Non-technical summary
In this study, we demonstrated that supplementation of the maternal diet with a particular fatty acid, 18:3 n-3, the precursor of the n-3 fatty acid family, modified intestinal permeability, probably via diet-induced neuroplastic changes of the enteric nervous system of newborn piglets. These findings suggest that feeding fatty acids of the n-3 family during pregnancy and lactation impact newborn intestinal barrier function. However, the beneficial versus harmful consequences of this increased intestinal permeability remain to be elucidated.
Abstract
Abstract
The intestinal epithelial barrier (IEB) plays a key role in the maintenance of gut homeostasis and the development of the immune system in newborns. The enteric nervous system (ENS), a key regulator of gastrointestinal functions, has been shown to be modulated by nutritional factors. However, it remains currently unknown whether maternal diet, in particular n–3 polyunsaturated fatty acids (n–3PUFAs), can impact upon the IEB in newborn piglets and whether the ENS is involved in this effect. Sows received either a control diet (lard based) or an n–3PUFA diet (linseed oil based) during gestation and lactation. Intestinal paracellular permeability was assessed in Ussing chambers on piglets at birth, 3, 7, 14, 21 and 28 postnatal days (PND). Basal jejunal permeability increased significantly and similarly in both groups until PND14 and decreased thereafter. However, at PND28, permeability was higher in n–3PUFA animals as compared to controls. In addition, a vasoactive intestinal peptide (VIP) receptor antagonist increased paracellular permeability in controls but not in n–3PUFA piglets. Conversely, atropine and hexamethonium decreased paracellular permeability in the n–3PUFA group but not in the control group. Moreover, the n–3PUFA diet increased the proportion of choline acetyltransferase (ChAT)-immunoreactive (IR) neurons and decreased the proportion of VIP-IR neurons in the submucosal plexus of piglet jejunum compared to controls. In addition, in primary culture of rat ENS, we showed that 20:5n–3 but not 18:3n–3 increased the proportion of ChAT-IR neurons and decreased the proportion of VIP-IR neurons. In conclusion, supplementation of the maternal diet with n–3PUFAs modified intestinal permeability probably via diet-induced neuroplastic changes in the ENS of newborn piglets.
Introduction
The intestinal epithelial barrier (IEB) plays a key role in the immune system development of newborns. The IEB is located at the interface between exogenous substances within the intestinal lumen (bacteria, pathogens, antigens …) and the immune system (Freier, 1989). After birth, individuals with a high intestinal permeability are at risk from excessive passage of toxins and development of inflammation (Israel, 1994). However, a high permeability seems also necessary to educate the immune system by regulating the passage of antigens involved in the development of oral tolerance (da Silva et al. 2002; Gebbers & Laissue, 2004; Zhang et al. 2010). Polyunsaturated fatty acids of the n–3 family (n–3PUFAs) are known for their beneficial effect on intestinal inflammatory disorders. A high 18:3n–3 maternal diet reduced the inflammation response in experimental colitis in rat pups (Jacobson et al. 2004). n–3PUFA supplementation also reduces the incidence of necrotizing enterocolitis in a murine model (Caplan & Jilling, 2001). Dietary n–3PUFAs are also beneficial for oral tolerance acquisition in mice (Harbige & Fisher, 2001). Moreover, supplementation of the maternal diet with n–3PUFAs during pregnancy and lactation decreased the risk of food allergy in infants with a family history of allergic disease (Furuhjelm et al. 2009).
n–3PUFAs’ beneficial effect upon intestinal inflammation could partly be accounted for by an effect upon the IEB. However, the effects of n–3PUFAs on intestinal permeability are not fully elucidated, as different models and different n–3PUFAs have led to conflicting results. In particular, while long chain PUFAs (20:5n–3 and 22:6n–3) increased paracellular permeability in Caco-2 monolayers (Usami et al. 2001, 2003), Rosella et al. (2000) showed that 20:5n–3 did not modify paracellular permeability in the same model. In contrast, n–3PUFAs (20:5n–3 and 22:6n–3) reduced IL-4 induced increase in permeability in human intestinal epithelial cell culture (T84) (Willemsen et al. 2008). In vivo, in adult rats, dietary n–3PUFAs (20:5n–3 and 22:6n–3) had no effect on paracellular jejunal permeability (Vine et al. 2002). In contrast, supplementation of the maternal diet with fish oil (20:5n–3 and 22:6n–3) increased paracellular permeability in rat colon at 15 days of age (Innis et al. 2010). This led to increased susceptibility to dinitrobenzene sulfonic acid-induced colitis later in life, at 3 months of age (Innis et al. 2010). Finally, besides this unclear effect of n–3PUFAs upon the IEB in adults, their impact on newborn IEB function has not yet been described in much detail.
The IEB function is influenced by various physiological stimuli such as nutrients, mediators from the immune system or the enteric nervous system (ENS) (Pacha, 2000; Keita & Söderholm, 2010). The ENS forms a neuronal network embedded within the gastrointestinal wall and divided into two plexuses: the myenteric and the submucosal plexus. Submucosal neurons innervate the mucosa and regulate absorption and secretion of fluid and electrolytes (Hens et al. 2000). More recently, the role of the ENS on the control of paracellular permeability has been demonstrated. In pigs, tetrodotoxin (TTX, a blocker of Na+ channels) increased basal jejunal paracellular permeability suggesting that a basal neuronal activity exerts an inhibitory tonus on intestinal permeability (Hayden & Carey, 2000). In vivo studies in animal models suggested that different neuromediators participate in this regulation (Hällgren et al. 1998; Hayden & Carey, 2000). In particular, vasoactive intestinal peptide (VIP) has been shown to decrease paracellular permeability in vivo and in vitro (Hällgren et al. 1998; Neunlist et al. 2003; Boudry et al. 2011). Conversely, acetylcholine (ACh) has been shown to increase the paracellular permeability of rat (Phillips et al. 1987), rabbit (Greenwood & Mantle, 1992), mice (Cameron & Perdue, 2007) and piglets (Boudry et al. 2011).
The development of the ENS is associated with a time-dependent change in the expression of major neuromediators within enteric neurons which occurs during the pre- and post-natal period (Newgreen & Young, 2002). In piglet small intestine, 12% of submucosal neurons are VIP-immunoreactive (IR) at birth; this proportion increases to 23% at PND3 (Van Haver et al. 2008) and reaches 50% at 6 weeks post-natal (Hens et al. 2000). Concerning the cholinergic population, in the rat colon, the proportion of choline acetyltransferase (ChAT)-IR neurons increases within the first 5 weeks post-natal from 2 to 13%, and is probably responsible for the development of colonic motility in rat pups (de Vries et al. 2010). By analogy with its impact upon motility, the post-natal maturation of VIP-IR and ChAT-IR neurons could also impact upon the regulation of the IEB function by the ENS. Furthermore, recent data suggest that endogenous (such as glial cell line-derived nerve growth factor) or exogenous (such as butyrate) factors induce neuroplastic changes in ENS and impact on GI functions (Rodrigues et al. 2010; Soret et al. 2010). However, whether n–3PUFAs can induce neuroplastic changes in the ENS and their functional impact upon gut permeability remain currently unknown.
The purpose of this study was therefore to investigate the effect of 18:3n–3 in the maternal diet on the development of intestinal permeability of newborn piglets and the possible involvement of n–3PUFA-induced modifications of the ENS.
