Abstract
Background
The evaluation of intravitreal bevacizumab treatment for delayed radiation maculopathy and papillopathy after irradiation for maxillary sinus cancer.
Case report
A patient with radiation maculopathy and papillopathy was treated with intravitreal bevacizumab (1.25 mg). Main outcome measures included fundus photography, angiography, and optical coherence tomography (OCT). Two weeks after intravitreal bevacizumab, visual acuity improved from 0.4 to 1.2. Fundus examination revealed decreased disc swelling, peripapillary hemorrhage, and macular edema. OCT demonstrated complete resolution of serous retinal detachment. At the 12-month follow-up, there was no exudation recurrence. No ocular or systemic side effects were observed.
Conclusion
Intravitreal bevacizumab can be used to treat radiation maculopathy and papillopathy. Antivascular endothelial growth factor therapy may decrease tissue injury associated with radiation vasculopathy.
Keywords: bevacizumab, radiation, maculopathy, papillopathy
Introduction
Radiation maculopathy and papillopathy are complications after radiotherapy for intracranial, skull base, and paranasal sinus tumors. Macular edema and serous retinal detachment associated with radiation maculopathy may lead to severe visual loss. Although the natural history of radiation papillopathy may be more favorable than previously assumed,1 any cases associated with underlying vascular disorders are more likely to be aggressive2 and there is still no evidence-based treatment for radiation papillopathy. More recently, there have been isolated reports of intravitreal bevacizumab (Avastin®; Genentech Inc, San Francisco, CA) for radiation optic neuropathy or maculopathy secondary to plaque radiotherapy.3–5 Here, a case of delayed radiation maculopathy and papillopathy secondary to irradiation for maxillary sinus cancer that was successfully treated with intravitreal bevacizumab is reported.
Case report
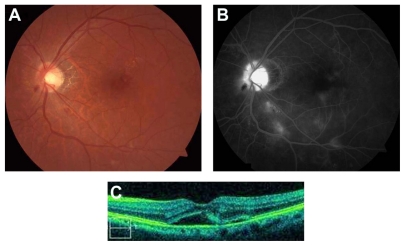
A 73-year-old male presented with acute decrease in vision in the left eye 6 years after irradiation therapy for maxillary sinus cancer. The patient had a history of irradiation with a dose of 50–55 Gy in 1.8–1.9 Gy fractions to the cancer without invasion into eye and orbit. Treatment was performed with an anterior and lateral pair of 45° wedged beams. The patient had no diabetes mellitus or vascular disease. On presentation, his best-corrected visual acuity was 1.2 OD and 0.4 OS. Ophthalmoscopy was normal in the right eye but showed optic disc swelling with peripapillary hemorrhage, retinal exudates, and intraretinal microangiopathy in the left eye (Figure 1A). The corresponding fluorescein angiogram (FA) revealed optic disc and macular edema, capillary nonperfusion, microaneurysms, and vascular leakage (Figure 1B). Optic coherence tomography (OCT) demonstrated serous retinal detachment (SRD) (Figure 1C).
Figure 1.
(A) Before bevacizumab treatment, the color fundus photograph shows optic disc swelling, peripapillary hemorrhage, retinal exudates and microangiopathy. (B) Fluorescein angiography demonstrates optic disc and macular edema, capillary nonperfusion, and microaneurysms. (C) Optical coherence tomography (OCT) reveals serous retinal detachment.
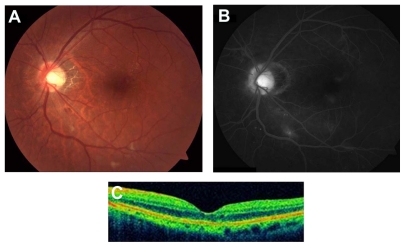
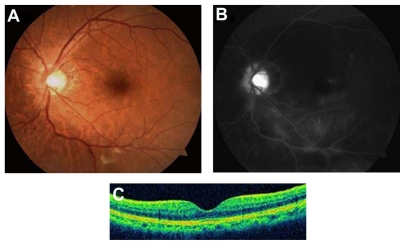
The patient was suspected to have radiation maculopathy and papillopathy in the left eye. Intravitreal bevacizumab (1.25 mg in 0.05 mL) was provided to reduce vascular hyperpermeability and intraretinal neovascularization. Two weeks after the administration, visual acuity improved to 20/20 with resolution of SRD. Two months after administration, a color photograph showed decreased optic disc swelling, hemorrhage, and exudates (Figure 2A). OCT demonstrated complete regression of SRD (Figure 2C). The corresponding FA revealed markedly reduced macular edema, decreased intraretinal microangiopathy, and vascular leakage (Figure 2B). These findings were maintained over 12 months and at the last follow-up (Figure 3A–C).
Figure 2.
(A) Two months after treatment with intravitreal bevacizumab, the color fundus photograph shows a decrease of optic disc swelling, peripapillary hemorrhage, retinal exudates and microangiopathy. (B) Fluorescein angiography demonstrates improvement of optic disc and macular edema, and microaneurysms. (C) Optical coherence tomography (OCT) reveals complete resolution of serous retinal detachment.
Figure 3.
(A) Twelve months after treatment, the color fundus photograph shows a well-defined optic disc margin with resolution of peripapillary hemorrhage. (B) Fluorescein angiography demonstrates persistent decreased microangiopathy and vascular leakage. (C) Optical coherence tomography (OCT) reveals sustained resolution of serous retinal detachment.
