Abstract
Background: Russia has one of the world’s fastest growing HIV epidemics and it has been largely concentrated among injection drug users (IDU). St Petersburg, Russia’s second largest city, is one of the country’s regions that has been most affected by the HIV epidemic. To monitor the current epidemic situation, we sought to estimate recent HIV incidence among IDU in St Petersburg. Methods: In a cross-sectional study of 691 IDU recruited during 2005–08, HIV incidence was estimated by two methods: a retrospective cohort analysis and BED capture enzyme immunoassay (EIA) results. Socio-demographic and behavioural correlates of incident infections and spatial patterns were examined. Results: In the retrospective cohort analysis, the incidence rate was estimated to be 14.1/100 person-years [95% confidence interval (CI) 10.7–17.6]. Using results of BED EIA and two correction formulas for known misclassification, incidence estimates were 23.9 (95% CI 17.8–30.1) and 25.5 (95% CI 18.9–32.0) per 100 person-years. Independent correlates of being recently infected included current unemployment (P = 0.004) and not having injected drugs in the past 30 days (P = 0.03). HIV incident cases were detected in all but one district in the city, with focal areas of transmission observed to be expanding. Conclusions: High HIV incidence among IDU in St Petersburg attests to continued growth of the epidemic. The need for expansion of HIV prevention interventions targeted to vulnerable populations throughout the city is urgent. These results also suggest that the BED EIA may over-estimate incidence even after correction for low specificity.
Keywords: human immunodeficiency virus, HIV, incidence, injection drug users, Russia
Introduction
The Russian Federation has experienced one of the fastest growing HIV epidemics observed anywhere in the world.1 The number of officially registered people with HIV increased from approximately 1000 to >438 000 during the 13-year period from 1996 to 2008.2 The current estimate of cumulative HIV cases in Russia is 940 000 with a possible range of 630 000–1 300 000, and the estimated adult prevalence now exceeds 1%.3 The epidemic has been largely concentrated among injection drug users (IDU) who comprise ~85% of the cumulative number of registered AIDS cases.1 HIV prevalence among IDU now exceeds 30% in several cities in Russia.4–6 Currently, there is the potential for a more generalized epidemic if transmission increases outside of high-risk core groups.4,5 With an estimated adult HIV prevalence now exceeding 1%, this seems increasingly plausible.3 This situation of high prevalence among core groups and increasing prevalence throughout the general population indicates that monitoring the future course of the HIV epidemic in Russia is an important priority.
Estimates of HIV incidence, the number of new infections that occur during a specified time interval, are generally more useful in monitoring epidemic trends than prevalence, though prevalence estimates are more readily available and thus commonly used. We are aware of only one study of HIV incidence in Russia, conducted in 2002–03, which recruited and followed 520 IDU in St Petersburg in a cohort study; the reported incidence rate was 4.5/100 person-years.7 This relatively high incidence is supported by prevalence data showing an increase among IDU in St Petersburg from <5% prior to 2001 to 30% in 2003 and ~50% in 2006.8–10 The scarcity of HIV incidence estimates may be due to the logistical and financial difficulties in following high-risk populations for long periods of time, indeed a formidable task for vulnerable populations most affected by HIV.
In recent years, several laboratory assays for detecting incident HIV infections in cross-sectional samples have been developed. One such assay is the BED capture enzyme immunoassay (EIA).11 By assessing antibody characteristics after seroconversion, recent infections can be distinguished from long-standing infections. In this particular assay, the proportion of HIV-1 specific IgG antibody in total IgG is measured with optical density (OD) readings. When used on specimens confirmed to be HIV positive by other tests, low OD readings tend to be found in people with recent infections. It has been shown that false positive results occur frequently with the BED EIA as some long-standing-infected individuals may have low OD readings for a variety of reasons and are thus misclassified. This low specificity of BED EIA produces over-estimates of HIV incidence and has called into question the utility of this assay for HIV incidence estimation.12,13 However, more recent work has produced correction formulas for use with BED EIA results that adjust estimates for misclassification due to sensitivity and specificity characteristics of the test.14,15 It has more recently been recognized that specificity of BED EIA may also depend on time since infection and thus can vary across populations by place, time and age groups;16 thus collecting data to estimate specificity in study populations may be useful for interpreting incidence estimates derived from BED EIA results. The use of such tests and related formulas has been applied in Africa, Western Europe and North America,17–20 but to the best of our knowledge not in Russia.
