Abstract
Background
Flower visiting insects provide a vitally important pollination service for many crops and wild plants. Recent decline of pollinating insects due to anthropogenic modification of habitats and climate, in particular from 1950's onwards, is a major and widespread concern. However, few studies document the extent of declines in species diversity, and no studies have previously quantified local abundance declines. We here make a quantitative assessment of recent historical changes in bumblebee assemblages by comparing contemporary and historical survey data.
Methodology/Principal Findings
We take advantage of detailed, quantitative historical survey data from the 1930's on bumblebee (Bombus spp.) abundances and species composition in red clover (Trifolium pratense) fields, an important floral resource and an attractant of all bumblebee species. We used the historical survey data as a pre-industrialization baseline, and repeated the same sampling protocol at nearly the same localities at present, hence setting up a historical experiment. We detected historical changes in abundances (bees/m2) of both workers (the “pollinatory units”) and queens (effective population size), in addition to species composition. In particular, long-tongued bumblebee species showed consistent and dramatic declines in species richness and abundances throughout the flowering season of red clover, while short-tongued species were largely unaffected. Of 12 Bombus species observed in the 1930's, five species were not observed at present. The latter were all long-tongued, late-emerging species.
Conclusions/Significance
Because bumblebees are important pollinators, historical changes in local bumblebee assemblages are expected to severely affect plant reproduction, in particular long-tubed species, which are pollinated by long-tongued bumblebees.
Introduction
Pollinators play a key role in natural and agricultural ecosystems, providing an important pollination service of wild plants and crops [1]. Serious concerns have been raised of a recent widespread decline of pollinating insects, potentially leading to a subsequent loss of insect-pollinated wild plants [2], [3] and/or substantial loss of agricultural productivity [4]. Although the existence of a global pollination crisis has been questioned [5], in recent years it has become a widespread perception and evidence is accumulating, that both wild and domesticated pollinators are in decline [6], [7]. Most notably, Biesmeijer et al. [8] presented convincing evidence of parallel decreases in species richness of pollinators (bees and hoverflies) and insect-pollinated plants at a national scale in England and the Netherlands. Agricultural intensification and habitat loss are often identified as the main drivers of pollinator decline [6], [9], [10], although pests and pathogens may also be important [11], [12], at least in some species and in certain regions (America) [13].
For bumblebees (Bombus Latreille spp.), which are among the most important and best known group of wild pollinators [14], [15], a long-term decline has been documented locally and regionally, in Europe, America and Asia [9], [13], [16], [17], [18]. In particular, local extinctions and decrease in range extension of some species are apparent from approximately the 1950's onwards [10], [17], [19], [20], [21], although population declines vary regionally [7], [17]. Whereas it is widely agreed that there has been a general decline in the diversity of bumblebees, quantitative documentation of historical changes are almost completely lacking to date [8], [17]. Exceptions include a few studies, in which historical declines of bumblebees are documented on the basis of natural history collections [13], [16]. Museum collections can provide important and accurate information regarding species numbers and distributions, however, they more rarely reflect differences in local abundances due to collector bias [20]. Furthermore, change in species composition of bumblebee assemblages was recently assessed from historical survey data [21]. While the latter study report proportional representation of species, to our knowledge, no studies have directly quantified historical changes in density (bees/m2), here termed abundance.
To date, no standardized and repeatable historical survey data have been presented, which may serve as a pre-industrialization baseline of pollinator abundances [8], [17]. However, in Scandinavia there is a long tradition of research on breeding of red clover (Trifolium pratense L.) [22], an important and widespread crop in Northern Europe before the intensification of agricultural production in the 1950's onwards [16]. Red clover is self-incompatible, and bumblebees, specifically long-tongued species, are the most important pollinating agents [22], [23]. Moreover, red clover is an important forage of bumblebees [22], [24], [25]. In February 1930, a prize was offered by the Royal Danish Academy of Sciences to investigate the importance of Bombus spp. in the pollination of red clover and the distribution of bumblebees and their nests in Denmark. The award winner, O. S. Skovgaard subsequently continued the study of bumblebees in red clover fields 1930–1934. In his publication [26], methods were described sufficiently for a similar study to be replicated at present, providing a unique retrospect to pre-industrialization.
The purpose of the current study is to compare present species composition and abundances of bumblebees in red clover fields to that of the study by Skovgaard from the 1930's. We use a similar sampling design to repeat the historical study at present, to make a quantitative assessment of changes in Danish Bombus populations in red clover fields in the last 80 years. We here report a significant change in local species composition and abundances of bumblebees. We found a marked decrease in abundances (in some cases even absence) of most long-tongued species at present, while short-tongued species remain unaffected.
