Abstract
INTRODUCTION
Faecal incontinence is a prevalent and important condition, with a range of treatment options.
Neuromodulation via sacral nerve stimulators is efficacious, but expensive and associated with complications due to device implantation. Peripheral neuromodulation via posterior tibial nerve stimulation (PTNS) has been assessed in urinary incontinence, but there is minimal evidence for its use in faecal incontinence and no literature from the UK. This retrospective review aimed to assess the efficacy of PTNS in faecal incontinence.
PATIENTS AND METHODS
Thirteen consecutive female patients with faecal incontinence of various causes (9 idiopathic, 3 obstetric, 1 surgery) underwent PTNS at a UK hospital. All were investigated with colonic imaging, anorectal physiology and endo-anal ultrasound. Prior treatments included physiotherapy (13), sphincteroplasty (3) biofeedback (3) and PTQ implants (1). PTNS was performed for 30 min, weekly for 12 weeks.
RESULTS
Median monthly episodes of incontinence of wind, liquid and solid reduced from 6, 10 and 18 respectively to 0 with 12 weeks' treatment (P < 0.05). Significant improvements in quality of life indices were also seen. At 1-month follow up, a sustained reduction in incontinence of wind was seen (0 episodes), with non-significant reductions of liquid and solid stool.
CONCLUSIONS
PTNS is a potentially efficacious, technically simple and minimally invasive alternative treatment modality for faecal incontinence. These early results are encouraging, but we await medium- and long-term follow-up, and a larger randomised trial comparing PTNS with alternative treatments and placebo.
Keywords: Neuromodulation, Stimulation, PTNS, Faecal, Incontinence
Faecal incontinence is an important and common multifac-torial disorder, experienced by up to 10% of adults with often profound consequences for their quality of life.13 The prevalence of major faecal incontinence in the adult population is estimated at 1.4%, with a further 1.7% experiencing minor incontinence.4 However, this is generally considered an underestimate5 and, as the population ages, faecal incontinence is expected to become yet more common.
Initial treatment options for faecal incontinence are usually conservative and include dietary modification, constipating medications, suppositories, physiotherapy/pelvic floor exercises and biofeedback.7,8 For those failing to respond, surgical procedures such as overlapping sphincteroplasty (for those with external anal sphincter defects) or, more recently, sacral nerve stimulators have shown benefit.9,10 Whilst the latter improves both faecal incontinence and attendant quality of life, these come at a high financial cost and risk of complications.10 Extreme cases of faecal incontinence with significant sphincter disruption may require dynamic graciloplasty or an artificial bowel sphincter.9,11
Peripheral neuromodulation of sacral nerve roots indirectly via posterior tibial nerve stimulation (PTNS) has been ialled in urinary incontinence.12,13 However, evidence for its use in faecal incontinence is limited to four small non-UK studies14–17 and a report.18 The aim of this study was to establish further the efficacy of PTNS in treating faecal incontinence.
Patients and Methods
Subjects
Thirteen consecutive patients with faecal incontinence of varying causes (9 idiopathic, 3 obstetric, 1 previous anorec-tal surgery) of at least 6 months' duration, in whom medical and non-invasive interventions (including pelvic floor physiotherapy and biofeedback) had failed, underwent PTNS. Prior treatments included physiotherapy (13), sphinctero-plasty (3) biofeedback (3) and PTQ implants (1). These were delivered more than 3 months prior to commencement of PTNS, and did not confer acceptable symptomatic improvement to the patient. Patients were drawn from one consultant's general colorectal clinic. Faecal incontinence was defined as the involuntary loss of flatus, liquid and solid stool19 and was confirmed by daily bowel diaries. All patients underwent colonoscopy or barium enema (demonstrating no structural abnormalities), and anorectal physiology/anal ultrasound before treatment. Subnormal anorectal physiology was demonstrated in seven patients; endo-anal ultrasound demonstrated damage/scarring in four patients with no defects amenable to surgical repair. Exclusion criteria comprised age under 18 years, coagulopathy, neuropathy, implanted pacemaker or cardiac defibrillator, and pregnancy or intention to become pregnant. This retrospective work was registered with the audit department of NUH.
