Non-technical summary
Specialized communication zones between neurons known as synapses undergo changes during memory formation. Noradrenaline is a neurotransmitter that is secreted in the brain when we are aroused or are exploring a new environment. β-Adrenergic receptors are activated by noradrenaline and can facilitate synaptic changes in the hippocampus, a brain structure critical for making new memories. We show that activation of β-adrenergic receptors generates long-lasting enhancements of synaptic strength that can facilitate plasticity at another group of synapses on the same post-synaptic cells. This research will further our understanding of how noradrenaline can facilitate the creation of associative memories.
Abstract
Abstract
Noradrenaline critically modulates the ability of synapses to undergo long-term plasticity on time scales extending well beyond fast synaptic transmission. Noradrenergic signalling through β-adrenergic receptors (β-ARs) enhances memory consolidation and can boost the longevity of long-term potentiation (LTP). Previous research has shown that stimulation of one synaptic pathway with a protocol that induces persistent, translation-dependent LTP can enable the induction of LTP by subthreshold stimulation at a second, independent synaptic pathway. This heterosynaptic facilitation depends on the regulation and synthesis of proteins. Recordings taken from area CA1 in mouse hippocampal slices showed that induction of β-AR-dependent LTP at one synaptic pathway (S1) can facilitate LTP at a second, independent pathway (S2) when low-frequency, subthreshold stimulation is applied after a 30 min delay. β-AR-dependent heterosynaptic facilitation requires protein synthesis as inhibition of mammalian target of rapamycin (mTOR), extracellular signal-regulated kinase (ERK), or translation, prevented homo- and heterosynaptic LTP. Shifting application of a translational repressor, emetine, to coincide with S2 stimulation did not block LTP. Heterosynaptic LTP was prevented in the presence of the cell-permeable cAMP-dependent protein kinase inhibitor, PKI. Conversely, the time window for inter-pathway transfer of heterosynaptic LTP was extended through inhibition of GluR2 endocytosis. Our data show that activation of β-ARs boosts the heterosynaptic expression of translation-dependent LTP. These results suggest that engagement of the noradrenergic system may extend the associative capacity of hippocampal synapses through facilitation of intersynaptic crosstalk.
Introduction
Noradrenaline (NA) is a neuromodulatory transmitter secreted in response to arousing or novel stimuli (Aston-Jones & Bloom, 1981; Sara & Segal, 1991; see Berridge & Waterhouse, 2003 for review). Activation of β-adrenergic receptors by NA engages signalling mechanisms which facilitate neuroplasticity (Harley et al. 1996; Gelinas & Nguyen, 2007) and memory genesis (Izquierdo et al. 1998; Straube et al. 2003; Lemon et al. 2009; reviewed in O'Dell et al. 2010). Stimulation of β-adrenergic receptors in the hippocampus, a brain structure required for memory formation (Scoville & Milner, 1957; Zola-Morgan et al. 1986; Eichenbaum, 2000), facilitates activity-dependent increases in synaptic strength (Thomas et al. 1996; Gelinas & Nguyen, 2005) known as long-term potentiation (LTP) (Bliss & Lomo, 1973; Bliss & Collingridge, 1993; Neves et al. 2008).
Previous research has shown that β-adrenergic receptors enhance LTP through regulation of protein synthesis (Walling & Harley, 2004; Gelinas & Nguyen, 2005; Gelinas et al. 2007). Translation regulation can serve as a priming mechanism for long-term synaptic changes in a cell-wide manner (Frey & Morris, 1997), including heterosynaptic metaplasticity (Abraham et al. 2001; Abraham et al. 2007). Similarly, heterosynaptic facilitation, a form of synaptic plasticity in which synaptic activity at one group of synapses initiates cellular mechanisms capable of facilitating synaptic strength at another group of synapses converging on the same postsynaptic cells, requires protein synthesis (Frey & Morris, 1997, 1998). As β-adrenergic receptors couple to signalling cascades implicated in translation regulation (Gelinas et al. 2007), we sought to determine if the β-adrenergic receptor agonist, isoproterenol (ISO), could enhance heterosynaptic facilitation in mouse hippocampus CA1.
We characterized the effects of β-adrenergic receptor activation on heterosynaptic facilitation of LTP using an in vitro, dual synaptic pathway protocol. In mouse hippocampus area CA1, two independent populations of synapses contacting the same postsynaptic cells were monitored to determine the effects of prior induction of homosynaptic β-AR-dependent LTP at one pathway (S1) on the subsequent induction of heterosynaptic long-term potentiation at a second pathway (S2). Herein, we have characterized the mechanisms through which β-ARs mediate heterosynaptic long-term potentiation.
Methods
Ethical approval
The experiments and methods of this paper were approved by the University Animal Policy and Welfare Committee (UAPWC) at the University of Alberta using guidelines approved by the Canadian Council on Animal Care (CCAC). Approximately 150 C57BL/6 mice (aged 7–12 weeks) were used for these experiments. Hippocampal tissue slices were harvested following cervical dislocation and decapitation in accordance with UAPWC and CCAC guidelines.
Electrophysiology
Transverse hippocampal slices (400 μm thick) were prepared as described by Nguyen & Kandel (1997). Briefly, following cervical dislocation and decapitation, the hippocampus was removed and sliced using a manual tissue chopper (Stoelting, Wood Dale, IL, USA). Slices were maintained in an interface chamber at 28°C and perfused at 1–2 ml min−1 with artificial CSF (ACSF) composed of the following (in mm): 124 NaCl, 4.4 KCl, 1.3 MgSO4, 1.0 NaH2PO4, 26.2 NaHCO3, 2.5 CaCl2 and 10 glucose, aerated with 95% O2 and 5% CO2. Slices were allowed to recover for 120 min prior to experiments. Extracellular field EPSPs (fEPSPs) were recorded with a glass microelectrode filled with ACSF (resistances, 2–3 MΩ) and positioned in the stratum radiatum of area CA1. fEPSPs were elicited by using two bipolar nickel–chromium electrodes placed in stratum radiatum to stimulate two separate sets of inputs converging onto the same postsynaptic population of neurons. Interpathway paired-pulse facilitation elicited by successive stimulation through the two electrodes at 75, 100, 150 and 200 ms intervals was used to test for independence of synaptic pathways during baseline acquisition and at the conclusion of experiments. Pathways were considered independent when no facilitation was observed following pairs of pulses. Stimulation intensity (0.08 ms pulse duration) was adjusted to evoke fEPSP amplitudes that were 40% of maximal size (Woo & Nguyen, 2003; Gelinas & Nguyen, 2007). Subsequent fEPSPs were elicited at the rate of once per minute at this ‘test’ stimulation intensity, with S1 stimulation preceding S2 stimulation by 200 ms.
After establishing a 20 min baseline recording, β-AR-dependent LTP was induced by applying one train of high-frequency stimulation (HFS; 100 Hz, 1 s duration at test strength) following a 10 min application of the β-AR agonist, isoproterenol (ISO; 1 μm). ISO was applied for an additional 5 min following HFS. Thirty minutes after HFS at S1, a LFS (5 Hz, 10 s duration) was applied to S2. Depotentiation (DPT) was induced using a previously established protocol consisting of 5 Hz, 3 min stimulation applied 15 min after LFS (Staubli & Lynch, 1990; Young & Nguyen, 2005). To assess the duration of activity-dependent synaptic changes, homosynaptic LFS (5 Hz 10 s) was applied followed by a 30 min or 1 h delay prior to heterosynaptic (1 × 100 Hz, 1 s paired with ISO) stimulation.
Drugs
The β-AR agonist (R(–)-isoproterenol(+)-bitartrate; Sigma, St Louis, MO, USA) was prepared daily as a concentrated stock solution at 1 mm, in distilled water and applied at a final concentration of 1 μm. The translation inhibitor emetine (EME; Sigma) was dissolved in DMSO to a stock concentration of 20 mm in distilled water. At lower concentrations than those used here (20 μm), EME blocked protein synthesis by >80% in hippocampal slices (Stanton & Sarvey, 1984). EME was perfused for 20 min prior to commencing experiments. The β-AR antagonist (±)-propranolol hydrochloride (PROP; Sigma) was prepared daily in distilled water as a 50 mm stock solution and was applied at a final concentration of 50 μm. A MEK inhibitor 2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one (PD98059) (50 μm; Sigma), was prepared in DMSO at a stock concentration of 10 mm and was applied 30 min prior to experiments. Rapamycin (Sigma), an mTOR inhibitor, was dissolved in DMSO to make a stock solution at 1 mm, diluted to 1 μm and was applied 30 min prior to experiments. The cell membrane-permeant PKA inhibitor 14-22 Amide (Calbiochem, La Jolla, CA, USA) was dissolved in ACSF (1 mm stock) and applied at 1 μm final concentration. An inhibitor of GluR2 endocytosis, the peptide Tat-GluR23Y, and a scrambled control peptide, Tat-GluR23S, were applied at 4 μm, 30 min prior to LFS and during the delay between weak and strong stimulation. Due to photosensitivity of the drugs, experiments were performed under dimmed light conditions. Drug experiments were interleaved with drug-free controls.