Monitoring trends in incidence among IDU in Russia is necessary to understand the possible future course of the epidemic, to assess and target current prevention and care needs, and to plan future prevention interventions including HIV vaccine research.21 The primary objective of the present analysis was to provide an updated estimate of HIV incidence among IDU in St Petersburg, Russia. Our secondary objective was to describe the epidemiology of HIV incident infections according to socio-demographic and behavioural correlates and spatial patterns.
Methods
Setting and study population
This cross-sectional study recruited IDU in St Petersburg, Russia from November 2005 through December 2008 as part of a multisite research project known as Sexual Acquisition and Transmission of HIV Cooperative Agreement Program (SATHCAP).22 Eligibility for inclusion in the present analysis included reporting a history of ever injecting drugs. Participants were recruited into the study using respondent-driven sampling, a chain referral sampling method that uses dual incentives and structured coupon disbursement procedures for peer referrals. Participants provided oral consent for participation in the study and written consent for blood storage. All study procedures were approved by institutional review boards at all participating sites.
Data collection and laboratory procedures
Participants completed structured interviews using computer assisted survey interviewing technology. The interview included questions about socio-demographic factors, injection drug use histories and risk behaviours and HIV testing histories. Participants provided blood specimens for HIV testing using commercially licensed ELISA kits from Genscreen HIV 1/2 (BioRad, France) and/or Vironostika HIV Uni-Form II plus 0 (Biomerieux, The Netherlands). Non-reactive specimens were considered to be HIV negative. Specimens that were reactive were confirmed with western blot assays using New Lav Blot HIV-1 (BioRad, France).
All confirmed HIV-positive specimens with sufficient remaining sample were tested with Calypte HIV-1 BED Incidence EIA according to package insert instructions (Calypte® Biomedical Corporation. Calypte® HIV-1 BED Incidence EIA, Cat. No. 98003 March 2007); a cut-off level for OD of ≤0.8 was used to classify specimens as recent infections.
Statistical analyses
Retrospective cohort
For our primary objective, HIV incidence was estimated using two methods. First, survey data including information about dates and results of past HIV tests were used to construct a retrospective cohort. To be included in this analysis, participants had to self-report that their last HIV test was negative so that the retrospective cohort consisted of a population that was known to be uninfected at baseline. Individuals in this group who were confirmed HIV positive at the enrolment study visit were considered new cases of HIV during the retrospective follow-up period. Assuming that HIV infections occurred at random during the follow-up period, we used the mid-point between the date of last reported negative HIV test and date of enrolment to calculate follow-up time. For individuals who remained HIV negative, follow-up times were calculated as the duration of time between the date of last reported negative HIV test and date of enrolment. The usual formula for incidence rates of the number of new cases divided by the total amount of person-time of follow-up was then applied to these data. Similar methodology has been used to estimate HIV incidence among male drug users in northern Thailand.23
BED EIA analysis
The second method for estimating incidence used the results of the BED EIA. To be eligible for BED EIA testing, participants had to test HIV positive on confirmatory western blot assay per the testing protocol. Due to increasing recognition of the frequency with which the BED EIA misclassifies long-standing infections as recent (high frequency of false positive results),12,13 corrected formulas for incidence estimation have been developed that adjust for misclassification due to sensitivity and specificity characteristics of the test.14,15 Spreadsheets that facilitate computation of corrected HIV incidence estimates are readily available from the assay developers (B. Parekh, personal communication). In these corrected formulas, we used an estimated mean seroconversion interval of 155 days in accordance with package insert guidelines. We also estimated specificity of the BED EIA for our study population by comparing BED EIA results with self-reported information about long-standing infections to determine a proportion in our sample that was misclassified. Among those who were confirmed HIV positive at enrolment and reported past testing, those who reported an HIV-positive diagnosis >155 days prior but were classified as recent according to BED EIA results were considered false positive results.
Correlates of incident infections
To address our second aim of describing the epidemiology of incident infections, we defined incident infections as those that were classified as recent by both BED EIA and self-report (i.e. not reporting an HIV-positive diagnosis >155 days prior). We excluded individuals for whom we had conflicting or missing HIV incident results. The primary analysis of interest was the comparison between HIV incident cases and participants who were HIV negative. Using logistic regression, we examined socio-demographic characteristics and injection-related risks as possible correlates. Socio-demographic variables included gender, age, education, employment, primary mode of transportation and homelessness. Injection-related risks included duration of use, type of drug injected (assessed in the past 6 months to capture possible variation over time), recent use, frequency of recent use and receptive and distributive sharing of syringes and sharing of other drug equipment (assessed in the past 30 days to increase validity of recall). Covariates were dichotomized at a priori meaningful cut-points. All covariates that were associated with HIV incidence in univariate models using the P < 0.05 criterion were then included in a multivariate model. Manual backward selection was used to remove covariates that were no longer significant at P < 0.05 level to arrive at the final, most parsimonious model.
