SUMMARY
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder that results in the death of motor neurons in the brain and spinal cord. The disorder generally strikes in mid-life, relentlessly leading to paralysis and death, typically 3–5 years after diagnosis. No effective treatments are available. Up to 10% of ALS is familial, usually autosomal dominant. Several causative genes are known and, of these, mutant superoxide dismutase 1 (SOD1) is by far the most frequently found, accounting for up to 20% of familial ALS. A range of human mutant SOD1 transgenic mouse strains has been produced, and these largely successfully model the human disease. Of these, the most widely used is the SOD1 mouse, which expresses a human SOD1 transgene with a causative G93A mutation. This mouse model is excellent for many purposes but carries up to 25 copies of the transgene and produces a great excess of SOD1 protein, which might affect our interpretation of disease processes. A variant of this strain carries a deletion of the transgene array such that the copy number is dropped to eight to ten mutant SOD1 genes. This ‘deleted’ ‘low-copy’ mouse undergoes a slower course of disease, over many months. Here we have carried out a comprehensive analysis of phenotype, including nerve and muscle physiology and histology, to add to our knowledge of this ‘deleted’ strain and give baseline data for future studies. We find differences in phenotype that arise from genetic background and sex, and we quantify the loss of nerve and muscle function over time. The slowly progressive pathology observed in this mouse strain could provide us with a more appropriate model for studying early-stage pathological processes in ALS and aid the development of therapies for early-stage treatments.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is the third most common neurodegenerative cause of adult death, after Alzheimer’s disease and Parkinson’s disease, and the lifetime risk of dying from ALS lies between 1/600 and 1/1000 (Boillee et al., 2006; Pasinelli and Brown, 2006). In ALS, the upper motor neurons, which run from the brain to the spinal cord, and the lower motor neurons, which extend from the spinal cord out to the muscles, degenerate, leading inexorably to paralysis and death (Boillee et al., 2006; Pasinelli and Brown, 2006; Schymick et al., 2007; Valdmanis and Rouleau, 2008). Intellect usually remains largely intact and, despite intensive research, no effective treatment is currently available. ALS usually presents as a focal weakness, with atrophy of muscles in the proximal limbs or body region, and this atrophy progressively spreads to distal muscle groups over time (Ravits et al., 2007b), with a gradient of loss of motor neurons from the site of onset (Ravits et al., 2007a).
Up to 10% of ALS is familial (fALS) (Dion et al., 2009), usually autosomal dominant. Mutations in the ubiquitously expressed enzyme superoxide dismutase 1 (SOD1) are causative in up to 23% of fALS (Birve et al., 2010; Deng et al., 1993; Rosen et al., 1993) and in up to 3% of sporadic ALS (sALS) (Pasinelli and Brown, 2006). In addition, a recent paper from Bosco, Brown and colleagues shows that misfolding of both wild-type and mutant SOD1 into a disease-specific and toxic conformer might underlie both fALS and sALS (Bosco et al., 2010). Several other genes are known to be causative of classical ALS, although these account for a lower percentage of cases than does mutant SOD1; these genes include mutant FUS [fused in sarcoma; also known as translated in liposarcoma (TLS)], TDP43 (TAR DNA-binding protein 43) and optineurin (Gitcho et al., 2008; Kabashi et al., 2008; Maruyama et al., 2010; Pasinelli and Brown, 2006; Sreedharan et al., 2008; Van Deerlin et al., 2008; Vance et al., 2009), and other genes remain to be found (Dion et al., 2009).
One of the most powerful approaches to understanding ALS and how mutations in ubiquitously expressed genes such as SOD1 cause neurodegeneration of specific subsets of neurons is to generate mouse models. Typically, an autosomal dominant disorder such as ALS is modelled by transgenic mice. Thus, in 1994, Gurney and colleagues created the SOD1G93A transgenic mouse line [Tg(SOD1*G93A)1Gur], which carries a mutant human SOD1 cDNA inserted randomly into the mouse genome; this line remains the most widely used model of human ALS (Gurney et al., 1994). Several other mutant SOD1 mouse models now exist that also model ALS; these models all have slightly different pathologies, including time to onset of symptoms and death (e.g. Bruijn et al., 1997; Jonsson et al., 2006; Nicholson et al., 2000).
The SOD1G93A transgenic mouse has proven to be an invaluable tool for studies of SOD1 ALS and the toxic gain of function acquired by the mutant protein. These mice carry a causative mutation (a glycine-to-alanine change at residue 93) in an array of ∼25 copies of the human transgene (Gurney et al., 1994), which are randomly inserted into mouse chromosome 12 (Achilli et al., 2005). This mouse model has an onset of paralysis at ∼90 days, accompanied by degenerative changes to motor neurons that compare well with human ALS pathology (Synofzik et al., 2010), and death by ∼135 days, depending on genetic background. The SOD1G93A mouse has been widely used for research ranging from basic molecular cell biology through to extensive drug trials (Gurney et al., 1994). This model was the first mutant SOD1 transgenic produced, and is freely available, which adds to its popularity.
However, although the SOD1G93A mouse has been a bedrock for ALS research and the most important and well-used model for well over a decade, there is some disquiet about its use because phenotypic features might arise from extreme SOD1 protein overexpression (up to 24-fold of the human protein compared with the endogenous mouse SOD1), rather than being the effect of the SOD1G93A mutation itself (Shibata, 2001). Indeed, wild-type human SOD1-overexpressing transgenic mice have abnormal phenotypes (Jaarsma et al., 2001) – although, to our knowledge, no effect on motor neurons has been reported. In addition, because SOD1 lies on human chromosome 21 and therefore is present in three copies in people with Down syndrome, SOD1 human wild-type overexpressing transgenics have been described as modelling phenotypic aspects of trisomy 21 (Avraham et al., 1988; Lalonde et al., 2004). Furthermore, because the development of therapeutics for human ALS is largely relevant to those with sporadic cases who are not at end-stage, and for those familial cases who are pre-symptomatic, it might be more appropriate for some studies to use a model with a lower mutant SOD1 protein level and less aggressive disease than found in the SOD1G93A mouse.
The Tg(SOD1*G93Adl)1Gur (SOD1G93Adl; also known as G1del) line seems to have arisen from a deletion in the transgene array of a SOD1G93A mouse in the breeding colony at the Jackson Laboratory (http://jaxmice.jax.org/strain/002300.html). SOD1G93Adl mice are estimated to carry ∼eight to ten copies of the human SOD1G93A transgene, and develop paralysis between 24 and 34 weeks of age (Alexander et al., 2004). In the United Kingdom, the humane endpoint for these mice is usually defined as the first sign of limb paralysis, or the loss of righting reflex as shown by a failure to right after laying the mouse on its side for 30 seconds, or loss of up to 20% bodyweight from the maximum adult weight; this endpoint is typically reached 2–10 weeks after symptom onset depending on genetic background (Alexander et al., 2004; Jaarsma et al., 2001; Lalonde et al., 2004; Muller et al., 2008; Teuling et al., 2008; Veldink et al., 2003; Vlug et al., 2005).
SOD1G93Adl mice have been reported to have a number of abnormalities, including gait and movement defects (Tucci et al., 2007; Wooley et al., 2005), altered axonal transport (Sasaki and Iwata, 1996; Zhang et al., 1997), fragmented Golgi apparatus in motor neurons (Stieber et al., 2000), accumulation of mutant SOD1 (Puttaparthi et al., 2003; Sasaki et al., 2005) in swollen and vacuolated mitochondria (Jaarsma et al., 2001; Sasaki et al., 2004; Sumi et al., 2006), neuronal and astrocytic hyaline inclusions (Shibata 2001; Sumi et al., 2006), loss of some synaptophysin staining (Zang et al., 2005), aberrant staining for parvalbumin and calbindin (Sasaki et al., 2006), aberrant p75NTR receptor expression in the spinal cord (Copray et al., 2003), aberrant heat shock protein levels (Maatkamp et al., 2004) and transcription factor levels (Vlug et al., 2005), and aberrant deposition of TDP43 protein (Sumi et al., 2009). These mice model human ALS and have a progressive loss of spinal motor neurons, astro- and microgliosis, degeneration of spinal interneurons, and degeneration of ascending and descending fibre tracks in the spinal cord; mutant SOD1 accumulates in the brain and spinal cord (Jaarsma et al., 2001; Teuling et al., 2008; Vlug et al., 2005).
In this study we present a comprehensive assessment of the SOD1G93Adl mouse, and the effects of genetic background on lifespan, motor neuron and muscle function, and pathology. To assess how well this mouse models human ALS, we also undertook longitudinal studies of disease progression in this animal to determine when the first motor deficits in different muscles appear and how weak the mice are at end-stage disease. In addition, because human ALS is thought to start focally and progress to other areas of the motor system, we tried to detect evidence for differential onset or progression between the left or right side of individual animals; we found no focal onset using the methods herein but, interestingly, we did see some asymmetry in motor unit survival in later stages of disease.
ALS research requires a range of different mouse models to understand different aspects of motor neuron degeneration; the SOD1G93Adl mouse is another excellent model of the human disease and is important to include in our arsenal in the fight to treat this relentless disorder.
RESULTS
Genetic background and gender effects in a SHIRPA screen
Male SOD1G93Adl mice on a C57BL/6J × SJL/J (C57BL/6J-SJL/J) hybrid background were backcrossed for at least three generations into four different inbred lines: C57BL/6J (‘B6’; backcrossed to generation N8), BALB/cAnNCrl, C3H/HeH and FVB/J. B6-BALB/c hybrids were also generated by crossing N8 C57BL/6 SOD1G93Adl males to BALB/c females. Mice of at least backcross N3 were used for all assays shown below except for the C57BL/6J line, where at least N8 mice were assessed.
Our five cohorts of mice (B6, BALB/c, C3H, FVB, B6-BALB/c) underwent SHIRPA analysis, a broadly based phenotypic screen, fortnightly from 12 weeks to at least 32 weeks of age. This test includes assessment of tremors, limb tone and grip strength, which, in the context of these ALS disease models, are taken as signs of motor neuron degeneration. SHIRPA also includes startle response analysis, which is another test that can relate to locomotor deficits. We assayed different cohort sizes of males and females for each strain – on BALB/c, C3H and FVB backgrounds we analysed five mice or fewer of each sex; because of the low numbers per sex analysed for these strains we only attempted to detect phenotypic sex bias on the B6 and B6-BALB/c strains (nevertheless, phenotype data for all strains broken down by sex is presented in supplementary material Tables S1, S2).