Posterior tibial nerve stimulation
All other interventions for faecal incontinence were ceased at least 1 month prior to commencing PTNS, with the exception of medications which continued unchanged. PTNS was performed by two clinical nurse specialists (HG and RR) using the Urgent® PC 200 Neuromodulation System (Uroplasty, Minnetonka, MN, USA), as part of a dedicated urinary and faecal incontinence clinic. Subjects underwent one 30-min session every week for 12 consecutive weeks in a UK community hospital, as previously described and representing the most common urological practice.20 Subjects lay supine without general or local anaesthesia with PTNS delivered by a needle electrode inserted three fingers cephalad to the medial malleolus, at a 60° angle towards the ankle joint to a depth of approximately 1 cm. Successful placement was confirmed by elicitation of digital plantar flexion or abduction. PTNS was undertaken for 15 min at the highest current (0-9 mA) not causing a motor response, at frequency of 20 Hz. After 15 min, the current was increased by 1 mA for a further 15 min.
Data collection
Patient and physiological data were gathered retrospectively using medical notes and a computer database. Outcome measures were episodes of incontinence, and incontinence and quality-of-life indices. Monthly episodes of faecal incontinence to wind, liquid and solid were generated by daily bowel diaries: month 0 (pre-treatment baseline), months 1-3 (during treatment) and month 4 (following treatment). Faecal incontinence and quality-of-life indices were also quantified by the Hospital Anxiety and Depression (HAD) Score, the International Consultation on Incontinence Questionnaire Anal Incontinence Symptoms and Quality of Life Module (ICIQ-B), and the Rockwood Faecal Incontinence Quality of Life Instrument (FIQOL), completed 4 weeks before and after treatment.
Statistical analysis
Statistical analysis was performed using SPSS® software v.17.0.0 (SPSS, Chicago, IL, USA). Kolmogorov-Smirnov test was used to assess the distribution of data; two-tailed independent t- and Wilcoxon signed ranks tests were used accordingly. A P-value < 0.05 was considered significant.
Results
Study population
Thirteen patients with a median age of 53 years (range, 34-80 years) were recruited. All were female. A total of 151 sessions of PTNS were delivered, with 12 patients completing the full 12-session course. One patient withdrew after 7 weeks' treatment citing a swollen and painful leg and was included on an intention-to-treat basis (with subsequent scores reverting to baseline).
Anal ultrasound and physiology
Anal ultrasound showed a degree of scarring of internal and/or external sphincters in four patients, although no distinct defects were amenable to repair. Subnormal physiology was demonstrated in seven patients. Mean resting pressure for the group was 37.8 mmH2O (normal, 50–80 mmH2O). Mean squeeze pressure was 73.4 mmH2O (normal, 100-140 mmH2O).
Faecal incontinence
Two patients were lost to follow up for the 1 month following treatment (month 4) and were excluded from analysis for this month only. Data were non-parametric and are expressed as median values (see Table 1) and presented in box plots (Fig. 1A-C).
Table 1.
PTNS and episodes of incontinence
| Wind | Liquid | Solid | ||
|---|---|---|---|---|
| Month 0 (baseline) | Median episodes (IQ range)a | 6 (0–17.5) | 10 (5–29.5) | 18 (0–30) |
| Month 1 | Median episodes (IQ range) | 0 (0–0) | 1 (0–9) | 4 (1–14) |
| P-valueb | P = 0.012 | P = 0.086 | P = 0.047 | |
| Month 2 | Median episodes (IQ range) | 0 (0–0) | 0 (0–1) | 0 (0–7) |
| P-value | P = 0.012 | P = 0.083 | P = 0.021 | |
| Month 3 | Median episodes (IQ range) | 0 (0–0) | 0 (0–4) | 0 (0–0) |
| P-value | P = 0.018 | P = 0.012 | P = 0.012 | |
| Month 4 (follow–up) | Median episodes (IQ range) | 0 (0–3) | 0 (0–5) | 1 (0–2) |
| P-value | P = 0.043 | P = 0.235 | P = 0.128 |
Interquartile range.
