Abstract
In this study, we investigated the effects of the ethanol extract of aerial parts of Raphanus sativus L. (ERL) on breast cancer cell proliferation and gene expression associated with cell proliferation and apoptosis in MDA-MB-231 human breast cancer cells. The MDA-MB-231 cells were cultured in the presence or absence of various concentrations (100, 200, or 300 µg/mL) of ERL. ERL significantly decreased cell proliferation after 48 h of incubation (P < 0.05). The protein and mRNA expression of ErbB2 were decreased significantly in a dose-dependent manner (P < 0.05). The protein expression of ErbB3 was decreased significantly at an ERL concentration of 300 µg/mL (P < 0.05), and mRNA expression of ErbB3 was decreased significantly in a dose-dependent manner (P < 0.05). The protein expression of Akt was decreased significantly at the ERL concentration of 200 µg/mL (P < 0.05), and the protein expression of pAkt was decreased significantly in a dose-dependent manner (P < 0.05). The mRNA expression of Akt was decreased significantly at the ERL concentration of 200 µg/mL ERL (P < 0.05). The protein and mRNA expression of Bax were increased significantly at ERL concentrations of 200 µg/mL or higher (P < 0.05). The protein expression of Bcl2 was increased significantly at ERL concentrations of 100 µg/mL or higher (P < 0.05), and mRNA expression of Bcl2 was increased significantly at an ERL concentration of 300 µg/mL (P < 0.05). In conclusion, we suggest that Raphanus sativus, L. inhibits cell proliferation via the ErbB-Akt pathway in MDA-MB-231 cells.
Keywords: Raphanus sativus L. ethanol extract, epidermal growth factor receptor, apoptosis, cell proliferation, MDA-MB-231 cell
Introduction
The World Health Organization estimates that the approximate 12.6 million new cancer cases that occurred in 2008 will be increased to 21.3 million in 2030 [1]. Cancer is one of the major causes of death worldwide, and its burden is growing. Cancer rates in Korea have continued to increase, and 178,816 new cases were diagnosed in 2008 [2]. Of those, a total of 12,659 new breast cancer cases were reported in 2008 [2]. Among women, breast cancer is the second most commonly diagnosed type of cancer annually, and it continues to be the most common form of cancer being treated from year to year [2].
The radish (Raphanus sativus L. leaf), which belongs to the cruciferae family, is a common edible leafy vegetable consumed in Korea [3]. In a previous study, we reported that the ethanol extract of the aerial parts of Raphanus sativus L. (ERL) contained 52.5 mg of polyphenols and total flavonoids per gram of dried leaf. This polyphenol and flavonoid content was superior to that of plums, Cornus fruits, persimmons, dried persimmons, and peeled sweet persimmons [4]. Beevi et al. [5] reported that the total phenolic content of ERL was comparable to other traditional rich sources, such as green tea and black tea. Studies have demonstrated that there is an association between the regular consumption of polyphenol-rich foods or beverages, including green tea, blueberry juice, and cranberry juice, and the prevention of cancer [6-8]. Therefore, plant polyphenols have received increasing attention due to their potential chemopreventive roles [9]. Polyphenols defend against free radical-induced toxicity by scavenging, metal chelating, and acting as antioxidants [10]. Several studies have reported on the antioxidant activities of radish leaves [5,11]. An in vitro study showed that R. sativus sprout extracts inhibited cell proliferation and induced apoptosis in cancer cells [12].
In breast cancer, the epidermal growth factor receptor (EGFR) is an important oncogene [13]. EGFR is composed of family members including ErbB1, ErbB2, ErbB3, and ErbB4. ErbB2 gene amplification has been associated with the development of breast cancer in animal models [14]. ErbB3 may act as a physiological substrate for the tyrosine kinase activities of ErbB2, as this phosphorylation is dependent on the formation of heterodimers with ErbB2 protein [15-17]. The amplification of the ErbB2 gene, or the overexpression of ErbB2 protein has been found in human breast cancer cells and has been associated with the unregulated growth of malignant cells [18]. Apoptosis is an important subject in cancer research because it induces a series of marked morphological changes that include cell contraction, plasma and nuclear membrane blebbing, chromatin condensation, organelle relocalization and compaction, and the formation of membrane enclosed particles containing intracellular material termed apoptotic bodies [19]. The Bcl2 family includes both proapoptotic proteins, such as Bax, Bad and Bak, and antiapoptotic proteins, such as Bcl2, Bcl-x1, Mcl-1, and Bcl-w [19]. Akt has been shown to directly phosphorylate Bad, which causes Bad to dissociate from Bcl2, losing its pro-apoptotic function and resulting in cell survival [20].
