Abstract
Purpose
Recent reports indicate that refractory central nervous system (CNS) metastases of non-small cell lung cancer (NSCLC) are improved by high-dose gefitinib or erlotinib administration. We describe a Japanese woman with NSCLC and CNS metastases who was resistant to 75 mg daily erlotinib, but the metastases were improved by 150 mg daily erlotinib. We investigated the plasma and CSF concentrations of erlotinib at each dose as well as the correlation between the plasma and CSF concentrations of erlotinib.
Methods
Including this patient, we administered 150 mg erlotinib daily to nine NSCLC patients with CNS metastases and measured the plasma and CSF concentrations just before administration on day 8. The concentrations were determined using high-performance liquid chromatography with ultraviolet detection.
Results
The plasma and CSF concentrations of erlotinib at a dose of 75 mg were 433 and 14 nM, respectively. The plasma and CSF concentrations of erlotinib at a dose of 150 mg were increased to 1,117 and 44 nM, respectively. The mean ± standard deviation of CSF concentrations and penetration rates were 106 ± 59 nM and 4.5 ± 1.5%, respectively. There was a good correlation (R 2 = 0.84) between plasma and CSF concentrations (P = 0.0005).
Conclusions
This study indicates that CSF concentrations of erlotinib depend on its plasma concentration. As seen in this patient, high CSF concentrations of erlotinib can be achieved by high-dose administration, and this finding suggests the efficacy of high-dose administration, especially to refractory CNS metastases of NSCLC patients.
Keywords: Non-small cell lung cancer, Epidermal growth factor receptor, Tyrosine kinase inhibitor, Erlotinib, Central nervous system metastasis, Cerebrospinal fluid
Introduction
Patients with non-small cell lung cancer (NSCLC) who have somatic activating mutations of the epidermal growth factor receptor (EGFR) gene (EGFR mutations) generally respond to EGFR-tyrosine kinase inhibitors (EGFR-TKIs, e.g., gefitinib and erlotinib) [1, 2]. Nevertheless, the majority of these patients experience eventual disease progression, despite an initial dramatic response to treatment. The central nervous system (CNS) is a common site of recurrence, which is thought to be due to penetration of the agents into the CNS [3, 4]. A previous report described refractory CNS metastases of NSCLC that were improved by high-dose gefitinib [5]. Several cases in which CNS metastases resistant to gefitinib were improved by erlotinib have been also reported [6]. In addition, in several articles, intermittent, high-dose erlotinib improved CNS metastases that were resistant to continuous, normal-dose erlotinib [7–9]. These challenges are based on the hypothesis that high cerebrospinal fluid (CSF) concentrations may be achieved by high-dose administration of EGFR-TKIs, with higher CSF concentrations achieved by erlotinib compared with gefitinib. We previously reported the CSF concentrations of erlotinib and its penetration rates in four NSCLC patients with CNS metastases [10]. In this article, we describe a Japanese woman with NSCLC and EGFR mutation having CNS metastases that are resistant to 75 mg daily erlotinib. The metastases were subsequently improved by 150 mg daily erlotinib. We investigated the plasma and CSF concentrations of erlotinib at each dose. Furthermore, including previous data, we investigated the correlation between plasma and CSF concentrations.