Wilcoxon signed ranks test.
Figure 1.
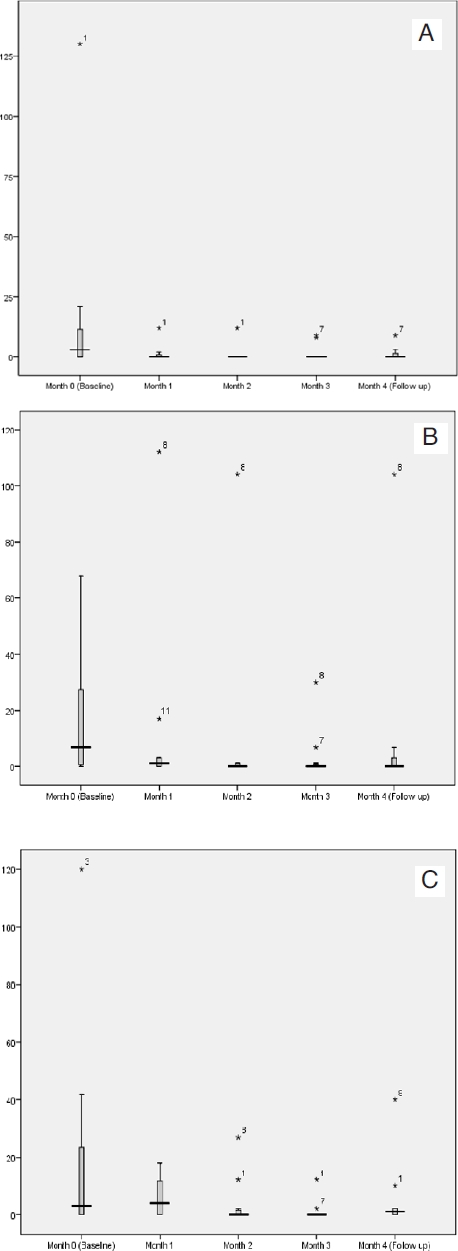
Episodes of incontinence of (A) wind, (B) liquid and (C) solid with PTNS. Boxes represent 1st to 3rd quartile. Horizontal line delineates the median. Error bars represent inner and outer fences (1.5 × interquartile range). Individual patient outliers are labelled.
Incontinence of wind
Median monthly episodes of incontinence of wind reduced significantly from a baseline of 6 to 0 (month 1), 0 (month 2) and 0 (month 3) with PTNS. At 1-month follow-up, this reduction was sustained at 0 episodes (month 4).
Incontinence of liquid
Median monthly episodes of incontinence of liquid reduced from a baseline of 10 to 1 (month 1; non-significant), 1 (month 2; non-significant) and 0 (month 3; significant). At 1-month follow-up, there were a median of 0 episodes (month 4; non-significant).
Incontinence of solid
Episodes of incontinence of solid reduced significantly from 18 to 4 (month 1), 0 (month 2) and 0 (month 3). At 1-month follow-up, a median of 1 episode was recorded (month 4; non-significant).
Quality-of-life parameters
Data were parametric and are expressed as mean values. One patient was lost to follow-up for post-treatment questionnaires. Mean changes and analysis were, therefore, based upon the remaining 12 patients. Significant improvements were seen in ICIQ-B bowel control (reducing from 19.75 before PTNS to 15.33 after) and quality of life (reducing from 22.33 to 17.58) and Rockwood life-style (increasing from 25.58 to 30.08), indicating improvements in overall ability to control bowel habit and quality of life. All other quality-of-life parameters improved marginally but non-sig-nificantly (Table 2A-C).