Data analysis
Axon Clampex (10.2) (Molecular Devices) was used for fEPSP analysis. To quantify changes in synaptic strength, the initial slope of the fEPSP was measured (Johnston & Wu, 1995). The average ‘baseline’ slope values were acquired over a period of 20 min before experimental protocols were applied. fEPSP slopes were measured at either 120 or 90 min after HFS or LFS for comparisons of LTP. Student's t test was used for statistical comparisons of mean fEPSP slopes between two groups, with a significance level of P < 0.05. For comparison of more than two groups, one-way ANOVAs were conducted followed by Tukey–Kramer tests for post hoc comparisons. The Welch correction was applied in cases in which the SDs of groups being compared were significantly different. All values shown are means ± SEM, with n the number of slices
Results
β-Adrenergic receptor activation facilitates heterosynaptic LTP in mouse CA1
In the first set of experiments, we tested the idea that β-adrenergic receptor activation primes heterosynaptic LTP in mouse CA1. Initially we assessed whether application of high-frequency stimulation (HFS; 100 Hz, 1 s) to one synaptic pathway (S1) could facilitate heterosynaptic LTP induced by low-frequency stimulation (LFS; 5 Hz, 10 s) applied 30 min later to a second, independent set of synapses. High-frequency stimulation generated potentiation which decayed to baseline in <2 h (Fig. 1A; fEPSPs 120 min after HFS were 111 ± 10%, n = 8). Low-frequency stimulation applied 30 min after HFS similarly induced decremental potentiation (fEPSPs 90 min after LFS were 103 ± 8%). These results suggest that neither 100 Hz nor 5 Hz stimulation alone is sufficient for initiating long-lasting (>2 h) LTP.
Figure 1. β-Adrenergic receptor activation primes heterosynaptic facilitation.
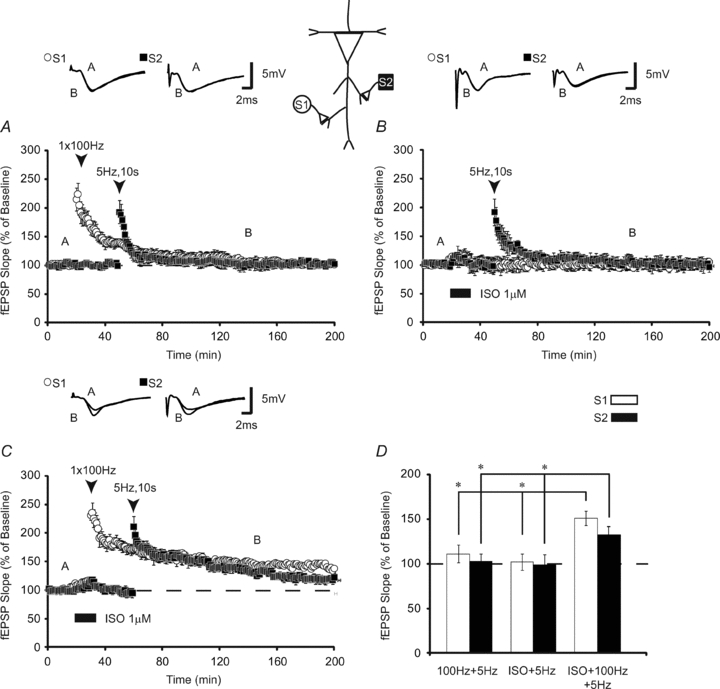
A, 100 Hz stimulation alone (open circles) induces transient (< 2 h) LTP which does not facilitate heterosynaptic LTP following low-frequency stimulation (5 Hz, 10 s; filled squares) at a second synaptic pathway (n = 8). LFS does not induce persistent changes in synaptic strength as fEPSPs at S2 returned to baseline in < 1 h. B, low-frequency stimulation 25 min after application of ISO alone has no long-lasting effects on synaptic transmission (n = 8), which shows that ISO alone is insufficient for facilitating heterosynaptic LTP. C, application of 1 × 100 Hz stimulation to S1 (open circles) paired with isoproterenol (ISO) facilitates the induction of LTP (filled squares) that can subsequently be captured by low-frequency stimulation at S2 (n = 10). D, summary histogram comparing fEPSP slopes obtained 120 min after HFS at S1 (open bars) and 90 min after LFS at S2 (filled bars). * indicates significant differences between treatment groups. Sample traces were taken 10 min after commencement of baseline recordings, 120 min after stimulation at S1 and 90 min after stimulation of S2. Results in D represent means ± SEM, *P < 0.05.
Next, we asked whether β-AR application alone would facilitate the induction of LTP by subthreshold stimulation applied heterosynaptically. Application of isoproterenol (ISO; 1 μm) for 15 min, 30 min before low-frequency stimulation (5 Hz, 10 s) at S2, failed to facilitate LTP at either S1 (fEPSPs were 102 ± 8% 120 min post-ISO application) or S2 (fEPSPs at S2 were 99 ± 9% 90 min after LFS, n = 8) (Fig. 1B). Thus, activation of β-ARs in the absence of HFS does not initiate heterosynaptic facilitation.
Previous research has demonstrated that pairing β-AR activation (ISO, 1 μm) with HFS (100 Hz, 1 s) induces long-lasting LTP which requires translation (Gelinas & Nguyen, 2005; Gelinas et al. 2007). Can prior induction of β-AR-dependent LTP facilitate heterosynaptic long-term potentiation? Pairing ISO (1 μm) with HFS homosynaptically (S1) induced LTP that was potentiated to 151 ± 8% (n = 10) of baseline 2 h post-stimulation (Fig. 1C). LFS applied 30 min later to S2 generated enhanced LTP (fEPSPs were 131 ± 7% of baseline 90 min after LFS; Fig. 1C). An ANOVA that compared fEPSPs 120 min after either 100 Hz alone, ISO alone or ISO + 100 Hz at S1 demonstrated significant differences between groups (F(2,25) = 9.77; P < 0.01). Subsequent Tukey–Kramer post hoc tests revealed that ISO + HFS generated homosynaptic LTP that was significantly greater than either 100 Hz or ISO alone (P < 0.05). When differences between heterosynaptic pathways stimulated with LFS following 100 Hz alone, ISO alone or ISO + 100 Hz applied homosynaptically were compared, a significant difference between groups was detected (F(2,25) = 4.86; P < 0.02). Tukey–Kramer post hoc tests showed that subthreshold LFS at S2 induced long-lasting LTP only when it was preceded by ISO + 100 Hz stimulation at S1 (P < 0.05; Fig. 1D). As heterosynaptic LTP was not facilitated by either ISO alone or 100 Hz HFS, these results show that pairing β-adrenergic receptor activation with 100 Hz HFS elicits stable LTP that can be transferred heterosynaptically in an activity-dependent manner.
Heterosynaptic facilitation elicited by ISO application requires β-adrenergic receptors
To determine if β-ARs are necessary for ISO-induced heterosynaptic facilitation, we co-applied a β-AR antagonist, propranolol (50 μm), overlapping with ISO and high-frequency stimulation. Induction of homosynaptic LTP was completely blocked in the presence of propranolol (Fig. 2A). fEPSPs in slices treated with propranolol were 109 ± 6% 120 min post-stimulation (n = 9), which was significantly less (P < 0.001) than control slices not exposed to propranolol (fEPSPs were 153 ± 8% 120 min post-HFS, n = 8) (Fig. 2B). Comparison of fEPSPs 120 min after stimulation at S1 revealed that propranolol applied overlapping with S1 stimulation also blocked the expression of heterosynaptic LTP (propranolol-treated slices, 102 ± 4%; controls, 122 ± 6%, P < 0.05; Fig. 2C).
Figure 2. β-ARs are required for heterosynaptic enhancement of LTP.
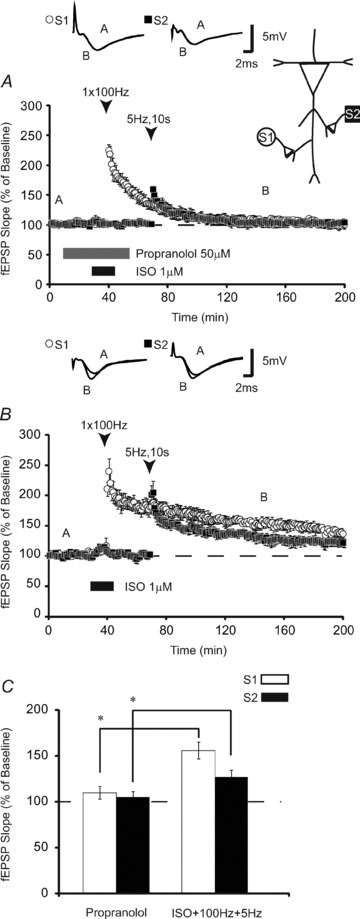
A, application of the β-AR antagonist, propranolol, overlapping with ISO blocked the induction of LTP at S1 (open circles) and prevented heterosynaptic LTP at S2 (filled squares) (n = 9). B, both homosynaptic β-AR-dependent LTP (open circles) and heterosynaptic LTP were significantly enhanced relative to slices treated with propranolol (n = 8). C, summary histogram comparing fEPSP slopes obtained 120 min after HFS at S1 (open bars) and 90 min after LFS (filled bars) at S2. Sample traces were taken 10 min after commencement of baseline recordings and 120 and 90 min after stimulation after HFS at S1 and LFS at S2, respectively. Results in C represent means ± SEM, *P < 0.05.
Protein synthesis is required for the β-AR-dependent heterosynaptic facilitation
As β-ARs gate LTP through translation regulation (Gelinas & Nguyen, 2005; Gelinas et al. 2007), we investigated whether heterosynaptic facilitation induced by activating β-adrenergic receptors requires protein synthesis. To test this, we applied a protein synthesis inhibitor, emetine (EME, 20 μm) 20 min prior to and overlapping with homosynaptic β-AR-dependent LTP. At this concentration, EME inhibits protein synthesis by >80% in hippocampal slices (Stanton & Sarvey, 1984). When HFS was paired with ISO in the presence of EME, homosynaptic LTP was significantly reduced (fEPSPs were 104 ± 9% of baseline 120 min post-HFS, n = 11) (Fig. 3A). Heterosynaptic LTP was similarly inhibited when emetine was applied overlapping with S1 (fEPSPs were 101 ± 5% 90 min after LFS Fig. 3A). This suggests that translation during β-AR-dependent LTP facilitates the subsequent induction of heterosynaptic LTP.
