Abstract
Remarkable advances have been made in recent years towards therapeutics for cognitive impairment in individuals with Down syndrome (DS) by using mouse models. In this review, we briefly describe the phenotypes of mouse models that represent outcome targets for drug testing, the behavioral tests used to assess impairments in cognition and the known mechanisms of action of several drugs that are being used in preclinical studies or are likely to be tested in clinical trials. Overlaps in the distribution of targets and in the pathways that are affected by these diverse drugs in the trisomic brain suggest new avenues for DS research and drug development.
Introduction
Trisomy 21 (Ts21) is among the most complex genetic conditions compatible with human survival past term. Ts21 results in Down syndrome (DS), which is the most common genetic cause for cognitive impairment, occurring at a frequency of ∼1 in 700 live births (Parker et al., 2010). Dosage imbalance of more than 300 genes (Hattori et al., 2000) that are present on the extra copy of chromosome 21 results in a wide range of clinical features, including hypotonia, speech and language impairment, congenital heart defects, and craniofacial dysmorphology (Delabar et al., 2006). Achieving improvements in cognitive ability that would expand the potential of people with DS to live more independently has been the goal of decades of research in the field.
Because of the inherent complexity of the genetic perturbation represented by Ts21, the availability of animal models that replicate some aspects of the condition has been pivotal to obtaining an increased understanding of the neurophysiological outcomes of trisomy. In this Perspective, we discuss the cognitive phenotypes of several mouse models, including Ts65Dn, Ts1Cje, Ts1Rhr, the recently described Ts1Yey, Ts2Yey and Ts3Yey strains, the ‘triple trisomy’ model (Ts1Yey;Ts2Yey;Ts3Yey) and Ts1Yah. We also describe the most widely used behavioral tests to measure cognitive traits in these models. Most, if not all, pending pharmacotherapies for DS have been tested in the Ts65Dn strain, which is often referred to as the ‘Down syndrome’ mouse. We discuss the mechanism of action of recently tested drugs that improve cognition in mouse models, including some that will soon enter clinical trials to test their ability to improve cognition in individuals with DS (Reeves and Garner, 2007).
Mouse models of DS
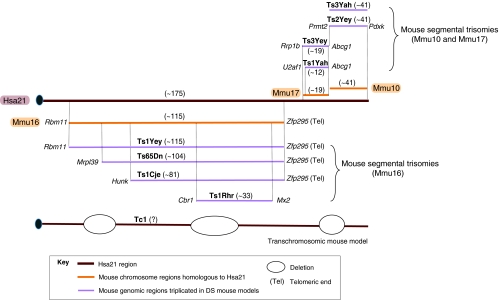
Mouse models of DS are based on conserved synteny between human chromosome 21 (Hsa21) and mouse chromosomes 16 (Mmu16), Mmu17 and Mmu10 (Pletcher et al., 2001). This relationship was revealed during the course of 20 years of mapping studies, culminating in comparative sequencing (Hattori et al., 2000; Francke et al., 1982; Lin et al., 1980; Waterston et al., 2002). A number of sequence analyses have been presented that approach a consensus in finding ∼175 genes that are highly conserved between Hsa21 and the mouse genome. The maps for frequently used models are shown in Fig. 1. As depicted, the Ts65Dn, Ts1Yey, Ts1Cje and Ts1Rhr strains are trisomic for segments of Mmu16 that are homologous to Hsa21, whereas Ts2Yey and Ts3Yah strains are trisomic for Mmu10 segments that are homologous to Hsa21, and Ts3Yey and Ts1Yah strains are trisomic for segments of Mmu17. Ts65Dn is the most widely studied mouse model and recapitulates some aspects of brain morphology and behavioral phenotypes observed in people with DS (Reeves et al., 1995). Ts1Cje and Ts1Rhr strains also show impairment in learning and memory, but phenotypes in these mice (which have fewer genes in trisomy) are generally less severe than in the Ts65Dn strain (Olson et al., 2007; Belichenko et al., 2009; Sago et al., 1998). Ts3Yey and Ts1Yah strains, which have triplicated regions of Mmu17, show some learning impairment, but their brain morphology has not been characterized (Yu et al., 2010b). Ts2Yey, which contains the Mmu10 region in triplicate, does not show impairment in the learning and memory tests assessed to date (Yu et al., 2010a). However, Ts1Yey;Ts2Yey;Ts3Yey (‘triple trisomy’) mice do recapitulate most of the behavioral features of the Ts65Dn strain (Yu et al., 2010a). Tc1 mice are transchromosomic (have one or more chromosomes or fragments transferred from a different species; in the case of Tc1 mice, this is Hsa21) and are mosaic for Hsa21-carrying cells – that is, the human chromosome is lost in a subset of cells from all tissues. They show mild learning impairment (O’Doherty et al., 2005). All cells in the Tc1 zygote are trisomic, and the exact point in lineage when a given cell or its neighbors become euploid cannot be determined. The percent of mosaicism varies from mouse to mouse, and among tissues in the same mouse. Several recent reviews describe mouse models of DS in detail (Brault et al., 2007; Gardiner, 2010; Kahlem et al., 2004; Moore and Roper, 2007).
Fig. 1.
Mouse models of Down syndrome (DS). Mouse orthologous regions to Hsa21 that are present on Mmu16, Mmu17 and Mmu10 are represented above and below Hsa21. The flanking genes found at the boundaries of the triplicated region in each model are depicted in italics; the approximate number of Hsa21 orthologous genes is shown in parentheses (not yet reported for the Tc1 strain). Mmu16 has the largest region of homology with Hsa21, and the Ts1Yey mouse [Dp(16Lipi-Zfp295)1Yey] contains the entire homologous region in trisomy. Ts65Dn, Ts1Cje and Ts1Rhr mice are segmental trisomies containing partial segments of Mmu16. The more recently developed segmental trisomic mouse strains Ts1Yah and Ts3Yey [Dp(17Abcg1-Rrp1b)1Yey] have three copies of the Hsa21-orthologous region of Mmu17, whereas Ts2Yey mice [Dp(10Prmt2-Pdxk)1Yey] and Ts3Yah mice duplicate the region on Mmu10. Tc1 is a transchromosomic mouse model bearing a mostly intact copy of Hsa21, producing trisomy for about 80% of the genes on that chromosome. Ts2Yey is the official alternate name for Dp(10Prmt2-Pdxk)1Yey on the MGI database, but has been referred to as Ts1Yey in print (Yu et al., 2010b). Combining the triplicated regions of Ts1Yey, Ts2Yey and Ts3Yey produces the triple trisomy model that is trisomic for all mouse orthologs of Hsa21 genes.
Strain background is an important consideration in the use of these models. Although most can be (and have been) completely inbred, the most widely used model, Ts65Dn, cannot be. Ts65Dn mice are maintained as an advanced intercross between C57BL/6 and C3H strains – that is, they have an average of 50% B6 alleles and 50% C3H alleles. One key difference is at the Pde6b (rd1) locus. C3H carries a common mutation in this gene that causes retinal degeneration in rd1/rd1 mice, resulting in blindness by ∼1 month of age (Keeler, 1924). A congenic C3H strain carrying only wild-type Pde6b alleles has been developed, and there is a simple molecular screen for homozygotes. Obviously, it is essential not to mix blind mice into experiments involving cognitive tests (Pittler and Baehr, 1991).
The availability of mice that are trisomic for different subsets of Hsa21-orthologous genes provides a powerful system to identify which genes contribute most to cognitive phenotypes (Lana-Elola et al., 2011). Because many of these complex phenotypes derive from the effects of multiple genes, one powerful approach is to ‘subtract’ the third copy by crossing a trisomic mouse to a genetically engineered strain that carries a null allele for the gene of interest (Salehi et al., 2006; Sussan et al., 2008) or even for a smaller segmental monosomy (Olson et al., 2004; Olson et al., 2007), thereby returning dosage for those genes to the normal two copies in the presence of the remaining trisomy. This allows a description of genes that are necessary and/or sufficient to produce a phenotype, as opposed to attempts to claim that a single gene ‘causes’ a complex phenotype of DS (Roper and Reeves, 2006).