A scientific evaluation of the antiproliferative and/or apoptotic effects of R. sativus L. leaves does not exist. Therefore, to understand the potential preventive effects of R. sativus L. leaf on human breast cancer, we investigated the expressions of ErbB2, ErbB3, Bcl2, Bax, and Akt as proliferation and apoptosis indices for a breast cancer cell line treated with ERL.
Materials and Methods
Reagent and chemicals
Aerial parts of radish (Raphanus sativus L.) were purchased fresh from Dongsu Farm, Yangpyeong-gun, Korea. They were washed thoroughly with distilled water and dried in an oven at 60℃. After grinding, the powder was extracted with 80% ethanol, and the extracts were filtered with filter paper (Whatman No. 2). The extracts were concentrated in a rotary evaporator at 45℃ and subsequently lyophilized and stored at -80℃ until use.
Dulbecco's modified Eagle's medium/Nutrient Mixture Ham's F12 (DMEM/F12) and streptomycin and penicillin were obtained from Gibco/BRL (Grand island, NY, USA). Antibodies for ErbB2, ErbB3, Bcl2, Bax, and pAkt were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) and antibody for Akt was purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). RIA-grade bovine serum albumin (BSA), transferrin, and other reagents were purchased from Sigma (St. Louis, MO, USA).
Cell culture
MDA-MB-231 human breast cancer cells were purchased from the American Type Culture Collection (Rockville, MD, USA). The cells were maintained in DMEM/F12 containing 100 ml/L of fetal bovine serum (FBS) with 100,000 U/L of penicillin and 100 mg/L of streptomycin. The medium was replaced every 2-3 days. To examine ERL on breast cancer cell proliferation, the MDA-MB-231 cells were plated in 24 well plates at a density of 2.5 × 104 cells/mL in DMEM/F12 supplemented with 10% FBS. After 48 h of incubation, monolayers were serum-starved with DMEM/F12 supplemented with 5 µg/mL of transferrin, 5 ng/mL of selenium, and 1 mg/mL of bovine serum albumin for 24 h. After serum starvation, the monolayers were treated and incubated in serum free medium (SFM) with vehicle (absolute ethanol, 0 ug/mL ERL) or 100, 200, or 300 µg/mL of ERL. The ERL was dissolved in absolute ethanol and diluted into appropriate concentrations for cell culture and treatment.
Viable cell numbers were estimated 0, 24, or 48 h after the cells were exposed to ERL using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay, as previously described [14]. The experiments were performed independently three times.
Western blotting analysis
MDA-MB-231 cells were plated in a 100 mm dish at a density of 1 × 105 cells/dish with DMEM/F12 supplemented with 10% FBS for 48 h. The cells were incubated in serum free medium for 24 h, after which they were incubated in the presence of ERL at concentrations of 0, 100, 200, or 300 µg/mL for 48 h. Then cell lysates were prepared as previously described [15]. The total cell lysates were resolved on a sodium dodecylsulfate 40-200 g/L polyacrylamide gel and then transferred to a polyvinylidene fluoride membrane (Millipore, Bedford, MA). Next, the blot was blocked for 1 h in 10 g/L of BSA in TBS-T (20 mmol/L Tris-Cl, pH 7.5, 150 mmol/L NaCl, 1 g/L Tween 20) or 50 g/L of milk TBS-T, after which it was incubated for 1 h with antibodies (ErbB2, ErbB3, Akt, pAkt, Bcl2, Bax). The blot was then incubated with antimouse or antirabbit HRP-conjugated antibody. Signals were detected by the enhanced chemiluminescence method using Super-Signal West Dura Extended Duration Substrate (Pierce, Rockford, IL). Finally, the relative abundance of each protein band was analyzed by scanning the exposed films densitometrically using an Image J Launcher (provided by NCBI).