Figure 3. Protein synthesis is necessary for heterosynaptic facilitation induced by β-ARs.
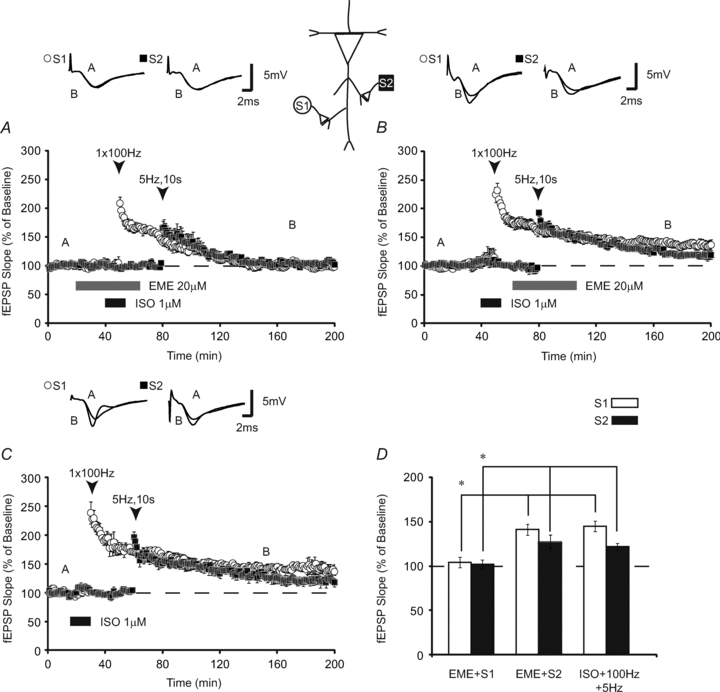
A, slices treated with the translation inhibitor, emetine (EME), during β-AR activation paired with high-frequency stimulation did not express LTP at S1 (open circles) or S2 (filled squares; n = 11). B, shifting emetine to coincide with low-frequency stimulation at S2 had no significant effect on LTP at either synaptic pathway, as LTP was still expressed (n = 9). C, LTP in the presence of emetine at S2 was similar in magnitude to translation inhibitor-free controls (n = 8). D, summary histogram comparing fEPSP slopes obtained 120 min after HFS (open bars) at S1 and 90 min after LFS at S2 (filled bars). * indicates significant differences between treatment groups. Sample traces were taken 10 min after commencement of baseline recordings and 120 min after HFS. Results in D represent means ± SEM, *P < 0.05.
Heterosynaptic transfer of LTP may be a process that is independent of protein synthesis as it is mediated by activation of specific kinases (Young et al. 2006). To determine if heterosynaptic transfer of LTP induction is independent of translation, we shifted the application of EME to coincide with S2 stimulation. If protein synthesis during homosynaptic LTP induction is sufficient for facilitating heterosynaptic LTP 30 min later, shifting application of EME to coincide with LFS at S2 should not prevent the expression of heterosynaptic LTP. Consistent with this hypothesis, bath application of EME during S2 stimulation failed to block either homosynaptic (fEPSPs were 141 ± 8% 120 min after HFS) or heterosynaptic LTP (fEPSPs were 128 ± 9% 90 min after LFS, n = 9) (Fig. 3B). An ANOVA that compared fEPSPs at S1 120 min after conjoint ISO application and HFS in the presence of emetine, during shifting of EME to overlap with LFS, or with no EME (Fig. 3C), demonstrated significant differences between groups (F(2,27) = 8.15; P < 0.01). Subsequent Tukey–Kramer post hoc tests revealed that emetine significantly inhibited LTP only when applied during HFS at S1 (P < 0.05). Comparisons of LTP at S2 (ANOVA; F(2,27) = 5.97; P < 0.02) revealed similar results in which heterosynaptic LTP was significantly reduced (P < 0.05) only when EME was applied during ISO + HFS at S1 (Fig. 3D). Comparisons between control and EME shift experiments revealed no difference between groups (P < 0.05). Thus, shifting the application of EME to coincide with S2 LFS did not block the expression of LTP at either synaptic pathway. Thus, similar to heterosynaptic mechanisms engaged by multiple trains of HFS, protein synthesis is required for the generation, but not the heterosynaptic transfer, of LTP. Our second pathway data also show that inhibition of protein synthesis does not affect basal synaptic transmission in hippocampal slices, consistent with previous reports (Krug et al. 1984; Frey et al. 1988; Nguyen et al. 1994; Scharf et al. 2002; Gelinas & Nguyen, 2005).
β-adrenergic receptors engage cap-dependent translation to facilitate heterosynaptic long-term potentiation
Stimulation of β-ARs with isoproterenol regulates protein synthesis at synapses through initiation of extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR) signalling pathways (Gelinas et al. 2007). We sought to determine if ERK and mTOR pathways, which are coupled to cap-dependent translation, are involved in the heterosynaptic facilitation initiated by β-adrenergic receptor activation.
We examined the effects of a MEK inhibitor, PD98059 (50 μm), on β-adrenergic receptor-mediated heterosynaptic LTP. Pairing 100 Hz stimulation with ISO in the presence of PD98059 inhibited LTP at both S1 (fEPSPs were 113 ± 9% 120 min after HFS) and S2 (fEPSPs were 98 ± 8% 90 min after LFS, n = 8) (Fig. 4A). Additionally, when the mTOR inhibitor, rapamycin (1 μm), was applied prior to and overlapping with induction of homosynaptic β-AR-LTP, expression of both homo- and heterosynaptic LTP was impaired (fEPSPs were reduced to 110 ± 12% 120 min after a HFS stimulation at S1 and 101 ± 6% 90 min after LFS at S2, n = 6) (Fig. 4B). An ANOVA that compared fEPSPs 120 min after conjoint ISO application and HFS in the presence of PD98059, rapamycin or no drug (Fig. 4C) showed significant differences between groups (F(2,21) = 6.79; P < 0.01). Subsequent Tukey–Kramer post hoc tests revealed that both PD98059 and rapamycin significantly inhibited LTP at S1 (P < 0.05; Fig. 4D). Furthermore, the PD98059 and rapamycin groups did not significantly differ from each other in their impairment of LTP (P > 0.05). An ANOVA comparing responses at our second synaptic pathway revealed differences between groups at S2 90 min following LFS (F(2,21) = 5.61; P < 0.02). When applied with S1, both PD98059 and rapamycin inhibited expression of heterosynaptic LTP at S2 (P < 0.05). Thus, ISO activates β-adrenergic receptors to establish LTP that requires downstream activation of both ERK and mTOR. Coupled with our emetine experiments, our data suggest that β-adrenergic receptor activation recruits cap-dependent translation to engage translation regulation capable of stabilizing LTP heterosynaptically.
Figure 4. β-AR-dependent heterosynaptic facilitation requires ERK and mTOR.
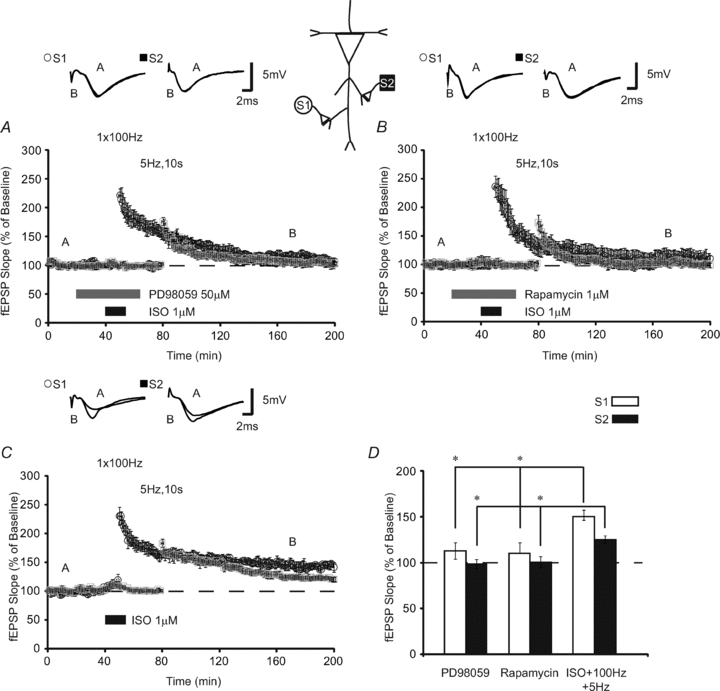
A, application of the MEK inhibitor, PD98059, overlapping with ISO and HFS at S1 prevented the induction of homosynaptic LTP (S1; open circles) as well as the subsequent expression of heterosynaptic LTP (S2; filled squares) (n = 8). B, slices treated with rapamycin (mTOR inhibitor) similarly exhibited decremental LTP at both S1 (open circles) and S2 (filled squares; n = 6). C, relative to drug-free controls (n = 8), fEPSPs in slices treated with PD98059 and mTOR were significantly less at both homo- and heterosynaptic inputs. D, summary histogram comparing fEPSP slopes obtained 120 min after HFS at S1 (open bars) and 90 min after LFS at S2 (filled bars). Sample traces were taken 10 min after commencement of baseline recordings and 120 min after HFS. Results in D represent means ± SEM, *P < 0.05.
