Abstract
Objectives
The aim of the study was to examine the additive effect of resistance training (RT) to a dietary education (DE) intervention on emerging coronary heart disease (CHD) risk factors, concentration of apolipoproteins B (apoB) and A-I (apoA-I), and Dietary Approaches to Stop Hypertension (DASH) Diet Index scores in overweight and obese older adults.
Patients and methods
This was an ancillary study of a randomized clinical trial held in the Fall of 2008 at the University of Rhode Island. Participants were overweight or obese subjects (mean body mass index [BMI] of 31.7 kg/m2) randomized into two groups, one participating in DE only (n = 12) and the other participating in DE plus RT (DERT) (n = 15). The intervention involved all subjects participating in 30 minutes of DE per week for 10 weeks. Subjects in the DERT group participated in an additional 40 minutes of RT three times per week for 10 weeks. Measurements taken were anthropometric (height, weight, waist circumference, and body composition using the BOD POD® [Body Composition System, v 2.14; Life Measurement Instruments, Concord, CA]), clinical (blood pressure), and biochemical (lipid profile and apoB and apoA-I concentrations), and the DASH Diet Index was used to measure diet quality.
Results
27 subjects (11 males, 16 females), with a mean age of 66.6 ± 4.3 years, were included in analyses. The DERT subjects had significantly better triacylglycerol and apoB concentrations and DASH Diet Index scores than the DE subjects post-intervention. Improvements were seen within the DE group in energy intake, fat-free mass, and systolic blood pressure and within the DERT group in body weight, percentage of body fat, BMI, diastolic blood pressure, and oxidized low-density lipoprotein (all P < 0.05).
Conclusion
The addition of RT effectively reduced CHD risk factors, body composition, and diet quality in overweight and obese older adults; DERT was more effective than DE alone in improving DASH Diet Index scores and lowering apoB concentrations but was not more effective in increasing apoA-I concentrations. Future research is needed to determine if apolipoproteins are superior to lipoprotein cholesterol concentrations in predicting CHD risk.
Keywords: physical activity, health education, lipids, DASH Diet Index
Introduction
Coronary heart disease (CHD) is the leading cause of death in the United States.1 Over 80% of CHD-related deaths occur in older adults (≥ 60 years old).2,3 Obesity-related dyslipidemia contributes to CHD and it is important to target as 78.4% of older men and 68.6% of older women are overweight.1,4 Dyslipidemia or elevated total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triacylglycerol (TAG), and/or decreased high-density lipoprotein cholesterol (HDL-C) levels are traditional CHD risk factors.4 Currently, LDL-C is the primary lipid measured to determine when to initiate lifestyle and/or pharmaceutical interventions.5,6 However, traditional measures fail to identify dyslipidemia contributing to CHD in approximately 22% of those with recommended LDL-C levels.7
New research suggests apolipoproteins B (apoB) and A-I (apoA-I), the primary apolipoproteins of LDL and HDL, respectively, may reveal CHD risk earlier than traditional measures.7,8 There is one apoB per very low-density lipoprotein (VLDL) and LDL particle in circulation.9,10 An increase or decrease in the number of LDL particles can be determined by apoB and this determines a different CHD risk than cholesterol concentrations alone.11 ApoA-I is unique to HDL particles. One of its primary roles is to activate lecithin-cholesterol acyltransferase, which esterifies free cholesterol released from non-hepatic tissue. Cholesterol removed from circulation via transport by HDL particles to the liver is utilized in bile acid production, leading to attenuated progression of atherosclerosis.5,12
The Dietary Approaches to Stop Hypertension (DASH) diet, which is high in fruits, vegetables, low-fat dairy, whole grains, nuts and fish, and low in total fat, cholesterol, and red meat,13,14 decreases TC and LDL-C levels.15 Compliance with the DASH diet and diet quality can be measured via the DASH Diet Index.13 Since the DASH diet reduces traditional CHD risk factors, it is essential to determine if it results in similar improvements in emerging risk factors such as elevated apoB concentrations, low apoA-I concentrations, as well as with other lifestyle modifications such as physical activity.16,17
While studies have shown that the combination of exercise and diet may have beneficial effects on health-related outcomes such as improvements in HDL-C and TAG levels, retention of muscle mass, and strength,18 no studies have examined the additive effect of resistance training (RT) to dietary education (DE) programs on emerging risk factors such as elevated apoB concentrations, low apoA-I concentrations and diet quality in overweight and obese older adults. Previous research has shown improvements in TC, TAG, and LDL-C levels19,20 with combined programs; however, this was not specific to RT. The addition of RT and DASH diet adherence may positively affect traditional and emerging risk factors. The primary aim of this study was to examine the additive effect of RT to a DE intervention (DERT) compared with DE alone on apoB and apoA-I concentrations, and the secondary aim was to examine the effect of the same intervention on diet quality.