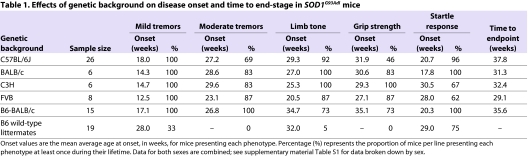
We found that all SOD1G93Adl mice on the five genetic backgrounds presented with mild tremors, and genetic background significantly affected the age at onset of tremors (P<0.001; Table 1, supplementary material Table S1). Limb-tone, grip-strength and startle-response deficits appeared in the majority of SOD1G93Adl mice on all genetic backgrounds and, again, age at onset was significantly affected by genetic background (P<0.001; Fig. 1A, Table 1). We also tested B6 wild-type littermates for comparison and found some effects, such as mild tremors occurring in different percentages of mice as they aged (Table 1). Overall, the B6 and B6-BALB/c cohorts tended to have later ages of onset and tended to have lower percentages of animals affected by individual phenotypes, compared with the BALB/c, C3H or FVB cohorts. All SOD1G93Adl C3H mice also presented with hind-limb clasping on the SHIRPA wire manoeuvre test by 14 weeks of age, whereas this was only rarely seen in any of the other transgenic SOD1G93Adl genetic backgrounds.
Table 1.
Effects of genetic background on disease onset and time to end-stage in SOD1G93Adl mice
Fig. 1.
Behavioural analysis of SOD1G93Adl on different genetic backgrounds. (A) SHIRPA analysis. Mice underwent modified SHIRPA once every 2 weeks from 12 weeks of age. Bars represent the average age at onset for tremors, limb tone, grip strength and startle response. Error bars represent s.e.m. Onset of all phenotypes is significantly different (log-rank P<0.001) when comparing between all SOD1G93Adl transgenic lines. (B) Survival. SOD1G93Adl mice reach the humane endpoint at different times, depending on genetic background (sex-averaged data; log-rank P<0.001).
A breakdown of the data by sex for all cohorts is given in supplementary material Table S1. Sex-matched cohorts were small for BALB/c, C3H and FVB mice, and so we would be unlikely to detect sex differences for these genetic backgrounds. However, greater than six mice per sex per genotype were assessed on the B6 and B6-BALB/c backgrounds, allowing us to detect effects of gender. On the B6 background we found significant effects of gender only for time to onset of moderate tremors (supplementary material Table S1). For the hybrid B6-BALB/c background, the onset of all traits was affected by sex, including tremors, limb tone, grip strength and startle response (supplementary material Table S1). In all but startle response, B6-BALB/c males presented with an earlier age at onset when compared with females.
Time to humane endpoint
The humane endpoint in this study, at which the mice were euthanised, was defined as loss of righting reflex for 30 seconds, or loss of 20% bodyweight, or the first sign of leg paralysis, whichever was reached soonest. We found that the majority of mice (∼92%) had to be culled because of leg paralysis, and most of the rest (∼8%) were culled because they reached 20% weight loss; both groups had identical mean survivals. No mice were culled because they were unable to right themselves. Age at humane endpoint was significantly different (P<0.001) on the different genetic backgrounds. The FVB background showed the shortest mean average time to endpoint, followed by BALB/c, C3H, hybrid B6-BALB/c and finally the B6 background, which presented the longest survival time (Fig. 1B, Table 1). Pairwise comparisons revealed significant differences (P<0.001) in survival time between the B6 mice and all other backgrounds except the B6-BALB/c hybrid; no significant differences were present between the BALB/c, C3H and FVB lines or between the B6 and hybrid B6-BALB/c backgrounds (supplementary material Table S2). Sex did not affect survival on the B6 background, but it had an effect on the B6-BALB/c background, with females reaching endpoint significantly later than males (supplementary material Table S1).
Disease progression in SOD1G93Adl mice on the C57BL/6J background
To gain a more detailed picture of disease progression in SOD1G93Adl transgenic mice, we focused on the C57BL/6J background, because this is standardly used in many laboratories (although note that we are not using a full congenic strain here).
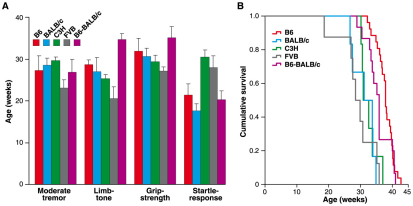
For grip strength analysis, we found that, compared with the wild-type age- and sex-matched littermates, significant differences occurred in female SOD1G93Adl B6 mice starting at 24 weeks of age, whereas males did not show significant differences from wild-type littermates until 28 weeks of age (Fig. 2A,B).
Fig. 2.
Grip-strength, startle-response and rotarod analysis of SOD1G93Adl B6 mice. (A) Female grip strength. At least five females per genotype were assessed for every time point except for week 20, when two SOD1G93Adl B6 females were assessed. Grip strength deteriorates in SOD1G93Adl females compared with wild-type littermates, with differences starting to appear from 24 weeks of age (ANOVA; 24 weeks: P=0.006; 26 weeks: P=0.073; 28 weeks: P=0.007; 30 weeks: P=0.001 and 32 weeks: P=0.003). The figure represents the average plus 2 s.e.m. per genotype and time point. (B) Male grip strength. At least five males per genotype were assessed for each time point except for week 20, when four wild-type males were assessed. Grip strength rapidly deteriorates in SOD1G93Adl males compared with wild-type littermates from 28 weeks of age (ANOVA; 28 weeks: P=0.005; 30 and 32 weeks: P<0.001). The figure represents the average plus 2 s.e.m. per genotype and time point. By 30 weeks of age the difference between grip strength of SOD1G93Adl B6 males and females is significant (P=0.020), as it is by 32 weeks of age (P=0.001). (C) Female rotarod. At least five females per genotype were assessed for each time point. No statistically significant differences in latency to fall were detected at any time point. The figure represents the average plus 2 s.e.m. per genotype and time point. (D) Male rotarod. At least five males per genotype were assessed for each time point. Rotarod performances deteriorate in SOD1G93Adl B6 males when compared with littermate controls, with significant differences appearing at 27 weeks (P=0.039) and 33 weeks (P=0.009) of age. The figure represents the average plus 2 s.e.m. per genotype and time point. (E) Startle response. 20 (8 females, 12 males) littermate controls and 13 (7 females, 6 males) SOD1G93Adl B6 mice were tested at 22 weeks of age (sex-averaged difference between wild types and SOD1G93Adl B6 littermates, P=0.011; females only P=0.025; males only P=0.232). Data are presented pooled by sex because no sex bias for any genotype was detected. Deficits on startle response were confirmed at 29 weeks of age (P=0.002, males only tested; data not shown). No differences are found in PPI (data not shown). Bars represent the average startle response per genotype. Error bars represent 2 s.e.m.
When we compared grip strength data from SOD1G93Adl B6 males and females, we found that, although grip-strength deficits occurred earlier in females, the rate of deterioration was more aggressive in males, suggesting a more rapid disease progression in males than in females (Fig. 2A,B). By 30 and 32 weeks of age the difference between SOD1G93Adl B6 males and females was significant, with males being more severely affected.
SOD1G93Adl B6 mice and wild-type littermates were tested by the accelerating rotarod (Fig. 2C,D). Suprisingly, we found no differences between wild-type and transgenic females at any time point, whereas significant differences were seen between males at week 27 and week 33.
As part of our SHIRPA analysis we tested all mice for qualitative startle deficits using a hand-held click-box and found significant differences from wild-type littermates for both male and female SOD1G93Adl B6 mice (Fig. 1A, Fig. 2E, Table 1, supplementary material Table S1). We investigated this further by quantitatively assessing startle response and pre-pulse inhibition (PPI) on wild-type and SOD1G93Adl B6 littermates at 22 and 29 weeks of age using commercial startle boxes (Med Associates, USA). These ages were chosen because 22 weeks is just after the onset of mild tremors and by 29 weeks the loss of limb tone and grip strength appears on the B6 background. We found a mild quantitative deficit in startle response at 22 weeks of age between wild-type and transgenic mice, with no significant differences between male and female SOD1G93Adl B6 mice. We went on to test PPI and found no differences between wild-type and transgenic mice at any time point in either sex-averaged or sex-separated data.
To determine whether the differences in startle response were due to hearing defects, SOD1G93Adl B6 mice and their wild-type littermate controls underwent auditory brainstem response analysis. At 22 weeks of age there was no difference between transgenic and non-transgenic littermates for hearing thresholds (only males tested; data not shown). Thus, the deficits in startle response might be due to the elicited motor response to a burst of noise. We note that male high copy number SOD1G93A transgenics [Tg(SOD1*G93A)1Gur/J] also present with startle-response deficits – at 95 days of age and end-stage – when compared with wild-type littermates (supplementary material Fig. S1).
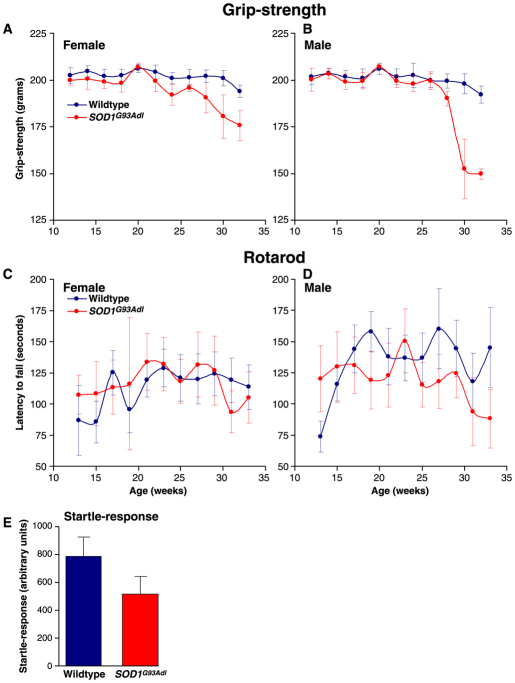
Assessment of hindlimb muscle function in SOD1G93Adl B6 mice at early and late stages of disease
The maximum force produced by the tibialis anterior (TA), extensor digitorum longus (EDL) and soleus muscles was established in both of the hindlimbs of male SOD1G93Adl B6 mice at early and late stages of disease, as defined by our longitudinal SHIRPA and grip-strength measurements. Isometric force measurements showed that, at early stages of disease (22 and 24 weeks of age), the maximum force produced by these muscles is not significantly different from that produced by the equivalent muscles in wild-type littermates (Fig. 3). However, in 34-week-old SOD1G93Adl mice, a late disease stage when obvious motor pathology was observed using behavioural assessment (Fig. 2), we found a reduction in muscle force in all three muscles examined (Fig. 3). Thus, by 34 weeks of age the TA muscle of SOD1G93Adl B6 mice is only capable of exerting ∼70% force of that produced by the TA of wild-type littermates (P<0.001). EDL and soleus muscles were also severely affected in SOD1G93Adl B6 mice at 34 weeks of age, with a reduction of muscle force of ∼50% in EDL (P<0.001) and a ∼20% soleus (P=0.1) compared with wild-type littermates.
Fig. 3.