Patients and methods
Patients
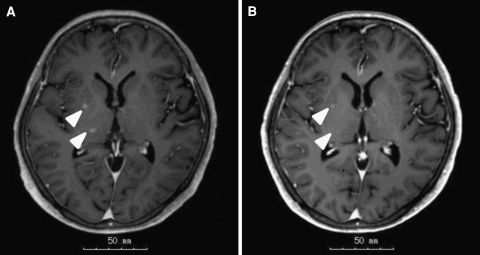
A 66-year-old Japanese woman was diagnosed with advanced lung adenocarcinoma and EGFR mutation (L858R) 3 years prior. Erlotinib had been administered as fourth line of treatment. Before the initiation of erlotinib, the patient had no CNS metastases. Although partial response (PR) was achieved, the erlotinib dose was reduced from 150 to 75 mg daily due to grade 3 fatigue. After progression-free survival (PFS) of 12 months, the patient experienced recurrent CNS metastases. The plasma and CSF concentrations of erlotinib just before administration of the 75-mg dose were 433 and 14 nM, respectively. The patient received whole-brain radiotherapy (WBRT), but the CNS metastases did not improve (Fig. 1a). The dose was subsequently increased to 150 mg daily; the plasma and CSF concentrations just before administration of erlotinib on day 8 erlotinib were 1,117 and 44 nM, respectively. Four weeks after the initiation of 150 mg administration of erlotinib, CNS metastases improved (Fig. 1b).
Fig. 1.
Brain magnetic resonance imaging (MRI) in the 66-year-old Japanese female patient. a Brain MRI after whole-brain radiotherapy demonstrated stable brain metastases (arrowheads). At this point, 75 mg daily erlotinib was administered. b Four weeks after initiation of 150 mg daily erlotinib, the metastases were improved (arrowheads)
We previously reported the plasma and CSF concentrations of four NSCLC patients with CNS metastases (cases 1–4) [10]. In addition to these patients, we investigated the plasma and CSF concentrations of four other NSCLC patients with CNS metastases (cases 5–8) (Table 1).
Table 1.
Patient characteristics
| Cases | Age (yr) | Sex | PS | Histology | EGFR gene | Smoking status | Previous chemotherapy regimen | Previous gefitinib (length of PFS) | Previous WBRT |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | F | 3 | Ad | NE | Never | 3 | Yes (10 months) | Yes |
| 2 | 82 | F | 4 | Ad | Wild type | Never | 0 | No | No |
| 3 | 70 | F | 3 | Ad | Wild type | Never | 1 | No | Yes |
| 4 | 86 | F | 3 | Ad | Ex 21; L858R | Never | 1 | Yes (7 months) | No |
| 5 | 67 | F | 1 | Ad | Ex 21; L858R | Never | 1 | No | No |
| 6 | 72 | M | 0 | Ad | Ex 21; L858R | Former | 1 | No | No |
| 7 | 81 | M | 1 | Ad | Ex 19 deletion | Former | 1 | Yes (46 months) | Yes |
| 8 | 85 | F | 2 | Ad | Ex 21; L858R | Never | 1 | No | Yes |
| a | 66 | F | 1 | Ad | Ex 21; L858R | Never | 3 | No | Yes |
PS performance status, EGFR epidermal growth factor receptor, PFS progression-free survival, WBRT whole-brain radiotherapy, F female, M male, Ad adenocarcinoma, NE not evaluated, Never never-smoker, Former former-smoker
aA 66-year-old Japanese female patient in this study
Methods
Blood and CSF samples were obtained just before the administration of 150 mg erlotinib on day 8 when steady-state plasma concentrations of erlotinib were assumed to be achieved. Plasma and CSF concentrations of erlotinib were determined using high-performance liquid chromatography with ultraviolet detection as previously reported [11]. Before the collection of samples and analysis, we obtained written informed consent from all patients.
Statistical analysis
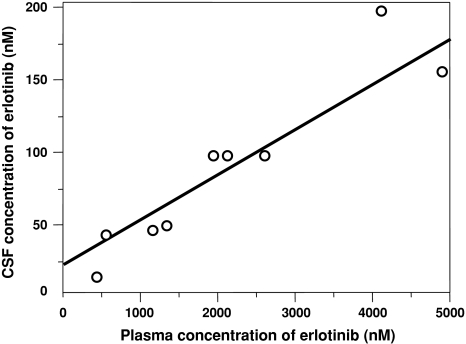
Linear regression was performed on the data in Fig. 2 by fitting a straight line. The R 2 value was used to measure the strength of the relationship between the plasma and CSF concentrations of erlotinib. The 150-mg daily erlotinib data from this 66-year-old Japanese female patient were analyzed, and the 75-mg daily erlotinib data were excluded. Linear regression analysis, performed using JMP 8 software (SAS Institute, Cary, NC, USA), was two-tailed, and P values less than 0.05 were considered statistically significant.