Heterosynaptically captured LTP is immune to depotentiation
Depotentiating (DPT) stimuli can lead to the reversal of LTP during a restricted time interval soon after LTP induction (Young & Nguyen, 2005). Importantly, LTP stabilized through translation-dependent mechanisms cannot be reversed by a DPT stimulus (O'Dell & Kandel, 1994; Woo & Nguyen, 2003; Young & Nguyen, 2005). If translation products generated during ISO + 100 Hz are captured at S2 following LFS, we hypothesized that this form of LTP should be immune to DPT. To test this, we paired ISO application with 100 Hz HFS, waited 30 min and applied 5 Hz for 10 s to a second independent synaptic pathway. Following a 15 min delay, LFS (5 Hz for 3 min) was given to S2 in an attempt to depotentiate established LTP. We found that LTP could not be persistently erased; following a reversal to below baseline, fEPSPs recovered to the previously potentiated values (Fig. 5A). fEPSPs were 129 ± 6% 90 min after LFS (n = 6), which was not significantly different from control slices not given a DPT stimulus (control fEPSPs were 124 ± 9% 90 min after S2 stimulation, n = 6, P > 0.05; Fig. 5B and C). To confirm that the depotentiating stimulus is sufficient for reversing previously potentiated LTP, we used a stimulation protocol which induces translation-independent LTP (1 × 100 Hz stimulation) (Duffy et al. 2001). Application of 5 Hz 3 min stimulation 15 min after brief HFS reversed LTP (Fig. 5D), which resulted in fEPSPs that were significantly reduced (108 ± 3% of baseline, n = 6) relative to slices tetanised with 100 Hz stimulation only (Fig. 5E; 132 ± 2%, n = 6; P < 0.05), when compared 60 min post-HFS.
Figure 5. Heterosynaptic LTP is immune to depotentiation.
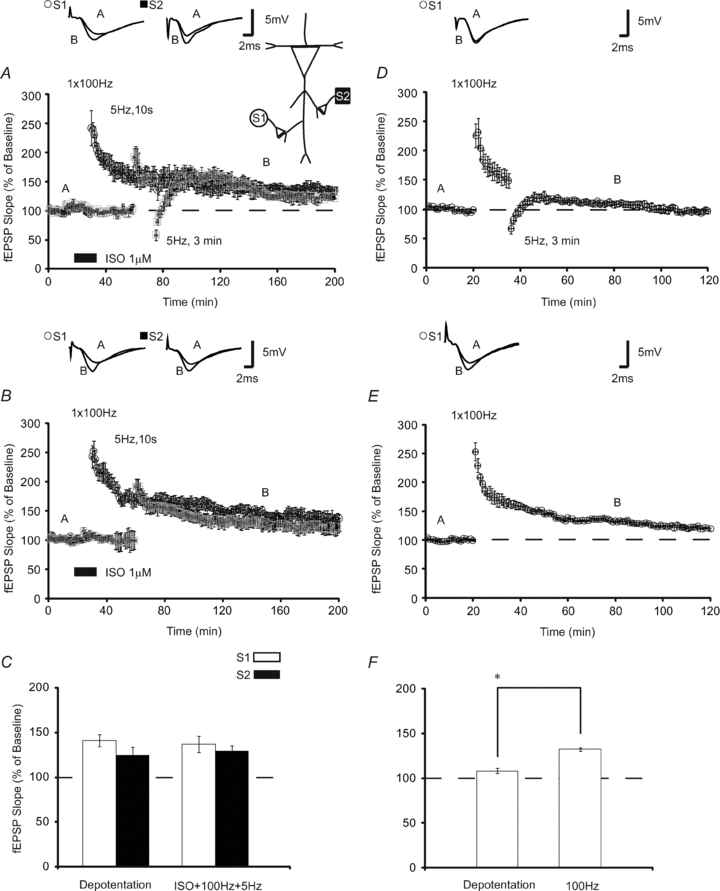
A, to determine if heterosynaptic LTP is immune to depotentiation (DPT), an activity-dependent reversal of established LTP, we applied a depotentiating stimulus (5 Hz, 3 min). Following induction of homosynaptic β-AR-dependent LTP (open circles; n = 6) at S1, LFS (5 Hz, 10 s) was applied to S2 (filled squares) 30 min later. Fifteen minutes after LFS at S2, a depotentiating stimulus (5 Hz, 3 min) was applied. Following an initial depression, LTP at S2 returned to previous potentiated levels which were not significantly different from slices not given a DPT stimulus (n = 6) (B). Homosynaptic LTP in which HFS was paired with ISO similarly was not significantly different when compared 2 h post-stimulation. C, summary histogram comparing fEPSP slopes obtained 120 min after HFS at S1 (open bars) and 90 min after LFS at S2 (filled bars). D, depotentiation induced with 5 Hz, 3 min is sufficient for reversing previously potentiated (100 Hz, 1 s) stimulation (n = 6). 100 Hz stimulation was followed by DPT stimuli which resulted in a reversal of LTP which was significantly reduced relative to LTP induced with 100 Hz alone (n = 6) (E and F). Sample traces were taken 10 min after commencement of baseline recordings and either 60 or 120 min after stimulation. Results in D and F represent means ± SEM.
Heterosynaptic transfer of β-adrenergic receptor-dependent LTP requires PKA
cAMP-dependent protein kinase (PKA) possesses properties required of a local synaptic activity indicator as it is localized at synapses, is activity dependent and inactivates with time (Huang et al. 2006; Young et al. 2006). Previous research has identified PKA as a mediator of heterosynaptic facilitation induced by repetitive HFS (Navakkode et al. 2004; Young et al. 2006). Does heterosynaptic transfer of LTP generated by pairing β-adrenergic receptor activation with HFS require PKA? To address this question, we paired ISO with HFS in the presence of a membrane-permeant inhibitor of PKA, PKI (1 μm). We found that LTP was intact following PKA inhibition, with S1 displaying potentiation of 152 ± 11% 120 min after HFS. Similarly, 5 Hz stimulation at S2 was also potentiated following PKA inhibition at S1; fEPSPs were potentiated to 126 ± 8% of baseline 90 min after stimulation (n = 8; Fig. 6A). Next, PKI was shifted to coincide with S2 stimulation. Homosynaptic LTP was not blocked when PKI was paired with S2 (fEPSPs were 148 ± 8% of baseline 120 min post-HFS; Fig. 6B). However, LTP was not expressed heterosynaptically (fEPSPs decayed to 102 ± 5% 90 min after LFS; n = 10) (Fig. 6B). An ANOVA comparing homosynaptic LTP induced in the presence of PKI with S1, paired with S2, or no drug revealed (Fig. 6C) no significant differences between groups (P > 0.05; Fig. 6D). An ANOVA comparing heterosynaptic LTP demonstrated that bath application of PKI only inhibited heterosynaptic LTP when it was present during LFS at S2 (F(2,23) = 6.19; P < 0.01). Subsequent Tukey–Kramer post hoc tests showed that LFS paired with PKI induced decaying LTP which was significantly less (P < 0.05) than either PKI with S1 or ISO + HFS controls (Fig. 6D). Slices treated with PKI homosynaptically were similar to ISO + HFS controls (P > 0.05). These data suggest that PKA is not required for plasticity protein generation, but is necessary for heterosynaptic transfer of LTP.
Figure 6. PKA is required for heterosynaptic transfer of LTP.
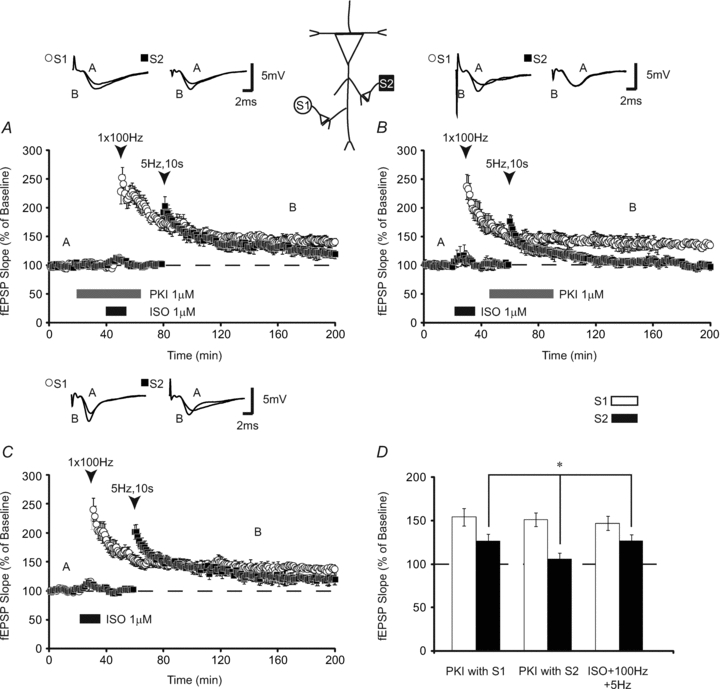
A, application of the membrane-permeant PKA inhibitor, PKI, overlapping with 1 × 100 Hz simulation paired with ISO (open circles), did not prevent the induction of homosynaptic LTP or the transfer of LTP to a second synaptic pathway (filled squares) (5 Hz, 10 s; n = 8). B, shifting PKI application to overlap with low-frequency stimulation prevented the heterosynaptic transfer of LTP to S2 (filled squares; n = 10). Heterosynaptic LTP was significantly reduced relative to slices treated with PKI during HFS or PKI-free controls. LTP at S1 was unaffected (open circles) as determined by comparisons with PKA inhibitor-free controls (C) (n = 6). D, summary histogram comparing fEPSP slopes obtained 120 min after HFS (open bars) at S1 and 90 min after LFS at S2. Sample traces were taken 10 min after commencement of baseline recordings and 120 min after S1 stimulation. Results in D represent means ± SEM, *P < 0.05.