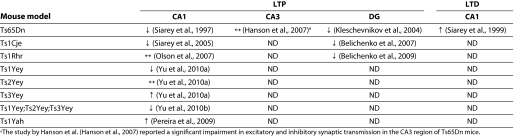
Aspects of brain function that map to different brain regions have been tested in these mouse models using a variety of tests. Some of the defects detected have parallels with cognitive phenotypes of DS. A common finding is the presence of impaired learning and memory. Synaptic plasticity, which is commonly measured by inducing long-term potentiation (LTP) in the hippocampus, is conventionally believed to be the physiological mechanism that underlies learning and memory. Many mouse DS models show reduced hippocampal LTP (Box 1).
Box 1. Electrophysiological measurements in the trisomic hippocampus.
Synaptic plasticity is the physiological response to neural activity, measured as changes in synaptic efficacy and excitability between presynaptic and postsynaptic neurons. Changes in synaptic strength can be triggered in vitro in brain slices with appropriate electrical stimulation of presynaptic neurons, which is converted to detectable electrophysiological or potentiation changes in postsynaptic neurons. Enhancement in potentiation that persists for long periods of time is called long-term potentiation (LTP) and can last for several minutes in a brain slice. Conversely, a persistent decrease in potentiation, or depression, is called long-term depression (LTD) (Martin et al., 2000; Neves et al., 2008). Most forms of LTP and LTD are glutamatergic and involve the activation of postsynaptic N-methyl-D-aspartate (NMDA) channels, but NMDA-independent mechanisms are also known (Katsuki et al., 1991; MacDonald et al., 2006).
LTP and LTD are thought to be the physiological basis for learning and memory. They conform to Hebbian rules – that is, the strength of the connection between pre- and post-synaptic neurons depends on their levels of activity. Synaptic connections can strengthen (when LTP is stimulated) or weaken (when LTD occurs) when the same type of stimulation is repeated; in other words, the synaptic connection recapitulates the original response following a particular stimulus (Hebb et al., 1994). The exact role of these conditions in learning or memory is a subject of ongoing investigation (Neves et al., 2008).
Given their proposed role in learning and memory, hippocampal LTP and LTD have been measured in many mouse models of DS, with contradictory findings by different groups. The method used to stimulate LTP gives variable results in trisomic mice, so equating the changes is not always justified (Costa and Grybko, 2005). LTP stimulation by theta burst stimulation (TBS) has been shown to be deficient in hippocampal slices from Ts65Dn mice, whereas there are contradictory reports about the results using high frequency stimulation (HFS). For example, Costa and Grybko did not find a deficit in LTP using HFS (Costa and Grybko, 2005), whereas others have reported a deficit using the technique (Siarey et al., 1999). The table below summarizes electrophysiological findings in the hippocampal regions of mouse models of DS. ↓, reduced; ↑, increased; ↔, similar, compared with euploid controls; ND, not determined.
Cognitive deficits in DS can be artificially conceptualized into those that are a product of development that result in functional, learning and memory deficits from birth, and those that are a function of age-related degenerative processes. The latter are accelerated in people with DS compared with the general population, especially the formation of the histopathological stigmata of Alzheimer’s disease (AD). Neurofibrillary tangles and plaques are evident in essentially every person with DS by the fourth decade of life, although a substantial fraction of people do not show a corresponding cognitive decline. In this review, we concentrate primarily on the changes that are evident early in life.
Behavioral tests
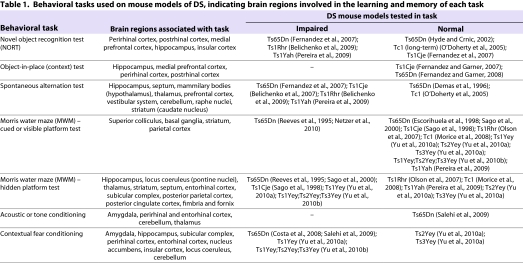
Behavioral tests can be used to assess defects in the function of specific brain regions, given their attributed roles in performing certain types of tasks (Table 1). Spatial and recognition learning and memory have been the most widely assessed in trisomic mice, using a variety of tasks that have differences in the degree of aversiveness. These tests have helped to define the brain regions that are especially affected in Ts65Dn mice and other models of DS, and in some cases have been successfully translated into behavioral tests that can be performed on people with DS. For example, behavioral tests in Ts65Dn mice originally pointed to deficits in hippocampal function that were subsequently implicated in children with DS (Edgin et al., 2010; Moran et al., 1996; Pennington et al., 2003; Reeves et al., 1995). Such tests can also be used to assess the efficacy of candidate drugs for DS (see later). Several important behavioral tests are described below.
Table 1.
Behavioral tasks used on mouse models of DS, indicating brain regions involved in the learning and memory of each task
Novel object recognition or object-in-place (context) test
The novel object recognition task (NORT) is a non-aversive test, meaning that it is not associated with a punishment for failing nor conducted under stress-inducing conditions. NORT is based on the innate tendency of rodents to prefer exploring novel objects over familiar ones. Mice are initially given an opportunity to habituate to an open arena, which later contains the objects that they are expected to explore. Habituation reduces the novelty of the arena compared with the objects. Mice are presented with two objects and allowed to explore for a short time. 24 hours later, one of the two familiar objects is replaced with a new object and the mice are again allowed to explore them. Normal mice tend to spend more time exploring a novel object compared with a familiar object, but mice with impaired recognition memory cannot discriminate between the two. Normal mice also remember if a familiar object has been moved to new position, which can be tested using the object-in-place test (Fernandez et al., 2007).
Ts65Dn and Ts1Rhr mice show impaired NORT performance in multiple versions of the test, whereas Ts1Cje mice can discriminate novel objects from familiar ones (Fernandez et al., 2007) (Table 1). These results are not readily explained by the genes that are triplicated in each model: Ts1Cje mice are trisomic for 80% of the genes in Ts65Dn mice and all genes in Ts1Rhr mice, yet Ts1Cje mice show no deficit in NORT. Ts1Yah mice, which have only 12 Hsa21 orthologous genes in triplicate, are impaired in NORT (Pereira et al., 2009). Tc1 mice, by contrast, are similar to euploid controls in the standard NORT paradigm (Morice et al., 2008).
Object recognition has been attributed to the dentate gyrus (DG) and to the CA1-CA3 regions of the hippocampus (Fernandez et al., 2007). However, the role of the hippocampus in recognition memory is controversial, and it is thought that the role of the hippocampus is in learning the context in which the objects are placed. This suggests that the hippocampus is necessary for a ‘what-where-when’ scenario or the object-in-place task, but not for NORT (Balderas et al., 2008). Interestingly, Ts65Dn mice perform normally in the object-in-place task (Fernandez and Garner, 2008). Furthermore, an earlier report using 10- to 12-month-old Ts65Dn mice found them to be normal in the object-in-place task and NORT (Hyde and Crnic, 2002). Perirhinal and insular cortices located in the temporal lobe and cerebral cortex of the brain, respectively, might make important contributions to NORT deficits in Ts65Dn mice for which the underlying mechanisms remain to be elucidated (Bermudez-Rattoni et al., 2005; Winters and Bussey, 2005).
Spontaneous alternation
Spontaneous alternation assesses a form of short-term working memory. In this test, mice are allowed to explore a T-shaped or Y-shaped maze. As in NORT, there are many variations of this test, and different variations might emphasize function of different brain regions. Also similar to NORT, free exploration versions of the test are based on the innate tendency of rodents to explore novelty, by visiting a different arm of the maze than the one just visited. Outcomes are measured as the percentage of times the mouse chooses to enter a different arm than the one it recently visited (alternations), divided by the possible number of alternations it could have made in the task. Among mouse models of DS, Ts65Dn, Ts1Cje, Ts1Rhr and Ts1Yah strains are most impaired, showing lower spontaneous alternation (50–60%) compared with control euploid mice (70–80%) (Belichenko et al., 2007; Fernandez et al., 2007; Pereira et al., 2009). Tc1 transchromosomic mice show comparable rates of alternation to euploid control mice (O’Doherty et al., 2005).
Morris water maze
Experiments involving some variation of the Morris water maze (MWM) test have been the most frequently used behavioral assessments of learning and memory in Ts65Dn mice. Significant differences in the performance of Ts65Dn mice compared with euploids in the MWM have been reported many times by many laboratories, lending credence to the conclusion that the mice are truly impaired.
