Abstract
Background
Yersinia pestis is the causative agent of plague. The two transcriptional regulators, PhoP and RovA, are required for the virulence of Y. pestis through the regulation of various virulence-associated loci. They are the global regulators controlling two distinct large complexes of cellular pathways.
Methodology/Principal Findings
Based on the LacZ fusion, primer extension, gel mobility shift, and DNase I footprinting assays, RovA is shown to recognize both of the two promoters of its gene in Y. pestis. The autoregulation of RovA appears to be a conserved mechanism shared by Y. pestis and its closely related progenitor, Y. pseudotuberculosis. In Y. pestis, the PhoP regulator responds to low magnesium signals and then negatively controls only one of the two promoters of rovA through PhoP-promoter DNA association.
Conclusions/Significance
RovA is a direct transcriptional activator for its own gene in Y. pestis, while PhoP recognizes the promoter region of rovA to repress its transcription. The direct regulatory association between PhoP and RovA bridges the PhoP and RovA regulons in Y. pestis.
Introduction
Yersinia pestis is one of the most dangerous bacterial pathogens. Humans infected with Y. pestis manifest three main forms: pneumonic, septicemic, and bubonic plagues, and it has a very high mortality rate without timely and effective antibiotic treatment [1]. There have been at least three plague pandemics in human history, including the Black Death, which accounted for the death of at least one-third of the European population between 1347 and 1353. Plague remains a great threat to public health because rodent plague epidemics are frequent in various natural plague foci, especially in Asia, America, and Africa, human plague infections are reported every year, and Y. pestis can possibly be used as a biowarfare or bioterrorism agent.
PhoP and PhoQ constitute a classic regulatory two-component system [2]. The sensor protein PhoQ responds to low environmental Mg2+, acidic pH, and host-secreted antimicrobial peptides, and then phosphorylates the response regulator PhoP. As a transcription factor, phosphorylated PhoP either activates or represses its target genes through binding to their promoter-proximal DNA regions. Intracellular growth of Y. pestis in macrophages occurs at early stages of systemic infection [3]. A phoP null mutant of Y. pestis showed reduced ability to survive in macrophages and human neutrophils, as well as under in vitro conditions of low pH, oxidative stress, high osmolarity, and antimicrobial peptides [4], [5], [6]; this mutant is slightly attenuated in mice [4]. As a global regulator, PhoP controls a very complex regulatory cascade in Y. pestis [7], [8], [9]. The PhoP regulons in Y. pestis and Salmonella enterica have considerable differences in terms of the functional changes in PhoP itself, as well as in the architecture of PhoP-dependent promoters. This allows the PhoP regulators to incorporate newly acquired genes into the ancestral regulatory circuits yet retain control of the core regulon members in these two bacteria [8], [9]. The proven direct PhoP targets in Y. pestis include several genes that function in detoxification, protection against DNA damage, resistance to antimicrobial peptides, and adaptation to magnesium limitation [7], especially the mgtCB and udg loci that encodes an Mg2+ transport system and a UDP-glucuronate decarboxylase for LPS modification, respectively, required for the replication of Y. pestis in macrophages [10]. These PhoP-dependent mechanisms used by Y. pestis contribute to intracellular growth of this pathogen.
As a member of the MarR/SlyA family of transcriptional regulators that control the virulence of multiple bacterial pathogens [11], RovA is required for the virulence of all three pathogenic yersiniae (Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica) through regulation of various virulence loci [12], [13], [14], [15], [16]. In Y. pseudotuberculosis and Y. enterocolitica, RovA stimulates the transcription of inv, which encodes an invasin that mediates translocation across the intestinal epithelium [14], [15], [16], [17]. However, the inv gene is naturally inactivated in Y. pestis due to the insertion of an IS200-like element within its coding region [18]. The rovA null mutant of Y. pestis is much more attenuated after subcutaneous inoculation than after intranasal or intraperitoneal route, indicating a more important role for RovA in subcutaneous infection than in the pneumonic or systemic one [12]. In Y. pestis, RovA stimulates the transcription of the psaEF, psaABC, and CUS-2 prophage loci [12]. The pH6 antigen encoded by psaABC acts as an antiphagocytic factor [19] and plays a more important role in bubonic plague than in the pneumonic and septicemic forms, closely mimicking the role for RovA [12]. The CUS-2 prophage is acquired by the Y. pestis ancestor and its genome forms an unstable episome in Antiqua and Medievalis, and a stably integrated one in Orientalis [20], [21]. The acquisition of this prophage does not correlate to flea transmission, but contributes to virulence in mice [20]. The RovA regulator still plays critical roles in the construction and functioning of the bacterial membrane, indicating the regulatory functions of RovA in antibiotic resistance and environmental adaptation [22].
The rovA gene transcribes with two distinct promoters, and the autoregulation of rovA has been established in Y. pseudotuberculosis, but whether both of the two promoters are dependent on RovA is unclear [17], [23]. This study indicates that the autoregulatory mechanism is also conserved in Y. pestis, and further discloses that RovA stimulates both of the two promoters. In addition, PhoP responses to low magnesium signals, and recognizes the promoter region of rovA to repress its transcription in Y. pestis. The direct regulatory association between PhoP and RovA bridges the two distinct complexes of the cellular pathways governed by the two regulators.
Materials and Methods
Bacterial strains
The wild-type (WT) Y. pestis biovar Microtus strain 201, avirulent to humans but highly virulent to mice, was isolated from Microtus brandti in Inner Mongolia, China, [24]. The base pairs 41 to 362 of rovA (432 bp in total length) or 41 to 631 of phoP (672 in total) were replaced with the kanamycin resistance cassette using the one-step inactivation method based on the lambda Red phage recombination system with the helper plasmid pKD46 [25]. This generated the rovA and phoP mutants of Y. pestis, designated as ΔrovA and ΔphoP, respectively. Chromosomal integration of the mutagenic cassette was confirmed by PCR and sequencing using oligonucleotides external to the integrated cassette. The elimination of pKD46 in the mutants was verified by PCR. All primers used in this study are listed in Table 1.
Table 1. Oligonucleotide primers used in this study.