Materials and Methods
Historical study, past data
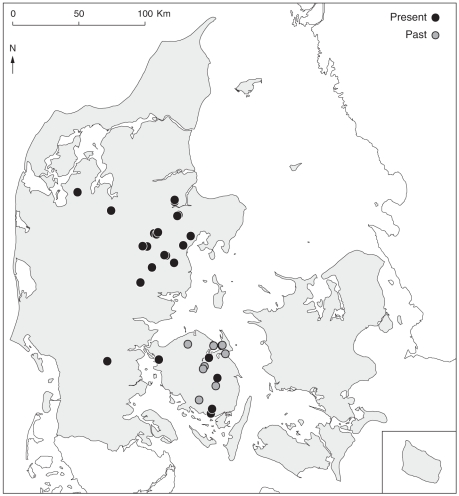
Skovgaard monitored nest-building bumblebees (excluding species of sub-genus Psithyrus) in a total of 25 red clover fields at 10 localities during five years (1930–34) on the island of Funen, Denmark (Figure 1). Varying numbers of study fields and locations were visited each year (Table 1). Except for three pairs of fields belonging to the same farm, all study fields of a given year were separated by several km. Observations included both early-flowering and late-flowering diploid red clover cultivars of Øtofte and Tystofte. Corolla tube length was approximately 9 mm [26]. Sampling was done from the middle of June until early September, sampling period differing among years (Table 1). Fields were observed before, at and after peak flowering of red clover, but mostly at peak flowering. Abundances of bumblebees peaked mostly in July (mean 23rd of July) for all study fields in all years. All bumblebee observations were done during the daytime (5 to 20 h).
Figure 1. Study sites.
Localities of red clover fields in the past [25] (grey) and the present study (black).
Table 1. Sampling effort in past and present field studies.
| Year | first day | last day | No fields | No localities | No recordsa |
| 1930 | 30 June | 03 Aug. | 3 | 3 | 4 |
| 1931 | 11 July | 10 Sep. | 4 | 3 | 9 |
| 1932 | 11 July | 12 Aug. | 7 | 7 | 14 |
| 1933 | 18 July | 26 July | 6 | 4 | 31 |
| 1934 | 17 June | 11 July | 5 | 2 | 12 |
| 2008 | 20 June | 06 Aug. | 12 | 12 | 42 |
| 2009 | 03 June | 20 Aug. | 17 | 16 | 99 |
| Total past | 25 | 10 | 70 | ||
| Total present | 29 | 19 | 141 | ||
For present observations, number of records represents aggregated data (observations aggregated within each observation day for each field).
In 1930–31, study plots were unequal in size, but generally covered a total of 900–1200 m2 per field, consisting of 2–3 sub-plots which represented different parts of the field. Sub-plots mostly encompassed six rows across the field, from one edge to the other. In 1932–34, bumblebees were counted in a total of 1000 m2 per study field (except one field, in which study plots were only 200 m2). No further details are reported on the spatial dimensions and location of the plot(s) within the field, but given Skovgaard's consideration of representing various parts of the field, including edge and center, we assume that several sub-plots were used. Flowering was estimated in the historical study [26] as % flowering (in late season as % withered flowers) or as “peak flowering” only for the first and last day of observation of each study field. Because bumblebee abundances changes through the flowering season of red clover [27], we only included observation days for which flowering estimates were reported in the analysis, i.e. 70 sampling records of a total of 119. We arbitrarily classified days of ≤30% flowering as early flowering season, “peak flowering” as mid season, and ≥70% withered as late flowering season. All past data were converted to density (Bombus individuals/m2) for the analysis.
Data collection, present data
Relevant permits for conducting field work in red clover fields were obtained. Because red clover is currently a minor crop and fields for seed production rare, we monitored bumblebee abundances in red clover fields in a total of 14 localities in Eastern Jutland, in addition to five localities on Funen, Denmark (Figure 1). The study included nearly all red clover seed fields in the two regions. The study encompassed a total of 29 fields: 12 fields were visited from the 20th of June to the 6th of August 2008, and 17 fields were visited from the 3rd of June to the 20th of August 2009. Fields were separated by at least 10 km, although three pairs of fields were only 1.5–2 km apart. Because most bumblebee species have a foraging range <500 m (although up to 2 km for B. terrestris) [28], [29], [30], [31], bumblebee faunas of different fields are expected to be independent. Study fields were all organically grown, a growing practice that more closely imitates agricultural practices of the 1930's (e.g. no pesticides and artificial fertilizers, small fields, more insect pollinated crops) than conventional management methods. Furthermore, all fields were grown for seed production, which ensured presence of flowers in the study fields. Currently, only red clover of the early-flowering cultivar, Milvus and the intermediate-late flowering Rajah (both diploid varieties), are used, but flowering time varied among fields due to cutting (which postponed flowering) and soil type (flowering earlier on sandy soils). Average corolla length of the most common cultivar, Rajah, is 8.83 mm [32], while corolla length of Milvus is unknown. Red clover flowered from early June to late August, although in most fields flowering peaked in the middle of July. Each red clover field was observed 2–4 (mean (SD) = 3.1 (0.4)) times during the flowering season of the field, preferably encompassing the beginning, middle and late flowering season.