Assessment of hindlimb muscle force production. (A) Characteristic traces obtained from TA muscles of wild-type mice (24–34 weeks of age), and 24-week- and 34-week-old and SOD1G93Adl B6 mice by stimulating the sciatic nerve by supramaximal single twitch and tetanic stimuli using variable frequencies for tetanic stimulation from 40 to 100 Hz. (B) Summary of tetanic force production by TA muscles in wild-type and SOD1G93Adl B6 mice at 22, 24 and 34 weeks of age. Maximum tetanic force of TA muscles was 137±12 g (n=10) in wild-type mice aged between 24 and 34 weeks, and 130±13 g (n=5; P=0.09) and 107±11 g (n=5; P=0.08) in 22- and 24-week old SOD1G93Adl littermates, respectively. At 34 weeks of age the TA muscle of SOD1G93Adl mice is only capable of exerting 33.8±5 g force, which is ∼70% less than the 137±12 g force produced by TA in wild-type littermates (**P<0.001). (C) Tetanic force production by the EDL and soleus muscles in wild-type and SOD1G93Adl B6 mice at 22, 24 and 34 weeks of age. The tetanic force of EDL was 30±1.9 g in wild-type mice, and 29.3±2.5 g (P=0.09) and 22.2±3.5 g (P=0.18) in SOD1G93Adl littermates at 22 and 24 weeks, respectively. In the soleus, the maximum force was 19±3.5 g in wild-type mice, and 20±1.9 g (P=0.24) and 12±4 g (P=0.13) in SOD1G93Adl littermates at 22 and 24 weeks, respectively. By 34 weeks of age, the EDL exerts 14.9±1.8 g force in SOD1G93Adl mice, compared with 30±1.9 g in wild-type littermates, a reduction of 50% (**P<0.001). At 34 weeks of age the soleus muscle in SOD1G93Adl mice exerts 14.8±2.8 g force, compared with 19±3.5 g in wild-type littermates, which is a reduction of ∼20% (P=0.1).
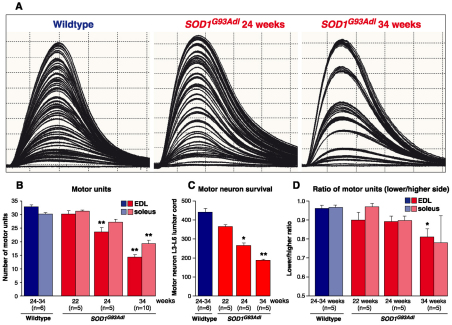
We assessed motor unit survival in SOD1G93Adl B6 mice at early stages of disease in order to have a readout of the number of functional motor neurons that survive and innervate individual muscles. At 22 weeks, there is no significant difference in the number of motor units innervating EDL and soleus muscles of wild-type and SOD1G93Adl B6 littermates, indicating that, at this stage, there has been no loss of functional motor units (Fig. 4A). However, although muscle force is still maintained in all muscle types investigated, in 24-week-old SOD1G93Adl B6 mice there was a significant reduction (P<0.001) in the number of motor units that innervate the EDL muscle, compared with wild type (Fig. 4B). Thus, even at this early stage of disease, 30% of motor units innervating the wild-type EDL muscle have already died in SOD1G93Adl B6 mice. By contrast, there was no loss of motor units in the soleus muscle of 24-week-old SOD1G93Adl B6 mice (Fig. 4B).
Fig. 4.
Physiological and morphological analysis of motor units and motor neuron survival. (A) Physiological analysis of the number of motor units innervating EDL muscles of wild-type mice (24–34 weeks of age), and 24- and 34-week-old SOD1G93Adl B6 mice was undertaken; typical traces are shown. Each twitch trace in the recordings represents a single motor unit. (B) The number of surviving motor units for EDL and soleus muscles in wild-type and SOD1G93Adl B6 mice at different stages of disease (22, 24 and 34 weeks of age) are summarised. No significant differences are found at 22 weeks of age. At 24 weeks, only 23±1.6 motor units survived in SOD1G93Adl B6 mice compared with 32.8±0.7 motor units in EDL muscles of wild-type littermates (P<0.001). By 34 weeks, only 14.2±0.9 motor units survived in the EDL muscles of SOD1G93Adl mice. (C) Morphological assessment of motor neuron survival in the L3-L6 region of the lumbar spinal cord was undertaken and the mean survival of motor neuron in the sciatic motor pool is shown. At 22 weeks of age, wild-type littermates had 439±23 motor neurons in the sciatic motor pool compared with 362±12 in 22-week-old SOD1G93Adl mice (P=0.571). By 24 weeks there was a significant decrease in motor neuron survival compared with wild-type littermate controls, and only 264±12 motor neurons survived in the SOD1G93Adl sciatic motor pool (P<0.05). By 34 weeks, the number of SOD1G93Adl surviving motor neurons was dramatically reduced and only 185±5.6 motor neurons were present in the sciatic motor pool (P<0.001). (D) Analysis of asymmetry in the number of surviving motor units between two sides in each animal is summarised. For each mouse, a ratio of the number of motor units in the weaker side over that on the stronger side was generated and compared with the wild-type group. *P<0.05; **P<0.001.
By 34 weeks of age, all three muscles were affected by disease in SOD1G93Adl B6 mice, with a clear difference in the vulnerability of different muscle types: fast twitch muscles such as the TA and EDL were more severely affected than muscles such as the soleus, which are composed largely of slow twitch fibres. The reduction in muscle force observed in muscles of 34-week-old mice was reflected by a significant reduction in motor unit survival in both EDL and soleus muscles; however, compared with wild-type littermates, ∼50% of motor units had died in the EDL, whereas ∼65% of motor units in the soleus had died (P<0.001 for both muscles; Fig. 4B).
Motor neuron survival in SOD1G93Adl B6 mice at early and late stages of disease
Following physiological assessment of muscle function, all experimental mice were perfused, and their spinal cords removed, sectioned and stained for Nissl. The number of surviving motor neurons present in the sciatic motor pool in the lumbar spinal cord was established. In 22-week-old SOD1G93Adl B6 mice, which show no deficits in muscle function, there was a small decrease in motor neuron survival compared with control wild-type littermates, although this did not reach significance (Fig. 4C). By 24 weeks, at which point we observed a reduction in motor unit survival but no loss of muscle force, there was a significant decrease in motor neuron survival compared with wild-type littermate controls (P<0.05). By 34 weeks, the number of surviving SOD1G93Adl motor neurons was dramatically reduced (P<0.001). Thus, in SOD1G93Adl B6 mice there is a 40% reduction in motor neuron survival at 24 weeks and a 60% reduction by 34 weeks compared with wild-type littermates (Fig. 4C).
Assessing asymmetry in motor unit survival during disease progression
Because the clinical presentation of ALS is frequently focal and asymmetric between the left and right hands and/or legs at the time of diagnosis, we also examined whether there are signs of this asymmetry in the SOD1G93Adl B6 mouse at the age of onset or during disease progression. The number of motor units in a given muscle gives a direct readout on the functionality of spinal motor neurons. Functional motor units are among the first affected in neuromuscular diseases and cannot be masked by compensatory mechanisms such as axonal sprouting and reinnervation that can preserve muscle-force production even when motor neurons are already lost.
As a measure of motor neuron function on each side of the spinal cord, the number of motor units was established in both of the hindlimbs for the EDL and soleus muscles of wild-type and SOD1G93Adl B6 mice at 22, 24 and 34 weeks of age. Then, for each animal, we determined the ratio of ‘the number of motor units on the side with the lowest counts’ over ‘the number of motor units on the side with the highest counts’. This ratio gives an indication of the difference in motor unit survival in EDL and soleus muscles between the two hindlimbs. A motor unit ratio of 1 indicates that both sides contained an equal number of motor units, and decreases in this ratio indicate an increasing difference between the two hindlimbs. The results are summarised in Fig. 4D. In wild-type animals motor unit ratios for both the EDL and soleus muscles are close to 1.0, indicating that there are no significant differences present between either sides. This symmetry in motor unit numbers is largely maintained in young SOD1G93Adl B6 mice, so that there were no significant differences between sides in either the EDL or soleus muscles at 22 and 24 weeks of age. However, at a late stage of disease, we observed some asymmetry in motor unit survival in EDL muscles of SOD1G93Adl B6 mice, so that EDL muscles on the more affected side contained only 80% of the motor units that were present in the less affected side (P=0.008).
Muscle pathology in SOD1G93Adl B6 mice
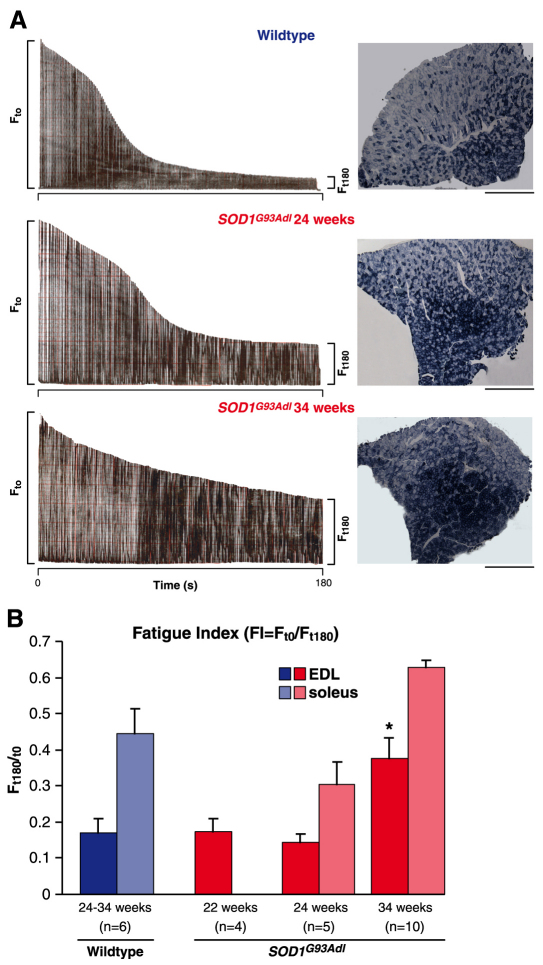
Progressive muscle pathology in SOD1G93Adl B6 mice also manifested as a change in the fatigue characteristics of EDL and soleus muscles. Fast twitch muscles such as the TA and EDL cannot normally maintain force when repeatedly stimulated and fatigue rapidly. This can be represented quantitatively by calculating a fatigue index (FI), in which the force produced at the end of a 3-minute period of stimulation is expressed as a ratio of the initial force produced; an FI approaching 1.0 indicates that a muscle is completely fatigue resistant. Thus, in wild-type mice, fast twitch muscles such as the TA and EDL have a low FI and slow twitch muscles such as the soleus have a higher FI (Fig. 5). However, in SOD1G93Adl B6 mice these muscle-specific fatigue characteristics change with disease progression. During the early stages of disease, at 22 and 24 weeks, the fatigue characteristics of both the EDL and soleus muscles remain unaltered in SOD1G93Adl mice but, by 34 weeks of age, there is a clear change in the fatigability of these muscles, with an increase in their fatigue resistance, reflected by a higher FI. Thus, wild-type littermate EDL and soleus muscles have an FI of 0.17±0.03 and 0.44±0.07, respectively, reflecting the differences in their fatigue resistance and muscle fibre phenotype, with soleus, a slow twitch muscle, being more fatigue resistant than the fast twitch EDL. In SOD1G93Adl B6 mice at 34 weeks, both muscles were more fatigue resistant than that of wild-type littermates, and the EDL had an FI of 0.37±0.05 (P=0.007) and soleus an FI of 0.62±0.02 (P=0.08, not significant; Fig. 5).