| Target | Primers (forward/reverse, 5'–3') |
| Construction of mutants | |
| rovA | TTGGAATCGACATTAGGATCTGATCTAGCACGATTAGTTCAGATTGCAGCATTACACG/CTCAAGCTTATCGATTAGGCCTGATAACACTGCAATTTCATGTAACGCACTGAGAAGC |
| phoP | ATGCGGGTTCTGGTTGTGGAAGATAACGCGTTGTTGCGTCAGTTGTGTCTCAAAATCTCTG/CTAGTTGACGTCAAAACGATATCCCTGACCACGAATAGTCGAAAGCCGCCGTCCCGTCAAG |
| Complementation of mutants | |
| phoP | GCGGGATCCCGTGAACATCGCCTATCGTC/GCGAAGCTTTGCCACTGTGCCAGACTG |
| rovA | GATCGATATCGCTCAGTTGCCGCCTTC/GATCGGATCCCTGCTGTGAATAAAGTCTTTGAAC |
| RT-PCR | |
| phoP | TTGTTGCGTCACCATCTG/GGCTTAACCCGTCTTCAC |
| rovA | TTACCACCAGAGCAATCACAG/ATCACGCCATCAACCTGTTC |
| LacZ fusion | |
| rovA | GCGGGATCCCGTTCGTTACTCTGCCCATC/GCGAAGCTTTTGTGATTGCTCTGGTGGTAAAC |
| Primer extension | |
| rovA | /GTATCCTCATTACCCAGCATCG |
| /GTGCTAGATCAGATCCTAATGTCG | |
| Protein expression | |
| rovA | GCGGGATCCTTGGAATCGACATTAGGATC/GCGGTCGACTTACTTAGTTTGTAATTGAATA |
| phoP | GCGGGATCCATGCGGGTTCTGGTTGTGG/GCGAAGCTTTTAGTTGACGTCAAAACGATATCCC |
| EMSA | |
| rovA | CGTTCGTTACTCTGCCCATC/TGTGATTGCTCTGGTGGTAAAC |
| TGCTCCCGACGCTAAGTG/TAGAAAATTTGTTCCCCTCGAC | |
| CTGAAAGCGAGGCGATGC/TCAGCCGATGGTCAATTAATGC | |
| DNase I footprinting | |
| rovA | TGCTCCCGACGCTAAGTG/TAGAAAATTTGTTCCCCTCGAC |
| CTGAAAGCGAGGCGATGC/TCAGCCGATGGTCAATTAATGC | |
| ACCAAATCTGAAAGCGAGGCG/GTGCTAGATCAGATCCTAATGTCG | |
A PCR-generated DNA fragment containing the rovA or phoP coding region with its promoter-proximal region (∼500 bp upstream the coding sequence) and transcriptional terminator (∼300 bp downstream) were cloned into the pACYC184 vector that harbors a chloramphenicol resistance gene (GenBank accession number X06403), as verified by DNA sequencing. The recombinant plasmid was subsequently introduced into ΔrovA and ΔphoP, yielding the complemented mutant strains C-rovA and C-phoP, respectively.
Bacterial growth
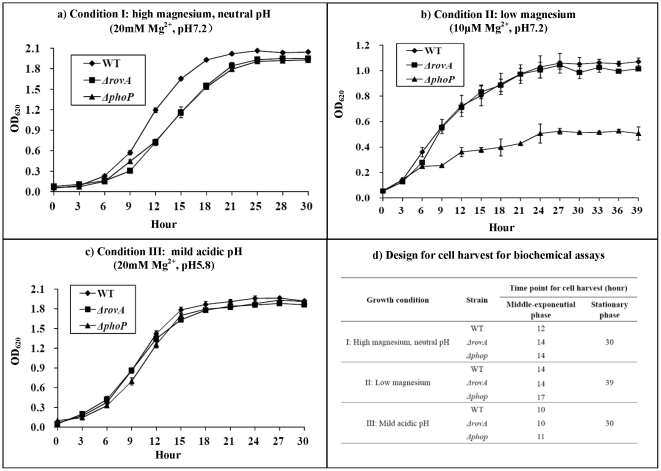
The original chemically defined TMH medium [26] [called “high magnesium, neutral pH” (I)] and its different modifications were used for Y. pestis cultivation. The 20 mM MgCl2 in the original TMH was changed to 10 µM to simulate the ‘low magnesium’ (II) condition. To simulate ‘mild acidic pH’ (III), the pH value of 7.2 in the original TMH was changed to 5.8. Overnight cell culture with an optical density (OD620) of about 1.0 in each medium was diluted 1∶20 into 18 ml of the corresponding fresh medium for further cultivation.
Cells were harvested at the middle-exponential or stationary phase for the followed primer extension or LacZ fusion assay. For cell harvest at the middle-exponential phase, bacteria were grown at 26°C with shaking at 230 rpm to enter the exponential phase; and then, half of the cell cultures were incubated at 37°C for 3 h and the remaining half were allowed to grow continuously at 26°C for 3 h. For harvest at the stationary phase, bacteria were grown at 26°C to enter the stationary phase; the cell cultures were then divided to grow at 37 and 26°C, respectively, for 3 h as above. The detailed time points for cell harvest were defined according to the bacterial growth curves (Fig. 1). The above cultures grown at 37 and 26°C were designated as “shift from 26 to 37°C (#)” and “26°C continuously (&),” respectively, so as to determine the effect of temperature on gene transcription.
Figure 1. Bacterial growth curves at 26°C.
Overnight cell culture with an OD620 value of about 1.0 in each medium was diluted 1∶20 into 18 ml of the corresponding fresh medium. Bacteria were then grown at 26°C with shaking at 230 rpm, and the OD620 values were monitored for each culture with a 3 or 4 h interval until the cultures reached the stationary growth phase (a, b, and c). Experiments were done with three biological replicates. Shown also is the design (d) for cell harvest for subsequent biochemical assays.
Primer extension assay
Total bacterial RNAs were extracted using the TRIzol Reagent (Invitrogen) [7], [25]. Immediately before harvesting, bacterial cultures were mixed with RNAprotect Bacteria Reagent (Qiagen) to minimize RNA degradation. RNA quality was monitored by agarose gel electrophoresis and RNA quantity was determined by spectrophotometry. For the primer extension assay [7], [25], an oligonucleotide primer complementary to a portion of the RNA transcript of rovA gene was employed to synthesize cDNAs from the RNA templates. About 10 µg of the total RNA from each strain was annealed with 1 pmol of [γ−32P] end-labeled reverse primer using a Primer Extension System (Promega) according to the manufacturer’s instructions. The same labeled primer was also used for sequencing with the fmol® DNA Cycle Sequencing System (Promega). The primer extension products and sequencing materials were concentrated and analyzed in a 6% polyacrylamide/8 M urea gel. The result was detected by autoradiography (Kodak film).
LacZ fusion and β-galactosidase assay
The 889 bp promoter-proximal DNA region of rovA was obtained by PCR with the ExTaq™ DNA polymerase (Takara) using Y. pestis 201 genome DNA as the template. PCR fragments were then directionally cloned into the EcoRI and BamHI sites of low-copy-number plasmid pRW50 that harbor a tetracycline resistance gene and a promoterless lacZ reporter gene [27]. Correct cloning was verified by DNA sequencing. An empty pRW50 plasmid was also introduced into each strain tested as the negative control. The Y. pestis strains transformed with the recombinant plasmids and the empty pRW50 plasmid were grown as previously described to measure the β-galactosidase activity in the cellular extracts using the β-Galactosidase Enzyme Assay System (Promega) [25]. Assays were performed with at least three biological replicates.
Purification of PhoP and RovA proteins
Preparation of the purified PhoP and RovA proteins were performed as previously described [7], [25]. The entire coding region of the phoP and rovA genes of strain 201 was directionally cloned into the BamHI and HindIII sites of plasmid pET28a (Novagen). The recombinant plasmid encoding the 6× His-tagged PhoP and RovA proteins (His-PhoP and His-RovA, respectively) were transformed into Escherichia coli BL21λDE3 cells. Expression of His-PhoP or His-RovA was induced by the addition of 1 mM IPTG (isopropyl-b-D-thiogalactoside). The overproduced proteins were purified under native conditions using an Ni-NTA Agarose Column (Qiagen). The purified protein was concentrated with the Amicon Ultra-15 centrifugal filter device (Millipore) and the protein purity was verified by SDS-PAGE.