The test is performed in a circular water tank of 90–150 cm in diameter, filled with water that has been made opaque by adding milk or non-toxic white paint. A transparent platform is submerged ∼2 cm below the water surface. A cued (visible) platform task, where mice learn to find the platform with a visible cue (such as a flag), is usually carried out as a control experiment to rule out factors that might affect results for reasons not involving visuospatial integration. The cued platform task can be solved by combining praxic and taxic strategies, for which mice learn a sequence of movements in response to a stimulus or a proximal cue (Fraenkel and Gunn, 1961). In the next phase (the hidden-platform task), the platform is not visibly tagged, and a complex spatial mapping strategy or locale strategy must be learned to find the platform (Morris, 1981; Redish and Touretzky, 1998). This requires the use of extra- and intra-maze cues to define the coordinates of the platform, the position of which is kept constant, while the starting position of the mice is changed in each trial to reduce taxic and/or praxic learning (Vorhees and Williams, 2006). Learning is evaluated using the latency time to find the platform, path length and/or type of trajectories (Morris, 1981; Petrosini et al., 1998; Vorhees and Williams, 2006).
A probe trial, in which the platform is removed and mice are allowed to explore the water maze for 1–3 minutes, is generally carried out a day after the last hidden-platform session. Normal mice spend a higher percentage of time in the quadrant that formerly contained the platform (Vorhees and Williams, 2006), compared with mice that have impaired learning and memory.
Most contemporary reports concur that trisomic mice are similar to euploid mice in the cued task (Bimonte-Nelson et al., 2003; Escorihuela et al., 1998; Rueda et al., 2008; Sago et al., 2000), but Ts65Dn, Ts1Cje and Ts1Yey mice show impaired learning and memory in the hidden-platform task (Table 1) (Li et al., 2007; Reeves et al., 1995; Sago et al., 1998). Ts1Rhr mice do not show impairment in the MWM in either cued or hidden-platform tasks (Olson et al., 2007). Mice in which all Hsa21 orthologous genes are trisomic (i.e. ‘triple trisomy’ mice) show impaired performance in the MWM (Yu et al., 2010a), but trisomy for only the Mmu10 or Mmu17 segments (Fig. 1) does not result in impairment in this task. In fact, Ts1Yah mice performed better in the hidden platform task than their euploid littermates (Pereira et al., 2009; Yu et al., 2010b). Surprisingly, Tc1 transchromosomic mice were also reported to do as well as euploid controls in a standard MWM paradigm (Morice et al., 2008). However, as mentioned earlier, the human transchromosome is lost in a substantial fraction of cells in Tc1 mice, and half or more euploid neurons might provide compensation for impairments in trisomic neurons (O’Doherty et al., 2005).
Stasko and Costa found that increasing stress causes deterioration in the performance of Ts65Dn mice in the MWM (Stasko and Costa, 2004). Differences in MWM results have been reported between sexes as well. In this regard, it is interesting to note that environmental enrichment, where mice are housed with toys and running wheels etc., has been shown to improve learning in female but not male Ts65Dn mice (Martinez-Cue et al., 2002).
Fear conditioning
In fear conditioning, a conditioned stimulus (CS) such as a tone and/or context is paired with an unconditioned stimulus (US), such as an electric shock that naturally generates a freezing response (i.e. fearful behavior). The CS can then induce a freezing response in the absence of the US, because normal mice remember and associate the two stimuli (Phillips and LeDoux, 1992; Wehner and Radcliffe, 2004). In this test, mice are habituated in a chamber for 3–5 minutes before being exposed to a tone, at the end of which they receive a mild foot shock (the US). A day later, the mice are tested separately for tone conditioning, in a different chamber (novel context) and exposed to the tone without receiving the shock. The following day, the mice are tested for contextual conditioning by being reintroduced to the initial chamber (conditioned context) and being exposed to the tone, again without receiving the shock. The freezing response on hearing the tone is evaluated in both the novel and conditioned context (Salehi et al., 2009). Both the tone and the context form components of the CS, but the association of each one with the US relies on different regions of the brain (Table 1). Acoustic (tone) fear conditioning is primarily dependent on the amygdala, with a lesser contribution by perirhinal, entorhinal and postrhinal cortices, and the thalamus (Campeau and Davis, 1995; Goosens and Maren, 2001; Kholodar-Smith et al., 2008). It has been proposed that the hippocampus has a more pronounced role in contextual discrimination, with important input from the cerebellum (Anagnostaras et al., 2001; Biedenkapp and Rudy, 2009; Sacchetti et al., 2002). However, the precise role of the hippocampus in fear conditioning is unresolved (Frankland et al., 1998).
Ts1Yey, Ts65Dn and triple trisomy mice (Ts1Yey;Ts2Yey;Ts3Yey) all show a reduced freezing response when reintroduced to the conditioned context compared with euploid littermates (Table 1) (Hyde and Crnic, 2001; Salehi et al., 2009; Yu et al., 2010a; Yu et al., 2010b). The Mmu10 and Mmu17 segmental trisomies, Ts2Yey and Ts3Yey, each show a normal response (Yu et al., 2010b). Ts65Dn mice do remember the association between the tone and the shock, and show a normal freezing response to acoustic conditioning (Salehi et al., 2009; Turner et al., 2001). This indicates that the amygdala function of pairing acoustic CS to the US is intact in Ts65Dn mice, whereas the function of pairing contextual CS to the US is not. However, whether the latter is a result of dysfunction in one or all of the brain regions – such as the hippocampus, entorhinal and perirhinal cortices, etc. – involved in the task is unclear from the present data (see Table 1).
Candidate drugs for DS
The identification of brain regions affected by trisomy in mice has been instrumental in the development of pharmacological approaches to correct underlying imbalances that disrupt cognitive functions in DS. In cases in which candidate drugs improve performance in the tests described above, mouse models provide the opportunity to assess physiological, molecular and structural changes that result from the treatment. In this way, mouse models remain a fundamental tool to rationalize the basis for treatment in humans. Indeed, essentially every proposed treatment for people has developed from results in Ts65Dn mice using the tasks described above. In this section, we review studies that have shed light on drug candidates for DS, discussing those that have effects on brain physiology, biochemistry and morphology, and briefly mention the drugs that will soon advance into clinical trials.
Drugs that influence synaptic plasticity
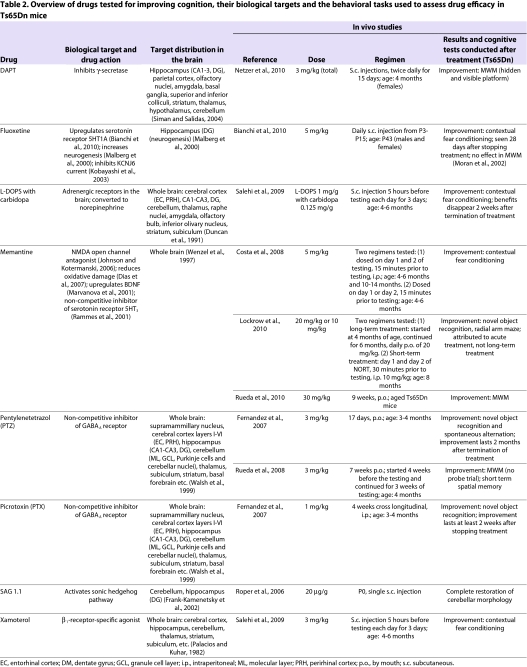
Several drugs have been tested on the basis of their remedial effects on synaptic plasticity, assessed by correction in LTP (Table 2). LTP is reduced in the CA1 and DG in hippocampal slices from Ts65Dn mice (Box 1). A key finding in this area was the demonstration that this reduced LTP occurred in part owing to an imbalance between excitatory and inhibitory inputs to the hippocampus. The balance could be returned to normal by the addition of drugs that inhibit γ-aminobutyric acid A (GABAA) receptors (which respond to the inhibitory neurotransmitter GABA) in both Ts65Dn and Ts1Cje mice (Belichenko et al., 2007; Hanson et al., 2007; Kleschevnikov et al., 2004). On the basis of these observations, drugs that inhibit GABAA receptors were tested for their ability to improve cognition. Picrotoxin (PTX) and pentylenetetrazol (PTZ) are antagonists that bind non-competitively to these receptors. In one study, Ts65Dn mice were treated for ∼2 weeks before cognitive testing in a modified NORT paradigm (Fernandez et al., 2007). Treatment with either PTX or PTZ completely reversed the NORT deficit observed in untreated Ts65Dn mice (Fernandez et al., 2007), and was subsequently demonstrated to overcome learning and memory deficits observed in the MWM test as well (Rueda et al., 2008). Remarkably, some cognitive improvement and even improved LTP was demonstrable for more than 2 months after the treatment was stopped (Fernandez et al., 2007). Several compounds that inhibit GABAA receptors are being developed for clinical trials.