Fig. 5.
Muscle pathology in SOD1G93Adl B6 mice. (A) Characteristic fatigue traces from 24- to 34-week-old wild-type and SOD1G93Adl mice, generated by tetanic stimulation of the fast EDL muscle over 180 seconds are shown on the left panels. Each tetanic contraction during this time is represented by a single line in the trace and the length of the line is relative to the force produced. The length of the force trace at Ft180 (time = 180 seconds) as a ratio of that at Ft0 gives a fatigue index (FI), i.e. FI=Ft180/Ft0. Wild-type mice lose a large proportion of force at the end of the 180-second stimulation period compared with the beginning of the trace; thus, wild-type muscles have a low FI. By contrast, in old SOD1G93Adl mice, the EDL muscle is less fatigable and this is indicated by the higher FI. On the right of each trace is a cross-section of the TA muscle obtained from wild-type mice, and 24- and 34-week-old SOD1G93Adl mice, stained for SDH. In the TA muscle there is a characteristic pattern of SDH staining, with the presence of darkly stained, oxidative type 1 fibres in the centre of the muscle and lightly stained, less oxidative and more likely to be fast type II fibres towards the outer regions of the muscle. Compared with wild-type mice, SDH staining in 24-week-old SOD1G93Adl mice shows that a large area in the centre of the muscle stains darkly for SDH, with a smaller outer region of the muscle staining lighter, indicating a transformation of a subset of muscle fibres into a slower phenotype. This transformation is even more apparent at 34 weeks in SOD1G93Adl mice. (B) Summary of FI in EDL and soleus muscles of wild-type and SOD1G93Adl B6 mice at different stages of disease, showing a functional consequence of the muscle fibre type shift shown in A. Wild-type littermate EDL and soleus muscles have an FI of 0.17±0.03 and 0.44±0.07, respectively. In SOD1G93Adl B6 mice at 34 weeks, both muscles are more fatigue resistant than that of wild-type littermates, and EDL has an FI of 0.37±0.05 (P=0.007) and soleus an FI of 0.62±0.02 (not significant; P=0.08). *P<0.05.
We next examined the histochemical properties of the TA and EDL muscles of SOD1G93Adl B6 mice. Slow oxidative muscle type I fibres stain intensely for succinate dehydrogenase (SDH), whereas lighter staining fibres have a low oxidative capacity and are likely to be fast IIa or IIb fibres. As shown in Fig. 5A (right panel), the majority of muscle fibres in the centre of the TA muscle of wild-type mice stain darkly for SDH, whereas the fibres in the outer regions of the muscle stain lightly. Surprisingly, despite the relatively minor functional deficits observed in TA muscles at 24 weeks in SOD1G93Adl B6 mice, staining of these TA muscles for SDH revealed that, even at this early stage, there are subtle alterations compared with the staining pattern observed in wild-type mice – as can be seen in Fig. 5, a larger proportion of muscle fibres in the middle region of the muscle stains darkly for SDH compared with wild type. This shift in the staining pattern of TA muscle fibres is even more apparent in 34-week-old SOD1G93Adl B6 mice, when most of the central region of the TA muscle consists exclusively of darkly stained fibres and lightly stained fibres are only present in the outer region of the TA muscle.
Spinal cord histopathology of SOD1G93Adl B6 mice, and the appearance of misfolded SOD1 and reactive gliosis
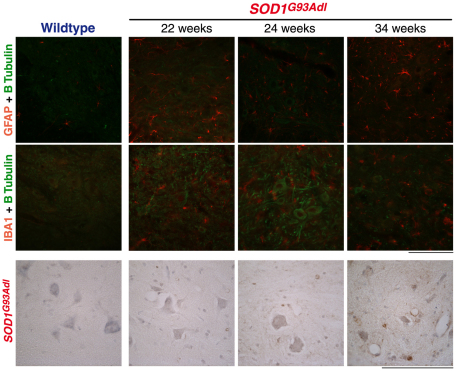
Spinal cord sections from SOD1G93Adl B6 mice at 22, 24 and 34 weeks of age were examined by using immunohistochemistry for the presence of misfolded SOD1 and for signs of astroglial and microglial activation. Staining of SOD1G93Adl B6 spinal cord for markers of astroglia (GFAP) and microglia (IBA1) revealed evidence of astroglial and microglial activation even at 22 weeks of age, when SOD1G93Adl mice have no loss of motor force, motor units or motor neurons (Fig. 6). Expression of GFAP and IBA1 increased with age and, by 34 weeks, there was a widespread expression of GFAP and IBA1 in the spinal cord of SOD1G93Adl B6 mice, indicating advanced astroglial and microglial reactions (Fig. 6). Spinal cord sections from SOD1G93Adl B6 mice at the same ages were also examined for the presence of misfolded SOD1 (Fig. 6). At 22 weeks of age we could not detect misfolded SOD1 and the pattern of staining was the same as that observed in spinal cord of wild-type littermates. However, just 2 weeks later, at 24 weeks of age, there were clear signs of misfolded SOD1 being present in the spinal cords of SOD1G93Adl B6 mice and, by 34 weeks, misfolded SOD1 became abundant (Fig. 6), as shown by staining with the SOD1 exposed dimer interface (SEDI) antibody, which exclusively reacts with misfolded SOD1 (Rakhit et al., 2007). This change in staining pattern indicates a profound pathology at this stage.
Fig. 6.
Glial reactions and the presence of misfolded SOD1 in SOD1G93Adl B6 spinal cord. Lumbar spinal cord sections from wild-type and SOD1G93Adl B6 mice at 22, 24 and 34 weeks were stained for markers that indicate pathology, such as GFAP (astrogliosis; top row, red), IBA1 (microglia; middle row, red) and using the SEDI antibody (DAB staining; bottom row). Wild-type spinal cord sections showed weak but specific immunoreactivity for both GFAP and IBA1; immunoreactivity for these markers was much stronger in SOD1G93Adl spinal cord even as early as 22 weeks of age, indicating the presence of glial activation. Misfolding of mutant SOD1 protein is also clearly detectable at 24 weeks using the SEDI antibody. Green staining in the top and middle row shows the localisation of neuronal cells (β-tubulin stain). Scale bars: 100 μm.
Immunogold labelling transmission electron microscopy
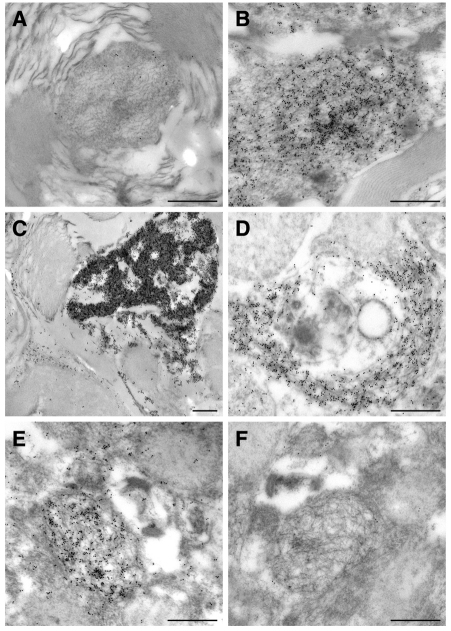
Spinal cord sections from end-stage SOD1G93Adl B6 mice at 39 weeks of age were examined using immunogold labelling for SOD1 and TDP43 followed by transmission electron microscopy (TEM) analysis. Low levels of anti-SOD1 labelling were consistently observed within axonal profiles of wild-type littermate control mice (Fig. 7A), but levels of anti-SOD1 labelling were much higher in the SOD1G93Adl B6 mice and labelling was commonly seen as fibrillar accumulations (Fig. 7B) or, very occasionally, as very dense aggregates within the axons (Fig. 7C). In the SOD1G93Adl B6 mice, spherical cytoplasmic inclusions of around 2–3 μm diameter, again fibrillar in nature, were quite common (Fig. 7D); no such inclusions were found in wild-type littermates. Examination of sections labelled for TDP43 showed the expected localisation to euchromatin regions within nuclei, as previously described in normal human brain neurons (Thorpe et al., 2008). Levels of labelling elsewhere were low, and the examination of serial sections of SOD1G93Adl B6 mice single-labelled for SOD1 and TDP43 revealed no association of TDP43 with SOD1-positive regions (e.g. Fig. 7E,F).
Fig. 7.
Electron microscopy analysis of SOD1G93Adl B6 spinal cord. Immunogold labelling TEM with anti-SOD1 (A-E) and anti-TDP43 (F) of wild-type littermate and SOD1G93Adl B6 spinal cord lumbar regions. (A) An axonal profile of a wild-type littermate control mouse, showing the typically low levels of anti-SOD1 labelling. (B) An axonal profile of a SOD1G93Adl B6 mouse showing the typically heavier levels of anti-SOD1 labelling. (C) An axonal profile of a SOD1G93Adl B6 mouse containing very dense aggregates that are heavily labelled for SOD1. (D) A cytoplasmic SOD1-positive inclusion with a fibrillar appearance in a SOD1G93Adl B6 mouse. (E,F) Serial sections of a SOD1G93Adl B6 spinal cord single-labelled for SOD1 (E) and TDP43 (F), showing the same inclusion that is both SOD1 positive and TDP43 negative. Scale bars: 0.2 μm (A,D); 0.5 μm (B,C,E,F).
DISCUSSION
Transgenic mice expressing mutations in the SOD1 gene have been invaluable tools to investigate disease mechanisms and possible therapeutic applications in ALS. However, failure to translate positive preclinical findings achieved in these mice into positive clinical trials in ALS patients has raised concerns about the relevance of SOD1 mouse models to ALS (as well as highlighted the need to carry out high-quality clinical trials) (Benatar, 2007; Scott et al., 2008). This has resulted in an increasing recognition that any disease model has to be thoroughly characterised and standard outcome measures applied in order to obtain reproducible and useful data (Ludolph et al., 2010). Therefore, in this study we carried out a comprehensive behavioural and physiological analysis of the SOD1G93Adl mouse, which is less widely used in ALS research than the ‘high-copy’ SOD1G93A mouse, in order to add to the standardised data available for this model and provide baseline information to help further its use in ALS research and therapeutic trials.
We have characterised the effects of genetic background and gender on a range of quantifiable behaviours and time to humane endpoint in the SOD1G93Adl mouse. We crossed the ‘low-copy’ transgene array onto five different backgrounds and determined that, although all animals tended to have similar phenotypic features (for example, tremors, grip-strength deficits), these occurred at different times in different percentages of mice, depending on the background inbred line. Overall, the C57BL/6J background delayed phenotype onset. Similarly, this background increased mean survival, which is in agreement with previous comparisons of the effects of C57BL/6, SJL/J and a hybrid C57BL/6-SJL/L background on SOD1G93Adl mice (Heiman-Patterson et al., 2005) and on SOD1G93A mice (Wooley et al., 2005). No differences in survival were found when comparing the C57BL/6J strain with the hybrid C57BL/6J-BALB/c line; this, together with the fact that no differences were found in survival between the FVB, C3H and BALB/c strains, suggests that the C57BL/6J genome carries modifier genes that act dominantly in delaying mutant SOD1-induced disease. Differences on average lifespan between the two most extreme lines, FVB and C57BL/6J, represent around 25% of the average lifespan of the C57BL/6J strain, highlighting the importance of taking the genetic background of this mutation into account, and highlighting that the loci responsible might be points for therapeutic intervention.