Gel mobility shift assay (EMSA)
The rovA promoter-proximal regions were amplified by PCR. For EMSA [7], [25], the 5' ends of DNA were labeled using [γ−32P] ATP and T4 polynucleotide kinase. DNA binding was performed in a 10 µl reaction volume containing binding buffer [1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 50 mM NaCl, 10 mM Tris-HCl (pH 7.5) and 0.05 mg/ml poly-(dI-dC)], labeled DNA (1000 to 2000 c.p.m/µl), and increasing amounts of the His-PhoP or His-RovA protein. Three controls were included in each EMSA experiment: 1) cold probe as specific DNA competitor (the same promoter-proximal DNA region unlabeled), 2) negative probe as nonspecific DNA competitor (the unlabeled coding region of the 16S rRNA gene), and 3) nonspecific protein competitor [rabbit anti-F1-protein polyclonal antibodies]. After incubation at room temperature for 30 min, the products were loaded onto a native 4% (w/v) polyacrylamide gel and electrophoresed in 0.5× TBE buffer for about 50 min at 220 V. Radioactive species were detected by autoradiography after exposure to Kodak film at −70°C.
DNase I footprinting
For DNase I footprinting [7], [25], the rovA promoter-proximal DNA regions with a single 32P-labeled end were PCR amplified with either the sense or antisense primer being end-labeled. The PCR products were purified using MinElute reaction cleanup columns (Qiagen). Increasing amounts of His-PhoP or His-RovA were incubated with the purified, labeled DNA fragment (2 to 5 pmol) for 30 min at room temperature, in a final 10 µl reaction volume containing the binding buffer used in EMSA. Before DNA digestion, 10 µl of Ca2+/Mg2+ solution (5 mM CaCl2 and 10 mM MgCl2) was added, followed by incubation for 1 min at room temperature. The optimized RQ1 RNase-Free DNase I (Promega) was then added to the reaction mixture, and the mixture was incubated at room temperature for 40 to 90 s. The reaction was quenched by adding 9 µl of stop solution (200 mM NaCl, 30 mM EDTA, and 1% SDS), followed by incubation for 1 min at room temperature. The partially digested DNA samples were extracted with phenol/chloroform, precipitated with ethanol, and analyzed in 6% polyacrylamide/8 M urea gel. Protected regions were identified by comparison with the sequence ladders. For sequencing, we used the fmol® DNA Cycle Sequencing System (Promega). The templates for sequencing were the same as the DNA fragments of DNase I footprinting assays. Radioactive species were detected as previously described.
Results
Mutation and complementation
Real-time RT-PCR experiments were performed to assess the relative mRNA levels of phoP and rovA in the corresponding WT, mutant, and complemented mutant strains. The phoP transcript was lacking in ΔphoP, but was restored in C-phoP relative to WT, and moreover similar results were observed for the rovA transcript in WT, ΔrovA, and C-rovA (data not shown). These data indicates the successful mutation and complementation of phoP and rovA.
To test whether the phoP or rovA mutation had the polar effect, the primer extension assays were conducted to detect the yield of the rovA primer extension product that represents the rovA transcriptional levels in the corresponding WT, mutant, and complemented mutant strains (Figure S1). As determined by several distinct methods (see below), the rovA gene was positively regulated by RovA under condition I, whereas it was under the negative control of PhoP under condition II. As determined by the primer extension assays herein, the rovA transcription under condition I was significantly repressed in ΔrovA relative to WT, and restored in C-rovA; its transcription under condition II was significantly elevated in ΔphoP relative to both C-phoP and WT (Figure S1). The rovA gene yielded almost the same transcriptional levels between the paired WT/ΔphoP or WT/ΔrovA strains. This complementation analysis confirmed that the observed PhoP or RovA-dependent transcription of rovA was due to the phoP or rovA mutation, respectively, rather than a polar mutation.
Growth of WT, ΔrovA, and ΔphoP
The growth curves of the WT, ΔrovA, and ΔphoP strains grown at 26°C under three different conditions I, II, and III were determined (Fig. 1). Under condition I, both ΔrovA and ΔphoP exhibited growth rates lower than WT (Fig. 1a). Under condition II, growth restriction was observed for ΔphoP rather than ΔrovA relative to WT (Fig. 1b). The three strains showed indistinguishable growth rates under condition III (Fig. 1c). For each strain, bacterial growth was impeded under suboptimal conditions II and III relative to the original condition I. In particular, bacterial cells exhibited very poor growth when each of strain was grown under condition II.
Bacterial cells were harvested at the middle-exponential or stationary phase for the following cell culture-related biochemical assays, and the time points for cell harvest were defined strictly according to the growth curves (Fig. 1d). It should be noted that bacterial cells grown under different conditions or those of different isogenic stains were harvested at the identical growth phase, rather than the identical optical density, which would devoid the secondary effects of growth rate or phase. In addition, temperature upshift from 26 to 37°C was designed prior to cell harvest, generating 26 (“26°C continuously”) and 37°C (“shift from 26 to 37°C”) grown cells.
Transcription of rovA under different temperatures
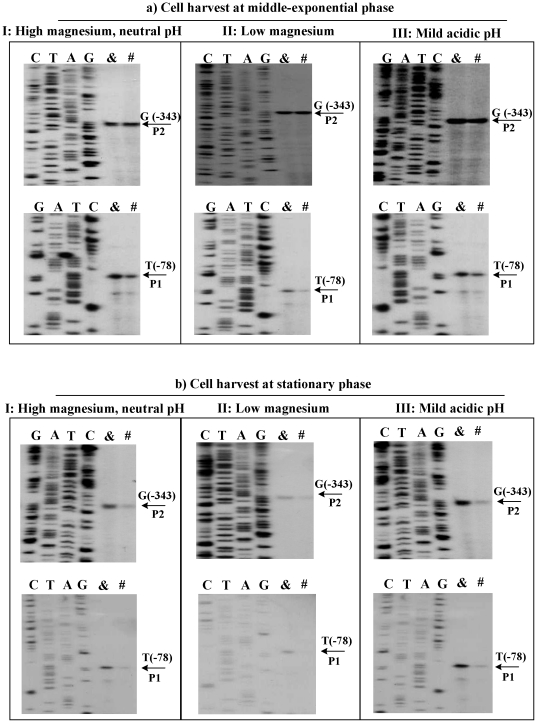
The primer extension experiments (Fig. 2) were then conducted to determine the yields of primer extension product of rovA (i.e., the relative rovA transcription levels, or the relative rovA promoter activities) in WT upon the above temperature upshift. The primer extension assay detected two transcriptional start sites located at 343 and 78 bp upstream of rovA (Fig. 2); therefore, two promoters (named P2 and P1, respectively) were transcribed for rovA. At the middle-exponential growth phase (Fig. 2a), the P2 promoter activity showed no obvious change upon the temperature upshift under conditions I, II, and III; yet, the P1 promoter activity decreased upon temperature upshift under all the three growth conditions. At the stationary growth phase (Fig. 2b), the promoter activities of both P2 and P1 decreased upon temperature upshift under all the three growth conditions. In conclusion, the temperature shift from 26 to 37°C triggered the down-regulation of both P2 and P1 promoters of rovA at the stationary growth phase. However, the down-regulatory effect occurred only for P2 at the middle-exponential growth phase.