Discussion
Radiation-induced retinopathy, maculopathy, and papillopathy are serious complications of head and neck radiotherapy. Takeda et al have suggested that radiation dose and area irradiated are the most important factors in the development of radiation retinopathy.6 The results in that study showed that 8/14 (57%) eyes that received 50 Gy or more, over 60% or more of the area of the retina, developed severe retinal complications. 6 Parsons et al also indicated that, in the dose range 45–50 Gy, 8/15 (53%) eyes studied developed retinopathy and there was an increased risk of injury among patients who received fractional doses of over 1.9 Gy.7 Kim et al reported that 63/93 (67.7%) patients who received 70 cobalt Gy equivalents (CGEs) developed papillopathy a median of 1.5 years after irradiation.1 Demizu et al found that the maximum dose to the optic nerve (>110 GyE3) was significant for the occurrence of radiation-induced optic neuropathy.8 Taking this into consideration, in this case with a dose of 50–55 Gy in 1.8–1.9 Gy fractions over 50% of the area of the retina, radiation damage to the macula and optic nerve might be mild and the clinical findings might support that view.
Intravitreal bevacizumab has been used in the treatment of age-related macular degeneration,9 retinal vein occlusion,10 and diabetic retinopathy11 as it inhibits the formation of abnormal blood vessels and reduces vascular hyperpermeability from vascular cell damage. Hopewell has noted that radiation injury in the central nervous system is a consequence of vascular rather than neural cell damage.12 Radiation papillopathy and maculopathy represent disc swelling, peripapillary hemorrhage, hard exudates, intraretinal edema, subretinal fluid, and capillary nonperfusion by a radiation- damaged microvasculature. Thus, intravitreal bevacizumab may be a reasonable choice for radiation papillopathy and maculopathy, although the severe forms may need more aggressive therapy.
Rapid and sustained resolution of radiation maculopathy and papillopathy by intravitreal bevacizumab may be a consequence of prompt reduction of vascular hyperpermeability and prolonged suppression of retinal neovascularization. A recent study has shown that intravitreous ranibizumab (Lucentis®; Genentech, San Francisco, CA) can be used for radiation maculopathy.13 Because ranibizumab is in the form of smaller molecules with rapid wash-out, the drug may need repeated monthly injections and consequently increase the opportunity for adverse events such as endophthalmitis and retinal detachment. In addition, the costs of ranibizumab are much higher than bevacizumab. Therefore, the longer acting and less expensive bevacizumab therapy may be best for the treatment of radiation vasculopathy, although risk–benefit comparisons are still needed.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kim IK, Lane AM, Egan KM, Munzenrider J, Gragoudas ES. Natural history of radiation papillopathy after proton beam irradiation of parapapillary melanoma. Ophthalmology. 2010;117(8):1617–1622. doi: 10.1016/j.ophtha.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Gragoudas ES, Li W, Lane AM, Munzenrider J, Egan KM. Risk factors for radiation maculopathy and papillopathy after intraocular irradiation. Ophthalmology. 1999;106(8):1571–1577. doi: 10.1016/S0161-6420(99)90455-4. [DOI] [PubMed] [Google Scholar]
- 3.Finger PT. Anti-VEGF bevacizumab (Avastin®) for radiation optic neuropathy. Am J Ophthalmol. 2007;143(2):335–338. doi: 10.1016/j.ajo.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Finger PT, Chin K. Anti-vascular endothelial growth factor bevacizumab (Avastin) for radiation retinopathy. Arch Ophthalmol. 2007;125(6):751–756. doi: 10.1001/archopht.125.6.751. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A, Muecke JS. Treatment of radiation maculopathy with intravitreal injection of bevacizumab (Avastin) Retina. 2008;28(7):964–968. doi: 10.1097/IAE.0b013e3181706302. [DOI] [PubMed] [Google Scholar]
- 6.Takeda A, Shigematsu N, Suzuki S, et al. Late retinal complications of radiation therapy for nasal and paranasal malignancies: relationship between irradiated-dose area and severity. Int J Radiat Oncol Biol Phys. 1999;44(3):599–605. doi: 10.1016/s0360-3016(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 7.Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Radiation retinopathy after external-beam irradiation: Analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30(4):765–773. doi: 10.1016/0360-3016(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 8.Demizu Y, Murakami M, Miyawaki D, et al. Analysis of vision loss caused by radiation-induced optic neuropathy after particle therapy for head-and-neck and skull-base tumors adjacent to optic nerves. Int J Radiat Oncol Biol Phys. 2009;75(5):1487–1492. doi: 10.1016/j.ijrobp.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 9.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113(3):363–372. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Iturralde D, Spaide RF, Meyerle CB, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: a short-term study. Retina. 2006;26(3):279–284. doi: 10.1097/00006982-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006;26(9):999–1005. doi: 10.1097/01.iae.0000247165.38655.bf. [DOI] [PubMed] [Google Scholar]
- 12.Hopewell JW. Radiation injury to the central nervous system. Med Pediatr Oncol. 1998;(Suppl 1):1–9. doi: 10.1002/(sici)1096-911x(1998)30:1+<1::aid-mpo1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.Finger PT, Chin KJ. Intravitreous ranibizumab (Lucentis) for radiation maculopathy. Arch Ophthalmol. 2010;128(2):249–252. doi: 10.1001/archophthalmol.2009.376. [DOI] [PubMed] [Google Scholar]