Neither the study of Heiman-Patterson and colleagues (Heiman-Patterson, 2005), nor our study here found sex differences in time to endpoint on the C57BL/6J background. However, we did find sex differences on time to endpoint on the hybrid C57BL/6J-BALB/c strain. Moreover, gender did affect the onset of phenotypes in our C57BL/6J and C57BL/6J-BALB/c hybrid lines, with males being generally affected by a more aggressive form of disease, which concurs with similar findings from studies looking at the effects of gender and exercise in SOD1G93A and SOD1G93Adl mice (Veldink et al., 2003).
As part of our behavioural study, we tested acoustic startle response in symptomatic SOD1G93Adl mice and their wild-type littermates, and found a significant difference, with the transgenic animals having an impaired response, although this was not affected by gender. This is in agreement with our data on the high-copy SOD1G93A transgenic mice, which also present with early startle-response deficits. We have shown that aberrant startle response is not due to hearing deficits in at least the male mice under test and so presumably is an indicator of underlying motor deficits. However, we did not detect deficits in pre-pulse inhibition. Thus, startle-response deficits could potentially be used as an early quantitative behavioural test in preclinical trials involving both high and low-copy SOD1G93A transgenic mice.
Our assessment of muscle function through studying the TA, EDL and soleus muscles showed the expected drop-off in function between early and late disease stages (22 weeks and 34 weeks, respectively). However, our study of motor unit number highlighted the differential vulnerability of these muscles and showed that the fast twitch TA and EDL muscles are more severely affected (earlier effect, greater loss of motor units) than the slow twitch soleus muscle. This selective vulnerability of fast fatigable muscles in ALS is thought to reflect a greater susceptibility of the specific motor neurons that innervate them – for example, to enhanced endoplasmic reticular stress (Pun et al., 2006; Saxena et al., 2009). The characteristics of TA and EDL muscles changed as disease progressed, and they became more fatigue resistant, which is also a feature of human ALS (Soraru et al., 2008; Telerman-Toppet and Coers, 1978). These changes in SOD1G93Adl mice are in line with our previously published data on the high-copy SOD1G93A mice, which also develop extensive spinal cord and muscle pathology by late disease stages (Kalmar et al., 2008; Kieran et al., 2004).
We observed significant loss of spinal cord motor neurons and extensive gliosis prior to any loss of motor function at 24 weeks in SOD1G93Adl B6 mice, which indicates that underlying pathology including protein misfolding is present for a prolonged period prior to the physiological manifestation of disease. Disease progression is slower in these mice than in the high-copy transgenics but, nevertheless, at a comparable later stage of disease (34 weeks), 58% of motor neurons had died compared with more than 70% in the high-copy SOD1G93A mice (at 120 days). Similar differences in the survival of functional motor units in the two strains of mice were also observed: thus, in SOD1G93Adl B6 mice at 34 weeks, 55% of motor units are lost compared to ∼70% in the high-copy SOD1G93A strain (Kalmar et al., 2008). This loss of functional motor units is very similar to that observed in ALS patients, in whom a 20–50% drop in motor unit numbers within a 4- to 6-month period has been reported (Gooch and Shefner, 2004; Wang et al., 2002).
Human ALS is often described as a disorder with a focal onset that spreads from the first site of weakness, and so we assessed the symmetry of disease in SOD1G93Adl B6 mice. We found no detectable evidence for asymmetry equivalent to a human focal onset by assessing force in EDL or soleus muscles at 22 and 24 weeks of age. However, perhaps surprisingly, we did find a significant difference in motor unit survival between the two hindlimbs in mice with late-stage disease, at 34 weeks of age. Whether this is a stochastic effect or has significance for disease processes remains to be determined. No such asymmetry in motor unit survival was observed in high-copy SOD1G93A mice at any stage of disease (data not shown).
Our histopathological study of SOD1G93Adl B6 spinal cord revealed early signs of astroglial and microglial activation, before the appearance of aberrant phenotypes. However, although the disease process is well underway by 22 weeks of age, we could not detect deposition of misfolded mutant SOD1 protein at this stage. However, just 2 weeks later, misfolded SOD1 could be detected in the spinal cord and so it is possible that the disease process might accelerate from this stage onwards. TEM of end-stage (39 weeks of age) SOD1G93Adl B6 axons detected fibrillary accumulations of SOD1 and rare dense aggregates. Spherical fibrillary inclusions that labelled with SOD1 were found in the cytoplasm. In agreement with Turner and co-workers, who used high-copy SOD1G93A mice (Gooch and Shefner, 2004; Turner et al., 2008; Wang et al., 2002), we found no evidence of colocalisation of TDP43 with SOD1 using single immunogold labelled serial sections followed by TEM. Moreover, TDP43 protein remained mainly nuclear in end-stage B6 SOD1G93Adl mice, with little evidence of cytoplasmic mislocalisation. A previous report by Sumi and co-workers (Sumi et al., 2009) reported low levels of TDP43 aggregates and TDP43 and SOD1 colocalisation by co-immunofluorescence in end-stage B6-SJL SOD1G93Adl mice. We were not able to reproduce these findings using end-stage B6 SOD1G93Adl mice, perhaps owing to the different techniques applied or variations in disease pathology from the different genetic backgrounds used in both studies.
Several other groups have looked at specific areas of pathology in SOD1G93Adl mice: Jaarsma and colleagues have documented brain pathology in SOD1G93Adl mice compared with mice expressing neuron-specific mutant SOD1 (Jaarsma et al., 2008); Son et al. have studied cytochrome c oxidase deficiency in SOD1G93Adl transgenics crossed to mice expressing the copper chaperone for SOD1 (Son et al., 2007); Sasaki and colleagues, and Puttaparthi and colleagues, have carried out structural and other studies of spinal cord aggregates (Puttaparthi et al., 2003; Sasaki et al., 2005; Son et al., 2007).
This strain has also been crossed to other mouse mutants to look for loci affecting disease progression. These studies include assessing the effects of the proteins SOD2/MnSOD (Muller et al., 2008), bicaudal D2 (which affects dynein-dynactin function) (Teuling et al., 2008), LMP2 (involved in immune-proteasome function) (Puttaparthi et al., 2007), hepatocyte growth factor (Kadoyama et al., 2007; Sun et al., 2002), BAX (Gould et al., 2006) and a microRNA that modulates the phenotype (Williams et al., 2009; Xia et al., 2006). These mice have also been crossed to animals expressing RNAi constructs to assess the therapeutic effects of this approach (Xia et al., 2006). Other studies with this animal have been carried out to assess conventional therapies, including creatine supplementation and riluzole treatment (Snow et al., 2003).
There is a small but increasing body of literature from groups working with SOD1G93Adl animals that shows that these mice are an important model for understanding early-stage pathological mechanisms and for testing therapies, both conventional and gene-therapy-based approaches, designed to tackle early-stage disease. Although mouse models are not perfect models of late-onset human disorders, such as ALS, they are clearly irreplaceable for both understanding genetic and cellular pathology, and as resources for finding and testing therapeutic strategies. SOD1G93Adl mice exist because of a fortuitous partial deletion of the transgene array, and succumb to a relatively rapid neurodegenerative disease in the last third of their lives, although disease progression is not as rapid as that observed in the high-copy SOD1G93A strain. This more gradual disease progression in SOD1G93Adl mice offers the opportunity not only to examine the very early stages of pathology, but also provides a longer therapeutic window in which the potential beneficial effects of novel agents might manifest. These mice seem to model human ALS well, including the progressive muscle weakness, motor neuron loss and protein deposition, and they provide us with another essential tool for understanding and ultimately treating human ALS.
METHODS
Mice
All experiments were performed under Licence from, and in agreement with, UK Home Office Regulations.
Male SOD1G93Adl mice on a hybrid C57BL/6J-SJL/J background were backcrossed for at least three generations onto C3H/HeH, FVB/J and BALB/cAnNCrl, and for at least eight generations onto C57BL/6J. To produce the hybrid C57BL/6J-BALB/c line, N8 C57BL/6J SOD1G93Adl males were backcrossed to BALB/cAnNCrl females. At least five mice per strain of generation N3 or later were tested for transgene copy number variation with no significant differences obtained (data not shown).
Experiments were performed blind to genotype. The humane endpoint was defined as first sign of limb paralysis, or loss of 20% body weight, or loss of the righting reflex for more than 30 seconds, whichever was reached soonest. Mice were checked daily for signs of paralysis and weighed weekly.
Behavioural analysis
SHIRPA methods are as described previously (Rogers et al., 1997; Rogers et al., 2001). Briefly, tremors were assessed qualitatively by observation in a viewing jar and recorded as no tremors (0), mild tremors (1) and moderate tremors (2). Limb-tone was assessed qualitatively by applying mild pressure to the hindlimbs of immobilised mice and response recorded as normal (2), poor (1) or none (0). Mice were subject to modified SHIRPA analysis every 2 weeks from 12 weeks of age. Cohort sizes of SOD1G93Adl mice for SHIRPA and survival analysis were: C57BL/6J, 11 females, 15 males; BALB/c, 3 females, 3 males; C3H, 4 females, 2 males; FVB, 5 females, 3 males; and B6-BALB/c, 9 females, 6 males. Wild-type littermate controls (ten females, nine males) from the C57BL/6J background were used as controls for the SOD1G93Adl B6 line. We used wild-type littermates and not wild-type SOD1 overexpressors as controls for the SOD1G93Adl B6 strain because the SOD1 protein expression levels of the available wild-type SOD1 overexpressor line have been reported to be around three times higher than that from SOD1G93Adl mice (Jonsson et al., 2006).
SOD1G93Adl B6 mice were subject to quantitative grip-strength assessment using a commercial apparatus (BioSeb, Chaville, France). Four-paw grip strength was measured following the manufacturer’s instructions. Measurements were taken twice per week every 2 weeks from 12 to 32 weeks of age, except for at 20 weeks of age, when no measurements were taken. The average of both measurements per time point was used per animal to obtain means per genotype for every time point. Cohort sizes for females per time point were: 12 weeks, 5 wild type and 5 SOD1G93Adl; 14, 16, 18, 22, 24 and 26 weeks, 12 wild type and 7 SOD1G93Adl; 28 and 30 weeks, 12 wild type and 6 SOD1G93Adl; and 32 weeks, 5 wild type and 5 SOD1G93Adl. The numbers of males used per time point were: 12 weeks, 5 wild type and 7 SOD1G93Adl; 14, 16, 18, 22, 24 and 26 weeks, 9 wild type and 12 SOD1G93Adl; 28 and 30 weeks, 9 wild type and 8 SOD1G93Adl; 32 weeks, 5 wild type and 7 SOD1G93Adl.