Table 2.
Overview of drugs tested for improving cognition, their biological targets and the behavioral tasks used to assess drug efficacy in Ts65Dn mice
Memantine is an open-channel antagonist of N-methyl D-aspartate (NMDA) receptors (Table 2) (Chen and Lipton, 2005) that has been reported to alleviate some of the symptoms of AD (Ferris, 2003). Ts65Dn mice show some sequelae of AD, including degeneration of forebrain cholinergic neurons and degeneration of norepinephrine (NE) inputs to hippocampus from locus coeruleus (LC) (Casanova et al., 1985; Salehi et al., 2006; Salehi et al., 2009; Weinshenker, 2008). Several groups have tested the efficacy of memantine in improving cognition in young adult Ts65Dn mice. Acute treatment prior to behavioral testing can improve learning in contextual fear conditioning (Costa et al., 2008) and NORT (Lockrow et al., 2010). However, Lockrow et al. reported that acute treatment on the day of testing is required even after long-term dosing (Table 2). Although known to be neuroprotective, memantine does not seem to protect against the age-related neurodegeneration in the basal forebrain and LC in Ts65Dn mice; however, treatment led to increased production of brain-derived neurotrophic factor (BDNF), as reported previously (Lockrow et al., 2010; Marvanova et al., 2001; Salehi et al., 2009). Extended treatment of aged mice with memantine improved their performance in the MWM, but whether acute treatment influences performance in this behavioral task has not been tested (Rueda et al., 2010).
Drugs that act on brain biochemistry
Humans with DS exhibit age-related neurodegeneration in many regions of the brain, including the LC and the basal forebrain (Casanova et al., 1985; Mann et al., 1985; Schochet et al., 1973). This results in a deficiency of important neurotransmitters such as NE and acetylcholine (Ach). Additionally, the increase in the expression of amyloid precursor protein (APP), which is present in three copies in both humans with DS and in Ts65Dn mice, causes a concomitant increase in APP-derived amyloid-β (Aβ) peptides, which form the most important component of amyloid plaques in AD (Dalakas et al., 1984; Glenner and Wong, 1984). Aβ might contribute to cognitive decline in DS by inducing synaptic depression in neurons that overexpress APP (i.e. all neurons in Ts65Dn mice) (Kamenetz et al., 2003) or oxidative-stress-induced neurotoxicity (Butterfield, 2002). Similarly to mouse models of AD, age-related degeneration of the LC and reduction of NE levels is seen in Ts65Dn mice, along with increased production of APP (Netzer et al., 2010; Salehi et al., 2009). However, Ts65Dn mice do not show neuritic plaques and neurofibrillary tangles (Reeves et al., 1995). Drug candidates that rescue NE deficiency or that target increased levels of APP have shown promising results in mouse models of DS.
L-threo-dihydroxyphenylserine (L-DOPS) corrects the deficiency in the production of NE in Ts65Dn mice (Table 2) (Salehi et al., 2009). L-DOPS is converted to NE by the enzyme aromatic L-amino acid decarboxylase (AAAD). Treatment with L-DOPS, when administered together with carbidopa, an inhibitor of peripheral AAAD activity that cannot cross the blood brain barrier, restores learning and memory in contextual fear conditioning tests in Ts65Dn mice. This improvement in cognition has been attributed to corrected NE-modulated adrenergic activation in the hippocampus; however, the enzyme AAAD is present in many brain regions in which the NE activity could affect cognition. Also, the LC is the source of NE for many other brain regions, including the cerebellum and the neocortex, which might also contribute to improved cognition in the presence of L-DOPS (Loughlin et al., 1986). A second drug that targets NE deficiency, xamoterol, specifically activates β1 adrenergic receptors (although NE activates all types of adrenergic receptors). Treatment with xamoterol also improves learning and memory in contextual fear conditioning in Ts65Dn mice (Salehi et al., 2009).
N-[(3,Difluorophenyl)acetyl]-L-alanyl-2-phenylglycine-1,1-dimethylethyl ester (DAPT) is an inhibitor of γ-secretase, the enzyme that cleaves APP (an important step in generating Aβ peptides). DAPT can reduce the production of Aβ peptides from APP in mice (Dovey et al., 2001). Treatment with DAPT reduces levels of Aβ peptides 40 and 42 in Ts65Dn mice, as well as reducing the amount of C-terminal fragments of APP. In line with the theory that increased levels of Aβ peptides contribute to cognitive decline in DS (Kamenetz et al., 2003), DAPT treatment also normalizes learning and memory in the MWM in female Ts65Dn mice (Netzer et al., 2010).
Degeneration of basal forebrain cholinergic neurons (BFCNs) has been also been found in Ts65Dn mice (Holtzman et al., 1996), which is correlated with deficient Ach release in the hippocampus during spontaneous alternation testing (Chang and Gold, 2008). To compensate for reduced Ach levels, mice were treated with physostigmine, an inhibitor of acetylcholine esterase that breaks down Ach. Acute treatment with physostigmine (50 μg/kg) 10 minutes prior to testing reverses deficits in 4-month-old Ts65Dn mice, but not in 10-month-old mice (Chang and Gold, 2008). This drug has not been tested for long-term effects or in other behavioral tasks.
Drugs that affect brain morphology
Parallels between the morphology of the trisomic brain in humans with DS and Ts65Dn mice include reduced cell number and volume in the hippocampal DG (Lorenzi and Reeves, 2006) and the notable decrease in overall size of the cerebellum along with a reduction in the number and density of its granule cell neurons (Baxter et al., 2000). As mentioned earlier, the number and size of BFCNs is also reduced in Ts65Dn mice (Holtzman et al., 1996). A final group of potential drugs consists of those that have effects on these morphological characteristics of the trisomic brain, usually by stimulating proliferation of neuronal populations that are depauperate. Fluoxetine (Prozac), injected daily from postnatal day 3 (P3) to P15, was shown to increase neurogenesis in the DG of male and female Ts65Dn mice by P15 (Bianchi et al., 2010). 28 days after stopping the treatment, providing time for the newly generated neurons to become incorporated in the circuitry, the treated mice showed improvement in contextual fear conditioning. Other molecular markers in the hippocampus that are known to be abnormal in Ts65Dn mice, such as the 5-hydroxytryptamine 1A (5HT1A) receptors, were also normalized to euploid levels following this treatment (Bianchi et al., 2010).
The cerebellum is smaller in Ts65Dn mice compared with euploid mice, reflecting observations in humans with DS. In Ts65Dn mice, a single dose of a sonic hedgehog agonist SAG 1.1 on the day of birth normalized the cerebellar volume and the deficit in granule cell number to the level observed in euploid littermate controls by P6 (Roper et al., 2006). We have found that the corrections mediated by SAG 1.1 persist into adulthood; however, we did not find any change in neuronal number in the DG following SAG treatment (I.D. and R.H.R., unpublished results).
Finally, the levels of nerve growth factor (NGF) in Ts65Dn mice were reduced in the medial septal region, which contains the BFCNs, because of a deficiency in retrograde transport of NGF to this region from the hippocampus (which is one of the sites of production). As a way to reverse or reduce the degeneration of BFCNs, NGF was administered intracerebroventricularly. This treatment reversed abnormalities in BFCN size and number as well as in cholinergic innervation (Cooper et al., 2001). However, behavioral effects of such a treatment have not yet been demonstrated.
Drugs that are likely to enter clinical trials
Several pharmaceuticals, including PTZ, L-DOPS and memantine, are in or will soon be in clinical trials to test their ability to improve cognition in people with DS. Some of these are true double-blinded placebo-controlled cross-over trials with sufficient numbers of participants to provide the statistical power to draw conclusions about efficacy. Others are pilot studies that should give an indication of efficacy to encourage the initiation of a fully powered trial. Notably, memantine has been approved by the European Union and by the U.S. Food and Drug Administration for the treatment of AD, and is the subject of a pilot study for DS currently (http://clinicaltrials.gov/ct2/results?term=costa+and+down+syndrome). If it is found to be safe in the DS population, it can be expected that memantine will be a prescribed drug for DS in the near future.