Methods
Animals and diets
The experimental protocol was designed in compliance with recommendations of French law (Decree: 2001-464 29/05/01) and EEC (86/609/CEE) for the care and use of laboratory animals under the certificate of authorization to experiment on living animals no. 35–69. Twelve (2 × 6) sows (Large White × Landrace, 241 ± 5 kg) and their piglets ((Large White × Landrace) × Pietrain) from the experimental herd of INRA (St Gilles, France) were used.
Isocaloric and isolipidic diets were formulated accordingly to nutrient and energy needs of pregnant and lactating sows. They were composed of a standard (no lipid added) gestation or lactation diet (Cooperl, Lamballe, France) supplied with 40 ppm α-tocopherol and either lard (control) or linseed oil (n–3 PUFA) (Valorex, France) (Table 1). Sows were separated into two groups at the 28th day of gestation: the first group received the control diet throughout the rest of gestation and lactation while the second received the n–3PUFA diet. Sows were given 2.8 kg day−1 of food during gestation and 7 kg day−1 during lactation, the transition from 2.8 to 7 kg day−1 being gradual within 1 week.
Table 1.
Composition of diets
| Control | n–3PUFA | |||
|---|---|---|---|---|
| Gestation | Lactation | Gestation | Lactation | |
| Ingredients (g kg−1) | ||||
| Wheat | 221 | 227 | 221 | 227 |
| Corn | 100 | 120 | 100 | 120 |
| Barley | 337 | 254 | 337 | 254 |
| Wheat bran | 155 | 100 | 155 | 100 |
| Soybean meal | 90 | 210 | 90 | 210 |
| Sugar beet pulp | 50 | — | 50 | — |
| Calcium carbonate | 19 | 11 | 19 | 11 |
| Dicalcium phosphate | 3 | 13 | 3 | 13 |
| Sodium chloride | 5 | 5 | 5 | 5 |
| Trace element and vitamin mix | 5 | 5 | 5 | 5 |
| Linseed oil | — | — | 15 | 55 |
| Lard | 15 | 55 | — | — |
| Fatty acid composition (g (kg diet)−1) | ||||
| SFA | 9.2 | 33.9 | 4.5 | 9.9 |
| MUFA | 7.7 | 27.0 | 5.4 | 13.6 |
| PUFA | 12.0 | 20.3 | 15.3 | 39 |
| n–6PUFA | 10.5 | 18.1 | 10.0 | 16.4 |
| 18:2n–6 | 10.4 | 17.9 | 10.0 | 16.4 |
| n–3PUFA | 1.5 | 2.0 | 5.3 | 22.6 |
| 18:3n–3 | 1.4 | 1.9 | 5.2 | 22.6 |
| 18:2n–6/18:3n–3 | 7.8 | 9.5 | 2.0 | 0.7 |
Sows were weighed every 3 weeks. Parturitions were not induced. The number of piglets was adjusted to 10–12 piglets 48 h after parturition, by removing the extra piglets from the litter. Piglets were weighed weekly, starting at birth.
Tissue collection
One piglet per litter was killed at birth before suckling (day 0), then at 3, 7, 14, 21 and 28 post-natal days (PND). Piglets were separated from the sow 1 h before killing. Each piglet was killed by electronarcosis and exsanguination. After opening the abdominal cavity, the proximal jejunum was collected. Segments of 20 cm were rinsed and placed in a Ringer bicarbonate solution (Na+ 145 mm, Cl− 128 mm, PO43− 0.32 mm, Ca+ 2 mm, Mg2+ 1 mm, HCO3− 25 mm, SO42− 1 mm, K+ 6.3 mm, pH 7.4) for immediate Ussing chamber experimentation. Segments of 10 cm were immediately frozen for the fatty acid composition and stored at −20°C until analysis. For RNA extraction, 100 mg of jejunum was placed into RNAlater (Applied Biosystems, France) at 4°C for 24 h and then maintained at −20°C for later analysis. For Western blot analysis, segments of 1 cm were immediately frozen and stored at −80°C until analysis. For histomorphometry and immunohistochemistry, segments of 5 cm were stripped of external muscle, stretched and placed in 4% formaldehyde for 4 h and stored in 0.1 m phosphate buffered saline (PBS) containing 0.01% NaN3 at 4°C.
Determination of fatty acid composition
Total lipids of the intestine were extracted with the method described by Folch et al. (1957) (chloroform and methanol; 2:1, v/v). Fatty acid composition was determined as previously described (de Quelen et al. 2010).
Ussing chamber experiments
Jejunal permeability
Segments of proximal jejunum were stripped of external muscle and mounted in Ussing chambers (0.6 cm2 exposed surface area) as previously described (Boudry et al. 2002). Tissues were bathed with a Ringer bicarbonate solution with d-glucose (16 mmol l−1) on the serosal side osmotically balanced by mannitol (16 mmol l−1) on the mucosal side (8 ml on each side). Bathing solutions were maintained at 39°C and gassed with 5% CO2–95% O2. Tissue viability was continuously monitored via transepithelial potential difference measurement every 30 min. Fluorescein-isothiocyanate conjugated dextran 4000 Da (FD4, Sigma, France) was used to examine intestinal permeability. FD4 (375 mg l−1) was added to the mucosal buffer 10 min after tissues had been mounted. Serosal samples (500 μl) were taken at 30 min intervals for 2 h and replaced with fresh buffer to maintain constant volume. The concentration of FD4 in the samples was measured with a fluorimeter (fluorimeter LB940 Mithras, Berthold) and fluxes expressed as nanogram per square centimetre per hour (ng cm−2 h−1).
Neuropharmacology of paracellular permeability
The general neurotoxin TTX (10−6m, Sigma), the neuropeptide VIP (10−6m, Sigma), the specific VIP receptor antagonist VIPra (VIP6-28 10−6m, Sigma), the acetylcholine receptor agonist carbachol (CCH, 10−3m, Sigma) or the specific acetylcholine receptor antagonists atropine (muscarinic antagonist, 10−5m, Sigma) and hexamethonium (nicotinic antagonist, 10−5m, Sigma) were added to the serosal side of the chamber before the addition of FD4 to the mucosal side, and the 2 h flux was determined.
Morphometry
Histological sections (5 μm) were stained with haematoxylin and eosin then examined under a light microscope (Nikon ECLIPSE E400, Nikon Instruments, France) and image analysis software (NIS-Elements AR3.0, Nikon Instruments). Villi height and area and crypt depth were measured in 15–20 well-oriented crypt-villus units per piglet.