Figure 2. Transcription of rovA upon temperature shift from 26 to 37°C.
Two oligonucleotide primers were designed to be complementary to the RNA transcript of rovA. The primer extension products were analyzed with 8 M urea−6% acrylamide sequencing gel. Lanes C, T, A, and G represent the Sanger sequencing reactions. Only the WT strain was tested to grow under conditions I, II and III, respectively. Bacterial cells were harvested at the middle-exponential (a) or stationary (b) phase. Temperature upshift was designed prior to cell harvest, generating two kinds of cultures: ‘26°C continuously’ (&) and ‘shift from 26 to 37°C’ ( #). Detected were the two promoters P1 and P2 located at 78 (nucleotide T ) and 343 (G) bp upstream of rovA, respectively. Images shown are representative of the results from at least three biological replicates.
For subsequent experiments, only bacterial cultures of “shift from 26 to 37°C” were analyzed, as 37°C is the temperature during human infections.
Autoregulation of RovA
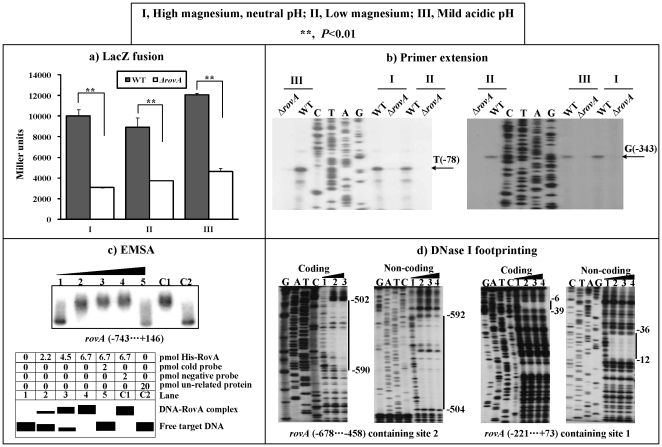
A rovA-lacZ fusion vector, containing the 889 bp promoter-proximal region of rovA and the promoterless lacZ, was transformed into both WT and ΔrovA to compare the rovA promoter activities in these two strains grown under conditions I, II, and III, respectively (Fig. 3a). Under all the three conditions, the expression of rovA significantly decreased in ΔrovA relative to WT. In addition, the primer extension experiments (Fig. 3b) were conducted to compare the yields of primer extension product of rovA in WT and ΔrovA; the activities of both P1 and P2 promoters were under the positive control of RovA under all the three growth conditions, which was consistent with the above lacZ fusion data. Interestingly, the rovA transcription in WT were up-regulated under condition III relative to the other two conditions (Fig. 2a and 2b), and thus mild acid appeared to stimulate the rovA expression.
Figure 3. RovA stimulated transcription of its own gene.
Only the bacterial cells of “shift from 26 to 37°C” at the middle-exponential growth phase were analyzed herein. a) LacZ fusion. A promoter-proximal region 743 bp upstream to 146 bp downstream of rovA was cloned into pRW50 containing a promoterless lacZ reporter gene, and then transformed into WT or ΔrovA to determine the β-galactosidase activity in cellular extracts. Shown are the rovA promoter activities (Miller units) in ΔrovA or WT grown under conditions I, II and III, respectively. b) Primer extension. The P1 and P2 promoters of rovA were detected in ΔrovA or WT grown as described above. Lanes C, T, A, and G represent the Sanger sequencing reactions. c) EMSA. The radioactively labeled DNA fragment from the 743rd bp upstream to the 146th bp downstream of rovA was incubated with increasing amounts of purified His-RovA protein, and then subjected to 4% (w/v) polyacrylamide gel electrophoresis. The band of free DNA disappeared with increasing amounts of His-RovA protein, and a retarded DNA band with decreased mobility turned up, which presumably represented the DNA-RovA complex. Shown on the lower side of the figure is the schematic representation of the EMSA design. d) DNase I footprinting. Labeled coding or non-coding DNA probes were incubated with increasing amounts of purified His-RovA (Lanes 1, 2, 3, and 4 containing 0, 4, 8, and 12 pmol, respectively), and subjected to DNase I footprinting assay. Lanes G, A, T, and C represent the Sanger sequencing reactions. The protected regions (vertical bars) are indicated on the right side of the image. The negative numbers indicate the nucleotide positions upstream of rovA.
The 889 bp promoter-proximal region of rovA tested in the LacZ fusion assay was amplified, radioactively labeled, and subjected to EMSA with a purified His-RovA protein (Fig. 3c). The results show that His-RovA is able to bind to this DNA fragment in a dose-dependent manner in vitro (Fig. 3c). As further determined by DNase I footprinting (Fig. 3d), the purified His-RovA protected two distinct regions upstream of rovA against DNase I digestion in a dose-dependent manner. These two footprints, located from 592 to 502 bp (RovA site 2) and from 39 to 6 bp (Site 1) upstream of rovA, respectively, were considered RovA sites. Taken together, RovA is able to recognize all the promoters of its own gene to stimulate their activity in Y. pestis.
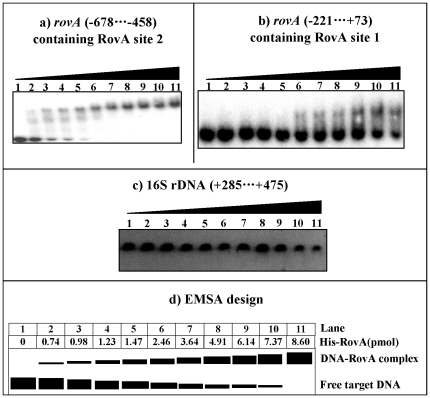
To test the affinity of RovA to Sites 1 and 2, EMSA was performed with two distinct rovA upstream DNA fragments containing Sites 1 and 2, respectively (Fig. 4). DNA retardation occurred at 0.74 pmol of His-RovA for the fragment containing Site 2 (Fig. 4a), whereas it was observed at 2.46 pmol for that containing Site 1 (Fig. 4b). This indicated that RovA had a much higher affinity to Site 2 than Site 1. The RovA proteins at all amounts used could not bind to the 16S rDNA fragment as the negative control, confirming the specificity of EMSA in this study (Fig. 4c).
Figure 4. Affinity of RovA to its Sites 1 and 2.
Labeled DNA fragments (a and b), which contained RovA Sites 1 and 2 respectively, were incubated with increasing amounts of purified His-RovA. The EMSA experiment was conducted with a coding region of the 16S rRNA gene (c) as the negative control. Also shown is the schematic representation of the EMSA design (d).
