Abstract
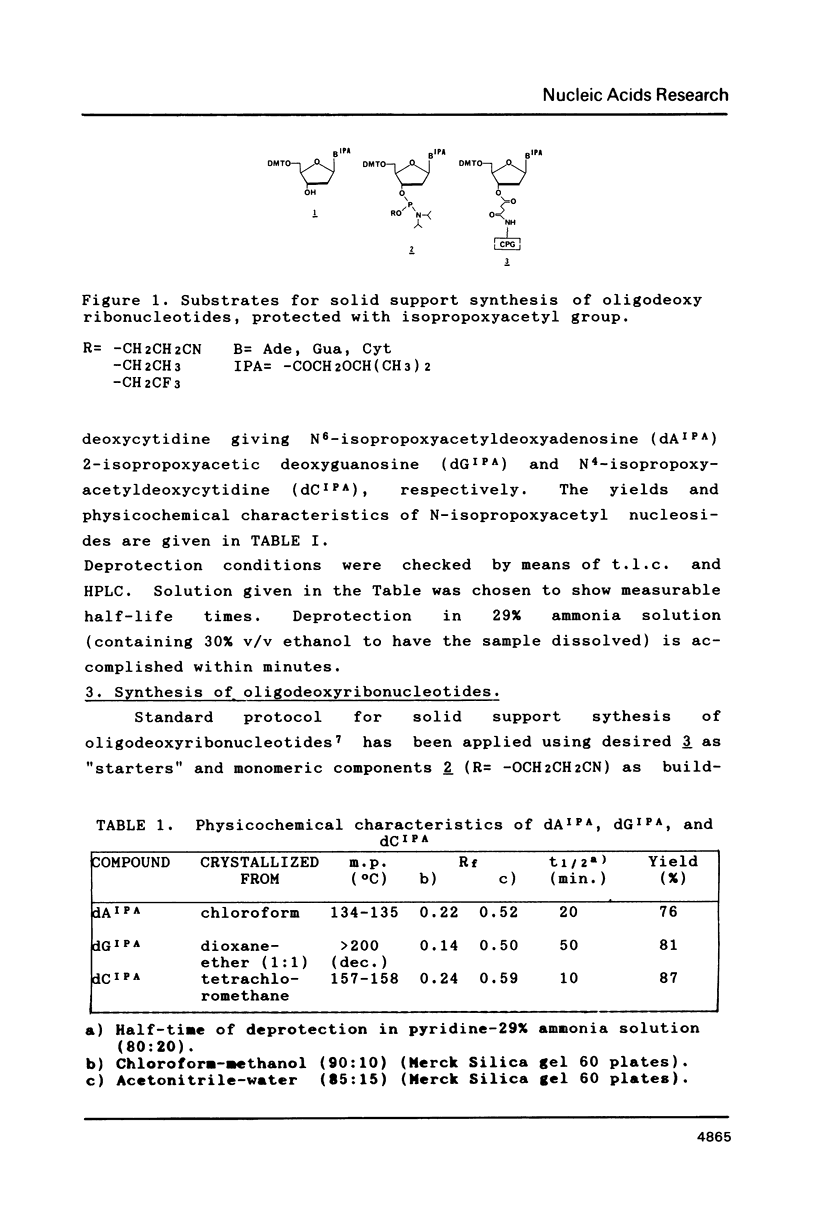
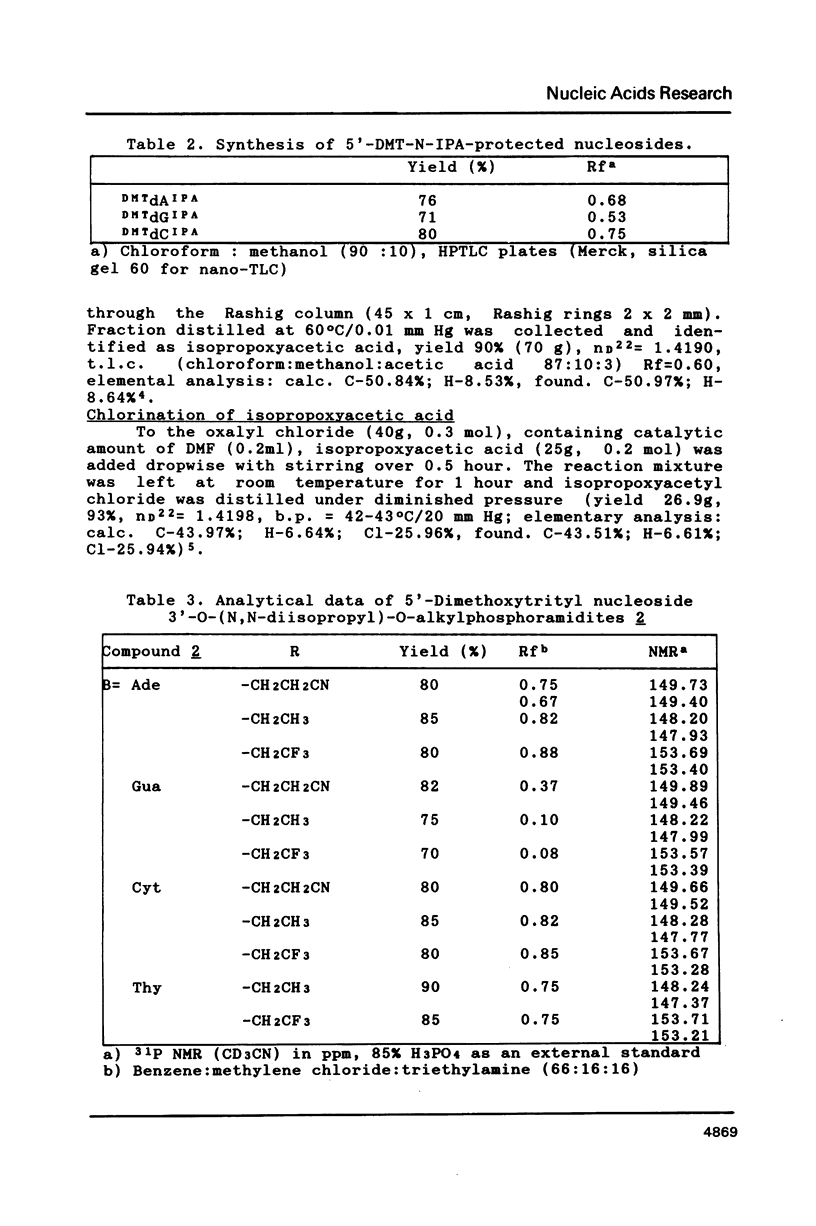
Isopropoxyacetic anhydride was successfully used for protection of exoaminofunctions of 2'-deoxyadenosine, -guanosine and -cytidine. N-isopropoxyacetylated nucleosides are stable under the conditions of the synthesis of oligodeoxyribonucleotides on the solid support. Removal of N-isopropoxyacetyl is much faster than that of commonly used benzoyl or isobutyryl groups viz. it is completed within the operation of cleavage of the oligodeoxyribonucleotide from the solid support. This observation enabled synthesis of -OCH2CH3 and -OCH2CF3 triesters, which hydrolyse partially or completely when standard deprotection conditions are applied.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gallo K. A., Shao K. L., Phillips L. R., Regan J. B., Koziolkiewicz M., Uznanski B., Stec W. J., Zon G. Alkyl phosphotriester modified oligodeoxyribonucleotides. V. Synthesis and absolute configuration of Rp and Sp diastereomers of an ethyl phosphotriester (Et) modified EcoRI recognition sequence, d[GGAA(Et)TTCC]. A synthetic approach to regio- and stereospecific ethylation-interference studies. Nucleic Acids Res. 1986 Sep 25;14(18):7405–7420. doi: 10.1093/nar/14.18.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letsinger R. I., Finnan J. L., Heavner G. A., Lunsford N. B. Letter: Phosphite coupling procedure for generating internucleotide links. J Am Chem Soc. 1975 May 28;97(11):3278–3279. doi: 10.1021/ja00844a090. [DOI] [PubMed] [Google Scholar]
- Schulhof J. C., Molko D., Teoule R. The final deprotection step in oligonucleotide synthesis is reduced to a mild and rapid ammonia treatment by using labile base-protecting groups. Nucleic Acids Res. 1987 Jan 26;15(2):397–416. doi: 10.1093/nar/15.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]