Material and methods
Study design
This ancillary study was based on a 10-week randomized clinical intervention examining the additive effects of DERT on emerging CHD risk factors and diet quality in 31 overweight and obese older adults. The primary study examined differences in physical functioning within and between DE and DERT.21 Primary dependent variables in this ancillary study were apoB and apoA-I; the secondary dependent variable was the DASH Diet Index score.
Subjects
After study procedures were explained and medical clearance was obtained, subjects read and signed an informed consent form that was approved by the Institutional Review Board of the University of Rhode Island. Inclusion criteria were as follows: (1) aged between 60 and 75 years; (2) not engaged in a regular exercise program (within the past 6 months); (3) body mass index (BMI) of 25–39.9 kg/m2; (4) stable weight (within 5%) for the past 3 months; (5) ability to safely engage in moderate exercise; and (6) medical clearance. Subjects were excluded if lipid-lowering medications were started ≤6 months prior to the study, if any other medications were started ≤3 weeks prior to the study, or if medications changed during the study.
Measurements
Anthropometric
Height was measured to the nearest 0.5 cm using a stadiometer (SECA 220; Seca North America East, Hanover, MD). Weight was measured to the nearest 0.1 kg using a digital read scale (part of the BIOPOD® system; Body Composition System; Life Measurement Instruments, Concord, CA). BMI was calculated in kg/m2. Body composition was measured using an air-displacement plethysmograph (part of the BIOPOD system).
Biochemical
A phlebotomist collected 12-hour fasting venous blood samples on 2 nonconsecutive days in 1 week. Briefly, whole blood was centrifuged (Eppendorf Centrifuge 5810R; North America, Inc, Westbury, NY) at 1500 × g for 20 minutes at 4°C to obtain plasma.22 A preservation cocktail was added and samples were aliquoted into separate labeled microcentrifuge tubes and stored at −80°C.
Concentrations of TC and TAG were determined enzymatically (Roche, Indianapolis, IN).23 Concentrations of HDL-C were measured with Roche Diagnostics-USA kits (Roche, Indianapolis, IN) after precipitation of apoB-containing lipoproteins using a dextran sulfate and magnesium chloride solution (Acros Organics, Morris Plains, NJ).24 Concentrations of LDL-C were determined by the Friedewald equation.25 Oxidized LDL (oxLDL) was determined using enzyme-linked immunosorbent assay kits (oxidized LDL assays kits; ALPCO, Salem, NH). Apolipoprotein concentrations were determined by immunoturbidimetric assays (Diazyme Apo A-1 and Apo B assays; Diazyme, Poway, CA).
Clinical
Blood pressure was measured after subjects were in a quiet, seated position for ≥5 minutes, using a standard aneroid sphygmomanometer (American Diagnostic Corporation, Hauppauge, NY). Resting blood pressure was taken twice and averaged.26
Questionnaires
Subjects completed a medical history, the Yale Physical Activity Survey (YPAS), and a food frequency questionnaire (FFQ). The YPAS is a validated measure of subjects’ physical activity levels.27 The FFQ, developed by the Nutrition Assessment Shared Resource of the Fred Hutchinson Cancer Research Center, assessed the previous month’s dietary intake and had multiple parts. The first part asked about food preparation and the types of fats consumed. The second part contained questions regarding usual frequency and amount of intake of 120 food items and beverages. The last three questions asked about the usual intake of fruits, vegetables, and added fats.
Diet quality
From the FFQ, diet quality was assessed using the DASH Diet Index.13 A value of 0.0, 0.5, or 1.0 was given for not meeting, almost meeting, or meeting each of the eleven components, respectively (Table 1). The maximum DASH Diet Index score is 11 and indicates total concordance with the DASH guidelines.13 Output from the FFQ gave frequencies and serving sizes of each item on the questionnaire. Foods were divided into eleven groups representing the eleven components, the servings were totaled, and servings per week or servings per day were calculated. Percentage of fat, percentage of saturated fat, and sodium (mg) did not require determinations of servings. All eleven components were then scored and totaled to calculate DASH Diet Index scores.