The acoustic startle response is characterised by an exaggerated flinching response to an unexpected auditory stimulus. This response can generally be attenuated when it is preceded by a weaker stimulus, the principle underlying PPI. A commercial apparatus (Med Associates, VE) was used according to the manufacturer’s instructions; methods are described in Mandillo et al. (Mandillo et al., 2008) and on the Empress database (http://empress.har.mrc.ac.uk). For the SOD1G93Adl B6 analysis, male and female wild-type and transgenic littermates were tested at 22 weeks of age (wild-type littermates: 8 females, 12 males; SOD1G93Adl B6: 7 females, 6 males). For the analysis of 29-week-old mice, only males were used (8 wild-type littermates, 7 SOD1G93Adl B6). For the SOD1G93A high-copy analysis, only male mice on a hybrid C57BL/6J-SJL/J background were used at two time points: 95 days (7 wild-type littermates, 8 SOD1G93Adl) and 120 days (4 wild-type littermates, 6 SOD1G93Adl).
SOD1G93Adl B6 mice were subject to an accelerating rotarod (Ugo Basile) paradigm three times per day, three times a week every 2 weeks from 13 to 33 weeks of age. The apparatus was set to increase speed from 4 to 38 revolutions per minute (rpm) in 5 minutes; mice staying for 5 minutes were scored the maximum 300 seconds. The average of all sessions per week was used per animal to obtain means per genotype for every time point. Cohort sizes for females per time point were: 13 weeks, 5 wild type and 10 SOD1G93Adl; 15 and 17 weeks, 12 wild type and 10 SOD1G93Adl; 19 weeks, 7 wild type and 5 SOD1G93Adl; 21, 23, 25, 27, 29, 31 and 33 weeks, 12 wild type and 10 SOD1G93Adl. Numbers of males used per time point were: 13 weeks, 8 wild type and 10 SOD1G93Adl; 15 and 17 weeks, 12 wild type and SOD1G93Adl; 19 weeks, 8 wild type and 10 SOD1G93Adl; 21, 23 and 25 weeks, 12 wild type and 10 SOD1G93Adl; 27 weeks, 8 wild type and 10 SOD1G93Adl; 29 weeks, 12 wild type and 5 SOD1G93Adl; 31 weeks, 12 wild type and 10 SOD1G93Adl; 33 weeks: 8 wild type and 10 SOD1G93Adl.
Physiological assessment of muscle function and motor unit number
For the physiological assessment of muscle function and motor unit survival, male wild-type and SOD1G93Adl B6 mice were examined at various stages of disease progression: 22 weeks (n=5, both genotypes) and 24 weeks (n=5, both genotypes) representing a presymptomatic and disease-onset stage, respectively, and 34 weeks, representing the late disease phase (n=10, both genotypes). Note that these were not littermates. The mice were deeply anaesthetised (4.5% chloral hydrate; 1 ml/100 g of body weight, intraperitoneally) and prepared for in vivo analysis of isometric muscle force. The distal tendons of the TA, EDL and soleus muscles in both hindlimbs were dissected free and attached by silk thread to isometric force transducers (Dynamometer UFI Devices, Welwyn Garden City, UK). The sciatic nerve was exposed and sectioned. The length of the muscles was adjusted for maximum twitch tension and the muscle and nerves were kept moist with saline throughout the recordings. All experiments were carried out at room temperature (23°C). Isometric contractions were elicited by stimulating the nerve to the TA, EDL or soleus using square-wave pulses of 0.02 milliseconds duration at supra-maximal intensity, via silver wire electrodes. Contractions were elicited by trains of stimuli at frequencies of 40, 80 and 100 Hz. The maximum tetanic tension was measured using a computer and appropriate software (Scope).
The number of motor units innervating the EDL and soleus muscles was determined by stimulating the motor nerve with stimuli of increasing intensity, resulting in stepwise increments in twitch tension due to successive recruitment of motor axons. The number of stepwise increments was counted to give an estimate of the number of functional motor units present in each muscle.
For EDL muscle, the fatigue characteristics of this normally fast, fatigable muscle were also examined. EDL muscle was stimulated at 40 Hz for 250 milliseconds every second and contractions were recorded with a pen recorder (Lectromed Multitrace 2). An FI was calculated as a measure of muscle fatigability: the decrease in tension after 3 minutes of stimulation was measured and the FI determined as initial tetanic tension/tetanic tension after 3 minutes of stimulation.
Morphological assessment of muscle and spinal cord
Following the physiological assessment of muscle function, the TA, EDL and soleus muscles were dissected and snap frozen in isopentane cooled in liquid nitrogen and were stored at −80°C until processing. Animals were then perfused transcardially with saline followed by fixative containing 4% paraformaldehyde (PFA). The spinal cords were then removed, postfixed in 4% PFA and cryopreserved in 30% sucrose overnight. Lumbar sections of fixed spinal cords (L2-L6) were cut on a freezing cryostat and 20-μm transverse sections were collected serially onto glass slides and subsequently stained for Nissl (gallocyanin) (Kellett, 1963). The number of Nissl-stained large motor neurons in L3-L6 lumbar sections (40 sections in total) was established. In these experiments, five mice were analysed from each experimental group.
Frozen muscle samples of TA muscles were cut on a cryostat at 12 μm. Serial cross-sections were collected on glass slides and stained for succinate dehydrogenase (SDH) activity to determine the oxidative capacity of the muscle fibres, as described previously (Boerio et al., 2008; Boerio et al., 2010).
Immunohistochemical detection of misfolded SOD1 and activated glia
The lumbar region of fixed spinal cords (L2-L6) were cut on a freezing cryostat and 10-μm transverse sections were collected serially onto glass slides. Sections were stored at −20°C until further analysis. Immunohistochemistry using diaminobenzidine (DAB) reaction was carried out for misfolded SOD1 (polyclonal rabbit SEDI antibody; kind gift of Janice Robertson, University of Toronto, Canada; used at 1:500), whereas astroglial and microglial reactions were visualised by immunofluorescence for GFAP (Cy-conjugated mouse monoclonal, Sigma; used at 1:1000) and IBA1 (rabbit polyclonal, Abcam; used at 1:500), respectively. In order to visualise neighbouring neuronal cells, a double labelling of sections was also carried out for the presence of βIII-tubulin, a neuronal marker (mouse monoclonal antibody by Covance; 1:1000).
Horseradish peroxidase reaction and DAB labelling
Sections were washed in PBS 0.1% Triton X-100 plus 3% H2O2 to block endogenous peroxides followed by a 1-hour incubation in a blocking solution: PBS containing 5% milk proteins and 3% normal goat serum. Sections were incubated at 4°C overnight in antibody solutions made up in blocking solution. A negative control slide was also prepared in which the primary antibody was omitted; these sections were incubated in blocking solution overnight. The next day, DAB-labelled sections were washed three times in PBS, and then incubated in secondary goat anti-rabbit antibody conjugated to biotin (Vector Laboratories; 1:100) for 2 hours at room temperature. Following a further three washes in PBS, the sections were incubated for 1 hour in avidin-biotin complex (Vector ABC kit). DAB (3,3′-diaminobenzidine solution; SIGMA, fast DAB) was used as a substrate to develop specific staining. Sections were then stained for Nissl (gallocyanin) to reveal Nissl-positive cells as a counterstain and the sections were then mounted using Dpx mounting media.
TRANSLATIONAL IMPACT.
Background
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease that usually strikes in mid-life, typically resulting in death 3–5 years after diagnosis. During the disease course, motor neurons that control movement die, leaving patients entirely paralysed but usually with intellect intact. There are no effective treatments for this disorder. Approximately 10% of ALS cases are genetic, usually autosomal dominant, and the main causative gene is superoxide dismutase 1 (SOD1). Other causative genes that affect fewer patients include TDP43, FUS and OPTN. Several strains of mutant human SOD1 transgenic mice successfully model human ALS. The first and most widely used model is the SOD1G93A mouse, which has ∼25 copies of the mutant transgene. This mouse has been invaluable for ALS research, but dies before 5 months of age owing to a highly aggressive motor neuron degenerative disease. In addition, it is thought that some aspects of its phenotype result from the massive overexpression of SOD1 protein per se. A spontaneous deletion resulted in a second mouse strain derived from the high-copy SOD1G93A transgenic mouse. This SOD1G93Adl strain carries eight to ten copies of the transgene and has a slower course of disease – end-stage is reached by about 34 weeks, depending on genetic background – which might make this a better model for studying the early stages of ALS pathology.
Results
In this paper, the authors set out to further characterise SOD1G93Adl mice, and to determine the effects of disease on nerve and muscle physiology. They find that genetic background and gender affect the phenotype, including the time to humane endpoint. Of the five backgrounds tested, the C57BL/6J mouse strain delayed disease onset. They introduce a new test for SOD1 transgenic mouse models, the startle-response, which is aberrant in these animals, although this is not due to hearing impairment. The authors also carry out a detailed assessment of muscle function and motor neuron pathology over time in these animals, and show the deposition of SOD1 protein, although no colocalisation with TDP43 is detected. Importantly, disease progression in these animals is comparable to the progression of ALS observed in humans.
Implications and future directions
As with most mouse models of human disease, no single model is ideal for all studies. In the case of ALS models, the more commonly studied high-copy SOD1G93A strain is considerably less expensive to maintain until end-stage than are the other models, which is one important reason why it is widely used. It has also been useful for studying late-stage ALS and for crossing to other mouse strains to identify disease-modifying loci. However, the low-copy SOD1G93Adl strain provides a superior system for studying the cellular and molecular pathology of early-stage disease, when treatments will be most valuable, and for testing novel therapies. The results of this study provide important physiological and genetic baseline data on this useful animal model of ALS and support the idea that this model should be used more widely.
Immunofluorescence for dual staining
Sections to be stained for IBA1 and tubulin immunofluorescence were blocked, and primary antibodies were applied overnight. The following day sections were incubated with a secondary anti-rabbit antibody conjugated to Alexa Fluor 568 (Molecular Probes) to develop IBA1 staining and also with an anti-mouse Alexa-Fluor-488 secondary antibody (Molecular Probes; 1:100) to develop staining for β-tubulin. GFAP- and β-tubulin-stained sections were first processed for β-tubulin immunoreactivity using the same secondary antibody (anti-mouse Alexa-Fluor-488 secondary antibody by Molecular Probes) as above and subsequently co-stained for a directly fluorolabelled anti-GFAP antibody (Sigma; 1:1000).
Immunogold labelling TEM
Whole spinal cords from three wild-type and three SOD1G93Adl B6 end-stage (39 weeks of age) littermates were initially fixed in 4% (w/v) formaldehyde/0.1% (v/v) glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.4. Lumbar regions of the spinal cords were subsequently dissected out, thoroughly rinsed in buffer, dehydrated in an ethanol series and embedded in Unicryl resin (British BioCell International, Cardiff, UK), all at 4°C throughout, as previously described (Thorpe et al., 1999; Thorpe et al., 2008). Thin sections were cut and immunogold labelled by established procedures (Thorpe et al., 1999; Thorpe et al., 2008). Rabbit polyclonal antibodies raised against human SOD1 (Santa Cruz Biotechnology) and TDP43 (ProteinTech Group, Chicago, IL) were used at 1 μg and 2 μg/ml IgG final concentrations, respectively. 10-nm gold-particle-conjugated goat anti-rabbit IgG secondary probe was obtained from British BioCell International (Cardiff, UK). Immunogold-labelled sections were post-stained in 2% (w/v) aqueous, 0.22 μm-filtered uranyl acetate for 1 hour. They were then systematically and thoroughly scanned for pathology in a Hitachi-7100 transmission electron microscope at 100 kV and images acquired with an axially mounted (2K×2K pixel) Gatan Ultrascan 1000 CCD camera (Gatan UK, Oxford, UK).