Fig. 2.
Correlation between plasma concentrations and cerebrospinal fluid (CSF) concentrations of erlotinib. A good correlation (R 2 = 0.84) was demonstrated (P = 0.0005)
Results
The clinical characteristics of all patients (n = 9) are summarized in Table 1. In CNS lesions, 7 of the 9 patients achieved PR (78%) and 5 of the 6 patients with EGFR mutations achieved PR (83%). Gefitinib was administered to three patients prior to erlotinib therapy; these patients achieved PR but had CNS recurrence after a long PFS of more than 6 months. Two of these three patients achieved PR after erlotinib therapy in spite of gefitinib resistance.
The plasma and CSF concentrations are summarized in Table 2. The mean ± standard deviation (SD) of CSF concentrations and penetration rates were 106 ± 59 nM and 4.5 ± 1.5%, respectively. As seen in Fig. 2, there was a good correlation (R 2 = 0.84) between plasma and CSF concentrations (P = 0.0005).
Table 2.
Plasma and cerebrospinal fluid concentrations of erlotinib, and central nervous system metastases response
| Case | Plasma concentration (nM) | CSF concentration (nM) | Penetration rate (%) | CNS responsea |
|---|---|---|---|---|
| 1 | 2,082 | 98 | 4.7 | Partial response |
| 2 | 4,059 | 202 | 5.0 | Stable disease |
| 3 | 4,922 | 156 | 3.2 | Partial response |
| 4 | 544 | 42 | 7.7 | Stable disease |
| 5 | 2,575 | 98 | 3.8 | Partial response |
| 6 | 1,314 | 47 | 3.5 | Partial response |
| 7 | 1,886 | 98 | 5.2 | Partial response |
| 8 | 5,376 | 172 | 3.2 | Partial response |
| (75 mg erlotinib)b | 433 | 14 | 3.3 | Progressive disease |
| (150 mg erlotinib)b | 1,117 | 44 | 3.9 | Partial response |
| Mean ± SDc | 2,653 ± 1,734 | 106 ± 59 | 4.5 ± 1.5 |
CSF cerebrospinal fluid, CNS central nervous system, SD standard deviation
aResponses were assessed by magnetic resonance imaging with use of the Response Evaluation Criteria in Solid Tumor version 1.1
bA 66-year-old Japanese female patient in this study
cWe analyzed the mean ± SD from all data except for the data from the 66-year-old Japanese female patient at a dose of 75 mg erlotinib
Discussion
This study demonstrated that there is a good correlation (R 2 = 0.84) between plasma and CSF concentrations of erlotinib (P = 0.0005), which indicates that high CSF concentrations can be achieved by high plasma concentrations. That is, high CSF concentrations can be achieved by high-dose erlotinib administration. CNS metastases of the 66-year-old Japanese female patient responded to 150 mg daily erlotinib despite resistance to 75 mg daily erlotinib, likely because of higher CSF concentrations. There are several reports based on the hypothesis that high CSF concentrations may be achieved by high-dose erlotinib administration [5–9], and our study supports this hypothesis.
The mean ± SD of CSF penetration rates were 4.5 ± 1.5%, and this result was similar to that of recent another study [12]. However, the rates of our study were ranged from 3.2 to 7.7% and those of another study ranged from 2.5 to 13.3%, which indicates that there seem to be difference in the penetration rate between individuals. Recently, it has been reported that some transporters restrict brain penetration of erlotinib [13, 14]. Polymorphisms of these transporters can be associated with this individual difference in the penetration rate.