Reverse transcriptase polymerase chain reaction
The MDA-MB-231 cells were treated with ERL using the same method as for western blotting analysis. Total RNA was isolated using Tri-reagent (Sigma), and cDNA was synthesized using 2 µg of total RNA with SuperScript II reverse transcriptase (lnvitrogen). For amplification of the cDNA, primers for ErbB2 (upstream primer, 5'-CAAGAGTGCACGGCAGAGT-3'; downstream primer, 5'-GCCTTACAATGTGGGCATG-3'; annealing at 72℃ for 30 sec with 45 cycles), ErbB3 (upstream primer, 5'-CAAGAGTGCACGGCAGAGT-3'; downstream primer, 5'-GCCTTACAATGTGGGCATG-3'; annealing at 72℃ for 30 sec with 34 cycles), Akt (upstream primer, 5'-CAACTTCTCTGTGGCGCAGTG-3'; downstream primer, 5'-GACAGGTGGAAGAACAGCTCG-3'; annealing at 72℃ for 1 min sec with 30 cycles), Bcl2 (upstream primer, 5'-TGTGGATGACTGAGTACCTGAAC-3'; downstream primer, 5'-AGCTTTGTTTCATGGAACATCACTGAC-3'; annealing at 72℃ for 90 sec with 30 cycles), and Bax (upstream primer, 5'- ATGGAGGGGTCCGGGGAG-3'; downstream primer, 5'-TGGAAGAAGATGGGCTGA-3'; annealing at 72℃ for 40 sec with 40 cycles) were used. The expression of human β-actin transcripts was examined as an internal control, as described previously [16]. The PCR products were separated on a 1% agarose gel and stained with ethidium bromide. The bands corresponding to each specific PCR product were quantified by densitometric scanning of the exposed film using the Bio-profile Bio-IL application (Vilber-Lourmat).
Statistical analysis
Statistical analyses were performed using Statistical Analysis System software (SAS Institute, Cary, NC, USA). The data were expressed as means with standard errors and analyzed via analysis of variance (ANOVA). Statistically significant differences among the means of groups were tested at α = 0.05 using Duncan's multiple range test.
Results
ERL inhibits cell proliferation in MDA-MB-231 cells
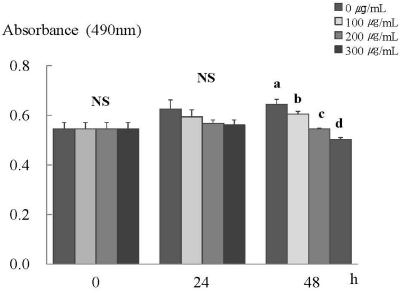
An MTT assay was performed to clarify the inhibiting effects of ERL on cancer cell proliferation. After 48 h of incubation with ERL, cell proliferation was decreased significantly in a dose-dependent manner (P < 0.05) (Fig. 1).
Fig. 1.
ERL inhibits cell proliferation in MDA-MB-231 cells. MDA-MB-231 cells were plated at a density of 2.5×104 cells/ml in a 24 well plate with DMEM/F12 supplemented with 10% FBS. Monolayers were then serum-starved with DMEM/F12 supplemented with 5 µg/ml transferrin, 5 ng/ml selenium, and 1 mg/ml bovine serum albumin for 24 h. After serum starvation, the monolayers were incubated in serum-free medium with 0, 100, 200, or 300 µg/mL ERL for 0, 24, or 48 h. Each bar represents the mean ± S.E. of three independent experiments. Different letters indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.