Table 1.
DASH Diet Index
| DASH variable | 0-point equivalent | 0.5-point equivalent | 1-point equivalent |
|---|---|---|---|
| Total grains (svgs/day) | <5 | 5–6 | ≥7 |
| Whole grains (svgs/day) | <1 | 1 | ≥2 |
| Vegetables (svgs/day) | <2 | 2–3 | ≥4 |
| Fruits (svgs/day) | <2 | 2–3 | ≥4 |
| Dairy foods (svgs/day) | <1 | 1 | ≥2 |
| Meats, poultry, fish (svgs/day) | >4 | 3 | ≤2 |
| Nuts, seeds, legumes (svgs/wk) | <2 | 2–3 | ≥4 |
| Fat (% kcal) | ≥33 | 31–32 | ≤30 |
| Saturated fat (% kcal) | ≥13 | 11–12 | ≤10 |
| Sweets (svgs/wk) | >8 | 6–7 | ≤5 |
| Sodium (mg) | >2401 | 1501–2400 | ≤1500 |
Note: DASH Diet Index scores range from 0 to 11.
Abbreviations: DASH, Dietary Approaches to Stop Hypertension; svgs, servings; wk, week.
Randomization
All subjects were randomly assigned to either the DE or the DERT group after baseline testing. After beginning the study, three subjects dropped out of the DE group; reasons for this were (1) dissatisfaction with the group assignment; (2) a health-related problem unrelated to study; and (3) subject unable to be contacted for follow-up testing. One subject in the DERT group was excluded from analyses due to a change in blood pressure medication. Twenty-seven subjects were included in the final analyses. DASH Diet Index scores are only presented on 25 subjects (DE, n = 11; DERT, n = 14) because two subjects did not complete the post-intervention FFQ. All measurements described were obtained at baseline and post-intervention.
DE intervention
All subjects participated in 30 minutes of DASH-based DE per week. A registered dietitian led the classes; session topics included an introduction to the DASH diet, interpreting nutrition labels, recognizing whole grains, and self-monitoring. The total fat goal was modified to ≤35% from the original goal of ≤27%; this was done to promote unsaturated fatty acids, due to their cardioprotective effects.28 Subjects were encouraged to engage in 30 minutes of physical activity on most days, as recommended by the American College of Sports Medicine29 and measured by the YPAS pre- and post-intervention. Each subject was given a personalized diet based on their basal metabolic rate (BMR) calculated by the Harris-Benedict equation.30 The BMR was multiplied by an activity factor of 1.2 for a sedentary lifestyle and was reduced by 10% to promote weight loss.21
RT intervention
The DERT group participated in both DE and RT sessions. The RT sessions were 40-minute supervised sessions on 3 nonconsecutive days per week. Six lower and upper body exercises, along with age-appropriate stretching, were performed at each session on Cybex® machines (Cybex International, Inc, Medway, MA) with each subject. Lower body exercises were the leg press, leg curl, and knee extension; Upper body exercises were the chest press, back row, and shoulder press. Each subject completed four sets of 8–12 repetitions and resistance was increased gradually over time. A team member monitoring each subject’s ability to maintain proper form and to complete 8–12 repetitions made adjustments to the resistance for each exercise on an individual basis. When a subject could complete more than 12 repetitions with proper form on a given exercise the resistance was typically increased in either the following set or the next session in order to have a repetition range of 8–12 repetitions with near-maximal effort. Additionally, given the population under study and that there were six exercises performed during the RT, the authors felt it would have been risky and an excessive testing burden on the subjects to perform one-repetition-maximum tests on all of the machines; thus, subjects were tested on a separate pneumatic leg press machine for assessing changes in lower body strength.