Spatial patterns
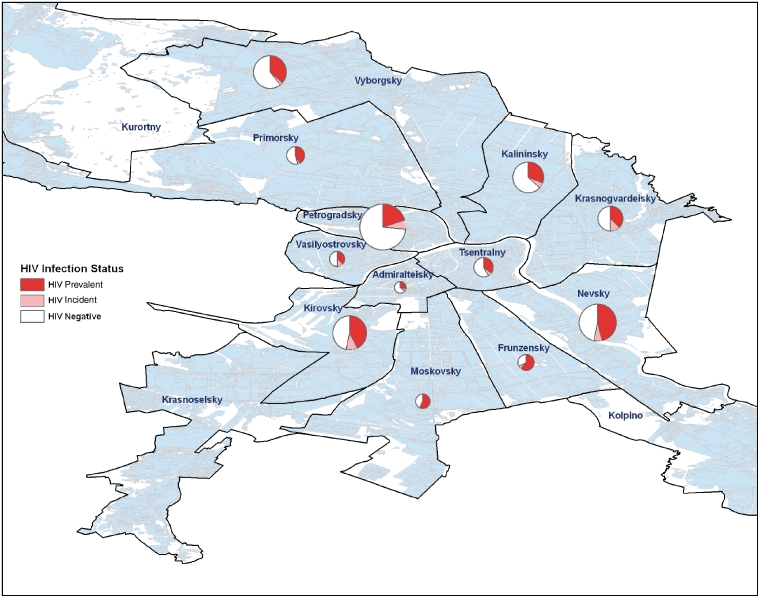
To describe spatial patterns of HIV incidence, self-reported metro stations closest to place of residence were aggregated to the district level for analysis. For each district, we mapped the proportions of participants that were HIV negative, HIV incident and HIV prevalent. Maps were created using ArcGIS/ArcMap 9.3.1 software (ESRI Corp, Redlands, CA, USA). Using likelihood ratio chi-square and Fisher’s exact tests, we compared the proportion of incident cases in each district to the proportion of incident cases in all other districts combined (e.g. the rest of the city). For this analysis, we excluded prevalent cases and the denominators included only HIV incident and negative cases.
Because we used RDS for recruitment of IDU, weighting procedures may be used in analysis to adjust for recruitment probabilities. Using a weighting procedure that took into account our modified RDS methodology, we found that the weighted and unweighted proportions for sex, age and HIV status in this sample did not differ substantially: unweighted estimates for proportions male <25 years of age and HIV infected were 74, 33 and 50%, respectively, compared to weighted estimates of 75, 32 and 49%, respectively. Therefore, we used unweighted estimates for all subsequent analyses.
Results
A total of 691 IDU was enrolled in this study from November 2005 to December 2008. A majority of participants was male (73%) and the median age was 29 years (range 18–53). The median duration of injection drug use was 8 years (range 1–36), and most participants reported injecting heroin (90%). The median number of injection drug episodes in the past 30 days was 20 (range 1–150), and a substantial proportion of participants reported either receptive or distributive syringe sharing in the past 30 days (31 and 30%, respectively). At enrolment, 301 were confirmed to be HIV positive for a sample prevalence of 43.6%.
Retrospective cohort
At the study visit, 540 (78%) individuals reported a previous HIV test date, of whom 322 reported their last test was negative and were thus eligible for inclusion in the retrospective cohort analysis. Of the 322, 65 tested positive at the enrolment study visit and the total amount of retrospective person-time since their last negative test and enrolment was 102.4 years. Using the mid-point assumption, these individuals contributed 51.2 person-years to follow-up for incidence estimation. Among the 257 individuals who remained HIV negative, the total amount of person-time between date of last negative test and enrolment was 408.7 years. HIV incidence was estimated to be 14.1 (65/[51.2 + 408.7]) per 100 person years [95% confidence interval (CI) 10.7–17.6].