LTP can be transferred heterosynaptically when LFS precedes HFS
Previous research has demonstrated that weak, subthreshold stimulation applied within a limited time window before strong LTP-inducing stimulation, can still be facilitated in a heterosynaptic manner (Frey & Morris, 1998). These experiments allow for determination of the temporal limitations for heterosynaptic facilitation. In the light of these data, we investigated whether our form of heterosynaptic facilitation can similarly be expressed in a ‘retrograde’ manner, with LFS (S1) preceding ISO paired with 100 Hz stimulation (S2). We observed that prior application of 5 Hz stimulation, which normally only induces a transient (<1 h) form of LTP, produced LTP lasting >2 h, provided that β-AR-dependent LTP was induced 30 min later (Fig. 7A) (fEPSPs were 124 ± 6% 120 min after 5 Hz, n = 6). When the duration between LFS and ISO + HFS was extended to 1 h, no transfer of LTP was observed (Fig. 7B; fEPSPs were 103 ± 4% 120 min after 5 Hz, n = 8). Comparisons between LFS applied 30 min or 1 h before heterosynaptic HFS showed that prior application of a weak stimulus could capture subsequently induced LTP, only at the 30 min interval. Mean fEPSP slopes from the 30 min delay group were substantially higher than the 1 h group (Fig. 7C; P < 0.02). β-AR-dependent homosynaptic LTP induced 30 min and 1 h after LFS were similar in magnitude (P > 0.05).
Figure 7. Prior LFS facilitates heterosynaptic transfer of LTP.
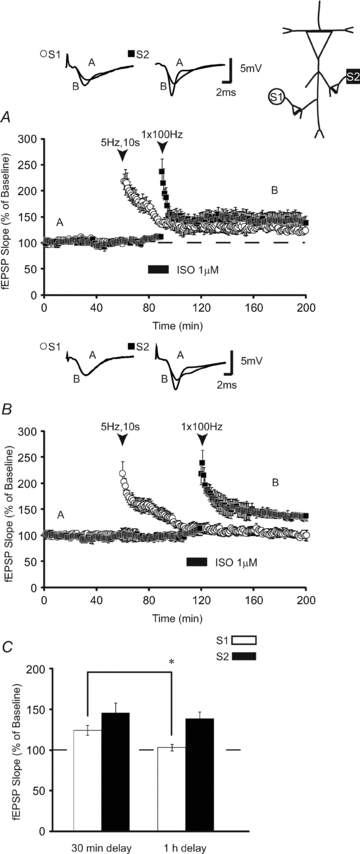
A, prior application of LFS (5 Hz, 10 s: S1; open circles), which normally induces transient LTP, enables long-lasting potentiation when β-AR LTP is induced heterosynaptically (S2; filled squares) 30 min later (n = 6). B, increasing the delay between LFS at S1 (open circles; n = 8) and HFS + ISO at S2 (S2; filled squares) to 1 h prevents the heterosynaptic transfer of long-lasting LTP. C, summary histogram comparing fEPSP slopes obtained 120 min after HFS (open bars) at S1 and LFS at S2 (filled bars). Sample traces were taken 10 min after commencement of baseline recordings and 120 min after low-frequency stimulation. * indicates a significant difference between treatment groups. Results in C represent means ± SEM, *P < 0.05.
Preventing GluR2 endocytosis extends the duration of the temporal window for heterosynaptic facilitation of LTP
Identification of mechanisms that could extend the time window for intersynaptic crosstalk is crucial for understanding the mechanisms required for heterosynaptic LTP. AMPA receptor trafficking and insertion have been identified as mechanisms involved in facilitating heterosynaptic long-term potentiation (Yao et al. 2008). To determine if GluR trafficking is involved in β-AR-dependent heterosynaptic facilitation, we asked whether preventing GluR internalization following LFS could increase the lifetime of the molecular activity trace. Using a membrane-permeant Tat-GluR23Y peptide which prevents endocytosis of GluR2, we tested the hypothesis that the duration of the time window for effective heterosynaptic transfer of LTP could be extended by blocking endocytosis of GluR2-containing receptors (thereby maintaining these receptors at the synapse). Tat-GluR23Y (4 μm) was applied 30 min prior to and 1 h after LFS (5 Hz, 10 s). Following a 1 h delay, LTP at a second pathway was induced by pairing ISO with HFS (100 Hz, 1 s). We found that fEPSPs were potentiated to 142 ± 11% (n = 4; Fig. 8A) 120 min after LFS. To determine if the effects of preventing GluR2 endocytosis facilitated the heterosynaptic expression of LTP, we conducted several control protocols. First, we applied the inert Tat-GluR23s scrambled control peptide to assess the effects of this peptide application. The Tat-GluR23s scrambled peptide failed to enhance the expression of homosynaptic LTP when induced prior to heterosynaptic β-AR-dependent LTP (fEPSPs were 99 ± 8% of baseline 120 min after LFS (n = 4; Fig. 8B). Next we determined if preventing GluR2 endocytosis with the active peptide is sufficient for enhancing the duration of LTP independent of β-AR stimulation. We applied active Tat-GluR23Y paired with 5 Hz, 10 s alone. Tat-GluR23Y coupled with 5 Hz stimulation induced transient LTP which returned to baseline in less than 2 h (fEPSPs were 103 ± 10% 120 min after LFS; n = 4; Fig. 8C). Finally, we applied Tat-GluR23Y with ISO alone to test whether prevention of GluA2 endocytosis paired with β-adrenergic receptor stimulation affected baseline transmission. Application of Tat-GluR23Y with ISO in the absence of stimulation induced a transient potentiation which returned to baseline in less than 30 min (fEPSPs were 103 ± 6% 90 min after the termination of drug application, n = 6; Fig. 8D). An ANOVA comparing either Tat-GluR23Y with LFS, Tat-GluR23Y paired with ISO and high-frequency stimulation, scrambled peptide Tat-GluR23s paired with heterosynaptic ISO + HFS or Tat-GluR23Y paired with ISO revealed a significant effect (F(3,17) = 6.76; P < 0.05; Fig. 8E). Tukey–Kramer post hoc tests demonstrated that only when active Tat-GluR23Y was paired with the induction of β-AR-LTP was the temporal window for heterosynaptic transfer of LTP extended to 1 h. There was no significant difference between the synaptic pathways receiving the strong stimulation protocol (fEPSPs were potentiated to 138 ± 10% 90 min after HFS in Tat-GluR23Y-treated slices and 153 ± 13% in slices exposed to the control scrambled peptide). However, both pathways receiving ISO + 100 Hz stimulation were significantly more potentiated than the non-stimulated control pathway in the LFS paired with Tat-GluR23Y experiment (P < 0.05). These findings show that blocking endocytosis of GluR2-containing receptors allows heterosynaptic transfer of persistent LTP to occur later than would normally be observed. The data also underscore the possibility that GluR2-containing receptors may serve as possible ‘tags’ to enable heterosynaptic capture of translation-dependent LTP.
Figure 8. Preventing GluR2 endocytosis extends the temporal window for transfer of heterosynaptic LTP.
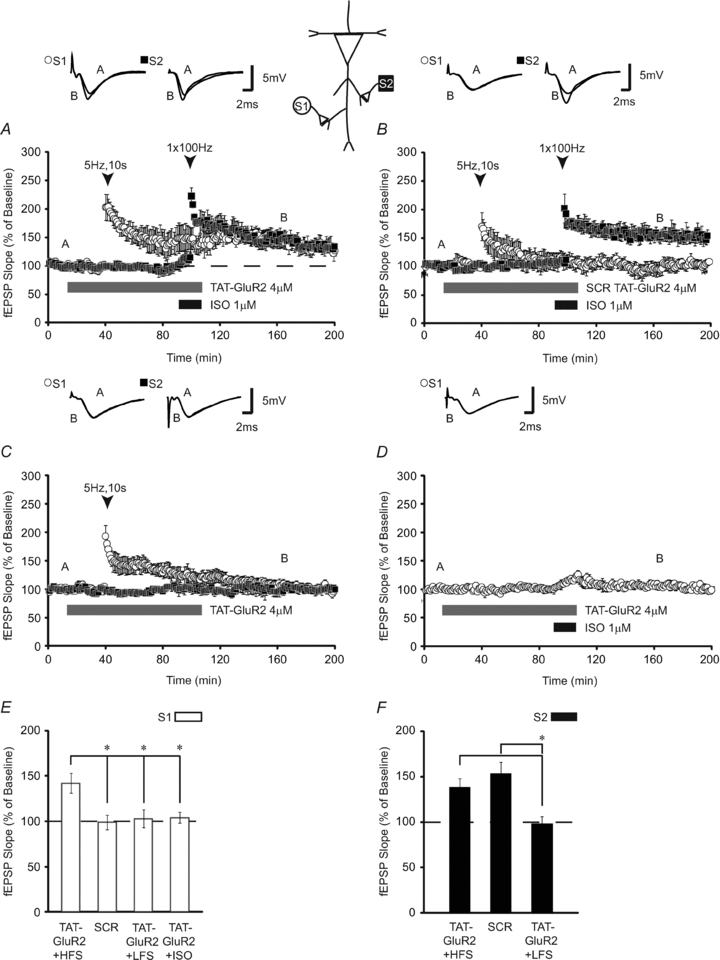
A, when GluR2 endocytosis is prevented through application of the Tat-GluR23Y peptide, LFS (5 Hz, 10 s: S1; open circles) still expresses LTP when HFS + ISO is applied 1 h later (n = 4). B, application of a scrambled, inert version of the peptide (Tat-GluR23s) failed to extend the time window for heterosynaptic transfer of LTP when ISO + HFS was delayed to 1 h post-LFS (n = 4). ISO + HFS generated homosynaptic, long-lasting LTP in the presence of both peptides, indicative of intact synaptic plasticity in both groups. C, application of Tat-GluR23Y peptide with 5 Hz stimulation fails to induce long-lasting LTP. When Tat-GluR23Y was applied prior to and overlapping with 5 Hz, 10 s stimulation transient LTP was induced which returned to baseline in < 2 h. D, pairing of ISO with Tat-GluR23Y does not have any significant effect on baseline synaptic responses. To test for effects on basal synaptic transmission, Tat-GluR23Y was paired with ISO application and basal synaptic responses were monitored. A transient, small (<20%) increase in synaptic potentiation was observed during ISO application. fEPSPs subsequently returned to baseline levels. E and F, summary histograms comparing fEPSP slopes obtained 120 min after LFS at S1 (E; open bars) and 90 min after HFS at S2 (F; filled bars). * indicates significant differences between groups. Sample traces were taken 10 min after commencement of baseline recordings and 120 min after stimulation. Results in E and F represent means ± SEM, *P < 0.05.