Immunohistochemistry and identification of cholinergic and VIPergic neuronal cell populations
Inner submucosal plexus were obtained by microdissection of the fixed proximal jejunum. They were then permeabilized for 24 h in PBS/NaN3 containing 0.5% Triton X-100 (Sigma, France) and 4% horse serum (Sigma). Submucosal plexuses were incubated overnight at 4°C with rabbit anti-protein gene product 9.5 (PGP9.5, 1:2000; Tebu-bio; France) and mouse anti-VIP (1:1000; Biogenesis, UK) or goat anti-ChAT (1:200; Millipore, France). After incubation with primary antibodies, tissues were incubated for 3h at room temperature with the following secondary antibodies coupled to fluorochromes: anti-rabbit IgG (for PGP9.5 revelation) conjugated to fluorescein isothiocyanate (1:200; Jackson ImmunoResearch Laboratories, Inc., USA) and either anti-mouse IgG (for VIP revelation) or anti-goat IgG (for ChAT revelation) both conjugated to carboxymethylindocyanine (1:500; Jackson ImmunoResearch). Pictures were acquired with a digital camera (Nikon DS-Ri1, Nikon Instruments, France) coupled to a fluorescence microscope (Nikon ECLIPSE E400) and analysed with image analysis software (NIS-Elements AR3.0, Nikon Instruments). The number of ChAT-IR or VIP-IR cells and PGP9.5-IR cells was counted in at least 10 ganglia per tissue and per condition. Data are expressed as the percentage of ChAT-IR or VIP-IR neurons normalized to the total number of PGP9.5-IR neurons.
Expression of neuronal marker, tight junction protein and cytokines by RT-PCR analysis
Total RNA was extracted from tissue as previously described (Boudry et al. 2009). Primers were designed using Primer Express Software (Applied Biosystems) or Primer Blast (NCBI) based on Sus scrofa published nucleotides sequences (Icare) and are described in Table 2. Real time RT-PCR was performed as previously described (Boudry et al. 2009).
Table 2.
Primer sequences used in real-time PCR reactions
| Gene | Protein name | Forward Primer | Reverse Primer | Accession no. |
|---|---|---|---|---|
| ChAT | Choline acetyl transferase | 5′-AGCTAGCCTTCTACAGGCTCCAT-3′ | 5′-CGCTCTCATAGGTAGGCACGA-3′ | J03021 |
| Claudin 1 | Claudin 1 | 5′-CGGCCCTTGAGCCGGTGCTA-3′ | 5′-TCTGCCCGGTGCTCTGCGAC -3′ | FJ873109 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase | 5′-CATCCATGACAACTTCGGCA-3′ | 5′-GCATGGACTGTGGTCATGAGTC-3′ | AF017079 |
| IL-1β | Interleukin-1β | 5′-GTGATGGCTAACTACGGTGACAA-3′ | 5′-CTCCCATTTCTCAGAGAACCAAG-3′ | M86725 |
| IL-6 | Interleukin-6 | 5′-GTCGAGGCTGTGCAGATTAGT-3 | 5′-TTCTGTGACTGCAGCTTATCC-3′ | M80258 |
| IL-10 | Interleukin-10 | 5′-CGGCGCTGTCATCAATTTCTG-3′ | 5′-CCCCTCTCTTGGAGCTTGCTA-3′ | L20001 |
| occludin | occludin | 5′-AGGTGCACCCTCCAGATTGGCT-3′ | 5′-AGCATTGGTCGAATGGGCGTC -3′ | FN400888 |
| S6 | 5′-GTTGGATCTCTTTTTCGTGGCGCC-3′ | 5′-TCCCACCACTGATTCGGACCACAT -3 | XM_003121890 | |
| TNFα | Tumour necrosis factor | 5′-ATCGGCCCCCAGAAGGAAGAG-3′ | 5′-GATGGCAGAGAGGAGGTTGAC-3′ | M29079 |
| VIP | Vasoactive intestinal peptide | 5′-GCCTCACGGAACTTCAGCACCC-3′ | 5′-AGTCTGTCCCTCAGAGCAGAAGGT-3′ | GU576974 |
| ZO-1 | Zonula occludens 1 | 5′-CGGCCCTTGAGCCGGTGCTA-3′ | 5′-TCTGCCCGGTGCTCTGCGAC -3′ | XM_003121672 |
Expression of tight junction proteins (zonula occludens 1, claudin 4 and occludin) by Western blot analysis
Samples of 100 mg of jejunum were homogenized in glass tubes containing 1ml of extracting buffer (1ml of tissue protein extraction reagent (Pierce, France), 1 mm of protease inhibitor cocktail (Sigma, France), 1 mm of sodium orthovanadate (Sigma) and 1 mm of phenylmethylsulfonyl fluoride (Sigma)) for 15 min on ice and centrifugated (10,000 g, 15 min, 4°C). Supernatants were collected and stored at −80°C. An aliquot of 30 μg protein was mixed with a loading buffer (0.5 m Tris-HCl, 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromophenol blue) and boiled for 5 min at 95°C before loading onto one-dimension SDS-PAGE gels (10%). For zonula occludens 1 (ZO-1) analysis, proteins were extracted using the Nucleospin RNA/Protein kit (Macherey-Nagel, Hoerdt, France) according to manufacturer's instructions with the following modifications. The protein pellets were resuspended in 1% SDS, sonicated and diluted 1:1 in PSB-tris(2-carboxyethyl)phosphine (TCEP) before incubation for 3 min at 95°C for complete protein dissolving and denaturation. Forty micrograms of proteins was loaded onto 4–8% SDS-polyacrylamide gels. After electrophoresis, proteins were transferred to a nitrocellulose membrane (Amersham Biotech, USA) and blocked in blocking buffer (0.1 m PBS, 0.05% Tween 20 (Sigma, France), 3% powdered milk). Blots were then incubated with different primary antibodies overnight at 4°C: mouse monoclonal anti-claudin4 (22 kDa, 1:200, Invitrogen, USA); rabbit monoclonal anti-actin (45 kDa, 1:2000, Sigma, USA); affinity purified rabbit anti-ZO-1 (220 kDa, 1:500, Zymed Laboratories, USA) or rabbit polyclonal anti-occludin (65 kDa, 1:250, Tebu-bio, France). After three washes with PBS, membranes were incubated for 2 h with secondary horseradish peroxidase conjugated antibodies raised against goat, mouse (1:10,000) or rabbit (1:5000) IgG (Santa Cruz Biotechnology, Inc., USA or ThermoFisher, France). Signals were revealed by the ECL Plus Western Blot detection kit (Amersham Biotech, USA). Autoradiograms were scanned, and densitometric values (arbitrary units) were determined using ImageJ or the ImageQuant software (Bio-Rad).
Effect of n–3 PUFAs on enteric neurons phenotype in cell culture
Primary enteric neuron cultures were prepared from the small intestine of embryos of rats (E15) as described by Chevalier et al. (2008). Cells were maintained in culture for 14 days, half of the medium (DMEM–F12 (1:1) containing 1% of N-2 supplement, Invitrogen, France) being replaced every 2 days. Cultures were then incubated with either 18:3n–3 (C18H29O2Na, Biovalley, France) or 20:5n–3 (C20H29O2Na, Sigma) at two concentrations (1 and 10 μm) for 48 h. n–3PUFAs were diluted in the culture medium. Ascorbic acid at 0.1 mm (Sigma) was added to the medium as antioxidant.