Bumblebee observations were carried out during the daytime (8 to 19 h) under favorable weather conditions, i.e. on days with no rain or strong wind. To mimic the historical study [25], bumblebee counts were carried out in three different sub-plots totaling 1000 m2 and representing different spatial parts of the study field. Currently, red clover is broad cast and, hence, it was not possible to select six rows as in the past [25]. The three sub-plots of 18×18 m each were placed on a line, one at the field edge, one at the centre of the field, and one in between. In every sub-plot, the observer walked slowly back and forth in rows, observing each red clover flower head approximately once, and recording the number, caste (workers, queens and males) and species of flower-visiting bumblebees. Workers and queens were distinguished based on size, as was presumably done in the historical study. Parasitic bumblebees (sub-genus Psithyrus) were excluded. Only bees alighting on the flower heads and collecting pollen or nectar were registered as flower-visitors. The observer walked through the sub-plots1-4 times on each sampling day, thereby sampling the abundance of bumblebees in 324–1296 m2.
On each sampling day, flowering was estimated by counting the number of red clover flower heads in three 1×1 m squares placed diagonally in each of the sub-plots. Because red clover fields flower for a prolonged period, we assumed that each field was visited at least once during peak flowering. Peak flowering was defined as the day of maximum flower head density of the 1×1 m squares (mean (SD)). Flowering of the field was categorized as early (if flowering before mid, and mean (SD) flower head density not overlapping with mid flowering), mid (maximum observed flower head density) or late flowering (if flowering after mid, and mean (SD) flower head density not overlapping with mid flowering).
Sample specimens of bumblebees were collected at all sites and all sampling days for later identification by a taxonomic expert (Henning Bang Madsen, Copenhagen University). Bee species data were classified and aggregated into long-tongued and short-tongued bumblebee species (Table 2). The recorded data from the same field and the same day of the present collections were aggregated, so that both the sampled area of past and present data were of the same order of magnitude and had the same level of aggregation.
Table 2. Total numbers of bumblebees observed in the red clover fields in the past and present.
| No workers | No queens | ||||
| Functional group | Bombus species | past | present | past | present |
| Long-tongued | B. hortorum | 1424 | 858 | 24 | 23 |
| B. pascuorum | 349 | 2307 | 6 | 19 | |
| B. muscorum | 122 | 236 | 3 | 0 | |
| B. distinguendus | 857 | 0 | 24 | 0 | |
| B. sylvarum | 52 | 0 | 2 | 0 | |
| B. veteranus | 121 | 0 | 5 | 0 | |
| B. ruderarius | 21 | 0 | 0 | 0 | |
| B. subterraneus | 17 | 0 | 2 | 0 | |
| Short-tongued | B. terrestris | 3906 | 13580 | 21 | 349 |
| B. lapidarius | 445 | 3499 | 12 | 98 | |
| B. hypnorum | 17 | 12 | 1 | 0 | |
| B. pratorum | 29 | 24 | 0 | 0 | |
| Total | 7360 | 20516 | 100 | 489 | |
Bumblebee species were classified as long-tongued or short-tongued on the basis of tongue lengths measured in [24]. In both past and present studies, individuals belonging to B. terrestris (L.) and the B. lucorum complex (B. lucorum L., B. magnus Vogt. and B. cryptarum (F.)) were recorded as one species (hereafter B. terrestris). These species are difficult to distinguish in the field, but functionally similar [53], [54]. Notice that the sampling intensity differed between past and present studies, and the observed numbers of bees are, hence, not directly comparable.
Statistical analysis
The aggregated numbers of observed long- and short-tongued bumblebee workers and queens were divided by the sampled area. Males were numerically rare in both past and present, and omitted from the analyses. Histograms of the area-corrected number of bumblebees showed that the area corrected number of bumblebees (bees/m2) was approximately exponentially distributed (Figure S1). Consequently, the change in the area-corrected number of observed long- and short-tongued bumblebees between the past and the present, respectively, were analysed under the assumption that the area corrected number of bees is exponentially distributed with the inverse of the mean area-corrected number of bees as the rate parameter. We used likelihood ratio tests on the rate parameters to test possible differences between the past and the present. Furthermore, we analysed changes in the observed species distribution assuming that the number of observed bee species are multinomial distributed, and differences were tested by the use of likelihood ratio tests on the vector of observation probabilities.
We tested for differences in abundance (bees/m2) and species composition (proportional representation of species) of bumblebee groups. Analyses were done for all species, in addition to long-tongued and short-tongued bumblebees and for queens and workers separately. For present data, differences among sub-plots at the edge, mid and centre of the field were tested. Queens were more common at the field edges (long-tongued: χ2 = 13.00, P<0.001; short-tongued: χ2 = 10.95, P<0.001), but always numerically rare. Abundances of workers did not differ significantly among sub-plots within a field on a given sampling day (P>0.01) (Figure S2). Only minor differences were found in species composition (Table S1). Hence, to enable comparison with past data, present data of sub-plots were aggregated within fields for each observation day. Differences between Jutland and Funen were tested for present data (all past data were from Funen). No regional differences (Jutland versus Funen) were found in abundances of bumblebees (P>0.01) (Figure S3), and only small differences in species composition (Table S2), justifying a comparison to past data from Funen.