BED EIA analysis
Of the 301 participants who were confirmed HIV positive at the enrolment study visit, 297 had sufficient specimen available and thus were tested with BED EIA. Of these, 58 were classified as recent infections. Using these results and two correction formulas for test performance including low specificity provided in an Excel spreadsheet by assay developers (B. Parekh, personal communication), we calculated HIV incidence estimates of 23.9 (95% CI 19.4–31.7) and 25.5 (95% CI 18.9–32.0) per 100 person-years. In a separate calculation, to estimate specificity of BED EIA for this study population, we considered 112 individuals who were confirmed HIV positive and reported past HIV testing, of whom 58 reported long-standing infections by self-reporting an HIV-positive diagnosis >155 days prior. Of those, 10 were classified as recent infections by BED EIA for a false positive rate of 17.2% (95% CI 8.9–29.1). We then excluded these known false positives (n = 10) from the 58 that were classified as recent infections by BED EIA, and used the correction formulas for HIV incidence considering 48 individuals recently infected; HIV incidence estimates were 18.7 (95% CI 13.4–24.0) and 20.0 (95% CI 14.3–25.6) per 100 person-years.
Correlates of incident infections
To describe the epidemiology of incident infections, 677 participants were included in the analysis (14 participants whose HIV status could not be determined were excluded, 10 because of conflicting BED EIA results and self-reported information and 4 because of insufficient specimen for BED EIA testing). Compared to HIV-negative participants (n = 390), those who were classified recent infections (n = 48) were significantly more likely to report not having any university education (P = 0.026), not currently being employed (P = 0.017) and not having injected drugs in the past 30 days (P = 0.039) (table 1). In the multivariate model, not currently being employed (OR = 3.4, 95% CI 1.5–7.9, P = 0.004) and not having injected drugs in the past 30 days (OR = 2.8, 95% CI 1.1–7.1, P = 0.03) remained significantly associated with HIV incidence. Because the association between not having injected drugs in the past 30 days and incident infection was unexpected, post hoc analyses were conducted on the seven participants who were classified as recently infected and reported not injecting in the past 30 days. Of these, four were male and three were female, five reported injection drug use in the past 6 months, two reported having a sex partner who was an IDU (one male, one female), three reported non-injection drug use in the past 30 days and none were MSM. One person reported being aware of HIV-positive serostatus due to a recent HIV test.
Table 1.
Characteristics of IDU recruited in St. Petersburg Russia, 2005–08, by HIV status, and comparison of HIV incident with HIV negative and HIV longstanding participants
| Characteristic | Total (n = 677a) | HIV incident, n (%) | HIV negative, n (%) | HIV prevalentc, n (%) | P-value for HIV incident vs. negative | P-value for HIV incident vs. prevalent |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 498 | 33 (69) | 300 (77) | 165 (69) | 0.214 | 0.969 |
| Female | 179 | 15 (31) | 90 (23) | 74 (31) | ||
| Age, years | ||||||
| ≤26 | 248 | 18 (38) | 143 (37) | 87 (36) | 0.920 | 0.885 |
| >26 | 428 | 30 (62) | 246 (63) | 152 (64) | ||
| Education | ||||||
| No/incomplete high school | 118 | 7 (15) | 65 (17) | 46 (19) | 0.026 | 0.144 |
| Completed high school | 190 | 21 (44) | 99 (25) | 70 (29) | ||
| Some university education | 368 | 20 (42) | 225 (58) | 123 (51) | ||
| Currently employed for pay | ||||||
| Yes | 220 | 10 (21) | 148 (38) | 62 (26) | 0.017 | 0.430 |
| No | 450 | 38 (79) | 238 (62) | 174 (74) | ||
| Primary mode of transport | ||||||
| Automobile | 95 | 6 (13) | 66 (17) | 23 (10) | 0.464 | 0.514 |
| Other | 580 | 41 (87) | 323 (83) | 216 (90) | ||
| Homeless during past year | ||||||
| Yes | 143 | 7 (15) | 80 (21) | 56 (23) | 0.362 | 0.249 |
| No | 533 | 40 (85) | 310 (79) | 183 (77) | ||
| Duration of injection drug use | ||||||
| ≤3 years | 120 | 10 (21) | 88 (23) | 22 (9) | 0.814 | 0.019 |
| >3 years | 548 | 37 (79) | 298 (77) | 213 (91) | ||
| Opioid use in past 6 months | ||||||
| Yes | 621 | 43 (93) | 358 (96) | 220 (95) | 0.430 | 0.619 |
| No | 29 | 3 (7) | 15 (4) | 11 (5) | ||
| Stimulant use in past 6 months | ||||||