After incubation of cultures with n–3PUFAs during 48 h, cells were fixed in 0.1 m PBS containing 4% paraformaldehyde for 3 h at room temperature. After washes with 0.1 m PBS, they were then permeabilized for 30 min with 0.1 m PBS containing 0.1% NaN3, 0.5% Triton X-100 (Sigma) and 4% horse serum (Sigma). Cells were then incubated sequentially with the primary antibodies for 1.5 h and the secondary antibodies for 30 min (with washes with 0.1 m PBS in between) in the following order: mouse anti-VIP (1:800; Biogenesis, USA) followed by donkey anti-mouse IgG conjugated to FluoProbes 488 (1:200; Interchim, France); goat anti-ChAT (1:200; Millipore) followed by anti-goat IgG conjugated to carboxymethylindocyanine (1:500; Jackson ImmunoResearch); and finally mouse anti-HuC/HuD (1:200; Invitrogen) and anti-mouse IgG conjugated to 7-amino-4-indodicarbocyanin (1:500; Jackson ImmunoResearch). Pictures were acquired with a digital camera (model DP71, Olympus, France) coupled to a fluorescence microscope (Olympus IX 50) and analysed with the Cell B software (Soft Imaging System, Olympus). To determine the general neurochemical phenotype, an average of 20 ganglia were analysed per well and per condition. Data are expressed as the percentage of ChAT-IR or VIP-IR neurons normalized to the total number of HU-IR neurons.
Statistical analysis
Data are expressed as mean values with the standard error of the mean. The significance of differences was determined using the Student's t test. For time course experiments in Ussing chambers, a two-way ANOVA was employed. Differences were considered significant for P≤ 0.05.
Results
Effect of n–3PUFAs in the maternal diet on piglet growth
The maternal diet had no effect on litter size (number of piglets per litter: 14.8 ± 1.3 for control group vs. 13.6 ± 1.4 for n–3PUFA, P > 0.05) or mean birth weight of live piglets (1.65 ± 0.1 for control group vs. 1.61 ± 0.09 kg for n–3PUFAs, P > 0.05). Average piglet daily weight gain from birth to PND28 was not different between the two groups (0.27 ± 0.01 for control group vs. 0.25 ± 0.01 kg day−1 for n–3PUFAs, P > 0.05).
Effect of n–3PUFAs in the maternal diet on the development of piglet jejunal permeability and structure
Basal jejunal permeability to FD4 increased until PND14 in both groups starting at birth (Fig. 1). After PND14, paracellular permeability decreased in both groups but was higher in n–3PUFA piglets compared to control piglets at PND28 (+76%, P < 0.05, Fig. 1). We next determined if the difference in intestinal permeability at PND28 could be due to difference in the mucosal structure of the jejunum. Villus height (262 ± 19 for control vs. 258 ± 15 μm for n–3PUFA, P > 0.05), villus area (41,190 ± 5600 for control vs. 43,666 ± 5500 μm2 for n–3PUFA, P > 0.05) and crypt depth (232 ± 18 for control vs. 229 ± 16 μm for n–3PUFA, P > 0.05) of piglet jejunum did not differ between the two groups. At PND28, real-time PCR analysis revealed that the n–3PUFA diet had no effect on jejunal mRNA relative expression of key tight junction proteins (P > 0.05, Table 3). Western blot analysis did not reveal any difference between dietary groups for claudin 4 expression (0.4 ± 0.05 for control vs. 0.5 ± 0.04 claudin4/β-actin for n–3PUFA, P > 0.05) and occludin expression (0.4 ± 0.1 for control vs. 0.3 ± 0.1 occludin/β-actin for n–3PUFA, P > 0.05). However, ZO-1 expression was increased in n–3PUFA piglet intestine compared to control animals (P < 0.05, Fig. 2). We also investigated whether n–3PUFA diet induced changes in cytokine expression in the jejunum. RT-PCR analysis revealed no difference in cytokines mRNA level (IL-10, IL-1β, IL-6 and TNFα, P > 0.05, Table 3).
Figure 1. Effect of n–3PUFAs in the maternal diet on the development of piglet jejunal permeability.
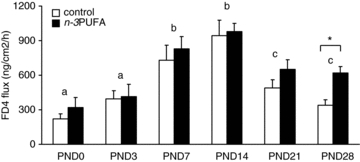
Basal jejunal permeability to FD4 was measured in Ussing chambers from birth to 28 post-natal days in control (open bar) and n–3PUFA (black bar) piglets. Bars are mean values with s.e.m. for six piglets. *Significantly different between the two groups, P < 0.05. a,b,c significantly different between days, irrespective of the diet, P < 0.05.
Table 3.
Levels of tight junction protein and cytokines mRNA in the jejunum of control and n–3PUFA piglets at PND28
| Control | n–3PUFA | |
|---|---|---|
| ZO-1a | 0.8 ± 0.3 | 2.2 ± 1.9 |
| Claudin-1a | 0.8 ± 0.4 | 1.4 ± 1.2 |
| Occludina | 0.6 ± 0.1 | 0.5 ± 0.03 |
| IL-10b | 1.5 ± 0.6 | 1.3 ± 0.6 |
| IL-1βb | 2.3 ± 0.8 | 2.6 ± 1.0 |
| IL-6b | 1.9 ± 1.6 | 4.3 ± 2.7 |
| TNFαb | 0.9 ± 0.3 | 1.5 ± 0.5 |
Normalized to S6 mRNA level
normalized to GAPDH mRNA level.
Figure 2. Effect of n–3PUFAs in the maternal diet on ZO-1 expression at PND28.
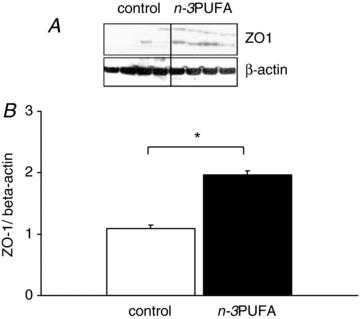
Expression of ZO-1 protein was measured in control (open bar) and n–3PUFA (black bar) piglets at PND28. A, typical blot example. B, quantification of protein density. Bars are mean values with s.e.m. for five piglets. *Significantly different between the two groups, P < 0.05.
Effect of n–3PUFAs in the maternal diet on fatty acid composition of piglet jejunum at PND28
Total lipid content and proportions of saturated fatty acid (SFA) and monounsaturated fatty acid (MUFA) were similar between the two groups (Table 4). There was no difference in the proportion of n–6PUFAs between the two groups but the proportion of n–3PUFAs in n–3PUFA piglets was greater than in controls (P < 0.05, Table 4). Within the n–3PUFA family, the jejunum of n–3PUFA piglets contained 10-fold more 18:3n–3 (P < 0.05, Table 4) and eightfold more 20:5n–3 (P < 0.05, Table 4) than that of control piglets. There was no difference between the two groups for 22:6n–3 (Table 4). Concerning the n–6PUFA family, proportions of 18:2n–6 and 20:4n–6 were similar between the two groups (Table 4).
Table 4.
Fatty acid composition of the jejunum of control and n–3PUFA piglets at PND28
| Control | n–3PUFA | |
|---|---|---|
| Total lipid content (g (100 g of tissue)−1) | 3.2 ± 0.9 | 3.4 ± 0.7 |
| Fatty acid composition (g (100g of total fatty acid)−1) | ||
| SFAs | 40.5 ± 1.7 | 34.6 ± 4.5 |
| MUFAs | 26.0 ± 4.5 | 21.3 ± 4.8 |
| PUFAs | 33.5 ± 6.0 | 44.3 ± 1.6* |
| n–6PUFA | 29.5 ± 3.2 | 26.5 ± 2.2 |
| 18:2n–6 | 21.1 ± 1.3 | 21.6 ± 0.4 |
| 20:4n–6 | 6.8 ± 1.8 | 4.3 ± 2.5 |
| n–3PUFA | 3.5 ± 0.3 | 17.4 ± 4.6* |
| 18:3n–3 | 1.1 ± 0.4 | 11.2 ± 4.0* |
| 20:5n–3 | 0.3 ± 0.2 | 2.4 ± 1.3* |
| 22:6n–3 | 0.9 ± 0.3 | 0.7 ± 0.3 |
| n–6PUFA/n–3PUFA | 8.4 ± 0.6 | 1.6 ± 0.5* |
SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid. Values are mean values ±s.e.m. for six piglets.