The effect of season (early, mid and late flowering) was tested for both past and present data. Generally, abundance and species composition of bumblebees were significantly affected by flowering season in both present and past. Hence, we tested whether aggregated data differed between past and present in early, mid and late season, respectively.
Results
Abundances
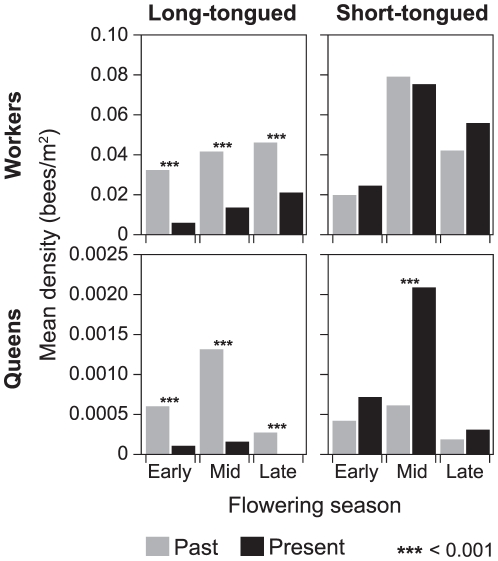
We currently observed a total of 20516 workers (16.6% long-tongued) and 489 queens (8.6% long-tongued), while Skovgaard observed 7360 workers (40.3% long-tongued) and 100 queens (66.0% long-tongued) in the past (Table 2). In all three periods during the flowering season of red clover, long-tongued workers and queens showed steep and significant abundance declines from past to present (Figure 2). In contrast, we generally found no significant difference in abundances for short-tongued bumblebees from past to present, and even significant increase in abundance of short-tongued queens in mid season (Figure 2). Overall, no significant historical abundance declines of all bumblebee workers or queens were detected (all P>0.01), possibly due to a general rarity of long-tongued species.
Figure 2. Historical change in bumblebee abundances.
Mean bumblebee abundance (bees/m2) of long tongued (left) and short tongued (right) workers (top) and queens (bottom) during the beginning, mid and late flowering season of the red clover fields in the past (grey) and at present (black).
Species composition
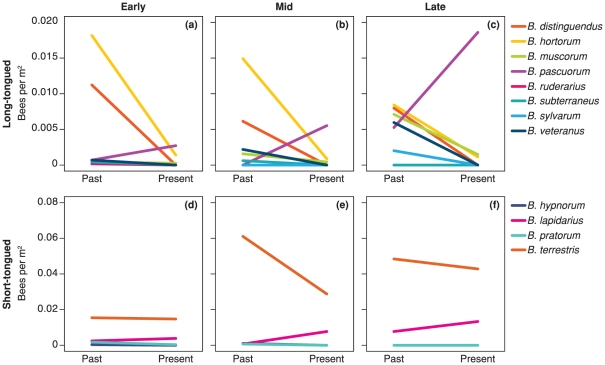
Species composition, i.e. proportional representation of species, differed significantly between past and present for both long-tongued and short-tongued species throughout the flowering season (all P<0.01). Generally, bumblebee assemblages at present had much lower abundances of long-tongued species (Figure 3). Skovgaard repeatedly observed 12 Bombus spp. in each study year in the past (13, if including B. soroeensis, for which no quantitative data were registered [25]). In contrast, only seven species were observed at present (2008 and 2009) despite a wider geographical range of study sites and a more intensive sampling effort (Table 1 and Figure 3). Five species had disappeared from past to present (B. distinguendus, B. sylvarum, B. veteranus, B. ruderarius, B. subterraneus). These were all long-tongued, late-emerging species. Only one long-tongued species, B. pascuorum, had increased, in particular in mid and late flowering season (Figure 3).
Figure 3. Historical change in bumblebee species composition.
Abundances (bees/m2) of long-tongued (top) and short-tongued (bottom) species and direction of change from past to present in early, mid and late flowering season of the red clover fields.
