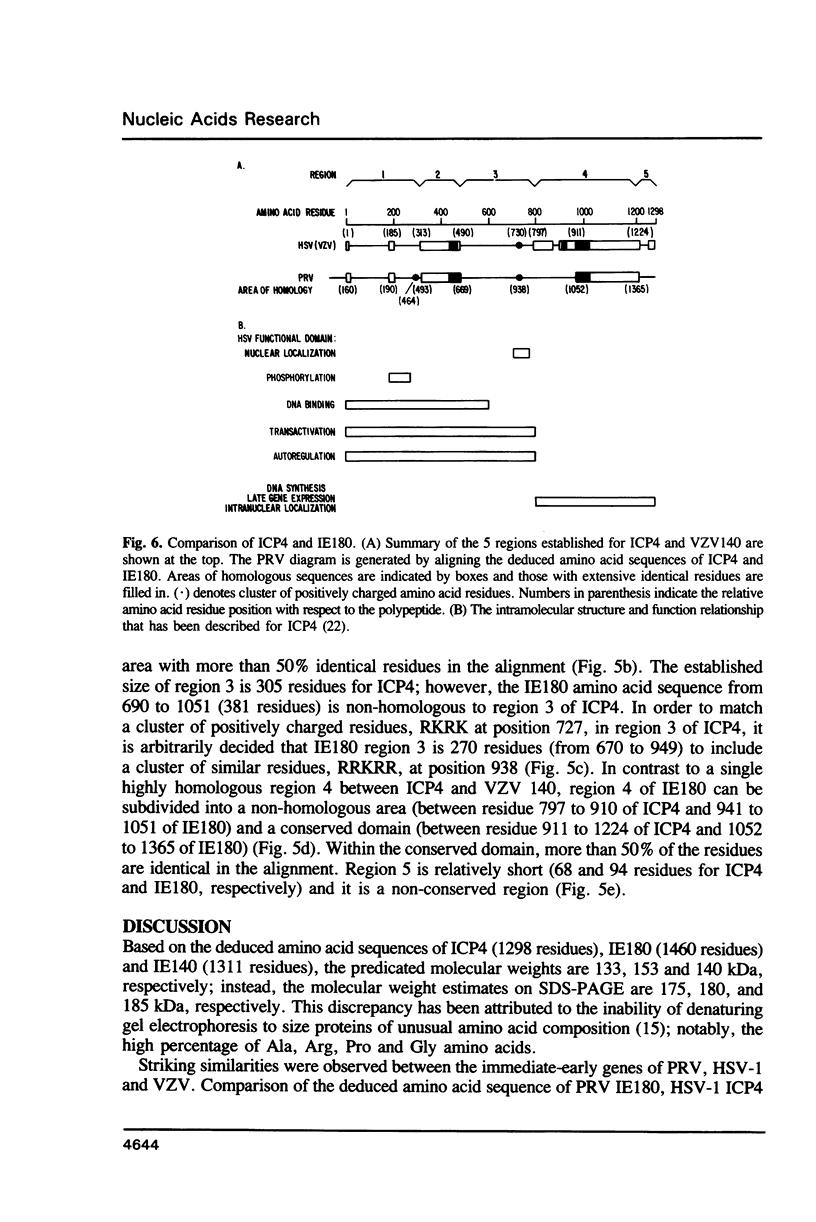
The complete DNA sequence coding for the immediate-early protein (IE180) of pseudorabies virus was determined. The coding region of IE180 is 4380 nucleotides for 1460 amino acid residues. G+C content of the non-coding portion of the IE gene is 70.3% while the G+C content of the coding portion is considerably higher at 80.1%. Correspondingly, codons consisting mainly of Gs and Cs are favoured. Clusters of amino acid homologies are observed among IE180 of pseudorabies virus, ICP4 of herpes simplex virus type-1 and IE140 of varicella-zoster virus, and are organized similarly in all three polypeptides. Functions exhibited by IE180 are assigned, tentatively, to structural domains of the molecule by analogy to the HSV-1 ICP4 polypeptide.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Kervina M., Kaplan A. S. Early functions of the genome of herpesvirus. V. Serological analysis of "immediate-early" proteins. Virology. 1975 Jun;65(2):355–362. doi: 10.1016/0042-6822(75)90041-0. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Veach R. A., Ihara S. Localization of the regions of homology between the genomes of herpes simplex virus, type 1, and pseudorabies virus. Virology. 1983 May;127(1):194–204. doi: 10.1016/0042-6822(83)90383-5. [DOI] [PubMed] [Google Scholar]
- Bronson D. L., Graham B. J., Ludwig H., Benyesh-Melnick M., Biswal N. Studies on the relatedness of herpes viruses through DNA-RNA hybridization. Biochim Biophys Acta. 1972 Jan 18;259(1):24–34. doi: 10.1016/0005-2787(72)90470-4. [DOI] [PubMed] [Google Scholar]
- Campbell M. E., Preston C. M. DNA sequences which regulate the expression of the pseudorabies virus major immediate early gene. Virology. 1987 Apr;157(2):307–316. doi: 10.1016/0042-6822(87)90273-x. [DOI] [PubMed] [Google Scholar]
- Cheung A. K. Fine mapping of the immediate-early gene of the Indiana-Funkhauser strain of pseudorabies virus. J Virol. 1988 Dec;62(12):4763–4766. doi: 10.1128/jvi.62.12.4763-4766.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlan C. A., Coulter C., Feldman L. T. Binding of the pseudorabies virus immediate-early protein to single-stranded DNA. J Virol. 1987 Jun;61(6):1855–1860. doi: 10.1128/jvi.61.6.1855-1860.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. DNA sequence of the major inverted repeat in the varicella-zoster virus genome. J Gen Virol. 1985 Feb;66(Pt 2):207–220. doi: 10.1099/0022-1317-66-2-207. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Location and orientation of homologous sequences in the genomes of five herpesviruses. J Gen Virol. 1983 Sep;64(Pt 9):1927–1942. doi: 10.1099/0022-1317-64-9-1927. [DOI] [PubMed] [Google Scholar]