| Yes | 121 | 8 (17) | 60 (16) | 53 (23) | 0.821 | 0.407 |
| No | 529 | 38 (83) | 313 (84) | 178 (76) | ||
| Injection drug use in past 30 days | ||||||
| Yes | 602 | 36 (84) | 348 (93) | 218 (94) | 0.039 | 0.020 |
| No | 48 | 7 (16) | 27 (7) | 14 (6) | ||
| Frequency of use in past 30 days | ||||||
| <30 | 364 | 19 (53) | 206 (60) | 139 (64) | 0.433 | 0.184 |
| ≥30 | 234 | 17 (47) | 140 (40) | 77 (36) | ||
| Receptive syringe sharingb | ||||||
| Yes | 175 | 12 (35) | 84 (25) | 79 (39) | 0.207 | 0.672 |
| No | 393 | 22 (65) | 248 (75) | 123 (61) | ||
| Distributive syringe sharingb | ||||||
| Yes | 162 | 14 (41) | 94 (29) | 54 (27) | 0.126 | 0.104 |
| No | 398 | 20 (59) | 235 (71) | 143 (73) | ||
| Sharing of drug equipmentb | ||||||
| Yes | 402 | 26 (76) | 229 (69) | 147 (72) | 0.378 | 0.622 |
| No | 166 | 8 (25) | 102 (31) | 56 (28) |
a: Totals may not add to 677 due to missing data
b: In the past 30 days
c: Excluding incident cases
Spatial patterns
Of 677 participants with valid HIV status as described above, 670 participants reported a valid metro stop closest to their place of residence and were thus included in spatial mapping. Participants were enrolled from 12 out of 15 city districts. In these 12 districts, the mean (SD) and median number of participants were 52 (44) and 45, respectively; the range was 14–155. Spatial mapping showed participants classified as recently infected in 11 of these 12 city districts, with the greatest proportions classified as recently infected in the eastern (Krasnogvardeisky, 18.5%) and central (Vasilyostorvsky, 20.0% and Kirovsky, 18.5%) districts of the city (figure 1). Only Kirovsky district had a significantly higher proportion of incident HIV cases compared to the rest of the city (P = 0.04, P > 0.05 for all other comparisons).
Figure 1.
Map of HIV incident, HIV prevalent and HIV negative participants among 670 IDU recruited in St Petersburg Russia 2005–08, by city district (size of circle proportionate to number of participants recruited from each district)
Discussion
Our findings reveal a very high HIV incidence rate among IDU in St Petersburg Russia, indicating that this vulnerable population remains at the core of HIV acquisition and transmission. Though our analysis revealed a range of possible estimates, from 14.1 to 25.5 new cases per 100 person-years (see below for discussion of this range), each of our estimates remain above acceptable standards in light of evidence that HIV prevention approaches for IDU have proven effective in reversing HIV epidemics in other parts of the world.24,25 This remarkably high estimate of HIV incidence is worrisome for the future course of the epidemic among IDU in Russia.
Comparison of our conservative estimate of 14.1/100 person-years to the previous estimate of 4.5/100 person-years in 2002–037 suggests the epidemic is continuing to expand. Though it is difficult to directly compare these estimates because of different study designs and estimation methodologies (the earlier lower incidence may have been influenced by prevention counselling provided to participants during the prospective cohort study), our estimates are evidence of continued HIV transmission during a time when other parts of the world have been able to contain the HIV epidemic among IDU. Thus, expansion of prevention efforts is urgently needed in St Petersburg. Our finding that participants who were unemployed were significantly more likely to be recently infected suggests that economically marginalized IDU may be at greater risk for HIV; future studies should investigate the degree to which economic vulnerability is associated with unsafe drug behaviours or being in a risky social network. In the meantime, HIV prevention efforts should focus on this highly vulnerable group. The only behavioural correlate we identified was not having injected in the past 30 days. This finding was unexpected and is not readily explainable. One possible explanation is that individuals may suspect their infection in the absence of HIV testing and reduce risk behaviours to prevent transmission. Another possible explanation is that individuals who inject sporadically and have periods of stopping and re-starting are at greater risk for infection. This finding could also be the result of type I statistical error in which the null hypothesis of no association is incorrectly rejected. Future work should seek to confirm or refute this finding.