Previously the authors reported21 that the DERT group increased their strength significantly and to a greater extent than those in the DE group. The 10-week duration was chosen as many physiologic outcomes have been shown to improve during this duration of intervention.31–33 Additionally, significant weight loss has previously been achieved in older adults with and without RT over 10 weeks or less.34,35
Statistical analysis
Data were analyzed using SPSS software (v 19.0 for Windows; SPSS Inc, Chicago, IL). When examining differences within groups, time served as the independent variable; when examining differences between groups, group served as the independent variable. Descriptive statistics were performed to assess normality. For data that were not normally distributed, outliers were removed or appropriate transformations were made. Continuous variables were expressed as mean ± standard deviation and categorical variables were expressed as frequencies. Independent samples t-tests were conducted to determine if there were significant differences between groups at baseline and post-intervention. Mixed between-within subjects analysis of variance (ANOVA) was conducted to examine if there were significant differences between groups over time. Partial eta squared values were obtained from the ANOVA test in order to determine the effect size of the interventions and were defined as 0.01 (small), 0.06 (medium), and 0.14 (large).36 Paired samples t-tests were conducted to determine differences within groups, from baseline to post-intervention. Statistical significance for all analyses was set at P < 0.05.
Results
The majority of subjects were female (59.2%) and obese (77.7%), and all identified themselves as white (Table 2). Results did not differ between overweight and obese subjects. Just over 25% of subjects were taking lipid-lowering medication (statin or fibrate) and 14.8% were taking antihypertensive medication. Using a chi-square analysis, there were no significant differences between the DE and DERT groups in the number of subjects taking lipid-lowering or antihypertensive medication.
Table 2.
Baseline descriptive data of all subjects
| Characteristics | Subjects (n) |
|---|---|
| Gender (M/F) | 11/16 |
| BMI (overweight/obese) | 6/21 |
| Medications | |
| Lipid-lowering | 7 |
| Blood pressure | 4 |
| Mean ± SD | |
| Age (years) | 66.6 ± 4.3 |
| Diet composition | |
| Calories | 1932.3 ± 1015.4 |
| Carbohydrate (%) | 51.0 ± 8.6 |
| Protein (%) | 17.1 ± 3.3 |
| Fat (%) | 31.1 ± 7.7 |
Notes: Overweight = BMI ≤ 29.9 kg/m2; obese = BMI ≥ 30 kg/m2.
Abbreviations: BMI, body mass index; F, female; M, male; SD, standard deviation.
Results for clinical and biochemical data are presented in Table 3. There were no significant differences between groups in all variables at baseline and no significant interaction between the interventions and time. The DERT subjects had lower apoB and TAG (P < 0.05) concentrations post-intervention than the DE subjects. The DERT group decreased oxLDL levels over time (P < 0.05). Only DE decreased systolic blood pressure and only DERT decreased diastolic blood pressure (both P < 0.05).
Table 3.
Clinical and biochemical data
| Clinical | DE (n = 12)
|
DERT (n = 15)
|
Effect size | ||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| Systolic BP (mmHg) | 135.0 ± 13.0 | 122.0 ± 17.0¥ | 130.0 ± 13.5 | 123.0 ± 7.1 | 0.055 |
| Diastolic BP (mmHg) | 79.0 ± 6.0 | 78.0 ± 4.6 | 78.0 ± 6.0 | 75.0 ± 5.4¥ | 0.034 |
| Biochemical | |||||
| Glucose (mg/dL)a | 104.1 ± 8.6 | 102.7 ± 12.0 | 99.9 ± 18.6 | 99.7 ± 18.1 | 0.002 |
| TC (mg/dL) | 199.1 ± 36.5 | 201.2 ± 35.6 | 176.7 ± 30.2 | 173.6 ± 36.6 | 0.018 |
| TAG (mg/dL)b | 140.9 ± 80.5 | 145.2 ± 83.4* | 97.7 ± 32.1 | 89.2 ± 32.9* | 0.040 |
| HDL-C (mg/dL)b | 56.4 ± 16.7 | 55.7 ± 17.5 | 55.3 ± 15.1 | 54.6 ± 16.6 | <0.001 |
| LDL-C (mg/dL) | 114.4 ± 26.0 | 116.5 ± 29.1 | 101.8 ± 29.7 | 101.1 ± 35.2 | 0.009 |
| oxLDL (ng/mL) | 394.6 ± 130.7 | 332.9 ± 87.3 | 474.6 ± 111.8 | 413.9 ± 124.4¥ | <0.001 |
| apoB (mg/dL) | 133.6 ± 19.6 | 131.7 ± 16.5* | 119.5 ± 23.9 | 114.6 ± 16.2* | 0.007 |
| apoA-I (mg/dL) | 187.3 ± 12.4 | 190.1 ± 16.3 | 188.2 ± 11.3 | 184.6 ± 16.8 | 0.056 |
Notes: Pre and post values expressed as mean ± standard deviation.