Effect of n–3PUFAs in the maternal diet on nervous regulation of piglet jejunal permeability at PND28
We next investigated if the difference in permeability at PND28 was associated with a difference in the nervous regulation of this permeability. TTX increased basal permeability in the control group (+25%, P < 0.05). In contrast, in the n–3PUFA group, TTX tended to decrease basal permeability (−20%, P = 0.07). VIPra increased basal permeability in the control group (+60%, P < 0.05) but had no effect in the n–3PUFA group (Fig. 3A). The mix of atropine and hexamethonium had no effect on basal permeability in the control group but decreased it in the n–3PUFA group (−30%, P < 0.05, Fig. 3B).
Figure 3. Effect of n–3PUFAs in the maternal diet on piglet jejunal permeability responses to VIP and acetylcholine antagonists at PND28.
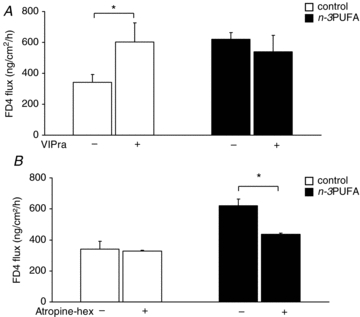
Jejunal permeability to FD4 of control (open bar) and n–3PUFA (black bar) piglets at PND28 was measured in Ussing chambers after addition of VIP antagonist (VIPra) (A) or cholinergic antagonists (mix of atropine and hexamethonium) (B). Bars are mean values with s.e.m. for six piglets. *Significantly different between basal condition and antagonist condition, P < 0.05.
VIP did not modify basal permeability in the control group but decreased it in the n–3PUFA group (−100%, P < 0.05, Fig. 4A). CCH increased basal permeability in both group (control, +100%, P < 0.05 and n–3PUFA, +50%, P < 0.05, Fig. 4B).
Figure 4. Effect of n–3PUFAs in the maternal diet on piglet jejunal permeability responses to VIP and acetylcholine agonists at PND28.
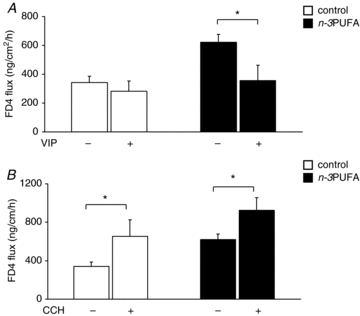
Jejunal permeability to FD4 of control (open bar) and n–3PUFA (black bar) piglets at PND28 was measured in Ussing chambers after addition of VIP (A) or carbachol (B). Bars are mean values with s.e.m. for six piglets. *Significantly different between basal condition and agonist condition P < 0.05.
Effect of n–3PUFAs in the maternal diet on the neurochemical phenotype of piglet jejunal inner submucosal plexus at PND28
We next determined the effect of the maternal diet on neurochemical phenotype of the inner submucosal plexus. The maternal n–3PUFA diet did not change the number of neurons per ganglion (69.4 ± 15.0 for control vs. 68.1 ± 5.0 for n–3PUFA, P > 0.05). However, it increased the proportion of ChAT-IR neurons (+17%, P < 0.05, Fig. 5A) and decreased the proportion of VIP-IR neurons (−18%, P < 0.05, Fig. 5B) in the inner submucosal plexus of piglet jejunum. This effect was associated with an increased ChAT mRNA expression in the n–3PUFA group (1.1 ± 0.3 for control vs. 1.9 ± 0.3 for n–3PUFA, P < 0.05) but not of VIP mRNA (0.7 ± 0.1 for control vs. 0.6 ± 0.1 for n–3PUFA, P > 0.05).
Figure 5. Effect of n–3PUFAs in the maternal diet on the neurochemical phenotype of the inner submucosal plexus of piglet jejunum at PND28.
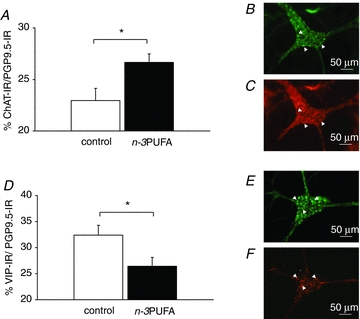
The proportion of cholinergic (A) and VIPergic neurons (D) was measured by immunohistochemistry in the inner submucosal plexus of control (open bar) and n–3PUFA (black bar) piglet jejunum at PND28. B and C, typical example of ChAT-immunostaining (B: PGP9.5- immunoreactivity, C: ChAT- immunoreactivity). E and F, typical example of VIP-immunostaining (E: PGP9.5-immunoreactivity, F: VIP-immunoreactivity). A and D: bars are mean values with s.e.m. for six piglets. *Significantly different between the two groups, P < 0.05; white arrowhead: IR-neuron.
Effect of n–3PUFAs on primary culture of enteric neurons
We next determined whether 18:3n–3 or its derivative, 20:5n–3, could modify the phenotype of enteric neurons in primary culture. n–3PUFAs did not modify neuronal cell death, since neuron specific enolase release was not different between the different culture conditions (data not shown). There was no difference in the proportion of cholinergic and VIPergic neurons when primary cultures were incubated with 18:3n–3 at any concentration (Fig. 6A and B). However, the proportion of ChAT neurons increased after incubation with 20:5n–3 at 10 μm (P < 0.05, Fig. 6A). The proportion of VIPergic neurons decreased after incubation with 20:5n–3 at 10 μm (P < 0.05, Fig. 6B).
Figure 6. Effect of n–3PUFAs on primary culture of enteric neurons.
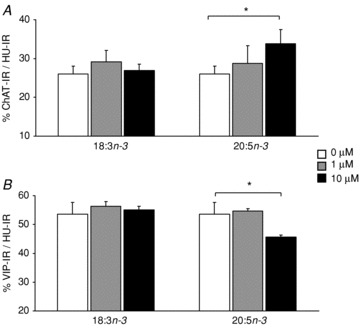
The proportion of cholinergic (A) and VIPergic (B) neurons was measured by immunohistochemistry in primary culture of rat enteric neurons after incubation for 48 h with 18:3n–3 or 20:5n–3 at 0 (open bar), 1 μm (grey bar) and 10 μm (black bar). Bars are mean values with s.e.m. for six primary cultures of enteric neurons. *Significantly different compared to 0 μm, P < 0.05.
Discussion
Our study demonstrated that supplementation of the maternal diet with 18:3n–3 modifies the development of IEB function of newborn piglets which exhibited increased jejunal permeability at PND28. This effect was correlated with modifications in the neurochemical phenotype of the ENS and consequently to modifications of the nervous regulation of intestinal permeability.