Discussion
Historical decline of pollinators: regional and local patterns
It is widely perceived that the regional bumblebee species richness and range extent of some species have declined historically across Europe [9], [10], [16], [18]. However, status and trends of pollinators are based mostly on red list assessments and studies of museum collections, while few quantitative data exist. The current study is one of the first accounts on the basis of historical survey data, which document local population declines in species richness and abundance of some species, but not others. Historically, rare long-tongued species of bumblebees were observed regularly in fields of red clover, albeit in low numbers, in addition to the historically abundant long-tongued B. distinguendus (whose common name in Danish is translated as the “Clover Bumblebee”) [26]. At present, only currently widespread and commonly occurring species were observed in the red clover fields. Bumblebee species of low abundance in red clover fields are common elsewhere (B. hypnorum in urban areas) or at other times during the season (B. pratorum in early summer) [14], [33]. However, none of the historically rare species were registered in the present investigation, nor was the historically common B. distinguendus observed, despite intensive sampling across the season at multiple locations. Historical trends in species composition of bumblebees of the current study are similar to that of a recent Swedish study, although some of the rare long-tongued species were registered at present in Sweden [21]. The agricultural landscape in Denmark, which is more intensively farmed, appears to be even more depauperate in long-tongued bumblebees, including B. hortorum, which is common in other habitats, e.g. gardens [33]. One exception to this pattern is the moderate to long-tongued generalist B. pascuorum, which has increased historically. Overall, for bumblebee communities of red clover fields of the current study, abundances in addition to species richness of long-tongued species have declined dramatically, while short-tongued species are largely unaffected. For long-tongued species, abundances of queens was found to decline an order of magnitude from the 1930's to the present, corresponding to a dramatic decline in effective population sizes. Findings of the present study are strong and direct evidence of local changes in species richness and abundances, which largely corroborate existing knowledge of larger scale regional changes of bumblebee species occurrences [15].
Possible causes of decline
Red clover is attractive for all bumblebee species, including rare and declining species [22], [24], [25], and hence the bumblebee assemblages observed in red clover fields is expected to be a good indicator of the regional species pool of bumblebees. The decline of some species of bumblebees and absence of others indicate that requirements of these species are not met at present in the modern agricultural landscape. There is a general agreement among bumblebee ecologists that historical changes in agricultural practices and land use are consistent drivers of bumblebee decline since the industrialization [18]. Adverse effects of historical changes in agricultural practices and land use include increased mortality due to pesticide application [34], pathogen spillover [11] and possible competition [27] from commercial bees, changes in landscape configuration leading to loss of hibernation and nesting sites [35], [36], [37], reduced sowing of leguminous crops (including red clover) and flower-rich meadows [16], [38], in addition to gaps in continuity of floral resources throughout colony life [30].
However, why some species have declined while others remain abundant is a long-standing question (e.g. [18], [24], [39], [40]). The most prevalent hypotheses include (1) diet specialization and decline of preferred food plants [9], [16], [24], [41] and (2) small geographical range size, reflecting a narrow climatic niche constrained by physiological tolerances [18], [39]. For both hypotheses, specificity is a key element, implying that specialized species are predisposed to decline and extirpation. Concordant with this pattern, the short-tongued species of the current study are all opportunistic in choice of food plants [26] and nesting sites [26], [37], while little is known about hibernation sites [35]. Furthermore, the by far most dominant bumblebees in red clover fields, the short-tongued Bombus terrestris and B. lucorum complex, have a relatively large foraging range, some studies reporting up to 1.5–2 km [30], [31]. Thus, these species may respond to the environment at larger spatial scales [42], and may be less vulnerable to e.g. habitat fragmentation and loss. In contrast, known foraging distances of other Bombus spp., including the long-tongued B. pascuorum, B. muscorum and B. hortorum, are limited to a few hundred meters [29], [31], [43].
Long-tongued bumblebee species have a strong preference for long-tubed flowers, including red clover [24], [38]. One hypothesis is that the historical decline of long-tongued bumblebees is linked to a general decline in red clover fields and other leguminous crops [16], [38], [44]. However, the rare and declining species B. veteranus, B. distinguendus and B. sylvarum are currently found in Denmark only at coastal meadows, which are highly diverse in plants and insects, but not specifically rich in red clover [45]. Thus, whereas the decline in availability of red clover fields may contribute to decline of long-tongued species, other factors are likely to be involved. For instance, the declining species also tend to emerge late in the season compared to stable species (this study, [40]). We lack knowledge about interspecific variation in habitat use and vulnerability during critical stages of the life cycle, including nest initiation, colony build-up and climax, reproduction and hibernation [14], [15]. Moreover, drivers of historical changes are difficult to assess because decline itself may influence observed patterns, e.g. present floral specialization may reflect absence or rarity of forage plants [18], [39]. Here, historical survey data may provide a reliable picture of the past.
Shifts in bumblebee functional groups and consequences for pollination
Bumblebees are, perhaps, the most important group of wild pollinators in Northern Europe, due to their ability to forage at low temperatures [46], capability of buzz pollination [47] and ability to handle complex flowers [48], their relatively long tongues compared to other bee species [14], [26], and generally broad floral diet despite preferences [39]. The changes in species composition and abundances of bumblebee workers in red clover fields result in a marked shift in composition of functional groups of bumblebees towards lower abundances of long-tongued bumblebees and stable abundances of short tongued species. Queens of the short-tongued Bombus terrestris have increased in mid season, perhaps due to niche space vacated by the dramatic decline of long-tongued bumblebee queens. Colonies of Bombus terrestris are among the most highly populated [19], which may further elevate the proportional representation of short-tongued workers. Because workers account for the vast majority of flower-visits by bumblebees [14], the compositional change of bumblebees may have severe implications for the pollination environment of plants [1]. At present, however, no historical abundance change of B. terrestris was observed.