Removed outlier (1) and log transformed for analyses;
log transformed for analyses;
difference between groups was P < 0.05.
Difference within groups was P < 0.05. Effect size expressed as partial eta squared (0.01 = small effect, 0.06 = moderate effect, 0.14 = large effect).
Abbreviations: apoB, apolipoprotein B; apoA-I, apolipoprotein A-I; BP, blood pressure; DE, dietary education; DERT, dietary education plus resistance training; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; oxLDL, oxidized low-density lipoprotein; TAG, triacylglycerol; TC, total cholesterol.
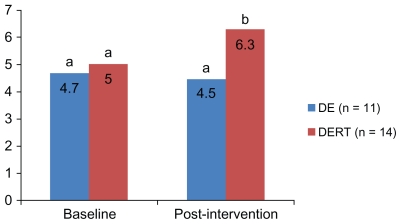
Figure 1 displays results for total DASH Diet Index scores at baseline and post-intervention. At baseline there were no significant differences between groups in total scores. There was a difference between groups post-intervention (P < 0.05).
Figure 1.
Dietary Approaches to Stop Hypertension Diet Index scores between groups at baseline and post-intervention.
Note: Different letters at one time point represent a significant difference between groups (P < 0.01).
Abbreviations: DE, dietary education; DERT, dietary education plus resistance training.
Additionally, energy intake and body composition were examined (Table 4). The DE group decreased total energy intake and fat-free mass (P < 0.05). The DERT group decreased body weight, percentage of body fat, BMI, and fat mass (P < 0.05). Although no differences were observed between groups in percentage of body fat, fat mass, and fat-free mass, effect sizes for the time by group interaction for these variables suggested a large effect of the interventions (effect sizes: 0.369, 0.267, and 0.342, respectively).
Table 4.
Energy balance and body composition
| Energy | DE (n = 12)
|
DERT (n = 15)
|
Effect size | ||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| Intake (kcals/day) | 1856.7 ± 614.8 | 1530.0 ± 351.8¥ | 1691.8 ± 554.8 | 1641.4 ± 605.4 | 0.088 |
| Expenditure (kcals/week) | 6620.0 ± 3880.0 | 11,658.0 ± 9567.0 | 6158.0 ± 2948.0 | 7935.0 ± 5009.0 | 0.070 |
| Body composition | |||||
| Weight (kg) | 87.2 ± 13.5 | 85.5 ± 13.7 | 87.1 ± 14.5 | 83.8 ± 12.6¥ | 0.075 |
| Body fat (%) | 40.2 ± 6.9 | 40.7 ± 6.4 | 41.4 ± 8.1 | 38.3 ± 9.1¥ | 0.369 |
| BMI (kg/m2) | 31.9 ± 3.3 | 31.3 ± 3.3 | 31.5 ± 3.8 | 30.4 ± 3.4¥ | 0.067 |
| Fat mass (kg) | 35.3 ± 9.2 | 35.1 ± 8.7 | 36.1 ± 8.9 | 32.0 ± 8.3¥ | 0.267 |
| Fat-free mass (kg) | 51.9 ± 9.0 | 50.5 ± 8.8¥ | 51.0 ± 11.3 | 51.8 ± 11.8 | 0.342 |
Notes: Pre and post values expressed as mean ± standard deviation.
Difference within groups was P < 0.05. Effect size expressed as partial eta squared (0.01 = small effect, 0.06 = moderate effect, 0.14 = large effect).
Abbreviations: BMI, body mass index; DE, dietary education; DERT, dietary education plus resistance training.
Discussion
This study is novel because it is the first to examine the additive effect of RT to a DE intervention on apoB and apoA-I concentrations and diet quality. Results for the aims of this study indicate improvements in apoB concentrations and diet quality with DERT while, unexpectedly, no differences were observed in apoA-I concentrations. Additionally, there were improvements in body composition with RT.