A major finding of our study is that basal jejunal permeability to FD4 was modified by the maternal diet with higher jejunal permeability in the n–3PUFA compared to control group at PND28. Few studies have investigated the effect of maternal diet on offspring IEB function. Our group recently described modification in mast-cell regulation of ileal barrier function in piglets by incorporation of n–3PUFAs in the maternal diet (Boudry et al. 2009). Moreover, a recent study demonstrated that supplementation of the maternal diet with long-chain n–3PUFAs during gestation and lactation increased paracellular permeability of rat colon at 15 days of age (Innis et al. 2010), which corroborates our results. Since the IEB function can be partly dependent upon mucosal structure of the jejunum and n–3PUFAs have been shown to affect epithelial cell turnover (Courtney et al. 2007), we checked if the difference observed in intestinal permeability at PND28 was due to difference in the mucosal morphology of the jejunum. We did not observe any difference between the two groups of piglets concerning villus height, villus area or crypt depth, suggesting that the cellular mechanisms involved in the increased permeability observed in the n–3PUFA group are not linked to the mucosal structure of the jejunum. At the tight junction protein level, mRNA expression of the main proteins was not affected by the maternal diet. Claudin 4 and occludin protein levels were also not affected. However, we observed a higher ZO-1 protein expression in the n–3PUFA group as compared to control animals. Although increase in ZO-1 expression is often associated with a reduced permeability, studies also pointed out the role of ZO-1 localisation (i.e. anchored to tight junctions or cytosolic) rather than protein quantity in the regulation of tight junction opening (McCall et al. 2009). Further work is therefore needed to precisely determine the localization of ZO-1 protein.
Another major finding of this study is that difference in basal permeability induced by the maternal diet is under the control of distinct neuronal pathways, as demonstrated by the use of different antagonists of the ENS. In the control group, VIPra increased basal intestinal permeability while the mix of atropine and hexamethonium did not modify it, suggesting an inhibitory VIPergic tonus (Neunlist et al. 2003). On the other hand, in the n–3PUFA group, VIPra had no effect on jejunal permeability but atropine and hexamethonium decreased it, suggesting a stimulatory cholinergic tonus (Cameron & Perdue, 2007). To our knowledge, this is the first report of an effect of the maternal diet on the functioning of the enteric nervous system of newborn. The modifications of nervous regulation of jejunal permeability corroborate the modifications in the neurochemical phenotype of the ENS, since the maternal 18:3n–3 diet increased the proportion of cholinergic neurons, while the proportion of VIPergic neurons decreased in the submucosal plexus of piglet jejunum. The development of ENS occurs during pre- and post-natal periods, with different patterns of development depending on the neuromediator considered. In particular, cholinergic neuronal population increased late in the post-natal period, between PND10 and 20, in rat myenteric plexus (de Vries et al. 2010). If the same phenomenon is true in piglets, this could explain why we observed the impact of maternal diet upon cholinergic phenotype only at PND28 in our study. The effect of diet-derived factors on the ENS phenotype and/or functions has already been reported. For example, a high cholesterol diet changes the cholinergic neurotransmission in the enteric nerves of the jejunum of squirrels which increased contractions of jejunal smooth muscle (Mathison & Shaffer, 2006). Butyrate increases the proportion of cholinergic neurons in the ENS and increases the cholinergic-mediated colonic muscle contractile response in rat (Soret et al. 2010). Introduction of enteral food increased the proportion of VIPergic neurons in piglet ENS (Van Haver et al. 2008). The only studies investigating the effect of n–3PUFAs on nervous regulation of intestinal function focused on ACh-induced ileal contractility in adult rats. These authors showed that dietary long chain n–3PUFAs (20:5n–3 and 22:6n–3 brought by fish oil in the diet) increased ACh-induced contractility of rat ileum (Patten et al. 2002) probably through altered sensitivity of muscarinic receptor (Patten et al. 2005). However these authors did not study the ENS phenotype while we clearly show a direct effect of n–3PUFA on the ENS.
To investigate the specific effect of each n–3PUFA on the enteric nervous system, we used primary culture of enteric neurons. Incubation of primary culture with 20:5n–3 increased the proportion of cholinergic neurons and decreased the proportion of VIPergic neurons, while incubation with 18:3n–3 had no effect. The maternal diet modified the fatty acid composition of the jejunum with higher proportion of 18:3n–3 and 20:5n–3 We can therefore speculate that in vivo 20:5n–3 mediated the modification of the phenotype of ENS. Further investigations will be necessary to elucidate the cellular and/or molecular mechanisms of action involved in the effect of 20:5n–3 upon the ENS. The mechanisms of action of long chain (LC)-PUFAs on neuronal cells still remain unknown. Several potential cellular pathways have been proposed. Indeed, by modifying composition of membrane phospholipids, LC-PUFAs can modulate activity of enzymes, transporters and receptors involved in neuronal functions. They can also be involved in alteration of neuronal signalling pathway by synthesis of bioactive mediators such as eicosanoid or resolvin. Finally, they can participate in regulation of gene expression involved in neuronal proliferation and differentiation by direct or indirect interaction with transcription factors such as proliferator-activated receptor or sterol regulatory element binding protein.
Despite the increased jejunal permeability at PND28, pro-inflammatory cytokine mRNA levels were not increased in the jejunum of n–3PUFA piglets. Several explanations can account for by this absence of inflammatory consequence of altered barrier function. First, n–3PUFAs are consensually known for their anti-inflammatory action, also at the intestinal level (Calder, 2009). The level of 20:5n–3, a precursor of potent anti-inflammatory prostaglandins, was increased in the jejunum of n–3PUFA piglets but also in their plasma (de Quelen et al. 2010). The immunosuppressive action of n–3PUFAs could therefore counteract any inflammatory process induced by the altered barrier function. Secondly, it has been demonstrated that vagus cholinergic nervous system attenuates the production of pro-inflammatory cytokines and inhibits inflammatory processes (Van Der Zanden et al. 2009). Acetylcholine inhibits cytokine release directly via the α-7 nicotinic ACh receptor (nAChR) expressed on macrophages and other immune cells (Van Der Zanden et al. 2009). Recent data also suggested an action of ACh on nicotinic receptor of colonic cell (Meregnani et al. 2011). We can hypothesized that the ongoing release of endogenous acetylcholine from enteric nerves in n–3PUFA piglets exerts an anti-inflammatory action through nicotinic receptors located on immune or epithelial cells, thereby counteracting the potential harmful effect of the altered barrier function. Lastly, whereas increased intestinal permeability during adulthood has been described as an aetiological factor of intestinal inflammatory conditions, such a paradigm might not completely apply in early life. Indeed, several papers show a correlation between increased permeability and maturation of the immune system (Gebbers & Laissue, 2004, Urao et al. 1996). Indeed, an enhanced passage of luminal contents (dietary antigen and commensal microflora) would participate in the development and maturation of the local immune system. The anti-inflammatory effect of n–3PUFAs and/or of ACh through nicotinic receptors would participate in this equilibrium between excessive inflammation versus maturation of the local immune system.
In conclusion, the ENS is highly plastic during the post-natal period and can adapt in a diet-dependent manner with functional consequences on mucosal barrier. Supplementation of the maternal diet with 18:3n–3 modified the neurochemical phenotype of the ENS, with the induction of cholinergic phenotype and modified the nervous regulation of jejunal permeability, with the induction of a cholinergic tonus. Both VIPergic and cholinergic regulation of epithelial barrier function have been shown to be TTX insensitive, suggesting a direct effect of ACh and VIP upon epithelial cells (Neunlist et al. 2003, Cameron & Perdue, 2007). Therefore, neuroplastic changes in neuronal ChAT or VIP expression by the maternal n–3PUFA diet would impact on enteric neuronal control of barrier function at the epithelial cell level. The functional consequence is increased jejunal permeability at 28 post-natal days with no effect on the inflammatory state of the mucosa.