Discussion
Our results indicate that β-adrenergic receptor activation can facilitate heterosynaptic long-term potentiation in the adult mouse hippocampus. Neuromodulators gate both local protein synthesis (Huber et al. 2000; Gelinas & Nguyen, 2005; Navakkode et al. 2007) and activation of second messengers that can support heterosynaptic facilitation. Previous research showed that application of a cAMP phosphodiesterase inhibitor, rolipram, enabled the transformation of heterosynaptic, transient LTP into translation-dependent long-lasting LTP, suggestive of a cAMP-mediated increase in plasticity proteins (Navakkode et al. 2004). As noradrenergic receptors can facilitate protein synthesis-dependent LTP (Straube et al. 2003; Gelinas & Nguyen, 2005) and long-term memory (Cahill et al. 1994; Walling & Harley, 2004; Hu et al. 2007;Kemp & Manahan-Vaughan, 2008), translation regulation is critically involved in long-term changes in synaptic strength required for memory formation. Our findings suggest that cap-dependent translation is required for this form of heterosynaptic facilitation as inhibition of protein synthesis, ERK and mTOR prevented the expression of homo- and heterosynaptic LTP. Here, upregulation of plasticity proteins through cap-dependent translation is sufficient for prolonging the duration of heterosynaptic LTP beyond 2 h.
The current study characterizes a novel heterosynaptic facilitation protocol which relies upon activation of β-ARs for its induction. We found that pairing β-adrenergic receptor activation with HFS induced LTP that could be transferred to a second synaptic pathway provided stimulation sufficient for sequestering LTP was applied within a finite time window (30 min). Additionally, once captured, heterosynaptic LTP could not be depotentiated. Depotentiation is an activity-dependent reversal of previously established LTP (O'Dell & Kandel, 1994; Woo & Nguyen, 2003; Young & Nguyen, 2005). However, if a depotentiating stimulus is applied after a finite time window during which translation-dependent mechanisms have been engaged to stabilize recently induced synaptic potentiation, LTP can no longer be depotentiated (O'Dell & Kandel, 1994; Woo & Nguyen, 2003). When we applied a depotentiating tetanus 15 min after LFS applied to our second synaptic pathway, LTP was not reversed. This suggests that once plasticity-related proteins generated by prior β-AR activation have been captured, heterosynaptic LTP is stabilized, thus conferring immunity to depotentiation.
How does β-adrenergic receptor activation facilitate heterosynaptic LTP?β-ARs regulate translation postsynaptically through initiation of ERK and mTOR pathways (Gelinas et al. 2007; Fig. 9). ERK and mTOR co-regulate cap-dependent translation through 4E-binding proteins (4E-BPs), which sequester and repress eukaryotic initiation factor 4E (eIF4E), which in turn forms part of the eIF4F initiation complex (Kelleher et al. 2004; Richter & Sonenberg, 2005; see Klann et al. 2004, Banko & Klann, 2008; Costa-Mattioli et al. 2009 for reviews). In the present study, application of emetine overlapping with β-AR-dependent LTP induction generated decremental LTP at both synaptic pathways, consistent with a necessity for translation regulation in the generation of plasticity proteins. Importantly, shifting application of emetine to coincide with S2 stimulation did not affect the expression of heterosynaptic LTP. These results are consistent with previous work which showed that proteins synthesized during strong S1 stimulation enable the induction of long-lasting LTP at S2 in a protein synthesis-independent manner (Frey & Morris, 1998).
Figure 9. Model for enhanced heterosynaptic long-term potentiation induced by β-AR-dependent LTP.
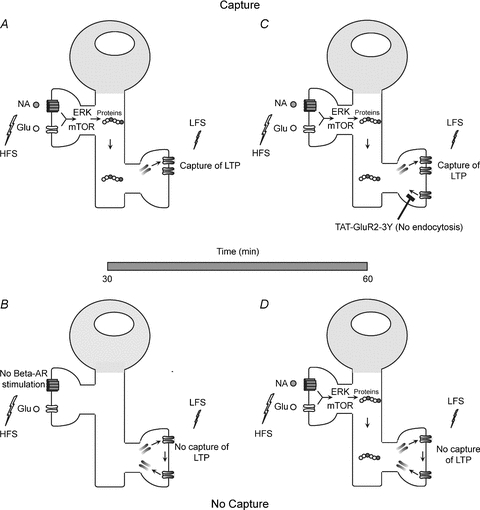
A, activation of β-ARs (shown here with the endogenous ligand noradrenaline; NA) paired with high-frequency stimulation (HFS) initiates intracellular signalling cascades that regulate protein synthesis (ERK, mTOR). ERK and mTOR converge on translational machinery to increase plasticity-related protein synthesis. These plasticity proteins can enhance heterosynaptic long-term potentiation by stabilizing AMPAR insertion induced by low-frequency stimulation. B, when β-AR-dependent LTP is not induced, LFS applied heterosynaptically induces a transient increase in AMPAR trafficking and insertion which, in the absence of protein synthesis upregulation is subsequently reversed through an endocytotic mechanism. Similarly, low-frequency stimulation applied prior to homosynaptic LTP induced with β-ARs does not capture LTP if the delay between stimuli is increased to 1 h (D). C, preventing GluR2 endocytosis following LFS extends the temporal window for heterosynaptic transfer of LTP. By stabilizing GluR2 in the membrane (with the Tat-GluR23Y peptide), subsequently generated plasticity proteins can promote increased synaptic strength during a more extended time window, thereby overcoming homeostatic mechanisms that would drive removal of synaptically localized AMPARs and weaken synaptic strength.
Recent evidence from in vivo experiments demonstrated that prior exposure of rats to novel experiences promoted the transformation of short-term memory into protein synthesis-dependent long-term memory in a form of ‘behavioural tagging’, provided that novelty exposure took place within a limited time frame (Moncada & Viola, 2007). Both noradrenaline and dopamine are released in the hippocampus in response to novelty, where it has been postulated that they serve as learning signals (Kitchigina et al. 1997; Harley, 2004). Given that both β-adrenergic (Walling & Harley, 2004; Harley et al. 2006; Lemon et al. 2009) and dopaminergic (Li et al. 2003) system activation induces cellular plasticity and boosts memory formation (Kemp & Manahan-Vaughn, 2008; Lemon & Manahan-Vaughn, 2006), these systems are well equipped for promoting the incorporation of new information into existing cellular networks representing previously encoded experiences.
In agreement with previous research, PKA was required for transfer of heterosynaptic LTP (Alarcon et al. 2006; Huang et al. 2006; Young et al. 2006). Inhibition of PKA failed to prevent homosynaptic LTP, consistent with previous experiments which demonstrated that isoproterenol paired with HFS induces PKA-independent homosynaptic LTP (Gelinas et al. 2008). Our present results suggest that translation regulation and gating of heterosynaptic facilitation occur through different mechanisms, with heterosynaptic transfer of LTP requiring PKA. What function does PKA serve in synaptic capture of LTP? PKA phosphorylates Ser845 of GluR1 which increases GluR insertion in response to synaptic activity (Esteban et al. 2003; Oh et al. 2006; Tenorio et al. 2010). Increased GluR trafficking could enhance synaptic capture both through boosting postsynaptic responses and providing additional slots for AMPAR insertion (Malinow, 2003). Previous data have established the dynamics of AMPAR subunit turnover at the synapse following the induction of LTP. Makino & Malinow (2009) have shown that GluR1 insertion is elevated immediately following the induction of LTP. These GluR1 receptors are subsequently replaced by GluR2-containing receptors during the expression of LTP which serves to maintain previously potentiated synaptic responses (Makino & Malinow, 2009). These data suggest that boosting the initial incorporation of GluR1 through Ser845 phosphorylation by PKA could increase the amount of postsynaptic AMPAR slots available for subsequent incorporation of GluR2, resulting in enhanced capture of LTP. Taken together, these results suggest that PKA activation may serve as a local synaptic indicator of previous synaptic activity following low-frequency stimulation.