Table 2.
| (A) Mean Hospital Anxiety and Depression Score and PTNS | |||
| Parameter | Before treatmentb | After treatment (n = 11)b | P-valuec |
| Anxiety (0–14)a | 13.00 | 11.42 | 0.226 |
| Depression (0–14) | 8.17 | 7.50 | 0.510 |
| (B) Mean ICIQB score and PTNS | |||
| Parameter | Before treatmentb | After treatment (n = 11)b | P-valuec |
| Bowel pattern (1–21)a | 8.58 | 7.58 | 0.209 |
| Bowel control (0–28) | 19.75 | 15.33 | 0.001 |
| Quality of life (0–26) | 22.33 | 17.58 | 0.007 |
| (C) Mean Rockwood score and PTNS | |||
| Parameter | Before treatmentb | After treatment (n = 11)b | P-valuec |
| Life-style (0–45)a | 25.58 | 30.08 | 0.028 |
| Coping (0–36) | 15.33 | 18.08 | 0.121 |
| Depression (0–20) | 9.75 | 11.33 | 0.121 |
| Embarrassment (0–18) | 9.50 | 10.42 | 0.460 |
Range of test.
Results are expressed as mean values.
Two-tailed paired t-test.
Discussion
Despite recent advances in management, faecal incontinence remains a common cause of profound social, economic and medical disability. Whilst non-invasive treatment modalities such as pelvic floor physiotherapy and biofeedback have been widely used, definitive evidence to support their use remains lacking.8 Until the advent of sacral nerve stimulators, further treatment for those with faecal incontinence was limited to medical or surgical therapy, with the latter's often significant risks.21 Sacral nerve stimulation (SNS) achieves complete continence in 41–75% of patients, with at least a 50% reduction in incontinence in 75–100%.10 However, in addition to undergoing an anaesthetic and invasive procedure, adverse events are seen in 12.8%, some of which (such as device infection and lead migration) mandate replacement or re-implantation in 6.7%.10 Furthermore, after approximately 8 years, device batteries must be replaced. By contrast, a wealth of evidence supports the efficacy, safety and cost-effectiveness of PTNS in treating urinary incontinence and associated disorders.22–27 It is hypothesised to access, indirectly, the same sacral nerve roots targeted in sacral nerve stimulation via the posterior tibial nerve, containing sensorimotor and autonomic fibres derived from the 4th and 5th lumbar and 1st to 3rd sacral roots. Technically simple to perform, there is no requirement for anaesthesia or insertion in the operating theatre. PTNS is estimated to cost less than a tenth that of sacral nerve stimulation,27 although often requiring a greater number of hospital attendances, the patient effect of which is yet to be established. The use of PTNS in faecal incontinence is, so far, limited to four non-UK small studies and a report.14–18 In 2003, Shafik et al.14 described a 78.3% reduction in idiopathic faecal incontinence scores in 1 month, limited to 32 patients incontinent of solid stool only, and with normal sphincter morphology and function. In 2005, Queralto et al.15 demonstrated a 60% improvement in faecal incontinence in 8 out of 10 patients with normal sphincter morphology again over a 4-week treatment period. Subsequently, PTNS was reported by Mentes et al.18 to improve faecal incontinence and quality of life in two patients with partial spinal cord injuries, and subjective improvements were shown by Vitton et al.16 in 5 out of 12 patients with inflammatory bowel disease. Most recently, De la Portilla et al.17 found improvements in 10 of 16 patients, with reductions in Wexner continence scores and associated improvements in quality of life. The mechanisms by which PTNS improves incontinence are not fully understood, but extrapolation from SNS would suggest both sensory and motor neuromodulatory effects. Such putative effects include alterations in rectal sensory perception, up-regulation of striated muscle function (allowing generation of increased maximum squeeze pressure), and a reduction in unwanted spontaneous anal relaxations and rectal contractions.14,17,28,29
Our study, the first description in the UK, found reductions in median episodes of incontinence of wind, liquid and solid stool from 6, 10 and 18, respectively, to 0 with 12 weeks' treatment. These reductions reached significance after just 4 weeks in the case of wind and solid stool. Short-term follow-up showed a sustained reduction in incontinence of wind (0 episodes), but non-significant reductions in incontinence of liquid (0) and solid (1) which may suggest that the improvements are short-lived. We also found improvements in the ICIQ-B bowel control score and some quality-of-life indices (ICIQ-B quality of life, Rockwood lifestyle), associated with minor non-significant improvements in Hospital Anxiety and Depression, ICIQ-B bowel pattern and Rockwood coping, depression and embarrassment scores; this must, however, be interpreted with caution due to any possible placebo effect.