Perspective
Understanding the bases for differences in cognitive impairment among different mouse models of DS will guide mechanism-based drug design. These models can be assessed using behavioral tests to detect specific deficits that implicate specific brain regions that should be tested in humans. There is a clear correlation between the number of genes that are orthologous to Hsa21 and the degree of behavioral abnormalities observed in mouse models (Table 1). For example, Ts1Cje mice are less affected than Ts65Dn mice, in that Ts1Cje mice have normal recognition memory and seem to be better at spatial tasks (Fernandez and Garner, 2007; Sago et al., 2000). However, these differences in behavior are related to specific genes: Ts1Rhr mice have impaired object recognition but normal spatial learning and memory, despite the fact that they are trisomic for about half the genes that are triplicated in Ts1Cje mice (Belichenko et al., 2009; Olson et al., 2007). Tc1 transchromosomic mice, which are trisomic for the majority of human genes from Hsa21, show normal spatial and recognition learning and memory, and are therefore dissimilar to Ts65Dn mice or to triple trisomy mice (Morice et al., 2008; O’Doherty et al., 2005; Yu et al., 2010a). Overall, it is important to note that the strength of these studies is not to compare human and mouse behavior, but to map affected brain regions by comparable trisomy in the two species.
Different subregions of the hippocampus have been attributed specific roles in different forms of spatial learning (Kesner et al., 2004; McHugh et al., 2007; Tsien et al., 1996). The normal performance of Ts65Dn mice in the object-in-place task demonstrates that some form of hippocampal function is intact in these mice (Fernandez and Garner, 2008). The behavioral phenotype of Ts1Yah mice is interesting because they perform normally in the complicated MWM task but are impaired in NORT, both of which require hippocampal contribution (Pereira et al., 2009). However, all of these tasks depend to some extent on multiple brain regions, and therefore the contribution of the hippocampus cannot be completely segregated from that of other regions in the final outcome. Therefore, regions such as the entorhinal and perirhinal cortices, the striatum and the cerebellum need to be studied in further detail to determine their contribution to the learning deficits in trisomy.
Basic research in this field is being translated into rational pharmacotherapies that aim to facilitate cognitive processes and expand life opportunities for people with DS. Although the known targets of the different classes of drugs discussed here are expressed in different regions of the trisomic brain, it is possible that different drugs act on some of the same pathways of learning, owing to interactions between target pathways. For example, PTZ, which binds non-competitively to GABAA receptors, can induce seizures in NE-deficient mice because NE normally modifies the activity of GABAA receptors through specific interactions. This indicates that GABA and NE activities have some form of feedback regulation whereby the deficiency of one can be compensated to some extent by the other. Indeed, NE-deficient mice do not normally have seizures but display seizures upon the addition of a strong epileptic, such as PTZ, which does not markedly affect normal mice (Szot et al., 1999; Tully et al., 2007; Weinshenker et al., 2001). In addition, memantine and DAPT might act by reducing excitotoxicity or abnormal currents through NMDA receptors that are caused by increased Aβ levels. A detailed electrophysiological study performed on Ts65Dn mice demonstrated a reduction in the efficacy of both inhibitory and excitatory connections in the hippocampal CA3 region (Hanson et al., 2007). Therefore, even drugs with seemingly diametrically opposed effects, such as reducing inhibitory connections (PTZ) or reducing excitatory connections (memantine), can improve cognition in Ts65Dn mice.
It might be appropriate to differentiate drug candidates for DS on the basis of short-term efficacy (i.e. corrections of physiologically stable states are achieved in the presence of drugs in the bloodstream) or long-term efficacy (i.e. treatment achieves close to a normal homeostatic steady state). In addition, correction of developmental processes, such as cerebellar cell number and volume, can also result in normalization that persists long after treatment. SAG 1.1, fluoxetine and possibly other neuroprotective drugs might also function in this manner.
A new era in the field of DS research and therapeutics is unfolding. Clinical trials to test drugs that aim to improve cognitive impairment – the most significant barrier to a normal lifestyle for people with DS – will commence soon. Studies of Ts65Dn mice have made an enormous contribution to the field, providing the basis for many potential treatments. The availability of additional genetic models of DS that more closely reflect the gene dosage in people is enabling us to refine our understanding of morphological, physiological or developmental deficiencies that lead to cognitive impairment in DS. Because the range of intellectual disability in people with DS varies from just below normal to significantly impaired and might encompass many different aspects of cognitive function and adaptive behavior, it is unlikely that a single drug will normalize brain function in all individuals. New mouse models with an expanded representation of the genetic basis for DS will open up new avenues for understanding the molecular basis of the various clinical features of DS. Trisomic mice will continue to be an essential tool for understanding and developing therapies to ameliorate disadvantageous features of DS.
Acknowledgments
This work was supported in part by a grant from the Down Syndrome Research and Treatment Foundation and by Award Number R01HD038384 from the National Institute of Child Health & Human Development (R.H.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health & Human Development or the National Institutes of Health.
Footnotes
COMPETING INTERESTS
The authors declare that they have no competing or financial interests.
REFERENCES
- Anagnostaras S. G., Gale G. D., Fanselow M. S. (2001). Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus 11, 8–17 [DOI] [PubMed] [Google Scholar]
- Balderas I., Rodriguez-Ortiz C. J., Salgado-Tonda P., Chavez-Hurtado J., McGaugh J. L., Bermudez-Rattoni F. (2008). The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn. Mem. 15, 618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter L. L., Moran T. H., Richtsmeier J. T., Troncoso J., Reeves R. H. (2000). Discovery and genetic localization of Down syndrome cerebellar phenotypes using the Ts65Dn mouse. Hum. Mol. Genet. 9, 195–202 [DOI] [PubMed] [Google Scholar]
- Belichenko N. P., Belichenko P. V., Kleschevnikov A. M., Salehi A., Reeves R. H., Mobley W. C. (2009). The “Down syndrome critical region” is sufficient in the mouse model to confer behavioral, neurophysiological, and synaptic phenotypes characteristic of Down syndrome. J. Neurosci. 29, 5938–5948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belichenko P. V., Kleschevnikov A. M., Salehi A., Epstein C. J., Mobley W. C. (2007). Synaptic and cognitive abnormalities in mouse models of Down syndrome: exploring genotype-phenotype relationships. J. Comp. Neurol. 504, 329–345 [DOI] [PubMed] [Google Scholar]
- Bermudez-Rattoni F., Okuda S., Roozendaal B., McGaugh J. L. (2005). Insular cortex is involved in consolidation of object recognition memory. Learn. Mem. 12, 447–449 [DOI] [PubMed] [Google Scholar]
- Bianchi P., Ciani E., Guidi S., Trazzi S., Felice D., Grossi G., Fernandez M., Giuliani A., Calza L., Bartesaghi R. (2010). Early pharmacotherapy restores neurogenesis and cognitive performance in the Ts65Dn mouse model for Down syndrome. J. Neurosci. 30, 8769–8779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedenkapp J. C., Rudy J. W. (2009). Hippocampal and extrahippocampal systems compete for control of contextual fear: role of ventral subiculum and amygdala. Learn. Mem. 16, 38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson H. A., Hunter C. L., Nelson M. E., Granholm A. C. (2003). Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav. Brain Res. 139, 47–57 [DOI] [PubMed] [Google Scholar]