Interestingly, increased synthesis of a constitutively active isoform of PKC, PKMζ, can prime heterosynaptic facilitation through upregulation of GluR2 (rat analogue for GluA2)-dependent AMPA receptor trafficking (Yao et al. 2008). Our data indicate that disrupting AMPAR internalization appeared to extend the temporal window for heterosynaptic transfer of LTP to 1 h. Recent data have implicated increased regulation of GluR2 trafficking in the maintenance of both LTP and memory (Serrano et al 2005; Migues et al. 2010). AMPARs form heterodimers which exist primarily in GluR1–GluR2 and GluR2–GluR3 configurations (Wenthold et al. 1996). Preventing the constitutive endocytosis of GluR2-containing AMPARs may extend the duration of synaptic tags by maintaining recently incorporated GluR1–GluR2 heterodimers at the synapse. We observed that maintaining GluR2 at the synapse extended the duration of the synaptic tag to 1 h. This result suggests that glutamatergic receptor trafficking determines the duration of synaptic tags. From a broader perspective, these data suggest that the temporal limits for associative memory formation are contingent upon the dynamics of experience-driven glutamate receptor trafficking. Synapses potentiated in response to environmental stimuli would be available for incorporation into cellular networks provided that AMPARs were trafficked to and maintained at the synapse (tagging), and stabilized through translation-dependent mechanisms initiated by neuromodulatory receptor stimulation (capture). Thus, GluR subunits may interact to facilitate synaptic plasticity, with GluR2 exocytosis acting to increase GluR levels to promote maintenance of LTP, and GluR1 acting to facilitate delivery of the receptors through phosphorylation, which has been correlated with reduced thresholds for LTP induction (Makino et al. 2011) and enhanced learning and memory (Hu et al. 2007).
Our results with the Tat-GluR23Y peptide, which inhibits GluR2 internalization, suggest that maintaining GluR2-containing AMPARs at the synapse is sufficient for prolonging the temporal window for intersynaptic crosstalk. The extension of the time window from 30 min to 1 h in the presence of Tat-GluR23Y peptide is in line with previously established time frames for heterosynaptic LTP (Frey & Morris, 1998) and LTD (Sajikumar & Frey, 2004). As preventing GluR2 endocytosis facilitated heterosynaptic LTP at 1 h, this suggests that activity-dependent GluR2 internalization may serve to define the effective temporal window for synaptic associativity across distinct synaptic pathways.
The role of homosynaptically induced translation products may be to stabilize primed AMPAR insertion following heterosynaptic stimulation (Fig. 9). Inhibiting GluR2 endocytosis appeared to extend the lifetime of the tag by maintaining synaptic incorporation of AMPARs, which would normally be removed in the absence of plasticity proteins. Activation of β-adrenergic receptors could facilitate heterosynaptic LTP through phosphorylation of AMPARs by PKA, in addition to increasing the generation of plasticity proteins which would enhance heterosynaptic facilitation. As heterosynaptic LTP maintenance continued beyond 1 h, Tat-GluR23Y-dependent enhancement of AMPAR surface expression appears to maintain potentiation until β-AR-dependent mechanisms are engaged to provide plasticity products capable of stabilizing long-term synaptic augmentation. Interestingly, application of Tat-GluR23Y appeared to increase the initial magnitude of synaptic potentiation following LFS relative to control peptide experiments (Fig. 8A and B). This could result from prevention of constitutive AMPA receptor endocytosis. Prevention of GluR2 endocytosis appears to maintain GluR2-containing AMPARs with no observed increase in basal synaptic transmission when the Tat-GluR23Y was applied alone (Fig. 8D). Increasing the pool of available AMPARs may prime these receptors for activity-dependent insertion into the postsynaptic membrane. These receptors could increase the capacity for AMPAR trafficking in response to synaptic stimulation (Hayashi et al. 2000; Ju et al. 2004). This would generate enhanced synaptic responses immediately following tetanisation. The elevated magnitude of the initial fEPSP potentiation observed in the presence of Tat-GluR23Y (Fig. 8A) is consistent with this hypothesis.
What would be expected if GluR1 receptor endocytosis was prevented? Most forms of LTP require the rapid insertion of GluR1 during induction (Makino & Malinow, 2009). Blocking GluR1 endocytosis could result in either increased basal synaptic responses or the saturation of synaptic weights. Interestingly, recent data using phosphomimetic knock-in mice suggest that increasing GluR1 phosphorylation lowers the threshold for inducing LTP without affecting membrane insertion of GluR1 (Makino et al. 2011). Although not observed in the Makino et al. (2011) study, increasing GluR1 phosphorylation has previously been reported to enhance GluR1 expression, which facilitated the induction of LTP (Oh et al. 2006). Importantly, most native receptors are GluR1–GluR2 heterodimers, and therefore, they are often trafficked together. However, the regulated endocytosis of this heterodimer is determined by sequences in the GluR2 tail, and therefore is GluR2 dependent, and not GluR1 dependent. As such, the endocytosis of heterodimers (both GluR1 and GluR2) will be inhibited by GluR2 tail peptides. Sequences in the GluR1 tail are only involved in constitutive endocytosis, but lack of signals for the regulated endocytosis and, thus, GluR1-derived peptide, may affect constitutive endocytosis, but not the regulated endocytosis, and therefore, will have little effect on LTP maintenance.
Our data suggest that preventing GluR2 endocytosis in conjunction with increased PKA activity, results in the synergistic facilitation of heterosynaptic LTP. Recent evidence has implicated GluR2 trafficking in the maintenance of LTP and memory (Migues et al. 2010). This process is mediated through mechanisms requiring the constitutively active PKC isoform, PKMζ (Sacktor, 2011). Activation of PKMζ increases N-ethylmaleimide-sensitive factor (NSF) activity which interferes with protein interacting with C-kinase 1 (PICK1)-mediated sequestration of GluR2 (Araki et al. 2010). Once activated, NSF liberates GluR2 from PICK1-mediated extrasynaptic pools, which allows for synaptic delivery of GluR2, resulting in potentiation (Yao et al. 2008). Our data are consistent with synaptic processes observed following the synthesis and phosphorylation of PKMζ. Preventing GluR2 endocytosis enhances levels of free GluR2, which can subsequently be driven into postsynaptic locations. An important mechanistic distinction is that in our protocol, PKA could substitute for PKMζ-dependent GluR2 trafficking by increasing the phosphorylation and synaptic delivery of GluR1, which is consistent with previous studies demonstrating a requirement for PKA in synaptic tagging (Young et al. 2006). It appears that endocytosis of GluR2-containing AMPARs may reset synapses which have not undergone translation-dependent potentiation back to basal states. which could be considered a form of tag degradation. Both PKMζ-mediated GluR2 regulation and PKA activation could prevent this tag degradation through the synaptic maintenance of GluR2 and priming of GluR1 insertion, respectively.
Our main finding was an enhancement of heterosynaptic long-term potentiation in CA1 following the induction of homosynaptic β-AR-dependent LTP. Our results suggest that increased noradrenergic signalling can prime heteroassociative processes requiring translation in a cell-wide manner. Synaptic protein synthesis may provide a mechanism for behavioural tagging, a process in which memory consolidation is enhanced by prior exposure to an open field and that requires translation (Moncada & Viola, 2007). Open field exposure is a mildly aversive stimulus which engages the neuromodulatory systems capable of upregulating translation (Thomas et al. 1996; Winder et al. 1999; Straube et al. 2003; Gelinas & Nguyen, 2005). Taken together, our results suggest that activation of noradrenergic receptors enhances heterosynaptic facilitation which, in turn, could provide a cellular mechanism for the remembrance of temporally spaced events during associative memory formation. Our results add β-adrenergic receptors to a growing list of neuromodulatory receptors that can engage cellular mechanisms capable of enhancing heterosynaptic facilitation.
Acknowledgments
This research was supported by grants from the Canadian Institutes of Health Research (to P.V.N. and Y.T.W.). S. Connor received a Graduate Scholarship from the Natural Sciences and Engineering Research Council of Canada and a Queen Elizabeth II Scholarship from the University of Alberta, and P. Nguyen is a Scientist of the Alberta Heritage Foundation for Medical Research. We thank Jonathan Wong, Kian Parseyan, Claire Gizowski and Rebecca McCourt for their technical assistance.
Glossary
Abbreviations
- 4E-BP
4E-binding proteins
- β-AR
β-adrenergic receptor
- CA1
cornus ammonis 1
- DPT
depotentiation
- eIF4E
eukaryotic initiation factor 4E
- eIF4F
eukaryotic initiation factor 4F
- EME
emetine
- ERK
extracellular signal-regulated kinase
- GluR
glutamate receptor
- HFS
high-frequency stimulation
- ISO
isoproterenol
- LFS
low-frequency stimulation
- LTD
long-term depression
- LTP
long-term potentiation
- MEK
mitogen-activated protein kinase kinase
- mTOR
mammalian target of rapamycin
- NA
noradrenaline
- NSF
N-ethylmaleimide sensitive factor
- PD98059
2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one
- PICK1
protein interacting with C-kinase 1
- PKA
cAMP-dependent protein kinase
- PKI
protein kinase A inhibitor
- PKM
protein kinase-M
- PROP
propranolol
Author contributions
All experiments were conducted by S.A.C. at the University of Alberta. All authors contributed to the conception, design, analysis and interpretation of data; to the initial drafting and revision of the intellectual content of this manuscript; and all authors have viewed and approved the final version of this manuscript.