Negative regulation of rovA by PhoP
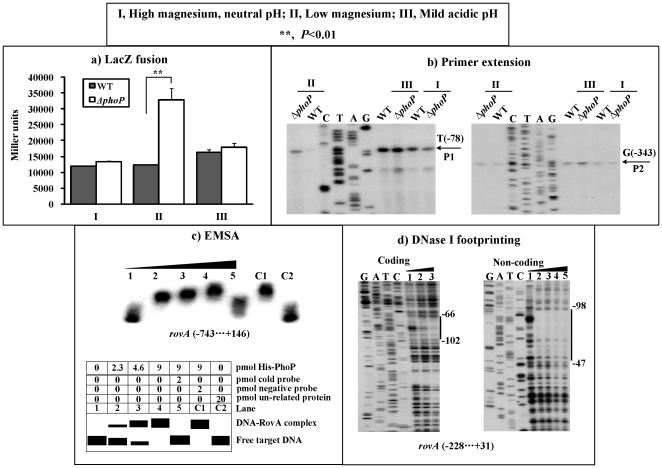
The rovA-lacZ fusion vector (Fig. 3a) was transformed into both WT and ΔphoP to compare the rovA promoter activities in the two strains grown under conditions I, II, and III (Fig. 5a). Under conditions I and III, there was no significant difference in the rovA promoter activities in the WT and ΔphoP strains. Under condition II, the expression of rovA was significantly enhanced in the ΔphoP relative to the WT. Further primer extension experiments for rovA (Fig. 5b) again detected the two promoters, P1 and P2, when the bacterial cells were grown under the three conditions. P1 activity was under the negative control of PhoP under condition II, and independent of this regulator under the other two growth conditions. PhoP had no effect on P2 activity under all the three conditions. The EMSA assay disclosed that the purified His-PhoP protein was able to bind to the 889 promoter-proximal region of rovA in a dose-dependent manner in vitro (Fig. 5c). Subsequent DNase I footprinting experiments (Fig. 5d) indicated that His-PhoP protected a single region located from 102 to 47 bp upstream of rovA. This footprint was considered the PhoP site. Therefore, the rovA transcription is negatively controlled by the PhoP regulator under the low magnesium conditions through the PhoP-promoter DNA association.
Figure 5. PhoP repressed rovA transcription.
Only the bacterial cells of “shift from 26 to 37°C” at the middle-exponential growth phase were analyzed herein. a) LacZ fusion. The rovA-lacZ fusion vector as described in Fig. 3a was transformed into WT or ΔphoP to determine the rovA promoter activity (Miller units) when the bacteria were grown under conditions I, II and III, respectively. b) Primer extension. The P1 and P2 promoters of rovA were detected in ΔphoP or WT grown as described above. Lanes C, T, A, and G represent the Sanger sequencing reactions. c) EMSA. The labeled promoter-proximal fragment of rovA as described in Fig. 3c was incubated with increasing amounts of purified His-PhoP. Shown on the lower side is the schematic representation of the EMSA design. Lanes C, T, A, and G represent the Sanger sequencing reactions. d) DNase I footprinting. The labeled DNA fragment from 228 bp upstream to 31 bp downstream of rovA was incubated with increasing amounts of His-PhoP. Lanes 1, 2, 3, 4 and 5 contained 0, 34.6, 41.5, 48.4 and 59.3 pmol, respectively. Vertical bars indicate the protected regions, whereas the negative numbers denote the nucleotide positions upstream of rovA. Lanes C, T, A, and G represent the Sanger sequencing reactions.
Promoter structure of rovA
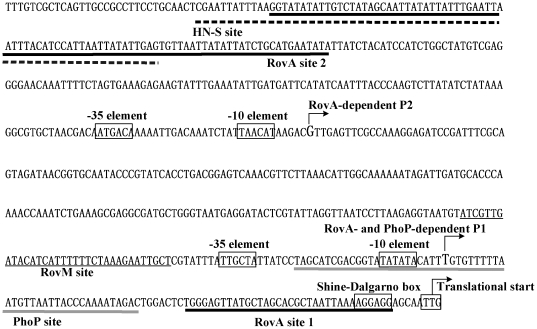
In this study, DNase I footprinting experiments precisely determined the PhoP and RovA sites for rovA. The primer extension assays mapped two promoters (P1 and P2) for rovA. Accordingly, the core promoter −10 and −35 elements for RNA polymerase recognition were predicted. Collection of data on the translation/transcription start sites, Shine-Dalgarno sequence (a ribosomal binding site in the mRNA), promoter −10 and −35 elements, as well as PhoP and RovA sites enabled us to depict the organization of PhoP and RovA-dependent promoters of rovA characterized herein (Fig. 6). The Y. pestis rovA promoter-proximal region is essentially identical to the Y. pseudotuberculosis one [28]. The two RovA sites within the rovA promoter-proximal region in Y. pestis are very similar to, although slightly different from, those determined in Y. pseudotuberculosis [17]. Y. pseudotuberculosis and Y. pestis have the same P2 promoter, but P1 in Y. pestis is 2 bp upstream of that in Y. pseudotuberculosis. The slight differences observed in the P1 sites and the RovA site sequences might be due to the personal inclination during DNA sequence reading.
Figure 6. Organization of the rovA promoter-proximal region.
The DNA sequence was derived from the genomic data of Y. pestis 91001 and the start codon was shown at the 3' terminal. The bent arrows indicate the two promoters P1 and P2 (transcription start sites). Predicted promoters −10 and −35 elements, and Shine-Dalgarno box are enclosed in boxes. The RovA, PhoP, H-NS, and RovM sites are underlined with different lines. The H-NS [17], [23] and RovM [36] sites are derived from those determined in Y. pseudotuberculosis, since the Y. pestis rovA promoter-proximal region is essentially identical to the Y. pseudotuberculosis one.
Discussion
Bacterial growth under magnesium-limitation conditions
The magnesium cation (Mg2+) is one of the essential elements for bacterial cell growth due to its function as a cofactor of enzymes. When grown under the condition II, Y. pestis cells exhibited poor growth, and moreover a extremely heavy restriction of growth was observed for ΔphoP. The Mg2+ transport systems are positively controlled by the Mg2+-responsive PhoP regulator in Y. pestis [7], [29], and the phoP mutation will impair the magnesium homeostasis of ΔphoP under Mg2+-limiting environments [2].
Regulation of rovA by growth temperature
The three pathogenic yersiniae Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica are ranked at different linkages in the evolution of the Yersinia genus [30]. The Y. pseudotuberculosis–Y. pestis clade diverged from Y. enterocolitica hundreds of millions of years ago, whereas Y. pestis from Y. pseudotuberculosis within thousands of years [31], [32]. Consistently, Y. pseudotuberculosis and Y. pestis are very divergent from Y. enterocolitica, but share a very high level of genomic homology with each other.