Quantitative comparison with the work of Shafik, Queralto, Mentes, Vitton, De La Portilla and colleagues is not possible due to the different methods of quantifying incontinence and populations studied, but our study seems to support their findings. Our study and that by De La Portilla are the first to use a 12-week rather than 4-week period of treatment in those with non-inflammatory faecal incontinence, in turn adopted from the urological evidence base.21,30 Whilst De La Portilla et al.17 did not subdivide the trends within this 12-week period, we found that, whilst improvement was seen earlier, statistical significance was reached only in the final month of treatment for liquid stool. Whilst this may be, in part, explained by the level of variance and power of the study, it suggests that the optimal number, timing and duration of PTNS sessions in faecal incontinence are certainly yet to be determined. This variance and power may also go some way to explaining the non-significant improvements seen for follow-up incontinence of liquid and stool (with median episodes of 0 and 1), and quality-of-life indices. Larger, prospective studies are needed to generate further evidence, including better establishing the duration of effect and correlation with physiological parameters. We were unable to assess the latter in this study, but findings have been disparate previously, with Shafik et al.14 and De La Portilla et al.17 demonstrating increased sphincter pressures in those treated with PTNS, a finding not supported by Queralto et al.15 Furthermore, such studies shall better allow assessment of important additional symptoms such as urgency and frequency which we were unable to assess, and determination of optimum regime and delivery mechanism. Importantly, PTNS has yet to be the subject of a placebo-controlled trial due to subjects' awareness of stimulation; however, the recent validation of a sham stimulation with TENS machines may help circumvent this.31
Urological studies have demonstrated PTNS to be associated only with occasional mild complications.32 One adverse event was reported during our study, a transient, painful, swollen leg. Although clinical examination was normal with no evidence of infection or thrombosis, it may be that use of a transcutaneous rather than percutaneous electrode may reduce risk still further. Whilst our follow-up period was short, medium-term follow-up in other studies suggests that the effects of PTNS may persist for 3-6 months after treatment14,17 and, furthermore, that they can be maintained with ‘top-up’ therapy, in a number of cases performed by the patients at home16,18 - an attractive economic and convenient possibility from a patient and provider perspective.
Conclusions
Our results, the first in the UK, add further weight to the small body of evidence suggesting that PTNS may represent a safe, cost-effective, technically simple and efficacious treatment for faecal incontinence. Where it might fit into treatment protocols remains to be seen; potentially, its use may obviate the need for more invasive procedures for those at high operative risk, provide a new treatment option for those unable to undergo more invasive procedures, and represent an interim control measure for those awaiting more definitive interventions. It might also be performed in the community. Such possibilities are particularly enticing as the population ages and faecal incontinence poses yet greater challenges to patients and health services alike. Larger, randomised, control studies comparing PTNS with placebo and alternative modalities shall better delineate its role.