Acknowledgments
Authors would like to thank Cécile Perrier, Véronique Romé, Armelle Cahu, Martine Fillaut, Philippe Aubert, Daniel Boutin, Yannick Surel, René Bouetard and Henri Renoult for laboratory analyses and technical help. The study was funded by INRA.
Glossary
Abbreviations
- CCH
carbachol
- ChAT
choline acetyltransferase
- ENS
enteric nervous system
- FD4
FITC-dextran 4000
- IEB
intestinal epithelial barrier
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IL-10
interleukin-10
- LC-PUFA
long-chain polyunsaturated fatty acid
- MUFA
monounsaturated fatty acid
- PGP9.5
protein gene product 9.5
- PND
post-natal day
- PUFA
polyunsaturated fatty acid
- SFA
saturated fatty acid
- TNFα
tumour necrosis factor α
- TTX
tetrodotoxin
- VIP
vasoactive intestinal peptide
- VIPra
VIP receptor antagonist
- ZO-1
zonula occludens 1
Author contributions
Experiments on animal were performed at UMR 1079 SENAH, Institut National de la Recherche Agronomique, St-Gilles, France. In vitro experiments were performed at the ‘Neuropathies of the Enteric Nervous System and Digestive Diseases’ Unit, U913, Institut National de la Santé et de la Recherche Médicale, Nantes, France. Study concept and design: F.D.Q, M.N. and G.B. Analysis and interpretation of data: F.D.Q, J.C., M.R.D, M.N. and G.B. Drafting of the article: F.D.Q, M.N. and G.B. Critical revision for important intellectual content: F.D.Q, J.C., M.R.D., J.M., M.N. and G.B. Final approval of the article: F.D.Q, J.C., M.R.D., J.M., M.N. and G.B. Author disclosures: the authors have no conflict of interest.
References
- Boudry G, Douard V, Mourot J, Lallès JP, Le Huërou-Luron I. Linseed oil in the maternal diet during gestation and lactation modifies fatty acid composition, mucosal architecture, and mast-cell regulation of the ileal barrier in piglets. J Nutr. 2009;139:1110–1117. doi: 10.3945/jn.108.102640. [DOI] [PubMed] [Google Scholar]
- Boudry G, Lallès JP, Malbert CH, Bobillier E, Sève B. Diet-related adaptation of the small intestine at weaning in pigs is functional rather than structural. J Pediatr Gastroenterol Nutr. 2002;34:180–187. doi: 10.1097/00005176-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Boudry G, Morise A, Sève B, Le Huërou-Luron I. Effect of milk formula protein content on intestinal barrier function in a porcine model of LBW neonates. Pediatr Res. 2011;69:4–9. doi: 10.1203/PDR.0b013e3181fc9d13. [DOI] [PubMed] [Google Scholar]
- Calder PC. Fatty acids and immune function: relevance to inflammatory bowel disease. Int Rev Immunol. 2009;28:506–534. doi: 10.3109/08830180903197480. [DOI] [PubMed] [Google Scholar]
- Cameron HL, Perdue MH. Muscarinic acetylcholine receptor activation increases transcellular transport of macromolecules across mouse and human intestinal epithelium in vitro. Neurogastroenterol Motil. 2007;19:47–56. doi: 10.1111/j.1365-2982.2006.00845.x. [DOI] [PubMed] [Google Scholar]
- Caplan MS, Jilling T. The role of polyunsaturated fatty acid supplementation in intestinal inflammation and neonatal necrotizing enterocolitis. Lipids. 2001;36:1053–1057. doi: 10.1007/s11745-001-0816-3. [DOI] [PubMed] [Google Scholar]
- Chevalier J, Derkinderen P, Gomes P, Thinard R, Naveilhan P, van den Berghe P, Neunlist M. Activity-dependent regulation of tyrosine hydroxylase expression in the enteric nervous system. J Physiol. 2008;586:1963–1975. doi: 10.1113/jphysiol.2007.149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney ED, Matthews S, Finlayson C, Di Pierro D, Belluzzi A, Roda E, Kang JY, Leicester RJ. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int J Colorectal Dis. 2007;22:765–776. doi: 10.1007/s00384-006-0240-4. [DOI] [PubMed] [Google Scholar]
- da Silva J, de Sousa M, Cara DC, Alvarez-Leite JI, Russo M, Vaz NM, de Faria AMC. Stimulation by food proteins plays a critical role in the maturation of the immune system. Int Immunol. 2002;15:447–455. doi: 10.1093/intimm/dxg043. [DOI] [PubMed] [Google Scholar]
- de Quelen F, Boudry G, Mourot J. Linseed oil in the maternal diet increases long chain-polyunsaturated fatty acid status of the foetus and the newborn during the suckling period in pigs. Br J Nutr. 2010;104:533–543. doi: 10.1017/S0007114510000772. [DOI] [PubMed] [Google Scholar]
- de Vries P, Soret R, Suply E, Heloury Y, Neunlist M. Post-natal development of myenteric neurochemical phenotype and impact upon neuromuscular transmission in the rat colon. Am J Physiol Gastrointest Liver Physiol. 2010;299:G539–547. doi: 10.1152/ajpgi.00092.2010. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Freier S. Development of humoral immunity in the alimentary system. In: Lebenthal E, editor. Human Gastrointestinal Development. New York: 1989. pp. 709–723. [Google Scholar]
- Furuhjelm C, Warstedt K, Larsson J, Fredriksson M, Böttcher MF, Fälth-Magnusson K, Duchen K. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr. 2009;98:1461–1467. doi: 10.1111/j.1651-2227.2009.01355.x. [DOI] [PubMed] [Google Scholar]
- Gebbers JO, Laissue JA. Bacterial translocation in the normal human appendix parallels the development of the local immune system. Ann NY Acad Sci. 2004;1029:337–343. doi: 10.1196/annals.1309.015. [DOI] [PubMed] [Google Scholar]
- Greenwood B, Mantle M. Mucin and protein release in the rabbit jejunum: effects of bethanecol and vagal nerve stimulation. Gastroenterology. 1992;103:496–505. doi: 10.1016/0016-5085(92)90839-q. [DOI] [PubMed] [Google Scholar]
- Hällgren A, Flemström G, Nylander O. Interaction between neurokinin A, VIP, prostanoids, and enteric nerves in regulation of duodenal function. Am J Physiol Gastrointest Liver Physiol. 1998;275:95–103. doi: 10.1152/ajpgi.1998.275.1.G95. [DOI] [PubMed] [Google Scholar]
- Harbige LS, Fisher BAC. Dietary fatty acid modulation of mucosally-induced tolerogenic immune responses. Proc Nutr Soc. 2001;60:449–456. doi: 10.1079/pns2001123. [DOI] [PubMed] [Google Scholar]
- Hayden UL, Carey HV. Neural control of intestinal ion transport and paracellular permeability is altered by nutritional status. Am J Physiol Regul Integr Comp Physiol. 2000;278:1589–1594. doi: 10.1152/ajpregu.2000.278.6.R1589. [DOI] [PubMed] [Google Scholar]