Although no differences were seen in lipoprotein cholesterol concentrations, differences were seen in apoB concentrations between groups post-intervention and in oxLDL levels over time with DERT, suggesting other components of lipoproteins may change besides cholesterol. Although not statistically significant, the decreases seen in oxLDL and apoB concentrations might have been due to the higher percentage of subjects meeting saturated fat (SFA) (≤10%) guidelines with the DASH Diet Index (data not shown). These findings are consistent with previous findings,37 reporting that subjects who consumed low-saturated-fat diets were less susceptible to LDL and apoB oxidation (P < 0.05). Altering total fat intake may extend beyond LDL-C only, including changes in particle number and size that may further decrease CHD risk. Unexpectedly, there was no change in apoA-I concentrations with DERT. A previous study38 reported that a 4%–5% decrease in body weight would be necessary to see significant changes in apoA-I concentrations. In this study, the DE group decreased their body weight by 1.9% and the DERT group decreased theirs by 3.7% over 10 weeks. DASH Diet Index scores differed between groups post-intervention. The addition of individualized, structured RT to DE might have provided additional social interaction and motivation that might have increased the DERT subjects’ self-efficacy for making changes. However, this study did not directly measure self-efficacy.
In addition to the primary and secondary aims, DERT resulted in improvements in overall body composition, evidenced by the decrease in BMI, percentage of body fat, and fat mass with DERT. The DE group decreased fat-free mass, suggesting that RT can positively influence body fat while preserving overall fat-free mass.
While some exercise modalities have been thoroughly explored in relation to CHD risk,16,17 the effects of RT on CHD risk has yet to be fully investigated. Compared with a control group, a RT group (mean age 73 ± 3 years) achieved lower LDL-C and TC after 10 weeks.18 Additionally, in Fahlman et al,18 the RT group demonstrated similar improvements in HDL-C and TAG levels compared with a group participating in aerobic training (AT), suggesting that RT may be as beneficial as AT in reducing risk. The additive effect of RT to DE has yet to be thoroughly explored as well. However, the decrease in TAG seen in this study is consistent with previous combined diet and exercise wellness programs in older adults.19,20
Despite the novel findings, there are some limitations. The participants were all Caucasian and therefore the data is not generalizable to all races. The sample size was small and the primary outcome that this research was powered on was to achieve a difference in functionality.21 To see significance in differences in the apoA-I and apoB concentrations, a total of 48 subjects would have been needed, but the primary study’s sample size was powered on expected differences in physical functioning.21 However, effect sizes are provided, and in the future, if a larger sample size were to be utilized, significant differences between groups in both apolipoproteins as well as other variables may be found. Also, the control group in this study represented a usual care group. In the future, a group receiving only RT and a strict control group would be beneficial. In addition, all dietary data were collected through self-report, which can lead to over-reporting of food intake. The FFQ requires subjects to estimate food portion sizes, which may be difficult if a person is not familiar with food measurements. Additional exercise outside the RT sessions was not monitored. It may be useful to have subjects utilize pedometers or accelerometers in the future. Lastly, a longer study or follow-up assessment may be useful to determine if this intervention has a prolonged effect and can truly initiate this change in lifestyle.
Despite these limitations, this research had several strengths. First, the DASH diet has reduced traditional CHD risk factors in previous studies, making it reasonable to hypothesize that it may also reduce emerging risk factors.15 Second, the DE classes were relatively small (2–6 subjects) which created a supportive learning environment, encouraged class discussions, and allowed more personalized diet information. Third, all RT sessions were one-on-one and with the same staff member each time, allowing individualized training sessions, high comfort level with study staff, and safe and effective increases in resistance. Lastly, subject attendance of both the DE and the RT sessions was high. Subjects in the DE group attended 85% of DE sessions, while DERT subjects attended 98% of DE sessions and 96% of RT sessions. As previous research has shown, better adherence is associated with better overall outcomes, and is a significant predictor of weight loss.39