References
- 1.Potter J. Bowel care in older people. Clin Med. 2003;3:48–51. doi: 10.7861/clinmedicine.3-1-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, et al. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999;42:1525–32. doi: 10.1007/BF02236199. [DOI] [PubMed] [Google Scholar]
- 3.Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, et al. Fecal Incontinence Quality of Life Scale: quality of life instrument for patients with fecal incontinence. Dis Colon Rectum. 2000;43:9–16. doi: 10.1007/BF02237236. discussion 16-7. [DOI] [PubMed] [Google Scholar]
- 4.Perry S, Shaw C, McGrother C, Matthews RJ, Assassa RP, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50:480–4. doi: 10.1136/gut.50.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johanson JF, Lafferty J. Epidemiology of faecal incontinence: the silent affliction. Am J Gastroenterol. 1996;91:33–6. [PubMed] [Google Scholar]
- 6.Bellicini N, Molloy PJ, Caushaj P, Kozlowski P. Fecal incontinence - a review. Dig Dis Sci. 2008;53:41–6. doi: 10.1007/s10620-007-9819-z. [DOI] [PubMed] [Google Scholar]
- 7.Madoff RD, Parker SC, Varma MG, Lowry AC. Faecal incontinence in adults. Lancet. 2004;364:621–32. doi: 10.1016/S0140-6736(04)16856-6. [DOI] [PubMed] [Google Scholar]
- 8.Norton C. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults (Review) Cochrane Database Syst Rev. 2006;3:CD002111. doi: 10.1002/14651858.CD002111.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Madoff RD. Surgical treatment options for fecal incontinence. Gastroenterology. 2004;126(Suppl 1):S48–54. doi: 10.1053/j.gastro.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Fraser C, Glazener C, Grant A, Graham M. Systematic review of the efficacy and safety of sacral nerve stimulation for faecal incontinence. London: National Institute for Health and Clinical Excellence; 2004. Review Body for Interventional Procedures. [Google Scholar]
- 11.Wong WD, Congliosi SM, Spencer MP, Corman ML, Tan P, et al. The safety and efficacy of the artificial bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum. 2002;45:1139–53. doi: 10.1007/s10350-004-6381-z. [DOI] [PubMed] [Google Scholar]
- 12.McGuire EJ, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129:78–9. doi: 10.1016/s0022-5347(17)51928-x. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura M, Sakurai T, Tsujimoto Y, Tada Y. Transcutaneous electrical stimulation for the control of frequency and urge incontinence. Hinyokika Kiyo. 1983;29:1053–9. [PubMed] [Google Scholar]
- 14.Shafik A, Ahmed I, El-Sibai O, Mostafa RM. Percutaneous peripheral neuro-modulation in the treatment of fecal incontinence. Eur Surg Res. 2003;35:103–7. doi: 10.1159/000069399. [DOI] [PubMed] [Google Scholar]
- 15.Queralto M, Portier G, Cabarrot PH, Bonnaud G, Chotard JP, et al. Preliminary results of peripheral transcutaneous neuromodulation in the treatment of idiopathic fecal incontinence. Int J Colorectal Dis. 2006;21:670–2. doi: 10.1007/s00384-005-0068-3. [DOI] [PubMed] [Google Scholar]
- 16.Vitton V, Damon H, Roman S, Nancey S, Flourié B, Mion F. Transcutaneous posterior tibial nerve stimulation for fecal incontinence in inflammatory bowel disease patients: a therapeutic option? Inflamm Bowel Dis. 2009;15:402–5. doi: 10.1002/ibd.20774. [DOI] [PubMed] [Google Scholar]
- 17.De La Portilla F, Rada R, Vega J, Gonazlez CA, Cisneros N, Maldonado VH. Evaluation of the use of posterior tibial nerve stimulation for the treatment of fecal incontinence: preliminary results of a prospective study. Dis Colon Rectum. 2009;52:1427–33. doi: 10.1007/DCR.0b013e3181a7476a. [DOI] [PubMed] [Google Scholar]