Since systemic chemotherapy is thought to play a limited role in CNS metastases because of the belief that the brain is a pharmacological sanctuary, patients with NSCLC who showed initial good responses to EGFR-TKIs often had recurrent CNS metastases. Considering CSF concentrations, high-dose EGFR-TKIs can be effective for such refractory metastases. Furthermore, the association between CNS responses/recurrence and CSF concentrations should be investigated in the future.
Conflicts of interest
We declare that we have no potential conflict of interest related to this article.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 2.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 3.Omuro AM, Kris MG, Miller VA, Franceschi E, Shah N, Milton DT, Abrey LE. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer. 2005;103:2344–2348. doi: 10.1002/cncr.21033. [DOI] [PubMed] [Google Scholar]
- 4.Lee YJ, Choi HJ, Kim SK, Chang J, Moon JW, Park IK, Kim JH, Cho BC. Frequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patients with nonsmall-cell lung cancer. Cancer. 2010;116:1336–1343. doi: 10.1002/cncr.24877. [DOI] [PubMed] [Google Scholar]
- 5.Jackman DM, Holmes AJ, Lindeman N, Wen PY, Kesari S, Borras AM, Bailey C, de Jong F, Jänne PA, Johnson BE. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006;24:4517–4520. doi: 10.1200/JCO.2006.06.6126. [DOI] [PubMed] [Google Scholar]
- 6.Katayama T, Shimizu J, Suda K, Onozato R, Fukui T, Ito S, Hatooka S, Sueda T, Hida T, Yatabe Y, Mitsudomi T. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol. 2009;4:1415–1419. doi: 10.1097/JTO.0b013e3181b62572. [DOI] [PubMed] [Google Scholar]
- 7.Dhruva N, Socinski MA. Carcinomatous meningitis in non-small-cell lung cancer: response to high-dose erlotinib. J Clin Oncol. 2009;27:31–32. doi: 10.1200/JCO.2008.21.0963. [DOI] [PubMed] [Google Scholar]
- 8.Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. 2010;99:283–286. doi: 10.1007/s11060-010-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hata A, Kaji R, Fujita S, Katakami N. High-dose erlotinib for refractory brain metastases in a patient with relapsed non-small cell lung cancer. J Thorac Oncol. 2011;6:653–654. doi: 10.1097/JTO.0b013e3181d899bb. [DOI] [PubMed] [Google Scholar]
- 10.Togashi Y, Masago K, Fukudo M, Terada T, Fujita S, Irisa K, Sakamori Y, Kim YH, Mio T, Inui K, Mishima M. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI-420 in patients with central nervous system metastases of non-small cell lung cancer. J Thorac Oncol. 2010;5:950–955. doi: 10.1097/JTO.0b013e3181dab0dd. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Siu LL, Moore MJ, Chen EX. Simultaneous determination of OSI-774 and its major metabolite OSI-420 in human plasma by using HPLC with UV detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;814:143–147. doi: 10.1016/j.jchromb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Masuda T, Hattori N, Hamada A, Iwamoto H, Ohshimo S, Kanehara M, Ishikawa N, Fujitaka K, Haruta Y, Murai H, Kohno N (2011) Erlotinib efficacy and cerebrospinal fluid concentration in patients with lung adenocarcinoma developing leptomeningeal metastases during gefitinib therapy. Cancer Chemother Pharmacol 67:1465–1469 [DOI] [PubMed]
- 13.Elmeliegy MA, Carcaboso AM, Tagen M, Bai F, Stewart CF. Role of ATP-binding cassette and solute carrier transporters in erlotinib CNS penetration and intracellular accumulation. Clin Cancer Res. 2011;17:89–99. doi: 10.1158/1078-0432.CCR-10-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Vries NA, Buckle T, Zhao J, Beijnen JH, Schellens JH, van Tellingen O (2010) Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Invest New Drugs [Epub ahead of print] [DOI] [PubMed]