Conclusion
The results of this ancillary study indicate that the addition of RT to a DE intervention is effective in reducing traditional and emerging CHD risk factors, diet quality, and body composition in white overweight and obese older adults. Although DERT was not more effective than DE at raising apoA-I concentrations, those participating in DERT had significantly lower apoB concentrations, DASH Diet Index scores, and TAG levels than those participating in DE alone. It may be useful to measure self-efficacy in future studies. Further research is needed with larger sample sizes to determine if this type of combined intervention is the most effective way to improve overall health and other lipoprotein components besides cholesterol concentrations.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM. Approach to lipoprotein management in 2001 National Cholesterol Guidelines. Am J Cardiol. 2002;90(8A):11i–21i. doi: 10.1016/s0002-9149(02)02631-0. [DOI] [PubMed] [Google Scholar]
- 3.Stanner S. Diet and lifestyle measures to protect the ageing heart. Br J Community Nurs. 2009;14:210–212. doi: 10.12968/bjcn.2009.14.5.42080. [DOI] [PubMed] [Google Scholar]
- 4.Hyre AD, Muntner P, Menke A, Raggi P, He J. Trends in ATP-III-defined high blood cholesterol prevalence, awareness, treatment and control among US adults. Ann Epidemiol. 2007;17(7):548–555. doi: 10.1016/j.annepidem.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Stipanuk M. Biochemical, Physiological, and Molecular Aspects of Human Nutrition. 2nd ed. Philadelphia (PA): Elsevier; 2006. [Google Scholar]
- 6.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 7.Superko HR, King S. Lipid management to reduce cardiovascular risk: a new strategy is required. Circulation. 2008;117(4):560–568. doi: 10.1161/CIRCULATIONAHA.106.667428. [DOI] [PubMed] [Google Scholar]
- 8.Superko HR. Advanced lipoprotein testing and subfractionation are clinically useful. Circulation. 2009;119(17):2383–2395. doi: 10.1161/CIRCULATIONAHA.108.809582. [DOI] [PubMed] [Google Scholar]
- 9.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358(9298):2026–2033. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 10.Sharrett AR, Ballantyne CM, Coady SA, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104(10):1108–1113. doi: 10.1161/hc3501.095214. [DOI] [PubMed] [Google Scholar]
- 11.Otvos J. Why cholesterol measurements may be misleading about lipoprotein levels and cardiovascular disease risk: clinical implications of lipoprotein quantification using NMR spectroscopy. J Lab Med. 2002;26:544–550. [Google Scholar]
- 12.Marcovina S, Packard CJ. Measurement and meaning of apolipoprotein AI and apolipoprotein B plasma levels. J Intern Med. 2006;259(5):437–446. doi: 10.1111/j.1365-2796.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 13.Folsom AR, Parker ED, Harnack LJ. Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. Am J Hypertens. 2007;20(3):225–232. doi: 10.1016/j.amjhyper.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harsha DW, Sacks FM, Obarzanek E, et al. Effect of dietary sodium intake on blood lipids: results from the DASH-sodium trial. Hypertension. 2004;43(2):393–398. doi: 10.1161/01.HYP.0000113046.83819.a2. [DOI] [PubMed] [Google Scholar]
- 15.Obarzanek E, Sacks FM, Vollmer WM, et al. Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) trial. Am J Clin Nutr. 2001;74(1):80–89. doi: 10.1093/ajcn/74.1.80. [DOI] [PubMed] [Google Scholar]
- 16.Holme I, Hostmark AT, Anderssen SA. ApoB but not LDL-cholesterol is reduced by exercise training in overweight healthy men: results from the 1-year randomized Oslo Diet and Exercise Study. J Intern Med. 2007;262(2):235–243. doi: 10.1111/j.1365-2796.2007.01806.x. [DOI] [PubMed] [Google Scholar]
- 17.Tani S, Nagao K, Anazawa T, et al. Coronary plaque regression and lifestyle modification in patients treated with pravastatin: assessment mainly by daily aerobic exercise and an increase in the serum level of high-density lipoprotein cholesterol. Circ J. 2010;74(5):954–961. doi: 10.1253/circj.cj-09-0705. [DOI] [PubMed] [Google Scholar]
- 18.Fahlman MM, Boardley D, Lambert CP, Flynn MG. Effects of endurance training and resistance training on plasma lipoprotein profiles in elderly women. J Gerontol A Biol Sci Med Sci. 2002;57(2):B54–60. doi: 10.1093/gerona/57.2.b54. [DOI] [PubMed] [Google Scholar]