References
- Abraham WC, Logan B, Wolff A, Benuskova L. Heterosynaptic” LTD in the dentate gyrus of anesthetized rat requires homosynaptic activity. J Neurophysiol. 2007;98:1048–1051. doi: 10.1152/jn.00250.2007. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Mason-Parker SE, Bear MF, Webb S, Tate WP. Heterosynaptic metaplasticity in the hippocampus in vivo: a BCM-like modifiable threshold for LTP. Proc Natl Acad Sci U S A. 2001;98:10924–10929. doi: 10.1073/pnas.181342098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon JM, Barco A, Kandel ER. Capture of the late phase of long-term potentiation within and across the apical and basilar dendritic compartments of CA1 pyramidal neurons: synaptic tagging is compartment restricted. J Neurosci. 2006;26:256–264. doi: 10.1523/JNEUROSCI.3196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y, Lin DT, Huganir RL. Plasma membrane insertion of the AMPA receptor GluA2 subunit is regulated by NSF binding and Q/R editing of the ion pore. Proc Natl Acad Sci U S A. 2010;107:11080–11085. doi: 10.1073/pnas.1006584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Klann E. Cap-dependent translation initiation and memory. Prog Brain Res. 2008;169:59–80. doi: 10.1016/S0079-6123(07)00004-0. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. β-Adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SN, Craddock KJ, Abel T, Nguyen PV. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn Mem. 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;38:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV. ERK and mTOR signaling couple β-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J Biol Chem. 2007;282:27527–27535. doi: 10.1074/jbc.M701077200. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen PV. β-Adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci. 2005;25:3294–3303. doi: 10.1523/JNEUROSCI.4175-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen PV. Neuromodulation of hippocampal synaptic plasticity, learning, and memory by noradrenaline. Cent Nerv Syst Agents Med Chem. 2007;7:17–33. [Google Scholar]
- Gelinas JN, Tenorio G, Lemon N, Abel T, Nguyen PV. β-Adrenergic receptor activation during distinct patterns of stimulation critically modulates the PKA-dependence of LTP in the mouse hippocampus. Learn Mem. 2008;15:281–289. doi: 10.1101/lm.829208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CW. Norepinephrine and dopamine as learning signals. Neural Plast. 2004;11:191–204. doi: 10.1155/NP.2004.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CW, Darby-King A, McCann J, McLean JH. β1-adrenoceptor or α1-adrenoceptor activation initiates early odor preference learning in rat pups: support for the mitral cell/cAMP model of odor preference learning. Learn Mem. 2006;13:8–13. doi: 10.1101/lm.62006. [DOI] [PubMed] [Google Scholar]
- Harley CW, Lalies MD, Nutt DJ. Estimating the synaptic concentration of norepinephrine in dentate gyrus which produces β-receptor mediated long-lasting potentiation in vivo using microdialysis and intracerebroventricular norepinephrine. Brain Res. 1996;710:293–298. doi: 10.1016/0006-8993(95)01443-8. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Huang T, McDonough CB, Abel T. Compartmentalized PKA signaling events are required for synaptic tagging and capture during hippocampal late-phase long-term potentiation. Eur J Cell Biol. 2006;85:635–642. doi: 10.1016/j.ejcb.2006.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH, Izquierdo LA, Barros DM, de Souza MM, Mello e Souza T. Short- and long-term memory are differentially regulated by monoaminergic systems in the rat brain. Neurobiol Learn Mem. 1998;69:219–224. doi: 10.1006/nlme.1998.3825. [DOI] [PubMed] [Google Scholar]
- Johnston D, Wu SM-S. Foundations of Cellular Neurophysiology. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. β-Adrenoreceptors comprise a critical element in learning-facilitated long-term plasticity. Cereb Cortex. 2008;18:1326–1334. doi: 10.1093/cercor/bhm164. [DOI] [PubMed] [Google Scholar]
- Kitchigina V, Vankov A, Harley C, Sara SJ. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci. 1997;9:41–47. doi: 10.1111/j.1460-9568.1997.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Klann E, Antion MD, Banko JL, Hou L. Synaptic plasticity and translation initiation. Learn Mem. 2004;11:365–372. doi: 10.1101/lm.79004. [DOI] [PubMed] [Google Scholar]
- Krug M, Lössner B, Ott T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull. 1984;13:39–42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Lemon N, Aydin-Abidin S, Funke K, Manahan-Vaughan D. Locus coeruleus activation facilitates memory encoding and induces hippocampal LTD that depends on β-adrenergic receptor activation. Cereb Cortex. 2009;19:2827–2837. doi: 10.1093/cercor/bhp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26:7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Makino Y, Johnson RC, Yu Y, Takamiya K, Huganir RL. Enhanced synaptic plasticity in mice with phosphomimetic mutation of the GluA1 AMPA receptor. Proc Natl Acad Sci U S A. 2011;108:8450–8455. doi: 10.1073/pnas.1105261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R. AMPA receptor trafficking and long-term potentiation. Philos Trans R Soc Lond B Biol Sci. 2003;358:707–714. doi: 10.1098/rstb.2002.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMζ maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010;13:630–634. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- Moncada D, Viola H. Induction of long-term memory by exposure to novelty requires protein synthesis: evidence for a behavioral tagging. J Neurosci. 2007;27:7476–7481. doi: 10.1523/JNEUROSCI.1083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navakkode S, Sajikumar S, Frey JU. The type IV-specific phosphodiesterase inhibitor rolipram and its effect on hippocampal long-term potentiation and synaptic tagging. J Neurosci. 2004;24:7740–7744. doi: 10.1523/JNEUROSCI.1796-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navakkode S, Sajikumar S, Frey JU. Synergistic requirements for the induction of dopaminergic D1/D5-receptor-mediated LTP in hippocampal slices of rat CA1 in vitro. Neuropharmacology. 2007;52:1547–1554. doi: 10.1016/j.neuropharm.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TVP. Synaptic plasticity, memory, and the hippocampus: A neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER. Brief theta-burst stimulation induces a transcription-dependent late phase of LTP requiring cAMP in area CA1 of the mouse hippocampus. Learn Mem. 1997;4:230–243. doi: 10.1101/lm.4.2.230. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Connor SA, Gelinas JN, Nguyen PV. Viagra for your synapses: enhancement of hippocampal long-term potentiation by activation of β-adrenergic receptors. Cell Signal. 2010;22:728–736. doi: 10.1016/j.cellsig.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell TJ, Kandel ER. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learn Mem. 1994;1:129–139. [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature. 2005;433:477–480. doi: 10.1038/nature03205. [DOI] [PubMed] [Google Scholar]
- Sacktor TC. How does PKMζ maintain long-term memory? Nat Rev Neurosci. 2011;12:9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Frey JU. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol Learn Mem. 2004;82:12–25. doi: 10.1016/j.nlm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Segal M. Plasticity of sensory responses of locus coeruleus neurons in the behaving rat: implications for cognition. Prog Brain Res. 1991;88:571–585. doi: 10.1016/s0079-6123(08)63835-2. [DOI] [PubMed] [Google Scholar]
- Scharf MT, Woo NH, Lattal KM, Young JZ, Nguyen PV, Abel T. Protein synthesis is required for the enhancement of long-term potentiation and long-term memory by spaced training. J Neurophysiol. 2002;87:2770–2777. doi: 10.1152/jn.2002.87.6.2770. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mζ maintains late-phase long-term potentiation. J Neurosci. 2005;25:1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM. Blockade of long-term potentiation in rat hippocampal CA1 region by inhibitors of protein synthesis. J Neurosci. 1984;4:3080–3088. doi: 10.1523/JNEUROSCI.04-12-03080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Lynch G. Stable depression of potentiated synaptic responses in the hippocampus with 1–5 Hz stimulation. Brain Res. 1990;513:113–118. doi: 10.1016/0006-8993(90)91096-y. [DOI] [PubMed] [Google Scholar]
- Straube T, Korz V, Balschun D, Frey JU. Requirement of β-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J Physiol. 2003;552:953–960. doi: 10.1113/jphysiol.2003.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenorio G, Connor SA, Guévremont D, Abraham WC, Williams J, O'Dell TJ, Nguyen PV. ‘Silent’ priming of translation-dependent LTP by ß-adrenergic receptors involves phosphorylation and recruitment of AMPA receptors. Learn Mem. 2010;17:627–638. doi: 10.1101/lm.1974510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Moody TD, Makhinson M, O'Dell TJ. Activity-dependent β-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron. 1996;17:475–482. doi: 10.1016/s0896-6273(00)80179-8. [DOI] [PubMed] [Google Scholar]
- Walling SG, Harley CW. Locus ceruleus activation initiates delayed synaptic potentiation of perforant path input to the dentate gyrus in awake rats: a novel β-adrenergic- and protein synthesis-dependent mammalian plasticity mechanism. J Neurosci. 2004;24:598–604. doi: 10.1523/JNEUROSCI.4426-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder DG, Martin KC, Muzzio IA, Rohrer D, Chruscinski A, Kobilka B, Kandel ER. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by β-adrenergic receptors. Neuron. 1999;24:715–726. doi: 10.1016/s0896-6273(00)81124-1. [DOI] [PubMed] [Google Scholar]
- Woo NH, Nguyen PV. Protein synthesis is required for synaptic immunity to depotentiation. J Neurosci. 2003;23:1125–1132. doi: 10.1523/JNEUROSCI.23-04-01125.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Frey JU, Sacktor TC. PKMζ maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci. 2008;28:7820–7827. doi: 10.1523/JNEUROSCI.0223-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JZ, Isiegas C, Abel T, Nguyen PV. Metaplasticity of the late-phase of long-term potentiation: a critical role for protein kinase A in synaptic tagging. Eur J Neurosci. 2006;23:1784–1794. doi: 10.1111/j.1460-9568.2006.04707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JZ, Nguyen PV. Homosynaptic and heterosynaptic inhibition of synaptic tagging and capture of long-term potentiation by previous synaptic activity. J Neurosci. 2005;25:7221–7231. doi: 10.1523/JNEUROSCI.0909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