Geographic spread of HIV throughout the city is another key finding of our study. Data from 2002 to 2003 showed significant clusters of incident cases in several central and eastern districts of the city (in Petrogradsky and on the border between Krasnogvardeisky and Nevsky, respectively).26 Our analysis also revealed a high proportion of incident cases in these areas, but we further observed incident cases spread more widely throughout the city in the vast majority of districts (11 of 12) from which we recruited participants, and new clusters eastward and southward in the Vasilyostrovsky and Kirovsky districts. Taken together, these data suggest that focal areas of HIV transmission are expanding throughout the city.
This analysis served another important purpose: it allowed us to compare HIV estimates in a cross-sectional sample between traditional retrospective cohort methods and a more recently developed serological assay. Even after adjusting for test performance, our estimates derived from the BED EIA were substantially higher (~80%) than retrospective cohort estimation. This is likely due to high frequency of misclassification of the BED EIA of long-standing infections as recently infected. High rates of false positive BED results have not been uncommon, and our estimated false positive rate of 17% fell within the range of estimates observed in other locations (1.7% in South Africa,19 5.2% in Zimbabwe,14 32.2% in Rwanda and Zambia17). The discrepancy in estimates derived from different methods could also be due to bias in the retrospective cohort estimate if, for example, recently infected individuals were systematically excluded from that analysis because they did not report a previous HIV test. While it is known that all three of these methods (two correction formulas and the cohort method) contain sources of error,27 a strength in using multiple methods as we have done here is to produce a range of estimates that can achieve more plausible results.
This study has several limitations that are important to consider. First, the clade of HIV circulating among IDUs in St Petersburg, subtype Afsu,28 is different from those used in the development of BED. Although the assay was designed to overcome problems associated with different subtypes,11 its sensitivity and specificity for subtype Afsu is unknown. Second, our study is subject to limitations that are inherent in the use of self-reported information regarding HIV testing histories including dates and results. In this setting where HIV testing coverage is sub-optimal and often not voluntary,10 infrequent testing and inaccurate reporting could influence our results. Our results could be biased if those who had never had an HIV test (22% of the sample) differed systematically from those who had been previously tested with respect to HIV status and examine covariates. Third, our findings are limited by our relatively small sample size. Use of the BED EIA for incidence estimation is recommended for use with large samples;12 thus our findings from a sample of 691 individuals produced a wide range of estimates for the false positive rate and did not permit estimation of how specificity varies for sub-populations16 or direct estimation of the mean seroconversion period, another important parameter for incidence estimation using BED EIA.14 However, our calculation of 95% CI for incidence estimates account for the size of our sample and still reflect high incidence estimates even at the lower bounds of confidence. Given the limitations associated with each method, a major strength of this analysis was the use of multiple methods to provide a range of estimates.
We conclude that the BED EIA may be a useful tool for estimating HIV incidence in cross-sectional studies when additional information to calculate adjustment parameters and measures of test performance is available. Further research is needed to critically assess the utility of the BED EIA in Russia. Much more pressing, the high estimates of incidence by all methods underscore the urgent need for expanded prevention efforts, both primary and secondary, to reduce the high HIV transmission in this setting, and prevention efforts will need to be widespread throughout the city. Methods of harm reduction that have been shown to reduce HIV transmission in IDU populations include increased syringe access, drug treatment and expanded anti-retroviral therapy. These programmes are not currently widely available in Russia.29,30 We hope that our research findings will be used to prompt prevention efforts on the scale needed to reverse one of the world’s most rapidly escalating HIV epidemics.
Funding
National Institutes of Health grants U01DA017387 (National Institute of Drug Abuse, PI R.H.); P30NIMH62294 (National Institute Mental Health, PI P. Cleary); D43TW001028 (Fogarty International Center, PI R. Dubrow) to Yale University.
Conflicts of interest: None declared.
Key points.
Russia has experienced one of the fastest growing HIV epidemics anywhere in the world, and it is concentrated among injection drug users (IDU).
Our findings reveal a very high HIV incidence rate among IDU in St Petersburg Russia, estimated to be between 14 and 25 new cases per 100 person-years.
Currently there is an urgent need for expanded prevention efforts, both primary and secondary, to reduce the high HIV transmission in this setting. Given that we identified focal areas of transmission to be expanding and limited behavioural risk factors, these efforts need to be widespread.