- 19.White K, Jacques PH. Combined diet and exercise intervention in the workplace: effect on cardiovascular disease risk factors. AAOHN J. 2007;55(3):109–114. doi: 10.1177/216507990705500303. [DOI] [PubMed] [Google Scholar]
- 20.Villareal DT, Miller BV, Banks M, Fontana L, Sinacore DR, Klein S. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr. 2006;84(6):1317–1323. doi: 10.1093/ajcn/84.6.1317. [DOI] [PubMed] [Google Scholar]
- 21.Avila JJ, Gutierres JA, Sheehy ME, Lofgren IE, Delmonico MJ. Effect of moderate intensity resistance training during weight loss on body composition and physical performance in overweight older adults. Eur J Appl Physiol. 2010;109(3):517–525. doi: 10.1007/s00421-010-1387-9. [DOI] [PubMed] [Google Scholar]
- 22.Lofgren I, Zern T, Herron K, et al. Weight loss associated with reduced intake of carbohydrate reduces the atherogenicity of LDL in premenopausal women. Metabolism. 2005;54(9):1133–1141. doi: 10.1016/j.metabol.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20(4):470–475. [PubMed] [Google Scholar]
- 24.Warnick GR, Nauck M, Rifai N. Evolution of methods for measurement of HDL-cholesterol: from ultracentrifugation to homogeneous assays. Clin Chem. 2001;47(9):1579–1596. [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 26.Delmonico MJ, Ferrell RE, Meerasahib A, et al. Blood pressure response to strength training may be influenced by angiotensinogen A-20C and angiotensin II type I receptor A1166C genotypes in older men and women. J Am Geriatr Soc. 2005;53(2):204–210. doi: 10.1111/j.1532-5415.2005.53104.x. [DOI] [PubMed] [Google Scholar]
- 27.Moore DS, Ellis R, Allen PD, et al. Construct validation of physical activity surveys in culturally diverse older adults: a comparison of four commonly used questionnaires. Res Q Exerc Sport. 2008;79(1):42–50. doi: 10.1080/02701367.2008.10599459. [DOI] [PubMed] [Google Scholar]
- 28.Kris-Etherton PM for American Heart Association Nutrition Committee. AHA Science Advisory: monounsaturated fatty acids and risk of cardiovascular disease. Circulation. 1999;100(11):1253–1258. doi: 10.1161/01.cir.100.11.1253. [DOI] [PubMed] [Google Scholar]
- 29.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine position stand: appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 30.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A. 1918;4(12):370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delmonico MJ, Kostek MC, Doldo NA, et al. Effects of moderate-velocity strength training on peak muscle power and movement velocity: do women respond differently than men? J Appl Physiol. 2005;99(5):1712–1718. doi: 10.1152/japplphysiol.01204.2004. [DOI] [PubMed] [Google Scholar]
- 32.Bottaro M, Machado SN, Nogueira W, Scales R, Veloso J. Effect of high versus low-velocity resistance training on muscular fitness and functional performance in older men. Eur J Appl Physiol. 2007;99(3):257–264. doi: 10.1007/s00421-006-0343-1. [DOI] [PubMed] [Google Scholar]
- 33.Manini TM, Clark BC, Tracy BL, Burke J, Ploutz-Snyder L. Resistance and functional training reduces knee extensor position fluctuations in functionally limited older adults. Eur J Appl Physiol. 2005;95:5–6. 436–446. doi: 10.1007/s00421-005-0048-x. [DOI] [PubMed] [Google Scholar]
- 34.Joseph LJ, Trappe TA, Farrell PA, et al. Short-term moderate weight loss and resistance training do not affect insulin-stimulated glucose disposal in postmenopausal women. Diabetes Care. 2001;24(11):1863–1869. doi: 10.2337/diacare.24.11.1863. [DOI] [PubMed] [Google Scholar]
- 35.Arguin H, Bouchard DR, Labonte M, et al. Correlation between the rate of weight loss and changes in body composition in obese postmenopausal women after 5 weeks: a pilot study. Appl Physiol Nutr Metab. 2008;33(2):347–355. doi: 10.1139/H08-004. [DOI] [PubMed] [Google Scholar]
- 36.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 37.Yu-Poth S, Etherton TD, Reddy CC, et al. Lowering dietary saturated fat and total fat reduces the oxidative susceptibility of LDL in healthy men and women. J Nutr. 2000;130(9):2228–2237. doi: 10.1093/jn/130.9.2228. [DOI] [PubMed] [Google Scholar]
- 38.Kralova Lesna I, Suchanek P, Kovar J, Poledne R. Life style change and reverse cholesterol transport in obese women. Physiol Res. 2009;58(Suppl 1):S33–38. doi: 10.33549/physiolres.931856. [DOI] [PubMed] [Google Scholar]
- 39.Hollis JF, Gullion CM, Stevens VJ, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35(2):118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]