- Hens J, Schrodl F, Brehmer A, Adriaensen D, Neuhuber W, Scheuermann DW, Schemann M, Timmermans JP. Mucosal projections of enteric neurons in the porcine small intestine. J Comp Neurol. 2000;421:429–436. [PubMed] [Google Scholar]
- Innis SM, Dai C, Wu X, Buchan AMJ, Jacobson K. Perinatal nutrition alters early intestinal development and programs the response to experimental colitis in young adult rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1376–1385. doi: 10.1152/ajpgi.00258.2010. [DOI] [PubMed] [Google Scholar]
- Israel EJ. Neonatal necrotizing enterocolitis, a disease of the immature intestinal mucosal barrier. Acta Paediatr Suppl. 1994;396:27–32. doi: 10.1111/j.1651-2227.1994.tb13238.x. [DOI] [PubMed] [Google Scholar]
- Jacobson K, Mundra H, Innis SM. Intestinal responsiveness to exprimental colitis in young rats is altered by maternal diet. Am J Physiol Gastrointest Liver Physiol. 2004;289:13–20. doi: 10.1152/ajpgi.00459.2004. [DOI] [PubMed] [Google Scholar]
- Keita AV, Söderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil. 2010;22:718–733. doi: 10.1111/j.1365-2982.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- Mathison R, Shaffer E. Increased cholinergic contractions of jejunal smooth muscle caused by a high cholesterol diet are prevented by the 5-HT agonist-tegaserod. BMC Gastroenterol. 2006;6:1–9. doi: 10.1186/1471-230X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall IC, Betanzos A, Weber DA, Nava P, Miller GW, Parkos CA. Effects of phenol on barrier function of a human intestinal epithelial cell line correlate with altered tight junction protein localization. Toxicol Appl Pharmacol. 2009;241:61–70. doi: 10.1016/j.taap.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meregnani J, Clarençon D, Vivier M, Peinnequin A, Mouret C, Sinniger V, Picq C, Job A, Canini F, Jacquier-Sarlin M, Bonaz B. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton Neurosci. 2011;160:82–89. doi: 10.1016/j.autneu.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche JP, Jarry A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol. 2003;285:1028–1036. doi: 10.1152/ajpgi.00066.2003. [DOI] [PubMed] [Google Scholar]
- Newgreen DF, Young HM. Enteric nervous system: development and developmental disturbances – Part 2. Pediatr Dev Pathol. 2002;5:329–349. doi: 10.1007/s10024-002-0002-4. [DOI] [PubMed] [Google Scholar]
- Pacha J. Development of intestinal transport function in mammals. Physiol Rev. 2000;80:1633–1667. doi: 10.1152/physrev.2000.80.4.1633. [DOI] [PubMed] [Google Scholar]
- Patten GS, Abeywardena MY, McMurchie EJ, Jahangiri A. Dietary fish oil increases acetylcholine- and eicosanoid-induced contractility of isolated rat ileum. J Nutr. 2002;132:2506–2513. doi: 10.1093/jn/132.9.2506. [DOI] [PubMed] [Google Scholar]
- Patten GS, Adams MJ, Dallimore JA, Abeywardena MY. Dietary fish oil dose-response effects on ileal phospholipid fatty acids and contractility. Lipids. 2005;40:925–929. doi: 10.1007/s11745-005-1453-6. [DOI] [PubMed] [Google Scholar]
- Phillips TE, Phillips TL, Neutra MR. Macromolecules can pass through occluding junctions of rat ileal epithelium during cholinergic stimulation. Cell Tissue Res. 1987;247:547–554. doi: 10.1007/BF00215748. [DOI] [PubMed] [Google Scholar]
- Rodrigues DM, Li AY, Nair DG, Blennerhassett MG. Glial cell line-derived neurotrophic factor is a key neurotrophin in the postnatal enteric nervous system. Neurogastroenterol Motil. 2010;23:e44–56. doi: 10.1111/j.1365-2982.2010.01626.x. [DOI] [PubMed] [Google Scholar]
- Rosella O, Sinclair A, Gibson PR. Polyunsaturated fatty acids reduce non-receptor-mediated transcellular permeation of protein across a model of intestinal epithelium in vitro. J Gastroenterol Hepathol. 2000;15:626–631. doi: 10.1046/j.1440-1746.2000.02215.x. [DOI] [PubMed] [Google Scholar]
- Soret R, Chevalier J, de Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1663–1666. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- Urao M, Teitelbaum DH, Drongowski RA, Coran AG. The association of gut-associated lymphoid tissue and bacterial translocation in the newborn rabbit. J Pediatr Surg. 1996;31:1482–1487. doi: 10.1016/s0022-3468(96)90160-8. [DOI] [PubMed] [Google Scholar]
- Usami M, Muraki K, Iwamoto M, Ohata A, Matsushita E, Miki A. Effect of eicosapentaenoic acid (EPA) on tight junction permeability in intestinal monolayer cells. Clin Nutr. 2001;20:351–359. doi: 10.1054/clnu.2001.0430. [DOI] [PubMed] [Google Scholar]
- Usami M, Komurasaki T, Hanada A, Kinoshita K, Ohata A. Effect of gamma-linolenic acid or docosaehxaenoic acid on tight junctionpermeability in intestinal monolayer cells and their mechanism by protein kinase C activation and/or eicosanoid formation. Nutrition. 2003;19:150–156. doi: 10.1016/s0899-9007(02)00927-9. [DOI] [PubMed] [Google Scholar]
- Van Der Zanden EP, Boeckxstaens GE, de Jonge WJ. The vagus nerve as a modulator of intestinal inflammation. Neurogastroenterol Motil. 2009;21:6–17. doi: 10.1111/j.1365-2982.2008.01252.x. [DOI] [PubMed] [Google Scholar]
- Van Haver ER, De Vooght L, Oste M, Sangild PT, Thymann T, Weyns AL, Van Ginneken CJ. Post-natal and diet-dependent increases in enteric glial cells and VIP-containing neurones in preterm pigs. Neurogastroenterol Motil. 2008;20:1070–1079. doi: 10.1111/j.1365-2982.2008.01160.x. [DOI] [PubMed] [Google Scholar]
- Vine DF, Charman SA, Gibson PR, Sinclair A, Porter CJH. Effect of dietary fatty acids on the intestinal permeability of marker drug compounds in excised rat jejunum. J Pharm Pharmacol. 2002;54:809–819. doi: 10.1211/0022357021779159. [DOI] [PubMed] [Google Scholar]
- Willemsen LEM, Koetsier MA, Balvers M, Beermann C, Stahl B, van Tol EAF. Polyunsaturated fatty acids support epithelial barrier integrity and reduce IL-4 mediated permeability in vitro. Eur J Nutr. 2008;47:183–191. doi: 10.1007/s00394-008-0712-0. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Chen X, Zheng PY, Luo Y, Lu GF, Liu ZQ, Huang H, Yang PC. Oral Bifidobacterium modulates intestinal immune inflammation in mice with food allergy. J Gastroenterol Hepathol. 2010;25:928–934. doi: 10.1111/j.1440-1746.2009.06193.x. [DOI] [PubMed] [Google Scholar]