Long-tongued bumblebees are important pollinators of several crops, including clovers (Trifolium spp.), field bean (Vicia faba), and a range of berries, fruits and vegetables [22]. Long-tongued species prefer to visit long-tubed flowers [49]. For red clover, which has the longest floral tube among Northern European plants, short-tongued bumblebees are not optimal pollinators and regularly act as non-pollinating nectar robbers [22], [26]. Decreasing seed yields have been reported, rendering red clover a crop of low profit [[21] (Sweden), C. Jørgensen and S. Oddershede, pers. com. (Denmark)].
Many wild plants are visited and pollinated predominantly or exclusively by bumblebees [15]. In plant-flower-visitor networks, which encompasses all co-occurring species of plants and visitors, bumblebees are suggested as important hubs [3], [50] or generalist core species [51], [52], which act as keystone pollinators in the system. Hence, species extinctions, local decline and shifts in functional group composition of bumblebees are expected to heavily impact the structural organization and functioning of natural pollination networks.
Results of the current study, thus, are strong direct evidence of extensive historical changes in abundance and species composition of local bumblebee populations in Denmark, changes, which have previously been assumed but scarcely documented. Although historical studies are highly variable in scientific quality, the use of appropriate historical survey data repeated at present, shows great potential for retrospective analyses, and hence “a look into the past”.
Supporting Information
Distributions of observed and expected abundances of bumblebees. Histograms of observed abundances of bumblebees (m−2) in mid season compared to the expected abundances under the assumption that data are exponential distributed. The same plots for early and late seasons were qualitatively similar.
(EPS)
Within-field differences in bumblebee abundances at present. Spatial differences in bumblebee abundances in sub-plots at the edge (light grey) middle (dark grey) and center (black) within fields. Likelihood ratio test of the effect of subplot: ** P<0.001.
(EPS)
Regional differences in bumblebee abundances at present. Regional differences in bumblebee abundances between Jutland (grey) and Funen (black). Likelihood ratio test of the effect of subplot: ** P<0.001.
(EPS)
Within-field differences in species composition of bumblebee assemblages at present. Total numbers of bumblebees observed in sub-plots at the edge, middle and center of the red clover fields in the present study.
(DOC)
Regional differences in species composition of bumblebee assemblages at present. Total numbers of bumblebees observed in Jutland and Funen in the present study.
(DOC)
Acknowledgments
D. Boll, K. Wermuth, A. M. Plejdrup, T. G. Sørensen and M. Thompsen are thanked for assistance in the field, and H. B. Madsen for identification of bumblebees. We furthermore thank S. Oddershede and C. Jørgensen for providing lists of red clover farmers, and for valuable discussions. Thank you to a large number of red clover farmers, who permitted us to work in their fields. We are grateful to M. Hagen, C. E. Kler and two anonymous referees for valuable comments on the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The project was financed by the Aarhus University Research Foundation (AUFF-F-2009-FLS-5-7). During the writing of the manuscript, YLD was supported by the Danish Natural Sciences Research Council (grant number 09-063874). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kremen C, Williams NM, Aizen MA, Gemmill-Herren B, LeBuhn G, et al. Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol Lett. 2007;10:299–314. doi: 10.1111/j.1461-0248.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 2.Kearns CA, Inouye DW, Waser NM. Endangered mutualisms: The conservation of plant-pollinator interactions. Annu Rev Ecol Syst. 1998;29:83–112. [Google Scholar]
- 3.Corbet SA. Conserving compartments in pollination webs. Conserv Biol. 2000;14:1229–1231. [Google Scholar]
- 4.Winfree R. Pollinator-dependent crops: An increasingly risky business. Curr Biol. 2008;18:R968–R969. doi: 10.1016/j.cub.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Ghazoul J. Buzziness as usual? Questioning the global pollination crisis. Trends Ecol Evol. 2005;20:367–373. doi: 10.1016/j.tree.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, et al. Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Murray TE, Kuhlmann M, Potts SG. Conservation ecology of bees: populations, species and communities. Apidologie. 2009;40:211–236. [Google Scholar]