- Brault V., Besson V., Magnol L., Duchon A., Hérault Y. (2007). Cre/loxP-mediated chromosome engineering of the mouse genome. Handb. Exp. Pharmacol. 178, 29–48 [DOI] [PubMed] [Google Scholar]
- Butterfield D. A. (2002). Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic. Res. 36, 1307–1313 [DOI] [PubMed] [Google Scholar]
- Campeau S., Davis M. (1995). Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J. Neurosci. 15, 2312–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M. F., Walker L. C., Whitehouse P. J., Price D. L. (1985). Abnormalities of the nucleus basalis in Down’s syndrome. Ann. Neurol. 18, 310–313 [DOI] [PubMed] [Google Scholar]
- Chang Q., Gold P. E. (2008). Age-related changes in memory and in acetylcholine functions in the hippocampus in the Ts65Dn mouse, a model of Down syndrome. Neurobiol. Learn. Mem. 89, 167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. S., Lipton S. A. (2005). Pharmacological implications of two distinct mechanisms of interaction of memantine with N-methyl-D-aspartate-gated channels. J. Pharmacol. Exp. Ther. 314, 961–971 [DOI] [PubMed] [Google Scholar]
- Cooper J. D., Salehi A., Delcroix J. D., Howe C. L., Belichenko P. V., Chua-Couzens J., Kilbridge J. F., Carlson E. J., Epstein C. J., Mobley W. C. (2001). Failed retrograde transport of NGF in a mouse model of Down’s syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proc. Natl. Acad. Sci. USA 98, 10439–10444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A. C., Grybko M. J. (2005). Deficits in hippocampal CA1 LTP induced by TBS but not HFS in the Ts65Dn mouse: a model of Down syndrome. Neurosci. Lett. 382, 317–322 [DOI] [PubMed] [Google Scholar]
- Costa A. C., Scott-McKean J. J., Stasko M. R. (2008). Acute injections of the NMDA receptor antagonist memantine rescue performance deficits of the Ts65Dn mouse model of Down syndrome on a fear conditioning test. Neuropsychopharmacology 33, 1624–1632 [DOI] [PubMed] [Google Scholar]
- Dalakas M. C., Fujihara S., Askanas V., Engel W. K., Glenner G. G. (1984). Nature of amyloid deposits in hypernephroma. Immunocytochemical studies in 2 cases associated with amyloid polyneuropathy. Am. J. Pathol. 116, 447–454 [PMC free article] [PubMed] [Google Scholar]
- Delabar J. M., Aflalo-Rattenbac R., Creau N. (2006). Developmental defects in trisomy 21 and mouse models. Scientific World Journal 6, 1945–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas G. E., Nelson R. J., Krueger B. K., Yarowsky P. J. (1996). Spatial memory deficits in segmental trisomic Ts65Dn mice. Behav. Brain Res. 82, 85–92 [DOI] [PubMed] [Google Scholar]
- Dias C. P., De Lima M. N. M., Presti-Torres J., Dornelles A., Garcia V. A., Scalco F. S., Guimaraes M. R., Constantino L., Budni P., Dal-Pizzol F., et al. (2007). Memantine reduces oxidative damage and enhances long-term recognition memory in aged rats. Neuroscience 146, 1719–1725 [DOI] [PubMed] [Google Scholar]
- Dovey H. F., John V., Anderson J. P., Chen L. Z., de Saint Andrieu P., Fang L. Y., Freedman S. B., Folmer B., Goldbach E., Holsztynska E. J., et al. (2001). Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J. Neurochem. 76, 173–181 [DOI] [PubMed] [Google Scholar]
- Duncan G. E., Little K. Y., Koplas P. A., Kirkman J. A., Breese G. R., Stumpf W. E. (1991). Beta-adrenergic receptor distribution in human and rat hippocampal formation: marked species differences. Brain Res. 561, 84–92 [DOI] [PubMed] [Google Scholar]
- Edgin J. O., Mason G. M., Allman M. J., Capone G. T., Deleon I., Maslen C., Reeves R. H., Sherman S. L., Nadel L. (2010). Development and validation of the Arizona Cognitive Test Battery for Down syndrome. J. Neurodev. Disord. 2, 149–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escorihuela R. M., Vallina I. F., Martinez-Cue C., Baamonde C., Dierssen M., Tobena A., Florez J., Fernandez-Teruel A. (1998). Impaired short- and long-term memory in Ts65Dn mice, a model for Down syndrome. Neurosci. Lett. 247, 171–174 [DOI] [PubMed] [Google Scholar]
- Fernandez F., Garner C. C. (2007). Object recognition memory is conserved in Ts1Cje, a mouse model of Down syndrome. Neurosci. Lett. 421, 137–141 [DOI] [PubMed] [Google Scholar]
- Fernandez F., Garner C. C. (2008). Episodic-like memory in Ts65Dn, a mouse model of Down syndrome. Behav. Brain Res. 188, 233–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F., Morishita W., Zuniga E., Nguyen J., Blank M., Malenka R. C., Garner C. C. (2007). Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat. Neurosci. 10, 411–413 [DOI] [PubMed] [Google Scholar]
- Ferris S. H. (2003). Evaluation of memantine for the treatment of Alzheimer’s disease. Expert Opin. Pharmacother. 4, 2305–2313 [DOI] [PubMed] [Google Scholar]
- Fraenkel G. S., Gunn D. L. (1961). The Orientation of Animals, Kineses, Taxes and Compass Reactions. New York: Dover Publications [Google Scholar]
- Francke U., De Martinville B., D’Eustachio P., Ruddle F. H. (1982). Comparative gene mapping: murine lambda light chain genes are located in region cen to B5 of mouse chromosome 16 not homologous to human chromosome 21. Cytogenet. Cell Genet. 33, 267–271 [DOI] [PubMed] [Google Scholar]
- Frank-Kamenetsky M., Zhang X. M., Bottega S., Guicherit O., Wichterle H., Dudek H., Bumcrot D., Wang F. Y., Jones S., Shulok J., et al. (2002). Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. J. Biol. 1, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland P. W., Cestari V., Filipkowski R. K., McDonald R. J., Silva A. J. (1998). The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav. Neurosci. 112, 863–874 [DOI] [PubMed] [Google Scholar]
- Gardiner K. J. (2010). Molecular basis of pharmacotherapies for cognition in Down syndrome. Trends Pharmacol. Sci. 31, 66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. (1984). Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem. Biophys. Res. Commun. 122, 1131–1135 [DOI] [PubMed] [Google Scholar]
- Goosens K. A., Maren S. (2001). Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn. Mem. 8, 148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. E., Blank M., Valenzuela R. A., Garner C. C., Madison D. V. (2007). The functional nature of synaptic circuitry is altered in area CA3 of the hippocampus in a mouse model of Down’s syndrome. J. Physiol. 579, 53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Fujiyama A., Taylor T. D., Watanabe H., Yada T., Park H. S., Toyoda A., Ishii K., Totoki Y., Choi D. K., et al. (2000). The DNA sequence of human chromosome 21. Nature 405, 311–319 [DOI] [PubMed] [Google Scholar]
- Hebb D. O., Martinez J. L., Glickman S. E. (1994). The organization of behavior-a neuropsychological theory. Contemp. Psychol. 39, 1018–1020 [Google Scholar]
- Holtzman D. M., Santucci D., Kilbridge J., Chua-Couzens J., Fontana D. J., Daniels S. E., Johnson R. M., Chen K., Sun Y., Carlson E., et al. (1996). Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome. Proc. Natl. Acad. Sci. USA 93, 13333–13338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L. A., Crnic L. S. (2001). Age-related deficits in context discrimination learning in Ts65Dn mice that model Down syndrome and Alzheimer’s disease. Behav. Neurosci. 115, 1239–1246 [PubMed] [Google Scholar]
- Hyde L. A., Crnic L. S. (2002). Reactivity to object and spatial novelty is normal in older Ts65Dn mice that model Down syndrome and Alzheimer’s disease. Brain Res. 945, 26–30 [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Kotermanski S. E. (2006). Mechanism of action of memantine. Curr. Opin. Pharmacol. 6, 61–67 [DOI] [PubMed] [Google Scholar]