- DeLuca N. A., Schaffer P. A. Physical and functional domains of the herpes simplex virus transcriptional regulatory protein ICP4. J Virol. 1988 Mar;62(3):732–743. doi: 10.1128/jvi.62.3.732-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L. T., Imperiale M. J., Nevins J. R. Activation of early adenovirus transcription by the herpesvirus immediate early gene: evidence for a common cellular control factor. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4952–4956. doi: 10.1073/pnas.79.16.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Treisman R., Maniatis T. Transcriptional activation of cloned human beta-globin genes by viral immediate-early gene products. Cell. 1983 Nov;35(1):137–148. doi: 10.1016/0092-8674(83)90216-7. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Ihara S., Feldman L., Watanabe S., Ben-Porat T. Characterization of the immediate-early functions of pseudorabies virus. Virology. 1983 Dec;131(2):437–454. doi: 10.1016/0042-6822(83)90510-x. [DOI] [PubMed] [Google Scholar]
- Jean J. H., Ben-Porat T., Kaplan A. S. Early functions of the genome of herpesvirus. 3. Inhibition of the transcription of the viral genome in cells treated with cycloheximide early during the infective process. Virology. 1974 Jun;59(2):516–523. doi: 10.1016/0042-6822(74)90461-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludwig H. O., Biswal N., Benyesh-Melnick M. Studies on the relatedness of herpesviruses through DNA-DNA hybridization. Virology. 1972 Jul;49(1):95–101. doi: 10.1016/s0042-6822(72)80010-2. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Brauer D. H. Complete DNA sequence of the short repeat region in the genome of herpes simplex virus type 1. Nucleic Acids Res. 1986 Feb 25;14(4):1727–1745. doi: 10.1093/nar/14.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson T., Everett R. D. Mutational dissection of the HSV-1 immediate-early protein Vmw175 involved in transcriptional transactivation and repression. Virology. 1988 Sep;166(1):186–196. doi: 10.1016/0042-6822(88)90160-2. [DOI] [PubMed] [Google Scholar]
- Pereira L., Wolff M. H., Fenwick M., Roizman B. Regulation of herpesvirus macromolecular synthesis. V. Properties of alpha polypeptides made in HSV-1 and HSV-2 infected cells. Virology. 1977 Apr;77(2):733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- Rakusanova T., Ben-Porat T., Himeno M., Kaplan A. S. Early functions of the genome of herpesvirus. I. Characterization of the RNA synthesized in cycloheximide-treated, infected cells. Virology. 1971 Dec;46(3):877–889. doi: 10.1016/0042-6822(71)90088-2. [DOI] [PubMed] [Google Scholar]
- Rand T. H., Ben-Porat T. Distribution of sequences homologous to the DNA of herpes simplex virus, types 1 and 2, in the genome of pseudorabies virus. Intervirology. 1980;13(1):48–53. doi: 10.1159/000149106. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley R. D., Woods R. D. Identification of a 17000 molecular weight antigenic polypeptide in transmissible gastroenteritis virus-infected cells. J Gen Virol. 1986 Jul;67(Pt 7):1419–1425. doi: 10.1099/0022-1317-67-7-1419. [DOI] [PubMed] [Google Scholar]