As previously shown [17], [33], two (P1 and P2) and three promoters are transcribed for rovA in Y. pseudotuberculosis and Y. enterocolitica, respectively, at room temperature (20 to 26°C), but a down-regulation was observed at 37°C. In addition to the mechanism of transcriptional regulation, the temperature control of rovA expression occur also at the post-transcriptional level in Y. pseudotuberculosis [34]. In this study, P1 and P2 were also detected for rovA in Y. pestis. The temperature shift from 26 to 37°C triggered the down-regulation of P1 promoter in Y. pestis at both stationary and middle-exponential growth phase. However, the down-regulation of P2 upon temperature upshift occurred only at the stationary phase rather than the middle-exponential one, indicating a growth phase-dependent effect for P2.
Regulation of rovA by transcriptional regulators
This study confirms that the autoregulation of rovA in Y. pestis is identical to that reported in Y. pseudotuberculosis, and further discloses that RovA stimulates the activity of both of the two promoters of its own gene in Y. pestis.
The nucleoid-associated protein H-NS silences target genes by selectively targeting their upstream DNA sequences with GC contents lower than that of the resident genome [35]. Similarly, Y. pseudotuberculosis H-NS binds to a long DNA region upstream of P2 (Fig. 6), and represses the rovA transcription [17], [23]. In addition, a LysR-type regulator RovM specifically binds to a short region closely upstream of the −35 element of P1 and far downstream of P2 (Fig. 6), and participates in the repression of rovA in Y. pseudotuberculosis [36]. Interestingly, the cooperation of RovM and H-NS is required for efficient silencing of rovA transcription [36]. It seems that the interaction of RovM and H-NS on the rovA promoter-proximal regions, which is accompanied by H-NS/RovM-DNA association, promotes the formation of a stable repressor complex to silence the rovA transcription [36].
The RovA Site 2 overlaps the H-NS site for rovA (Fig. 6), and RovA alleviates the H-NS-mediated repression of rovA by antagonizing the H-NS-promoter DNA association [17], [23]. The RovA Site 2 is upstream of the −35 elements of both P1 and P2, and thus the transcriptional activation of P1 and P2 by RovA is a Class I stimulation dependent on the RNA polymerase α subunit C-terminal domain (αCTD) for function [23], [37]. With the development of RovA autostimulation, the cellular RovA reaches a certain level; in this case, the low-affinity Site 1 is occupied by RovA. Notably, Site 1 is downstream of both P1 and P2, and accordingly the RovA Site 1 association blocks the entry of the RNA polymerase, which destroys the “endless” RovA-mediated activation of rovA transcription [23], [37], [38]. This RovA concentration-dependent regulation of its own gene allows the bacterium to finely modulate the cellular RovA levels for the most favorable production of RovA-dependent virulence factors [23], [37], [38].
As shown in this study, the PhoP regulator recognizes a single site within the rovA promoter-proximal region, and negatively controls the rovA transcription under magnesium-limiting conditions. It is further confirmed that PhoP as the responsive regulator of the PhoP/PhoQ two-component system responds to low magnesium signals [7], [8], [9] rather than magnesium-rich or acidic pH conditions. An 18-bp PhoP box sequence (TGTTTAWN4 TGTTTAW), which is consisted of a direct repeat of the hepta-nucleotide consensus (underlined), has been established previously in Y. pestis [7]. This box consensus represents the conserved signals for PhoP recognition in Y. pestis. Herein, a PhoP box-like sequence (TGTGTTT TTAATGTTAAT ) is found in the PhoP site for rovA. Notably, the promoter activity of P1, but not P2, is dependent on PhoP. The PhoP site overlaps the −10 region of the P1 promoter, and thus the PhoP-promoter association is thought to block RNA polymerase-DNA association, thereby repressing the transcription of rovA. This mode of regulator-promoter DNA interaction for transcriptional repression is frequently observed in transcriptional repressors, such as Fur [39] and Zur [40] in Y. pestis. PhoP and RovA control distinct complexes of cellular pathways, especially including those involved in virulence and host-adaptation [7], [8], [9], [12], [17], [41]. The two regulons governed by PhoP and RovA, respectively, have evolved to merge into a single global regulatory circuit, due to the direct transcriptional association between PhoP and RovA.
The rovA upstream DNA regions are identical in Y. pestis and Y. pseudotuberculosis, and moreover all the four regulators (RovA, PhoP, H-NS, and RovM) involved in the regulation of rovA are extremely conserved in these two bacteria. Therefore, the mechanisms that regulate rovA discussed above are conserved in these two bacteria.
S. typhimurium has the homolgous gene (named slyA) of rovA. SlyA and PhoP formed a complex positive feedback circuit in S. typhimurium [42]. The slyA transcription is activated by the PhoP/PhoQ system under low Mg2+ conditions [43], [44], and PhoP footprints the slyA upstream region [43], which indicating that PhoP stimulates slyA directly through PhoP-promoter DNA association. H-NS binds to the phoP upstream region to silence the transcription of phoPQ operon under high Mg2+ conditions [45]. PhoP binds to the phoP upstream region and activates (i.e., autoregulates) the phoP transcription under low Mg2+ conditions [46], [47]. SlyA also footrpints with the phoP upstream region, and competes with H-NS since they share the same footprint that is adjacent to the PhoP site within the phoP upstream region [42]. Thus, the association between the phoP upstream region and SlyA will facilitate PhoP binding to the PhoP site by reducing the inhibitory activity of the H-NS protein [42]. Whether Y. pestis employs a regulatory feedback circuit involving in RovA and PhoP/PhoQ needs to be elucidated. In addition to Y. pestis and Y. pseudotuberculosis, the genus Yersinia still contains another pathogenic species, i.e., Y. enterocolitica, which shows a widely genetic diversity from the Y. pseudotuberculosis/Y. pestis clade. The DNA upstream of rovA in Y. enterocolitica differs greatly from the relevant Y. pseudotuberculosis/Y. pestis DNA. The Y. enterocolitica rovA gene is transcribed with the P1 and P2 promoters that are identical to those in Y. pseudotuberculosis/Y. pestis, and moreover a third promoter P3 downstream of P1 is detected. The differences in the promoters result in significantly lower levels of rovA transcription in Y. enterocolitica. H-NS binds to two regions upstream of rovA to repress the rovA transcription in Y. enterocolitica. H-NS shows much lower affinities for either of the two sites than for the reported single Y. pseudotuberculosis/Y. pestis site. RovA stimulates the rovA transcription in Y. enterocolitica although the lack of observable RovA binding to the Y. enterocolitica promoter. RovM binds to a single region upsteam of rovA to repress the rovA transcription, as reported for Y. pseudotuberculosis. Together, the cis-acting DNA region of rovA has undergone great genetic variation between Y. enterocolitica and Y. pseudotuberculosis/Y. pestis, which will lead to the remodeling in the mechanisms for controlling the rovA transcription, although the relevant trans-acting factors (H-NS, RovA, and RovM) are highly conserved in the three pathogenic yersiniae.