Statistical analysis
Log-rank analysis was used to compare age at onset of SHIRPA endophenotypes between all lines. An ANOVA test was used to compare wild-type with SOD1G93Adl cohorts per time point for rotarod, grip-strength and startle-response analysis. The Mann-Whitney test was used to compare two groups: either wild-type with SOD1G93Adl cohorts, as well as lower versus higher number of surviving motor units innervating hindlimb muscles. For the statistical analysis of motor neuron survival and muscle force measurements, the Mann-Whitney U test was employed. Two-tailed tests were used in all instances and significance level was set at P<0.05.
Supplementary Material
Acknowledgments
We thank Ray Young for graphics and Michelle Stewart for mouse colony management. This work was supported by the UK Medical Research Council (A.A.-A., R.K., C.R., A.P., M.H., E.M.C.F.), the Motor Neurone Disease Association (A.A.-A., T.R., P.J., L.G., E.M.C.F.), the Thierry Latran Foundation (A.A.-A., L.G., E.M.C.F.), The Brain Research Trust (L.G., E.M.C.F.) and University College London Hospitals Charities CRDC grant (B.K.).
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
A.A.-A., L.G. and E.M.C.F. conceived the project and, with B.K., S.E., T.R., P.J., M.H. and J.R.T., designed the experiments. A.A.-A., B.K., S.E., T.R., P.J., R.K., C.R., A.P., A.G. and J.R.T. performed the experiments. A.A.-A., B.K., L.G., J.R.T. and E.M.C.F. wrote the manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.007237/-/DC1
REFERENCES
- Achilli F., Boyle S., Kieran D., Chia R., Hafezparast M., Martin J. E., Schiavo G., Greensmith L., Bickmore W., Fisher E. M. (2005). The SOD1 transgene in the G93A mouse model of amyotrophic lateral sclerosis lies on distal mouse chromosome 12. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 6, 111–114 [DOI] [PubMed] [Google Scholar]
- Alexander G. M., Erwin K. L., Byers N., Deitch J. S., Augelli B. J., Blankenhorn E. P., Heiman-Patterson T. D. (2004). Effect of transgene copy number on survival in the G93A SOD1 transgenic mouse model of ALS. Brain Res. Mol. Brain Res. 130, 7–15 [DOI] [PubMed] [Google Scholar]
- Avraham K. B., Schickler M., Sapoznikov D., Yarom R., Groner Y. (1988). Down’s syndrome: abnormal neuromuscular junction in tongue of transgenic mice with elevated levels of human Cu/Zn-superoxide dismutase. Cell 54, 823–829 [DOI] [PubMed] [Google Scholar]
- Benatar M. (2007). Lost in translation: treatment trials in the SOD1 mouse and in human ALS. Neurobiol. Dis. 26, 1–13 [DOI] [PubMed] [Google Scholar]
- Birve A., Neuwirth C., Weber M., Marklund S. L., Nilsson A. C., Jonsson P. A., Andersen P. M. (2010). A novel SOD1 splice site mutation associated with familial ALS revealed by SOD activity analysis. Hum. Mol. Genet. 19, 4201–4206 [DOI] [PubMed] [Google Scholar]
- Boerio D., Hogrel J. Y., Lefaucheur J. P., Wang F. C., Verschueren A., Pouget J., Carrera E., Kuntzer T. (2008). Stimulus-response curve of human motor nerves: multicenter assessment of various indexes. Neurophysiol. Clin. 38, 31–38 [DOI] [PubMed] [Google Scholar]
- Boerio D., Kalmar B., Greensmith L., Bostock H. (2010). Excitability properties of mouse motor axons in the mutant SOD1(G93A) model of amyotrophic lateral sclerosis. Muscle Nerve 41, 774–784 [DOI] [PubMed] [Google Scholar]
- Boillee S., Vande Velde C., Cleveland D. W. (2006). ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52, 39–59 [DOI] [PubMed] [Google Scholar]
- Bosco D. A., Morfini G., Karabacak N. M., Song Y., Gros-Louis F., Pasinelli P., Goolsby H., Fontaine B. A., Lemay N., McKenna-Yasek D., et al. (2010). Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat. Neurosci. 13, 1396–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn L. I., Becher M. W., Lee M. K., Anderson K. L., Jenkins N. A., Copeland N. G., Sisodia S. S., Rothstein J. D., Borchelt D. R., Price D. L., et al. (1997). ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18, 327–338 [DOI] [PubMed] [Google Scholar]
- Copray J. C., Jaarsma D., Kust B. M., Bruggeman R. W., Mantingh I., Brouwer N., Boddeke H. W. (2003). Expression of the low affinity neurotrophin receptor p75 in spinal motoneurons in a transgenic mouse model for amyotrophic lateral sclerosis. Neuroscience 116, 685–694 [DOI] [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P., et al. (1993). Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science 261, 1047–1051 [DOI] [PubMed] [Google Scholar]
- Dion P. A., Daoud H., Rouleau G. A. (2009). Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nat. Rev. Genet. 10, 769–782 [DOI] [PubMed] [Google Scholar]
- Gitcho M. A., Baloh R. H., Chakraverty S., Mayo K., Norton J. B., Levitch D., Hatanpaa K. J., White C. L., III, Bigio E. H., Caselli R., et al. (2008). TDP-43 A315T mutation in familial motor neuron disease. Ann. Neurol. 63, 535–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooch C. L., Shefner J. M. (2004). ALS surrogate markers. MUNE. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 5 Suppl. 1, 104–107 [DOI] [PubMed] [Google Scholar]
- Gould T. W., Buss R. R., Vinsant S., Prevette D., Sun W., Knudson C. M., Milligan C. E., Oppenheim R. W. (2006). Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J. Neurosci. 26, 8774–8786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H. X., et al. (1994). Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 [DOI] [PubMed] [Google Scholar]
- Heiman-Patterson T. D., Deitch J. S., Blankenhorn E. P., Erwin K. L., Perreault M. J., Alexander B. K., Byers N., Toman I., Alexander G. M. (2005). Background and gender effects on survival in the TgN(SOD1-G93A)1Gur mouse model of ALS. J. Neurol. Sci. 236, 1–7 [DOI] [PubMed] [Google Scholar]
- Jaarsma D., Rognoni F., van Duijn W., Verspaget H. W., Haasdijk E. D., Holstege J. C. (2001). CuZn superoxide dismutase (SOD1) accumulates in vacuolated mitochondria in transgenic mice expressing amyotrophic lateral sclerosis-linked SOD1 mutations. Acta Neuropathol. 102, 293–305 [DOI] [PubMed] [Google Scholar]
- Jaarsma D., Teuling E., Haasdijk E. D., De Zeeuw C. I., Hoogenraad C. C. (2008). Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J. Neurosci. 28, 2075–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson P. A., Graffmo K. S., Andersen P. M., Brannstrom T., Lindberg M., Oliveberg M., Marklund S. L. (2006). Disulphide-reduced superoxide dismutase-1 in CNS of transgenic amyotrophic lateral sclerosis models. Brain 129, 451–464 [DOI] [PubMed] [Google Scholar]
- Kabashi E., Valdmanis P. N., Dion P., Spiegelman D., McConkey B. J., Velde C. V., Bouchard J. P., Lacomblez L., Pochigaeva K., Salachas F., et al. (2008). TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat. Genet. 40, 572–574 [DOI] [PubMed] [Google Scholar]
- Kadoyama K., Funakoshi H., Ohya W., Nakamura T. (2007). Hepatocyte growth factor (HGF) attenuates gliosis and motoneuronal degeneration in the brainstem motor nuclei of a transgenic mouse model of ALS. Neurosci. Res. 59, 446–456 [DOI] [PubMed] [Google Scholar]
- Kalmar B., Novoselov S., Gray A., Cheetham M. E., Margulis B., Greensmith L. (2008). Late stage treatment with arimoclomol delays disease progression and prevents protein aggregation in the SOD1 mouse model of ALS. J. Neurochem. 107, 339–350 [DOI] [PubMed] [Google Scholar]
- Kellett B. S. (1963). Gallocyanin-chrome alum: a routine stain for Nissle substance in paraffin sections. J. Med. Lab. Technol. 20, 196–198 [PubMed] [Google Scholar]
- Kieran D., Kalmar B., Dick J. R., Riddoch-Contreras J., Burnstock G., Greensmith L. (2004). Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat. Med. 10, 402–405 [DOI] [PubMed] [Google Scholar]
- Lalonde R., Dumont M., Paly E., London J., Strazielle C. (2004). Characterization of hemizygous SOD1/wild-type transgenic mice with the SHIRPA primary screen and tests of sensorimotor function and anxiety. Brain Res. Bull. 64, 251–258 [DOI] [PubMed] [Google Scholar]
- Ludolph A. C., Bendotti C., Blaugrund E., Chio A., Greensmith L., Loeffler J. P., Mead R., Niessen H. G., Petri S., Pradat P. F., et al. (2010). Guidelines for preclinical animal research in ALS/MND: a consensus meeting. Amyotroph. Lateral Scler. 11, 38–45 [DOI] [PubMed] [Google Scholar]
- Maatkamp A., Vlug A., Haasdijk E., Troost D., French P. J., Jaarsma D. (2004). Decrease of Hsp25 protein expression precedes degeneration of motoneurons in ALS-SOD1 mice. Eur. J. Neurosci. 20, 14–28 [DOI] [PubMed] [Google Scholar]
- Mandillo S., Tucci V., Holter S. M., Meziane H., Banchaabouchi M. A., Kallnik M., Lad H. V., Nolan P. M., Ouagazzal A. M., Coghill E. L., et al. (2008). Reliability, robustness, and reproducibility in mouse behavioral phenotyping: a cross-laboratory study. Physiol. Genomics 34, 243–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H., Morino H., Ito H., Izumi Y., Kato H., Watanabe Y., Kinoshita Y., Kamada M., Nodera H., Suzuki H., et al. (2010). Mutations of optineurin in amyotrophic lateral sclerosis. Nature 465, 223–226 [DOI] [PubMed] [Google Scholar]
- Muller F. L., Liu Y., Jernigan A., Borchelt D., Richardson A., Van Remmen H. (2008). MnSOD deficiency has a differential effect on disease progression in two different ALS mutant mouse models. Muscle Nerve 38, 1173–1183 [DOI] [PubMed] [Google Scholar]
- Nicholson S. J., Witherden A. S., Hafezparast M., Martin J. E., Fisher E. M. (2000). Mice, the motor system, and human motor neuron pathology. Mamm. Genome 11, 1041–1052 [DOI] [PubMed] [Google Scholar]
- Pasinelli P., Brown R. H. (2006). Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat. Rev. Neurosci. 7, 710–723 [DOI] [PubMed] [Google Scholar]
- Pun S., Santos A. F., Saxena S., Xu L., Caroni P. (2006). Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat. Neurosci. 9, 408–419 [DOI] [PubMed] [Google Scholar]