Acknowledgements
The authors thank staff at Biomedical Centre who conducted data collection, Dr Bharat Parekh for assistance in analysis and interpretation of BED results, and all participants who contributed their time and experience to make this study possible. The authors also thank the SATHCAP Steering Committee for useful guidance and support throughout this project. We also acknowledge support from other SATHCAP sites (U01DA017373 – William Zule, RTI International; U01DA017377 – Martin Y. Iguchi and Sandra Berry, RAND Corporation; U01DA017378 – Lawrence Ouellet, University of Illinois, Chicago; U01DA017394 – Steve Shoptaw and Pamina Gorbach, University of California, Los Angeles). These data were presented previously at 17th Biennial Meeting of the International Society for Sexually Transmitted Disease Research (2007, Seattle Washington) and 17th Conference on Retroviruses and Opportunistic Infections (2010, San Francisco CA, USA).
References
- 1.World Health Organization. Russian Federation: summary country profile for HIV/AIDS treatment scale-up, 2005. (22 November 2009, date last accessed) Available at: http://www.who.int/hiv/HIVCP_RUS.pdf.
- 2.AIDS Foundation East-West. Officially registered HIV cases in Russian Federation 1 January 1987–31 July 2008 as reported by the Russian Federal AIDS Center. [(22 November 2008, date last accessed)]. Available at: http://www.afew.org.
- 3.World Health Organization and UNAIDS. Russian Federation: Epidemiological fact sheet on HIV and AIDS. [(18 June 2010, date last accessed)]. Available at: http://apps.who.int/globalatlas/predefinedReports/EFS2008/full/EFS2008_RU.pdf.
- 4.Niccolai LM, Shcherbakova IS, Toussova OV, et al. The potential for bridging of HIV transmission in the Russian Federation: sex risk behaviors and HIV prevalence among drug users (DUs) and their non-DU sex partners. J Urban Health. 2009;86:131–43. doi: 10.1007/s11524-009-9369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowndes CM, Renton A, Alary M, et al. Conditions for widespread heterosexual spread of HIV in the Russian Federation: implications for research, monitoring and prevention. Int J Drug Policy. 2003;14:45–62. [Google Scholar]
- 6.Rhodes T, Lowndes CM, Judd A, et al. Explosive spread and high prevalence of HIV infection among injecting drug users in Togliatti City, Russia. AIDS. 2002;16:F25–F31. doi: 10.1097/00002030-200209060-00002. [DOI] [PubMed] [Google Scholar]
- 7.Kozlov AP, Shaboltas AV, Toussova OV, et al. HIV incidence and factors associated with HIV acquisition among injection drug users in St. Petersburg Russia. AIDS. 2006;20:901–6. doi: 10.1097/01.aids.0000218555.36661.9c. [DOI] [PubMed] [Google Scholar]
- 8.Abdala N, Carney JM, Durante AJ, et al. Estimating the prevalence of syringe-borne and sexually transmitted diseases among injection drug users in St. Petersburg, Russia. Int J STD AIDS. 2003;14:697–703. doi: 10.1258/095646203322387965. [DOI] [PubMed] [Google Scholar]
- 9.Shaboltas AV, Toussova OV, Hoffman IF, et al. HIV prevalence, social demographic and behavioral correlates and recruitment methods among injection drug users in St. Petersburg, Russia. J Acquir Immunune Defic Syndr. 2006;41:657–63. doi: 10.1097/01.qai.0000220166.56866.22. [DOI] [PubMed] [Google Scholar]
- 10.Niccolai LM, Toussova OV, Verevochkin SV, et al. High HIV prevalence, suboptimal HIV testing, and low knowledge of HIV-positive serostatus among injection drug users in St. Petersburg Russia. AIDS Behav. 2010;14:932–41. doi: 10.1007/s10461-008-9469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parekh BS, Kennedy MS, Dobbs T, et al. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res Hum Retroviruses. 2002;18:295–307. doi: 10.1089/088922202753472874. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention, Global Program on AIDS, Surveillance and Survey and the Laboratory Working Groups. Interim recommendations for the use of the BED capture enzyme immunoassay for incidence estimation and surveillance, 21 November 2006. [(30 September 2009, date last accessed)]. Available at: http://www.cdc.gov/nchstp/od/GAP/docs/surveillance.