Supporting Information
Primer extension assay for validation of non-polar mutation. The rovA or phop null mutant (ΔrovA or Δphop, respectively) was generated from the wild-type strain 201 (WT), and then the corresponding complemented mutant strain (C-rovA or C-phop, respectively) was constructed. As determined by several distinct methods (see the text of manuscript), the P1 promoter of rovA was positively regulated by RovA when the bacteria were grown in the original TMH medium, but negatively controlled by PhoP when grown in the TMH containing 10μM MgCl2. Herein, an oligonucleotide primer, which was complementary to the RNA transcript of rovA, was employed to detect the primer extension product that represented the relative P1 promoter activity in the corresponding strains. The primer extension products were analyzed with 8 M urea−6% acrylamide sequencing gel. Lanes C, T, A, and G represent the Sanger sequencing reactions. Shown on the right side of the image is the transcription start site (nucleotide T, corresponding to the P1 promoter) that was located at 78 bp upstream of rovA. The P1 promoter was significantly repressed in ΔrovA relative to both C-rovA and WT gown in the original TMH; yet, it was significantly enhanced in ΔphoP relative to both C-phoP and WT grown in the TMH containing 10μM MgCl2 The P1 promoter was transcribed at almost the same level in every paired WT and complemented mutant. These results confirmed that the phoP or rovA mutation was nonpolar.
(TIF)
Acknowledgments
The English writing of the manuscript was polished by EnPapers.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Financial support for this study came from the National Natural Science Foundation of China (30930001, 30900823, and 30771179) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Perry RD, Fetherston JD. Yersinia pestis–etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lukaszewski RA, Kenny DJ, Taylor R, Rees DG, Hartley MG, et al. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect Immun. 2005;73:7142–7150. doi: 10.1128/IAI.73.11.7142-7150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oyston PC, Dorrell N, Williams K, Li SR, Green M, et al. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect Immun. 2000;68:3419–3425. doi: 10.1128/iai.68.6.3419-3425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hitchen PG, Prior JL, Oyston PC, Panico M, Wren BW, et al. Structural characterization of lipo-oligosaccharide (LOS) from Yersinia pestis: regulation of LOS structure by the PhoPQ system. Mol Microbiol. 2002;44:1637–1650. doi: 10.1046/j.1365-2958.2002.02990.x. [DOI] [PubMed] [Google Scholar]
- 6.O'Loughlin JL, Spinner JL, Minnich SA, Kobayashi SD. Yersinia pestis two-component gene regulatory systems promote survival in human neutrophils. Infect Immun. 2010;78:773–782. doi: 10.1128/IAI.00718-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li YL, Gao H, Qin L, Li B, Han YP, et al. Identification and characterization of PhoP regulon members in Yersinia pestis biovar Microtus. BMC Genomics. 2008;9:143. doi: 10.1186/1471-2164-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez JC, Shin D, Zwir I, Latifi T, Hadley TJ, et al. Evolution of a bacterial regulon controlling virulence and Mg(2+) homeostasis. PLoS Genet. 2009;5:e1000428. doi: 10.1371/journal.pgen.1000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez JC, Groisman EA. Transcription factor function and promoter architecture govern the evolution of bacterial regulons. Proc Natl Acad Sci U S A. 2009;106:4319–4324. doi: 10.1073/pnas.0810343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabenstein JP, Fukuto HS, Palmer LE, Bliska JB. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infection and immunity. 2006;74:3727–3741. doi: 10.1128/IAI.00255-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellison DW, Miller VL. Regulation of virulence by members of the MarR/SlyA family. Curr Opin Microbiol. 2006;9:153–159. doi: 10.1016/j.mib.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Cathelyn JS, Crosby SD, Lathem WW, Goldman WE, Miller VL. RovA, a global regulator of Yersinia pestis, specifically required for bubonic plague. Proc Natl Acad Sci U S A. 2006;103:13514–13519. doi: 10.1073/pnas.0603456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dube PH, Handley SA, Revell PA, Miller VL. The rovA mutant of Yersinia enterocolitica displays differential degrees of virulence depending on the route of infection. Infect Immun. 2003;71:3512–3520. doi: 10.1128/IAI.71.6.3512-3520.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagel G, Lahrz A, Dersch P. Environmental control of invasin expression in Yersinia pseudotuberculosis is mediated by regulation of RovA, a transcriptional activator of the SlyA/Hor family. Molecular microbiology. 2001;41:1249–1269. doi: 10.1046/j.1365-2958.2001.02522.x. [DOI] [PubMed] [Google Scholar]
- 15.Revell PA, Miller VL. A chromosomally encoded regulator is required for expression of the Yersinia enterocolitica inv gene and for virulence. Molecular microbiology. 2000;35:677–685. doi: 10.1046/j.1365-2958.2000.01740.x. [DOI] [PubMed] [Google Scholar]
- 16.Ellison DW, Lawrenz MB, Miller VL. Invasin and beyond: regulation of Yersinia virulence by RovA. Trends in microbiology. 2004;12:296–300. doi: 10.1016/j.tim.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Heroven AK, Nagel G, Tran HJ, Parr S, Dersch P. RovA is autoregulated and antagonizes H-NS-mediated silencing of invasin and rovA expression in Yersinia pseudotuberculosis. Molecular microbiology. 2004;53:871–888. doi: 10.1111/j.1365-2958.2004.04162.x. [DOI] [PubMed] [Google Scholar]
- 18.Simonet M, Riot B, Fortineau N, Berche P. Invasin production by Yersinia pestis is abolished by insertion of an IS200-like element within the inv gene. Infect Immun. 1996;64:375–379. doi: 10.1128/iai.64.1.375-379.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang XZ, Lindler LE. The pH 6 antigen is an antiphagocytic factor produced by Yersinia pestis independent of Yersinia outer proteins and capsule antigen. Infect Immun. 2004;72:7212–7219. doi: 10.1128/IAI.72.12.7212-7219.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derbise A, Chenal-Francisque V, Pouillot F, Fayolle C, Prevost MC, et al. A horizontally acquired filamentous phage contributes to the pathogenicity of the plague bacillus. Mol Microbiol. 2007;63:1145–1157. doi: 10.1111/j.1365-2958.2006.05570.x. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Dai E, Cui Y, Li M, Zhang Y, et al. Different region analysis for genotyping Yersinia pestis isolates from China. PLoS ONE. 2008;3:e2166. doi: 10.1371/journal.pone.0002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang F, Ke Y, Tan Y, Bi Y, Shi Q, et al. Cell membrane is impaired, accompanied by enhanced type III secretion system expression in Yersinia pestis deficient in RovA regulator. PLoS One. 2010;5.e12840 doi: 10.1371/journal.pone.0012840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran HJ, Heroven AK, Winkler L, Spreter T, Beatrix B, et al. Analysis of RovA, a transcriptional regulator of Yersinia pseudotuberculosis virulence that acts through antirepression and direct transcriptional activation. J Biol Chem. 2005;280:42423–42432. doi: 10.1074/jbc.M504464200. [DOI] [PubMed] [Google Scholar]