- Puttaparthi K., Wojcik C., Rajendran B., DeMartino G. N., Elliott J. L. (2003). Aggregate formation in the spinal cord of mutant SOD1 transgenic mice is reversible and mediated by proteasomes. J. Neurochem. 87, 851–860 [DOI] [PubMed] [Google Scholar]
- Puttaparthi K., Van Kaer L., Elliott J. L. (2007). Assessing the role of immuno-proteasomes in a mouse model of familial ALS. Exp. Neurol. 206, 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhit R., Robertson J., Vande Velde C., Horne P., Ruth D. M., Griffin J., Cleveland D. W., Cashman N. R., Chakrabartty A. (2007). An immunological epitope selective for pathological monomer-misfolded SOD1 in ALS. Nat. Med. 13, 754–759 [DOI] [PubMed] [Google Scholar]
- Ravits J., Laurie P., Fan Y., Moore D. H. (2007a). Implications of ALS focality: rostral-caudal distribution of lower motor neuron loss postmortem. Neurology 68, 1576–1582 [DOI] [PubMed] [Google Scholar]
- Ravits J., Paul P., Jorg C. (2007b). Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology 68, 1571–1575 [DOI] [PubMed] [Google Scholar]
- Rogers D. C., Fisher E. M., Brown S. D., Peters J., Hunter A. J., Martin J. E. (1997). Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm. Genome 8, 711–713 [DOI] [PubMed] [Google Scholar]
- Rogers D. C., Peters J., Martin J. E., Ball S., Nicholson S. J., Witherden A. S., Hafezparast M., Latcham J., Robinson T. L., Quilter C. A., et al. (2001). SHIRPA, a protocol for behavioral assessment: validation for longitudinal study of neurological dysfunction in mice. Neurosci. Lett. 306, 89–92 [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O’Regan J. P., Deng H. X., et al. (1993). Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 [DOI] [PubMed] [Google Scholar]
- Sasaki S., Iwata M. (1996). Impairment of fast axonal transport in the proximal axons of anterior horn neurons in amyotrophic lateral sclerosis. Neurology 47, 535–540 [DOI] [PubMed] [Google Scholar]
- Sasaki S., Warita H., Murakami T., Abe K., Iwata M. (2004). Ultrastructural study of mitochondria in the spinal cord of transgenic mice with a G93A mutant SOD1 gene. Acta Neuropathol. 107, 461–474 [DOI] [PubMed] [Google Scholar]
- Sasaki S., Warita H., Murakami T., Shibata N., Komori T., Abe K., Kobayashi M., Iwata M. (2005). Ultrastructural study of aggregates in the spinal cord of transgenic mice with a G93A mutant SOD1 gene. Acta Neuropathol. 109, 247–255 [DOI] [PubMed] [Google Scholar]
- Sasaki S., Warita H., Komori T., Murakami T., Abe K., Iwata M. (2006). Parvalbumin and calbindin D-28k immunoreactivity in transgenic mice with a G93A mutant SOD1 gene. Brain Res. 1083, 196–203 [DOI] [PubMed] [Google Scholar]
- Saxena S., Cabuy E., Caroni P. (2009). A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat. Neurosci. 12, 627–636 [DOI] [PubMed] [Google Scholar]
- Schymick J. C., Talbot K., Traynor B. J. (2007). Genetics of sporadic amyotrophic lateral sclerosis. Hum. Mol. Genet. 16 Spec No. 2, R233–R242 [DOI] [PubMed] [Google Scholar]
- Scott S., Kranz J. E., Cole J., Lincecum J. M., Thompson K., Kelly N., Bostrom A., Theodoss J., Al-Nakhala B. M., Vieira F. G., et al. (2008). Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph. Lateral Scler. 9, 4–15 [DOI] [PubMed] [Google Scholar]
- Shibata N. (2001). Transgenic mouse model for familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation. Neuropathology 21, 82–92 [DOI] [PubMed] [Google Scholar]
- Snow R. J., Turnbull J., da Silva S., Jiang F., Tarnopolsky M. A. (2003). Creatine supplementation and riluzole treatment provide similar beneficial effects in copper, zinc superoxide dismutase (G93A) transgenic mice. Neuroscience 119, 661–667 [DOI] [PubMed] [Google Scholar]
- Son M., Puttaparthi K., Kawamata H., Rajendran B., Boyer P. J., Manfredi G., Elliott J. L. (2007). Overexpression of CCS in G93A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc. Natl. Acad. Sci. USA 104, 6072–6077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soraru G., D’Ascenzo C., Nicolao P., Volpe M., Martignago S., Palmieri A., Romeo V., Koutsikos K., Piccione F., Cima V., et al. (2008). Muscle histopathology in upper motor neuron-dominant amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 9, 287–293 [DOI] [PubMed] [Google Scholar]
- Sreedharan J., Blair I. P., Tripathi V. B., Hu X., Vance C., Rogelj B., Ackerley S., Durnall J. C., Williams K. L., Buratti E., et al. (2008). TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319, 1668–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieber A., Gonatas J. O., Collard J., Meier J., Julien J., Schweitzer P., Gonatas N. K. (2000). The neuronal Golgi apparatus is fragmented in transgenic mice expressing a mutant human SOD1, but not in mice expressing the human NF-H gene. J. Neurol. Sci. 173, 63–72 [DOI] [PubMed] [Google Scholar]
- Sumi H., Nagano S., Fujimura H., Kato S., Sakoda S. (2006). Inverse correlation between the formation of mitochondria-derived vacuoles and Lewy-body-like hyaline inclusions in G93A superoxide-dismutase-transgenic mice. Acta Neuropathol. 112, 52–63 [DOI] [PubMed] [Google Scholar]
- Sumi H., Kato S., Mochimaru Y., Fujimura H., Etoh M., Sakoda S. (2009). Nuclear TAR DNA binding protein 43 expression in spinal cord neurons correlates with the clinical course in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 68, 37–47 [DOI] [PubMed] [Google Scholar]
- Sun W., Funakoshi H., Nakamura T. (2002). Overexpression of HGF retards disease progression and prolongs life span in a transgenic mouse model of ALS. J. Neurosci. 22, 6537–6548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synofzik M., Fernandez-Santiago R., Maetzler W., Schols L., Andersen P. M. (2010). The human G93A SOD1 phenotype closely resembles sporadic amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 81, 764–767 [DOI] [PubMed] [Google Scholar]
- Telerman-Toppet N., Coers C. (1978). Motor innervation and fiber type pattern in amyotrophic lateral sclerosis and in Charcot-Marie-Tooth disease. Muscle Nerve 1, 133–139 [DOI] [PubMed] [Google Scholar]
- Teuling E., van Dis V., Wulf P. S., Haasdijk E. D., Akhmanova A., Hoogenraad C. C., Jaarsma D. (2008). A novel mouse model with impaired dynein/dynactin function develops amyotrophic lateral sclerosis (ALS)-like features in motor neurons and improves lifespan in SOD1-ALS mice. Hum. Mol. Genet. 17, 2849–2862 [DOI] [PubMed] [Google Scholar]
- Thorpe J. R., Rulten S. L., Kay J. E. (1999). Binding of a putative and a known chaperone protein revealed by immunogold labeling transmission electron microscopy: a suggested use of chaperones as probes for the distribution of their target proteins. J. Histochem. Cytochem. 47, 1633–1640 [DOI] [PubMed] [Google Scholar]
- Thorpe J. R., Tang H., Atherton J., Cairns N. J. (2008). Fine structural analysis of the neuronal inclusions of frontotemporal lobar degeneration with TDP-43 proteinopathy. J. Neural Transm. 115, 1661–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci V., Achilli F., Blanco G., Lad H. V., Wells S., Godinho S., Nolan P. M. (2007). Reaching and grasping phenotypes in the mouse (Mus musculus): a characterization of inbred strains and mutant lines. Neuroscience 147, 573–582 [DOI] [PubMed] [Google Scholar]
- Turner B. J., Baumer D., Parkinson N. J., Scaber J., Ansorge O., Talbot K. (2008). TDP-43 expression in mouse models of amyotrophic lateral sclerosis and spinal muscular atrophy. BMC Neurosci. 9, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdmanis P. N., Rouleau G. A. (2008). Genetics of familial amyotrophic lateral sclerosis. Neurology 70, 144–152 [DOI] [PubMed] [Google Scholar]
- Van Deerlin V. M., Leverenz J. B., Bekris L. M., Bird T. D., Yuan W., Elman L. B., Clay D., Wood E. M., Chen-Plotkin A. S., Martinez-Lage M., et al. (2008). TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 7, 409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C., Rogelj B., Hortobagyi T., De Vos K. J., Nishimura A. L., Sreedharan J., Hu X., Smith B., Ruddy D., Wright P., et al. (2009). Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldink J. H., Bar P. R., Joosten E. A., Otten M., Wokke J. H., van den Berg L. H. (2003). Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul. Disord. 13, 737–743 [DOI] [PubMed] [Google Scholar]
- Vlug A. S., Teuling E., Haasdijk E. D., French P., Hoogenraad C. C., Jaarsma D. (2005). ATF3 expression precedes death of spinal motoneurons in amyotrophic lateral sclerosis-SOD1 transgenic mice and correlates with c-Jun phosphorylation, CHOP expression, somato-dendritic ubiquitination and Golgi fragmentation. Eur. J. Neurosci. 22, 1881–1894 [DOI] [PubMed] [Google Scholar]
- Wang F. C., Bouquiaux O., De Pasqua V., Delwaide P. J. (2002). Changes in motor unit numbers in patients with ALS: a longitudinal study using the adapted multiple point stimulation method. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 3, 31–38 [DOI] [PubMed] [Google Scholar]
- Williams A. H., Valdez G., Moresi V., Qi X., McAnally J., Elliott J. L., Bassel-Duby R., Sanes J. R., Olson E. N. (2009). MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science 326, 1549–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley C. M., Sher R. B., Kale A., Frankel W. N., Cox G. A., Seburn K. L. (2005). Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle Nerve 32, 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X., Zhou H., Huang Y., Xu Z. (2006). Allele-specific RNAi selectively silences mutant SOD1 and achieves significant therapeutic benefit in vivo. Neurobiol. Dis. 23, 578–586 [DOI] [PubMed] [Google Scholar]
- Zang D. W., Lopes E. C., Cheema S. S. (2005). Loss of synaptophysin-positive boutons on lumbar motor neurons innervating the medial gastrocnemius muscle of the SOD1G93A G1H transgenic mouse model of ALS. J. Neurosci. Res. 79, 694–699 [DOI] [PubMed] [Google Scholar]
- Zhang B., Tu P., Abtahian F., Trojanowski J. Q., Lee V. M. (1997). Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J. Cell Biol. 139, 1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.