- 8.Biesmeijer JC, Roberts SPM, Reemer M, Ohlemüller R, Edwards M, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- 9.Goulson D, Lye GC, Darvill B. Decline and conservation of bumble bees. Annu Rev Entomol. 2008;53:191–208. doi: 10.1146/annurev.ento.53.103106.093454. [DOI] [PubMed] [Google Scholar]
- 10.Kosior A, Celary W, Olejniczak P, Fijal J, Krol W, et al. The decline of the bumble bees and cuckoo bees (Hymenoptera: Apidae: Bombini) of Western and Central Europe. Oryx. 2007;41:79–88. [Google Scholar]
- 11.Otterstatter MC, Thomson JD. Does pathogen spillover from commercially reared bumble bees threaten wild pollinators? PLoS One. 2008;3:9. doi: 10.1371/journal.pone.0002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stout JC, Morales CL. Ecological impacts of invasive alien species on bees. Apidologie. 2009;40:388–409. [Google Scholar]
- 13.Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, et al. Patterns of widespread decline in North American bumble bees. P Natl Acad Sci USA. 2010;108:662–667. doi: 10.1073/pnas.1014743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benton T. Bumblebees: The natural history and identification of the species found in Britain. London: Collins; 2006. 580 [Google Scholar]
- 15.Goulson D. Bumblebees: their behaviour and ecology. Oxford: Oxford University Press; 2003. 235 [Google Scholar]
- 16.Rasmont P, Mersch P. Première estimation de la dérive faunique chez les bourdons de la Belgique (Hymenoptera, Apidae). Ann Soc Roy Zool Bel. 1988;118:141–147. [Google Scholar]
- 17.Williams PH, Osborne JL. Bumblebee vulnerability and conservation world-wide. Apidologie. 2009;40:367–387. [Google Scholar]
- 18.Williams P, Colla SR, Xie Z. Bumblebee vulnerability: common correlates of winners and losers across three continents. Conserv Biol. 2009;23:931–940. doi: 10.1111/j.1523-1739.2009.01176.x. [DOI] [PubMed] [Google Scholar]
- 19.Free JB, Butler CG. Bumblebees. London: Collins; 1959. [Google Scholar]
- 20.Grixti JC, Wong LT, Cameron SA, Favret C. Decline of bumble bees (Bombus) in the North American Midwest. Biol Conserv. 2009;142:75–84. [Google Scholar]
- 21.Bommarco R, Lundin O, Smith HG, Rundlöf M. Drastic historic shifts in bumble-bee community composition in Sweden. P Roy Soc B Bio. 2011 doi: 10.1098/rspb.2011.0647. doi: 10.1098/rspb.2011.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Free JB. Insect pollination of crops. London: Academic Press; 1993. 684 [Google Scholar]
- 23.Holm SN. The utilization and management of bumble bees for red clover and alfalfa seed production. Annu Rev Entomol. 1966;11:155–182. [Google Scholar]
- 24.Goulson D. Causes of rarity in bumblebees. Biol Conserv. 2005;122:1–8. [Google Scholar]
- 25.Carvell C, Roy DB, Smart SM, Pywell RF, Preston CD, et al. Declines in forage availability for bumblebees at a national scale. Biol Conserv. 2006;132:481–489. [Google Scholar]
- 26.Skovgaard OS. Rødkløverens bestøvning, humlebier og humleboer. Det Kongelige Danske Videnskabernes Selskabs Skrifter Naturvidenskabelig og Matematisk Afdeling Række. 1936;96:1–140. [Google Scholar]
- 27.Wermuth K, Dupont YL. Effects of field characteristics on abundance of bumblebees (Bombus spp.) and seed yield in red clover fields. Apidologie. 2010;41:657–666. [Google Scholar]
- 28.Kreyer D, Oed A, Walther-Hellwig K, Frankl R. Are forests potential landscape barriers for foraging bumblebees? Landscape scale experiments with Bombus terrestris agg. and Bombus pascuorum (Hymenoptera, Apidae). Biol Conserv. 2004;116:111–118. [Google Scholar]
- 29.Knight ME, Martin AP, Bishop S, Osborne JL, Hale RJ, et al. An interspecific comparison of foraging range and nest density of four bumblebee (Bombus) species. Mol Ecol. 2005;14:1811–1822. doi: 10.1111/j.1365-294X.2005.02540.x. [DOI] [PubMed] [Google Scholar]
- 30.Osborne JL, Martin AP, Carreck NL, Swain JL, Knight ME, et al. Bumblebee flight distances in relation to the forage landscape. J Anim Ecol. 2008;77:406–415. doi: 10.1111/j.1365-2656.2007.01333.x. [DOI] [PubMed] [Google Scholar]
- 31.Walther-Hellwig K, Frankl R. Foraging habitats and foraging distances of bumblebees, Bombus spp. (Hymenoptera, Apidae), in an agricultural landscape. J Appl Entomol. 2000;124:299–306. [Google Scholar]
- 32.Brødsgaard CJ, Hansen H. Pollination of red clover in Denmark. Danish Institute of Agricultural Sciences, Ministry of Food, Agriculture and Fisheries. 2002. pp. 3–49. DIAS report no 71 DIAS report no 71.