- Kahlem P., Sultan M., Herwig R., Steinfath M., Balzereit D., Eppens B., Saran N. G., Pletcher M. T., South S. T., Stetten G., et al. (2004). Transcript level alterations reflect gene dosage effects across multiple tissues in a mouse model of down syndrome. Genome. Res. 14, 1258–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetz F., Tomita T., Hsieh H., Seabrook G., Borchelt D., Iwatsubo T., Sisodia S., Malinow R. (2003). APP processing and synaptic function. Neuron 37, 925–937 [DOI] [PubMed] [Google Scholar]
- Katsuki H., Kaneko S., Tajima A., Satoh M. (1991). Separate mechanisms of long-term potentiation in two input systems to CA3 pyramidal neurons of rat hippocampal slices as revealed by the whole-cell patch-clamp technique. Neurosci. Res. 12, 393–402 [DOI] [PubMed] [Google Scholar]
- Keeler C. E. (1924). The inheritance of a retinal abnormality in white mice. Proc. Natl. Acad. Sci. USA 10, 329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner R. P., Lee I., Gilbert P. (2004). A behavioral assessment of hippocampal function based on a subregional analysis. Rev. Neurosci. 15, 333–351 [DOI] [PubMed] [Google Scholar]
- Kholodar-Smith D. B., Allen T. A., Brown T. H. (2008). Fear conditioning to discontinuous auditory cues requires perirhinal cortical function. Behav. Neurosci. 122, 1178–1185 [DOI] [PubMed] [Google Scholar]
- Kleschevnikov A. M., Belichenko P. V., Villar A. J., Epstein C. J., Malenka R. C., Mobley W. C. (2004). Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. J. Neurosci. 24, 8153–8160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Washiyama K., Ikeda K. (2003). Inhibition of G protein-activated inwardly rectifying K+ channels by fluoxetine (Prozac). Br. J. Pharmacol. 138, 1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lana-Elola E., Watson-Scales S. D., Fisher E. M. C., Tybulewicz V. L. J. (2011). Down syndrome: searching for the genetic culprits. Dis. Model. Mech. 4, 586–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Yu T., Morishima M., Pao A., LaDuca J., Conroy J., Nowak N., Matsui S., Shiraishi I., Yu Y. E. (2007). Duplication of the entire 22.9 Mb human chromosome 21 syntenic region on mouse chromosome 16 causes cardiovascular and gastrointestinal abnormalities. Hum. Mol. Genet. 16, 1359–1366 [DOI] [PubMed] [Google Scholar]
- Lin P. F., Slate D. L., Lawyer F. C., Ruddle F. H. (1980). Assignment of the murine interferon sensitivity and cytoplasmic superoxide dismutase genes to chromosome 16. Science 209, 285–287 [DOI] [PubMed] [Google Scholar]
- Lockrow J., Boger H., Bimonte-Nelson H., Granholm A. C. (2010). Effects of long-term memantine on memory and neuropathology in Ts65Dn mice, a model for Down syndrome. Behav. Brain Res. 221, 610–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi H. A., Reeves R. H. (2006). Hippocampal hypocellularity in the Ts65Dn mouse originates early in development. Brain Res. 1104, 153–159 [DOI] [PubMed] [Google Scholar]
- Loughlin S. E., Foote S. L., Grzanna R. (1986). Efferent projections of nucleus locus coeruleus: morphologic subpopulations have different efferent targets. Neuroscience 18, 307–319 [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Jackson M. F., Beazely M. A. (2006). Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit. Rev. Neurobiol. 18, 71–84 [DOI] [PubMed] [Google Scholar]
- Malberg J. E., Eisch A. J., Nestler E. J., Duman R. S. (2000). Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 20, 9104–9110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D. M., Yates P. O., Marcyniuk B., Ravindra C. R. (1985). Pathological evidence for neurotransmitter deficits in Down’s syndrome of middle age. J. Ment. Defic. Res. 29, 125–135 [DOI] [PubMed] [Google Scholar]
- Martin S. J., Grimwood P. D., Morris R. G. (2000). Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 23, 649–711 [DOI] [PubMed] [Google Scholar]
- Martinez-Cue C., Baamonde C., Lumbreras M., Paz J., Davisson M. T., Schmidt C., Dierssen M., Florez J. (2002). Differential effects of environmental enrichment on behavior and learning of male and female Ts65Dn mice, a model for Down syndrome. Behav. Brain Res. 134, 185–200 [DOI] [PubMed] [Google Scholar]
- Marvanova M., Lakso M., Pirhonen J., Nawa H., Wong G., Castren E. (2001). The neuroprotective agent memantine induces brain-derived neurotrophic factor and trkB receptor expression in rat brain. Mol. Cell. Neurosci. 18, 247–258 [DOI] [PubMed] [Google Scholar]
- McHugh T. J., Jones M. W., Quinn J. J., Balthasar N., Coppari R., Elmquist J. K., Lowell B. B., Fanselow M. S., Wilson M. A., Tonegawa S. (2007). Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science 317, 94–99 [DOI] [PubMed] [Google Scholar]
- Moore C. S., Roper R. J. (2007). The power of comparative and developmental studies for mouse models of Down syndrome. Mamm Genome 18, 431–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran T. H., Reeves R., Smith D., Fisher E. (1996). Ain’t misbehavin’ – it’s genetic! Nat. Genet. 12, 115–116 [DOI] [PubMed] [Google Scholar]
- Moran T. H., Capone G. T., Knipp S., Davisson M. T., Reeves R. H., Gearhart J. D. (2002). The effects of piracetam on cognitive performance in a mouse model of Down’s syndrome. Physiol. Behav. 77, 403–409 [DOI] [PubMed] [Google Scholar]
- Morice E., Andreae L. C., Cooke S. F., Vanes L., Fisher E. M., Tybulewicz V. L., Bliss T. V. (2008). Preservation of long-term memory and synaptic plasticity despite short-term impairments in the Tc1 mouse model of Down syndrome. Learn. Mem. 15, 492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. G. M. (1981). Spatial localization does not require the presence of local cues. Learn. Motiv. 12, 239–260 [Google Scholar]
- Netzer W. J., Powell C., Nong Y., Blundell J., Wong L., Duff K., Flajolet M., Greengard P. (2010). Lowering beta-amyloid levels rescues learning and memory in a Down syndrome mouse model. PLoS ONE 5, e10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G., Cooke S. F., Bliss T. V. (2008). Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat. Rev. Neurosci. 9, 65–75 [DOI] [PubMed] [Google Scholar]
- O’Doherty A., Ruf S., Mulligan C., Hildreth V., Errington M. L., Cooke S., Sesay A., Modino S., Vanes L., Hernandez D., et al. (2005). An aneuploid mouse strain carrying human chromosome 21 with Down syndrome phenotypes. Science 309, 2033–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L. E., Richtsmeier J. T., Leszl J., Reeves R. H. (2004). A chromosome 21 critical region does not cause specific down syndrome phenotypes. Science 306, 687–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L. E., Roper R. J., Sengstaken C. L., Peterson E. A., Aquino V., Galdzicki Z., Siarey R., Pletnikov M., Moran T. H., Reeves R. H. (2007). Trisomy for the Down syndrome ‘critical region’ is necessary but not sufficient for brain phenotypes of trisomic mice. Hum. Mol. Genet. 16, 774–782 [DOI] [PubMed] [Google Scholar]
- Palacios J., Kuhar M. J. (1982). Beta adrenergic receptor localization in rat brain by light microscopic autoradiography. Neurochem. Int. 4, 473–490 [DOI] [PubMed] [Google Scholar]
- Parker S. E., Mai C. T., Canfield M. A., Rickard R., Wang Y., Meyer R. E., Anderson P., Mason C. A., Collins J. S., Kirby R. S., et al. (2010). Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res. A Clin. Mol. Teratol. 88, 1008–1016 [DOI] [PubMed] [Google Scholar]
- Pennington B. F., Moon J., Edgin J., Stedron J., Nadel L. (2003). The neuropsychology of Down syndrome: evidence for hippocampal dysfunction. Child Dev. 74, 75–93 [DOI] [PubMed] [Google Scholar]
- Pereira P. L., Magnol L., Sahun I., Brault V., Duchon A., Prandini P., Gruart A., Bizot J. C., Chadefaux-Vekemans B., Deutsch S., et al. (2009). A new mouse model for the trisomy of the Abcg1-U2af1 region reveals the complexity of the combinatorial genetic code of down syndrome. Hum. Mol. Genet. 18, 4756–4769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosini L., Leggio M. G., Molinari M. (1998). The cerebellum in the spatial problem solving: a co-star or a guest star? Prog. Neurobiol. 56, 191–210 [DOI] [PubMed] [Google Scholar]