- 24.Zhou D, Tong Z, Song Y, Han Y, Pei D, et al. Genetics of metabolic variations between Yersinia pestis biovars and the proposal of a new biovar, microtus. J Bacteriol. 2004;186:5147–5152. doi: 10.1128/JB.186.15.5147-5152.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhan L, Han Y, Yang L, Geng J, Li Y, et al. The cyclic AMP receptor protein, CRP, is required for both virulence and expression of the minimal CRP regulon in Yersinia pestis biovar microtus. Infect Immun. 2008;76:5028–5037. doi: 10.1128/IAI.00370-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Straley SC, Bowmer WS. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Robh MS, Busby SJ. The Escherichia coli cAMP receptor protein bound at a single target can activate transcription initiation at divergent promoters: a systematic study that exploits new promoter probe plasmids. Biochem J. 2002;368:835–843. doi: 10.1042/BJ20021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chain PS, Carniel E, Larimer FW, Lamerdin J, Stoutland PO, et al. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A. 2004;101:13826–13831. doi: 10.1073/pnas.0404012101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou D, Han Y, Qin L, Chen Z, Qiu J, et al. Transcriptome analysis of the Mg2+-responsive PhoP regulator in Yersinia pestis. FEMS Microbiol Lett. 2005;250:85–95. doi: 10.1016/j.femsle.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 30.Chen PE, Cook C, Stewart AC, Nagarajan N, Sommer DD, et al. Genomic characterization of the Yersinia genus. Genome Biol. 2010;11:R1. doi: 10.1186/gb-2010-11-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achtman M, Zurth K, Morelli G, Torrea G, Guiyoule A, et al. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A. 1999;96:14043–14048. doi: 10.1073/pnas.96.24.14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skurnik M, Peippo A, Ervela E. Characterization of the O-antigen gene clusters of Yersinia pseudotuberculosis and the cryptic O-antigen gene cluster of Yersinia pestis shows that the plague bacillus is most closely related to and has evolved from Y. pseudotuberculosis serotype O:1b. Mol Microbiol. 2000;37:316–330. doi: 10.1046/j.1365-2958.2000.01993.x. [DOI] [PubMed] [Google Scholar]
- 33.Lawrenz MB, Miller VL. Comparative analysis of the regulation of rovA from the pathogenic yersiniae. J Bacteriol. 2007;189:5963–5975. doi: 10.1128/JB.00528-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herbst K, Bujara M, Heroven AK, Opitz W, Weichert M, et al. Intrinsic thermal sensing controls proteolysis of Yersinia virulence regulator RovA. PLoS Pathog. 2009;5:e1000435. doi: 10.1371/journal.ppat.1000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoebel DM, Free A, Dorman CJ. Anti-silencing: overcoming H-NS-mediated repression of transcription in Gram-negative enteric bacteria. Microbiology. 2008;154:2533–2545. doi: 10.1099/mic.0.2008/020693-0. [DOI] [PubMed] [Google Scholar]
- 36.Heroven AK, Dersch P. RovM, a novel LysR-type regulator of the virulence activator gene rovA, controls cell invasion, virulence and motility of Yersinia pseudotuberculosis. Mol Microbiol. 2006;62:1469–1483. doi: 10.1111/j.1365-2958.2006.05458.x. [DOI] [PubMed] [Google Scholar]
- 37.Ishihama A. Functional modulation of Escherichia coli RNA polymerase. Annu Rev Microbiol. 2000;54:499–518. doi: 10.1146/annurev.micro.54.1.499. [DOI] [PubMed] [Google Scholar]
- 38.Heroven AK, Bohme K, Rohde M, Dersch P. A Csr-type regulatory system, including small non-coding RNAs, regulates the global virulence regulator RovA of Yersinia pseudotuberculosis through RovM. Mol Microbiol. 2008;68:1179–1195. doi: 10.1111/j.1365-2958.2008.06218.x. [DOI] [PubMed] [Google Scholar]
- 39.Gao H, Zhou D, Li Y, Guo Z, Han Y, et al. The iron-responsive Fur regulon in Yersinia pestis. J Bacteriol. 2008;190:3063–3075. doi: 10.1128/JB.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Qiu Y, Gao H, Guo Z, Han Y, et al. Characterization of Zur-dependent genes and direct Zur targets in Yersinia pestis. BMC Microbiol. 2009;9:128. doi: 10.1186/1471-2180-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cathelyn JS, Ellison DW, Hinchliffe SJ, Wren BW, Miller VL. The RovA regulons of Yersinia enterocolitica and Yersinia pestis are distinct: evidence that many RovA-regulated genes were acquired more recently than the core genome. Mol Microbiol. 2007;66:189–205. doi: 10.1111/j.1365-2958.2007.05907.x. [DOI] [PubMed] [Google Scholar]
- 42.Song H, Kong W, Weatherspoon N, Qin G, Tyler W, et al. Modulation of the regulatory activity of bacterial two-component systems by SlyA. J Biol Chem. 2008;283:28158–28168. doi: 10.1074/jbc.M801058200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norte VA, Stapleton MR, Green J. PhoP-responsive expression of the Salmonella enterica serovar typhimurium slyA gene. Journal of bacteriology. 2003;185:3508–3514. doi: 10.1128/JB.185.12.3508-3514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi Y, Latifi T, Cromie MJ, Groisman EA. Transcriptional control of the antimicrobial peptide resistance ugtL gene by the Salmonella PhoP and SlyA regulatory proteins. The Journal of biological chemistry. 2004;279:38618–38625. doi: 10.1074/jbc.M406149200. [DOI] [PubMed] [Google Scholar]
- 45.Kong W, Weatherspoon N, Shi Y. Molecular mechanism for establishment of signal-dependent regulation in the PhoP/PhoQ system. J Biol Chem. 2008;283:16612–16621. doi: 10.1074/jbc.M800547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soncini FC, Vescovi EG, Groisman EA. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J Bacteriol. 1995;177:4364–4371. doi: 10.1128/jb.177.15.4364-4371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lejona S, Aguirre A, Cabeza ML, Garcia Vescovi E, Soncini FC. Molecular characterization of the Mg2+-responsive PhoP-PhoQ regulon in Salmonella enterica. J Bacteriol. 2003;185:6287–6294. doi: 10.1128/JB.185.21.6287-6294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer extension assay for validation of non-polar mutation. The rovA or phop null mutant (ΔrovA or Δphop, respectively) was generated from the wild-type strain 201 (WT), and then the corresponding complemented mutant strain (C-rovA or C-phop, respectively) was constructed. As determined by several distinct methods (see the text of manuscript), the P1 promoter of rovA was positively regulated by RovA when the bacteria were grown in the original TMH medium, but negatively controlled by PhoP when grown in the TMH containing 10μM MgCl2. Herein, an oligonucleotide primer, which was complementary to the RNA transcript of rovA, was employed to detect the primer extension product that represented the relative P1 promoter activity in the corresponding strains. The primer extension products were analyzed with 8 M urea−6% acrylamide sequencing gel. Lanes C, T, A, and G represent the Sanger sequencing reactions. Shown on the right side of the image is the transcription start site (nucleotide T, corresponding to the P1 promoter) that was located at 78 bp upstream of rovA. The P1 promoter was significantly repressed in ΔrovA relative to both C-rovA and WT gown in the original TMH; yet, it was significantly enhanced in ΔphoP relative to both C-phoP and WT grown in the TMH containing 10μM MgCl2 The P1 promoter was transcribed at almost the same level in every paired WT and complemented mutant. These results confirmed that the phoP or rovA mutation was nonpolar.
(TIF)