- 33.Dupont YL, Madsen HB. Humlebier. Natur og Museum. 2010;1:1–36. [Google Scholar]
- 34.Marletto F, Patetta A, Manino A. Laboratory assessments of pesticide toxicity to bumblebees. B Insectol. 2003;56:155–158. [Google Scholar]
- 35.Skovgaard OS. Humlebiarternes bopladser og overvintringssteder. Tidsskrift for Planteavl. 1943;47:287–305. [Google Scholar]
- 36.Osborne JL, Martin AP, Shortall CR, Todd AD, Goulson D, et al. Quantifying and comparing bumblebee nest densities in gardens and countryside habitats. J Appl Ecol. 2008;77:406–415. [Google Scholar]
- 37.Kells AR, Goulson D. Preferred nesting sites of bumblebee queens (Hymenoptera : Apidae) in agroecosystems in the UK. Biol Conserv. 2003;109:165–174. [Google Scholar]
- 38.Goulson D, Hanley ME. Distribution and forage use of exotic bumblebees in South Island, New Zealand. New Zeal J Ecol. 2005;28:225–232. [Google Scholar]
- 39.Williams P. Does specialization explain rarity and decline British bumblebees? - A response to Goulson et al. Biol Conserv. 2005;122:33–43. [Google Scholar]
- 40.Fitzpatrick U, Murray TE, Paxton RJ, Breen J, Cotton D, et al. Rarity and decline in bumblebees - A test of causes and correlates in the Irish fauna. Biol Conserv. 2007;136:185–194. [Google Scholar]
- 41.Kleijn D, Raemakers I. A retrospective analysis of pollen host plant use by stable and declining bumble bee species. Ecology. 2008;89:1811–1823. doi: 10.1890/07-1275.1. [DOI] [PubMed] [Google Scholar]
- 42.Gabriel D, Sait SM, Hodgson JA, Schmutz U, Kunin WE, et al. Scale matters: the impact of organic farming on biodiversity at different spatial scales. Ecol Lett. 2010;13:858–869. doi: 10.1111/j.1461-0248.2010.01481.x. [DOI] [PubMed] [Google Scholar]
- 43.Hagen M, Wikelski M, Kissling WD. Space use of bumblebees (Bombus spp.) revealed by radio-tracking. PLoS One. 2011;6:e19997. doi: 10.1371/journal.pone.0019997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goulson D, Hanley ME, Darvill B, Ellis JS, Knight ME. Causes of rarity in bumblebees. Biol Conserv. 2005;122:1–8. [Google Scholar]
- 45.Madsen HB. Bumblebees. In: Wind P, editor. The Danish red list. Aarhus: The National Environmental Research Institute, Aarhus University [2004]; 2009. 39 Available: http://redlist.dmu.dk. [Google Scholar]
- 46.Corbet SA, Fussell M, Ake R, Fraser A, Gunson C, et al. Temperature and the pollinating activity of social bees. Ecol Entomol. 1993;18:17–30. [Google Scholar]
- 47.Buchmann SL. Bees use vibration to aid pollen collection from non-poricidal flowers. J Kansas Entomol Soc. 1985;58:517–525. [Google Scholar]
- 48.Heinrich B. Bumblebee economics. Cambridge, Mass. and London: Harvard University Press; 1979. [Google Scholar]
- 49.Fussell M, Corbet SA. Flower usage by bumble-bees: A basis for forage plant management. J Appl Ecol. 1992;29:451–465. [Google Scholar]
- 50.Olesen JM, Bascompte J, Dupont YL, Jordano P. The modularity of pollination networks. P Natl Acad Sci USA. 2007;104:19891–19896. doi: 10.1073/pnas.0706375104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Memmott J, Waser N, Price MV. Tolerance of pollination networks to species extinctions. P Roy Soc Lond B Bio. 2004;271:2605–2611. doi: 10.1098/rspb.2004.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alarcón R, Waser NM, Ollerton J. Year-to-year variation in the topology of a plant-pollinator interaction network. Oikos. 2008;117:1796–1807. [Google Scholar]
- 53.Hammer K, Holm SN. Danske humlebier og snyltehumler. Natur og Museum. 1970;14:3–21. [Google Scholar]
- 54.Bertsch A, Schweer H, Titze A. Discrimination of the bumblebee species Bombus lucorum, B. cryptarum and B. magnus by morphologica characters and male labial gland secretions (Hymenoptera: Apidae). Beitr Ent. 2004;54:365–386. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distributions of observed and expected abundances of bumblebees. Histograms of observed abundances of bumblebees (m−2) in mid season compared to the expected abundances under the assumption that data are exponential distributed. The same plots for early and late seasons were qualitatively similar.
(EPS)
Within-field differences in bumblebee abundances at present. Spatial differences in bumblebee abundances in sub-plots at the edge (light grey) middle (dark grey) and center (black) within fields. Likelihood ratio test of the effect of subplot: ** P<0.001.
(EPS)
Regional differences in bumblebee abundances at present. Regional differences in bumblebee abundances between Jutland (grey) and Funen (black). Likelihood ratio test of the effect of subplot: ** P<0.001.
(EPS)
Within-field differences in species composition of bumblebee assemblages at present. Total numbers of bumblebees observed in sub-plots at the edge, middle and center of the red clover fields in the present study.
(DOC)
Regional differences in species composition of bumblebee assemblages at present. Total numbers of bumblebees observed in Jutland and Funen in the present study.
(DOC)