- 13.UNAIDS Reference Group on Estimates, Modeling, and Projections. Statement on the use of the BED assay for the estimation of HIV-1 incidence for surveillance or epidemic monitoring. [(30 September 2009, date last accessed)]. Available at: http://data.unaids.org/pub/EPISlides/2006/Statement_BED_Policy_13Dec05_en.pdf. [PubMed]
- 14.Hargrove JW, Humphrey JH, Mutasa K, et al. Improved HIV-1 incidence estimates using the BED capture enzyme immunoassay. AIDS. 2008;22:511–8. doi: 10.1097/QAD.0b013e3282f2a960. [DOI] [PubMed] [Google Scholar]
- 15.McDougal JS, Parekh BS, Peterson ML. Comparison of HIV Type 1 incidence observed during longitudinal follow-up with incidence estimated by cross-sectional analysis using the BED capture enzyme immunoassay. AIDS Res Hum Retroviruses. 2006;22:945–52. doi: 10.1089/aid.2006.22.945. [DOI] [PubMed] [Google Scholar]
- 16.Hallett TB, Ghys P, Bärnighausen T, et al. Errors in ‘BED’-derived estimates of HIV incidence will vary by place, time and age. PLoS ONE. 2009;4:e5720. doi: 10.1371/journal.pone.0005720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karita E, Price M, Hunter E, et al. Investigating the utility of the HIV-1 BED capture enzyme immunoassay using cross-sectional and longitudinal seroconverter specimens from Africa. AIDS. 2007;21:403–8. doi: 10.1097/QAD.0b013e32801481b7. [DOI] [PubMed] [Google Scholar]
- 18.Rehle T, Shisana O, Pillay V, et al. National HIV incidence measures – new insights into the South African epidemic. S Afr Med J. 2007;97:194–9. [PubMed] [Google Scholar]
- 19.Bärnighausen T, Wallrauch C, Welte A, et al. HIV incidence in rural South Africa: comparison of estimates from longitudinal surveillance and cross-sectional cBED assay testing. PLoS One. 2008;3:e3640. doi: 10.1371/journal.pone.0003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–9. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beyrer C, Baral S, Shaboltas A, et al. The feasibility of HIV vaccine efficacy trials among Russian injection drug users. Vaccine. 2007;25:7014–6. doi: 10.1016/j.vaccine.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothenberg R, Jenkins R, Lambert E. Special issue: Sexual Acquisition and Transmission of HIV Cooperative Agreement Program (SATHCAP), July 2009: commentary. J Urban Health. 2009;86S1:144–8. doi: 10.1007/s11524-009-9374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawichai S, Celentano DD, Vongchak T, et al. HIV voluntary counseling and testing and HIV incidence in male injecting drug users in Northern Thailand. J Acquir Immune Defic Syndr. 2006;41:186–93. doi: 10.1097/01.qai.0000179431.42284.3e. [DOI] [PubMed] [Google Scholar]
- 24.Hurley SF, Jolley DJ, Kaldor JM. Effectiveness of needle-exchange programmes for prevention of HIV infection. Lancet. 1997;349:1797–800. doi: 10.1016/S0140-6736(96)11380-5. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine. Preventing HIV infection among injecting drug users in high risk countries: an assessment of the evidence, 2006. [(13 March 2007, date last accessed)]. Available at: http://www.iom.edu/Object.File/Master/37/074/11731_brief.pdf. [DOI] [PubMed]
- 26.Heimer R, Barbour R, Shaboltas AV, et al. Spatial distribution of HIV prevalence and incidence among injection drug users in St. Petersburg: implications for HIV transmission. AIDS. 2008;22:123–30. doi: 10.1097/QAD.0b013e3282f244ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brookmeyer R. Should biomarker estimates of HIV incidence be adjusted? AIDS. 2009;23:485–91. doi: 10.1097/QAD.0b013e3283269e28. [DOI] [PubMed] [Google Scholar]
- 28.Nabatov AA, Masharsky AE, Verevochkin SV, et al. The rate of epidemiological and virological changes during the transition from nascent to concentrated HIV epidemic stage in the former Soviet Union countries. AIDS Res Human Retroviruses. 2007;23:183–92. doi: 10.1089/aid.2006.0006. [DOI] [PubMed] [Google Scholar]
- 29.The future of harm reduction programmes in Russia [editorial] Lancet. 2009:374–1213. doi: 10.1016/S0140-6736(09)61761-X. [DOI] [PubMed] [Google Scholar]
- 30.International Harm Reduction Development Program (IHRD) of the Open Society Institute. Harm Reduction Developments 2008: Countries with Injection-Driven HIV Epidemics. New York: 2008. [Google Scholar]