- 18.Mentes BB, Yüksel O, Aydin A, Tezcaner T, Leventoglu A, Aytaç B. Posterior tibial nerve stimulation for faecal incontinence after partial spinal injury: preliminary report. Tech Coloproctol. 2007;11:115–9. doi: 10.1007/s10151-007-0340-3. [DOI] [PubMed] [Google Scholar]
- 19.Counihan TC, Madoff RD. Faecal incontinence. In: Fazio VW, Church JM, Delaney CP, editors. Current Therapy in Colon and Rectal Surgery, 2nd edn. Philadelphia, PA: Elsevier Mosby; 2005. pp. 105–12. [Google Scholar]
- 20.van Balken MR. Percutaneous tibial nerve stimulation: the Urgent PC device. Expert Rev Med Devices. 2007;4:693–8. doi: 10.1586/17434440.4.5.693. [DOI] [PubMed] [Google Scholar]
- 21.Brown SR, Nelson RL. Surgery for faecal incontinence in adults. Cochrane Database Syst Rev. 2007;(2):CD001757. doi: 10.1002/14651858.CD001757.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Vandoninck V, Van Balken MR, Finazzi Agro E, Petta F, Caltagirone C, Heesakkers JP, et al. Posterior tibial nerve stimulation in the treatment of urge incontinence. Neurourol Urodyn. 2003;22:17–23. doi: 10.1002/nau.10036. [DOI] [PubMed] [Google Scholar]
- 23.Congregado RB, Pena OXM, Campoy MP, Leon DE, Leal LA. Peripheral afferent nerve stimulation for treatment of lower urinary tract irritative symptoms. Eur Urol. 2004;45:65–9. doi: 10.1016/j.eururo.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 24.van der Pal F, van Balken MR, Heesakkers JP, Debruyne FM, Kiemeney LA, Bemelmans BL. Correlation between quality of life and voiding variables in patients treated with percutaneous tibial nerve stimulation. BJU Int. 2006;97:113–6. doi: 10.1111/j.1464-410X.2006.05860.x. [DOI] [PubMed] [Google Scholar]
- 25.van Balken MR, Vergunst H, Bemelmans BL. Prognostic factors for successful percutaneous tibial nerve stimulation. Eur Urol. 2006;49:360–5. doi: 10.1016/j.eururo.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Van Balken MR, Vandoninck V, Messelink BJ, Vergunst H, Heesakkers JP, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol. 2003;43:158–63. doi: 10.1016/s0302-2838(02)00552-3. [DOI] [PubMed] [Google Scholar]
- 27.Klinger HC, Pycha A, Schmidbauer J, Marberger M. Use of the peripheral neuromodulation of the S3 region for treatment of detrusor overactivity: a urodynamic-based study. Urology. 2000;56:766–71. doi: 10.1016/s0090-4295(00)00727-5. [DOI] [PubMed] [Google Scholar]
- 28.Vaizey CJ, Kamm MA, Turner IC, Nicholls RJ, Woloszko J. Effects of short term sacral nerve on anal and rectal function in patients with anal incontinence. Gut. 1999;44:407–12. doi: 10.1136/gut.44.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michelsen HB, Buntzen S, Krogh K, Laurberg S. Rectal volume tolerability and anal pressures in patients with fecal incontinence treated with sacral nerve stimulation. Dis Colon Rectum. 2006;49:1039–44. doi: 10.1007/s10350-006-0548-8. [DOI] [PubMed] [Google Scholar]
- 30.Finazzi Agro E, Campagna A, Sciobica F, Petta F, Germani S, Zuccala A, et al. Posterior tibial nerve stimulation: is the once-a-week protocol the best option? Minerva Urol Nefrol. 2005;57:119–123. [PubMed] [Google Scholar]
- 31.Peters K, Carrico D, Burks F. Validation of a sham for percutaneous tibial nerve stimulation (PTNS) Neurourol Urodyn. 2009;28:58–61. doi: 10.1002/nau.20585. [DOI] [PubMed] [Google Scholar]
- 32.van Balken MR, Vandoninck V, Gisolf KW, Vergunst H, Kiemeney LA, et al. Posterior tibial nerve stimulation as neuromodulative treatment of lower urinary tract dysfunction. J Urol. 2001;166:914–8. doi: 10.1097/00005392-200109000-00025. [DOI] [PubMed] [Google Scholar]