- Phillips R. G., LeDoux J. E. (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 106, 274–285 [DOI] [PubMed] [Google Scholar]
- Pittler S. J., Baehr W. (1991). Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc. Natl. Acad. Sci. USA 88, 8322–8326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletcher M. T., Wiltshire T., Cabin D. E., Villanueva M., Reeves R. H. (2001). Use of comparative physical and sequence mapping to annotate mouse chromosome 16 and human chromosome 21. Genomics 74, 45–54 [DOI] [PubMed] [Google Scholar]
- Rammes G., Rupprecht R., Ferrari U., Zieglgansberger W., Parsons C. G. (2001). The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neurosci. Lett. 306, 81–84 [DOI] [PubMed] [Google Scholar]
- Redish A. D., Touretzky D. S. (1998). The role of the hippocampus in solving the Morris water maze. Neural Comput. 10, 73–111 [DOI] [PubMed] [Google Scholar]
- Reeves R. H., Garner C. C. (2007). A year of unprecedented progress in Down syndrome basic research. Ment. Retard. Dev. Disabil. Res. Rev. 13, 215–220 [DOI] [PubMed] [Google Scholar]
- Reeves R. H., Irving N. G., Moran T. H., Wohn A., Kitt C., Sisodia S. S., Schmidt C., Bronson R. T., Davisson M. T. (1995). A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat. Genet. 11, 177–184 [DOI] [PubMed] [Google Scholar]
- Roper R. J., Reeves R. H. (2006). Understanding the basis for Down syndrome phenotypes. PLoS Genet. 2, e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper R. J., Baxter L. L., Saran N. G., Klinedinst D. K., Beachy P. A., Reeves R. H. (2006). Defective cerebellar response to mitogenic Hedgehog signaling in Down [corrected] syndrome mice. Proc. Natl. Acad. Sci. USA 103, 1452–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda N., Florez J., Martinez-Cue C. (2008). Chronic pentylenetetrazole but not donepezil treatment rescues spatial cognition in Ts65Dn mice, a model for Down syndrome. Neurosci. Lett. 433, 22–27 [DOI] [PubMed] [Google Scholar]
- Rueda N., Llorens-Martin M., Florez J., Valdizan E., Banerjee P., Trejo J. L., Martinez-Cue C. (2010). Memantine normalizes several phenotypic features in the Ts65Dn mouse model of Down syndrome. J. Alzheimers Dis. 21, 277–290 [DOI] [PubMed] [Google Scholar]
- Sacchetti B., Baldi E., Lorenzini C. A., Bucherelli C. (2002). Cerebellar role in fear-conditioning consolidation. Proc. Natl. Acad. Sci. USA 99, 8406–8411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sago H., Carlson E. J., Smith D. J., Kilbridge J., Rubin E. M., Mobley W. C., Epstein C. J., Huang T. T. (1998). Ts1Cje, a partial trisomy 16 mouse model for Down syndrome, exhibits learning and behavioral abnormalities. Proc. Natl. Acad. Sci. USA 95, 6256–6261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sago H., Carlson E. J., Smith D. J., Rubin E. M., Crnic L. S., Huang T. T., Epstein C. J. (2000). Genetic dissection of region associated with behavioral abnormalities in mouse models for Down syndrome. Pediatr. Res. 48, 606–613 [DOI] [PubMed] [Google Scholar]
- Salehi A., Delcroix J. D., Belichenko P. V., Zhan K., Wu C., Valletta J. S., Takimoto-Kimura R., Kleschevnikov A. M., Sambamurti K., Chung P. P., et al. (2006). Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron 51, 29–42 [DOI] [PubMed] [Google Scholar]
- Salehi A., Faizi M., Colas D., Valletta J., Laguna J., Takimoto-Kimura R., Kleschevnikov A., Wagner S. L., Aisen P., Shamloo M., et al. (2009). Restoration of norepinephrine-modulated contextual memory in a mouse model of Down syndrome. Sci. Transl. Med. 1, 7ra17. [DOI] [PubMed] [Google Scholar]
- Schochet S. S., Jr, Lampert P. W., McCormick W. F. (1973). Neurofibrillary tangles in patients with Down’s syndrome: a light and electron microscopic study. Acta Neuropathol. 23, 342–346 [DOI] [PubMed] [Google Scholar]
- Siarey R. J., Stoll J., Rapoport S. I., Galdzicki Z. (1997). Altered long-term potentiation in the young and old Ts65Dn mouse, a model for Down Syndrome. Neuropharmacology 36, 1549–1554 [DOI] [PubMed] [Google Scholar]
- Siarey R. J., Carlson E. J., Epstein C. J., Balbo A., Rapoport S. I., Galdzicki Z. (1999). Increased synaptic depression in the Ts65Dn mouse, a model for mental retardation in Down syndrome. Neuropharmacology 38, 1917–1920 [DOI] [PubMed] [Google Scholar]
- Siarey R. J., Villar A. J., Epstein C. J., Galdzicki Z. (2005). Abnormal synaptic plasticity in the Ts1Cje segmental trisomy 16 mouse model of Down syndrome. Neuropharmacology 49, 122–128 [DOI] [PubMed] [Google Scholar]
- Siman R., Salidas S. (2004). Gamma-secretase subunit composition and distribution in the presenilin wild-type and mutant mouse brain. Neuroscience 129, 615–628 [DOI] [PubMed] [Google Scholar]
- Stasko M. R., Costa A. C. (2004). Experimental parameters affecting the Morris water maze performance of a mouse model of Down syndrome. Behav. Brain Res. 154, 1–17 [DOI] [PubMed] [Google Scholar]
- Sussan T., Yang A., Li F., Ostrowski M., Reeves R. H. (2008). Trisomy protects against ApcMin-mediated tumors in mouse models of Down syndrome. Nature 451, 73–75 [DOI] [PubMed] [Google Scholar]
- Szot P., Weinshenker D., White S. S., Robbins C. A., Rust N. C., Schwartzkroin P. A., Palmiter R. D. (1999). Norepinephrine-deficient mice have increased susceptibility to seizure-inducing stimuli. J. Neurosci. 19, 10985–10992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien J. Z., Huerta P. T., Tonegawa S. (1996). The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338 [DOI] [PubMed] [Google Scholar]
- Tully K., Li Y., Tsvetkov E., Bolshakov V. Y. (2007). Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc. Natl. Acad. Sci. USA 104, 14146–14150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C. A., Presti M. F., Newman H. A., Bugenhagen P., Crnic L., Lewis M. H. (2001). Spontaneous stereotypy in an animal model of Down syndrome: Ts65Dn mice. Behav. Genet. 31, 393–400 [DOI] [PubMed] [Google Scholar]
- Vorhees C. V., Williams M. T. (2006). Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh L. A., Li M., Zhao T. J., Chiu T. H., Rosenberg H. C. (1999). Acute pentylenetetrazol injection reduces rat GABAA receptor mRNA levels and GABA stimulation of benzodiazepine binding with No effect on benzodiazepine binding site density. J. Pharmacol. Exp. Ther. 289, 1626–1633 [PubMed] [Google Scholar]
- Waterston R. H., Lindblad-Toh K., Birney E., Rogers J., Abril J. F., Agarwal P., Agarwala R., Ainscough R., Alexandersson M., An P., et al. (2002). Initial sequencing and comparative analysis of the mouse genome. Nature 420, 520–562 [DOI] [PubMed] [Google Scholar]
- Wehner J. M., Radcliffe R. A. (2004). Cued and contextual fear conditioning in mice. Curr. Protoc. Neurosci. Chapter 8, Unit 8 5C. [DOI] [PubMed] [Google Scholar]
- Weinshenker D. (2008). Functional consequences of locus coeruleus degeneration in Alzheimer’s disease. Curr. Alzheimer. Res. 5, 342–345 [DOI] [PubMed] [Google Scholar]
- Weinshenker D., Szot P., Miller N. S., Palmiter R. D. (2001). Alpha(1) and beta(2) adrenoreceptor agonists inhibit pentylenetetrazole-induced seizures in mice lacking norepinephrine. J. Pharmacol. Exp. Ther. 298, 1042–1048 [PubMed] [Google Scholar]
- Wenzel A., Fritschy J. M., Mohler H., Benke D. (1997). NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J. Neurochem. 68, 469–478 [DOI] [PubMed] [Google Scholar]
- Winters B. D., Bussey T. J. (2005). Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J. Neurosci. 25, 52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., Li Z., Jia Z., Clapcote S. J., Liu C., Li S., Asrar S., Pao A., Chen R., Fan N., et al. (2010a). A mouse model of Down syndrome trisomic for all human chromosome 21 syntenic regions. Hum. Mol. Genet. 19, 2780–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., Liu C., Belichenko P., Clapcote S. J., Li S., Pao A., Kleschevnikov A., Bechard A. R., Asrar S., Chen R., et al. (2010b). Effects of individual segmental trisomies of human chromosome 21 syntenic regions on hippocampal long-term potentiation and cognitive behaviors in mice. Brain Res. 1366, 162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]