ERL suppresses protein and mRNA expressions of ErbB2, ErbB3, and Akt in MDA-MB-231 cells
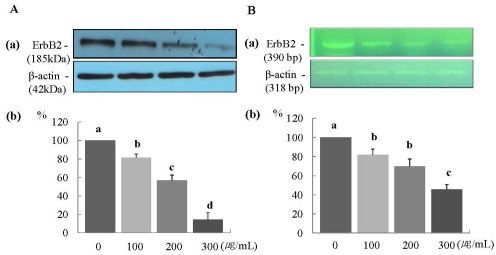
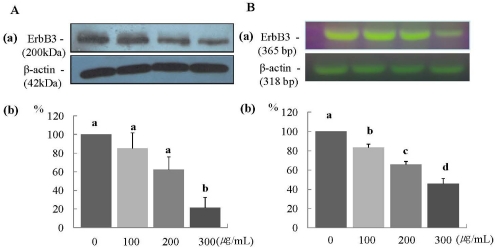
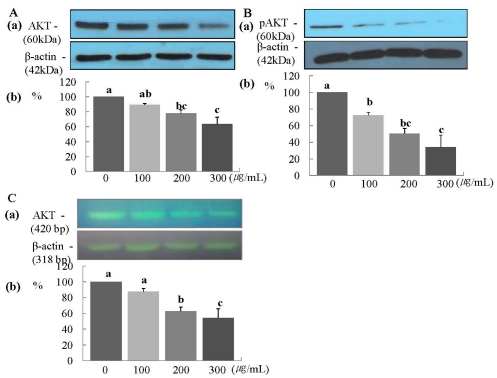
The protein expression of ErbB2 was decreased significantly in a dose-dependent manner (P < 0.05) (Fig. 2A), and the mRNA expression of ErbB2 was decreased significantly at ERL concentrations of 100 µg/mL and higher (P < 0.05) (Fig. 2B). The protein expression of ErbB3 was decreased significantly at an ERL concentration of 300 µg/mL (P < 0.05) (Fig. 3A), and the mRNA expression of ErbB3 was decreased significantly in a dose-dependent manner (P < 0.05) (Fig. 3B). The protein expression of AKT and its active form pAKT was decreased significantly in a dose-dependent manner (P < 0.05) (Fig. 4A and 4B). The mRNA expression of ErbB3 was decreased significantly in a dose-dependent manner (P < 0.05) (Fig. 4C).
Fig. 2.
ERL reduces protein and mRNA expression of ErbB2 in MDA-MB-231 cells. For ErbB2 protein expression, MDA-MB-231 cells were plated in a 100 mm dish at a density of 1×105 cells/dish with DMEM/F12 supplemented with 10% FBS for 48 h. The cells were then incubated in serum free medium for 24 h, after which they were incubated in the presence of ERL at concentrations of 0, 100, 200, or 300 µg/mL for 48 h. Equal amounts of cell lysates (30 µg) were then resolved by SDS-PAGE, transferred to a membrane, and probed with ErbB2 (A). For ErbB2 mRNA expression, the cells were cultured in serum-free medium with ERL at concentrations of 0, 100, 200, or 300 µg/mL for 48 h. Total RNA was isolated and RT-PCR was performed (B). a) Photographs of the bands, which are representative of three independent experiments. b) Quantitative analysis of the bands. Each bar represents the mean ± S.E. of three independent experiments. Different letters indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.
Fig. 3.
ERL reduces protein and mRNA expression of ErbB3 in MDA-MB-231 cells. For ErbB3 protein expression, MDA-MB-231 cells were seeded in a 100 mm dish at a density of 1×105 cells/dish with DMEM/F12 supplemented with 10% FBS for 48 h. The cells were then incubated in serum free medium for 24 h, after which they were incubated in the presence of ERL at concentrations of 0, 100, 200, or 300 µg/mL for 48 h. Equal amounts of cell lysates (30 µg) were then resolved by SDS-PAGE, transferred to a membrane, and probed with ErbB3 (A). For ErbB3 mRNA expression, the cells were cultured in serum-free medium with ERL at concentrations of 0, 100, 200, or 300 µg/mL for 48 h. Total RNA was isolated and RT-PCR was performed (B). a) Photographs of the bands, which are representative of three independent experiments. b) Quantitative analysis of the bands. Each bar represents the mean ± SE of three independent experiments. Different letters indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.
Fig. 4.
ERL reduces protein and mRNA expression of Akt MDA-MB-231 cells. For Akt and pAkt protein expression, MDA-MB-231 cells were seeded in a 100 mm dish at a density of 1×105 cells/dish with DMEM/F12 supplemented with 10% FBS for 48 h. The cells were then incubated in serum free medium for 24 h, after which they were incubated in the presence of ERL at concentrations of 0, 100, 200, or 300 µg/mL for 48 h. Equal amounts of cell lysates (30 µg) were then resolved by SDS-PAGE, transferred to a membrane, and probed with Akt (A) and pAkt (B). For Akt mRNA expression, the cells were cultured in serum-free medium with ERL at concentrations of 0, 100, 200, or 300 µg/mL for 48 h. Total RNA was isolated and RT-PCR was performed (C). a) Photographs of the bands, which are representative of three independent experiments. b) Quantitative analysis of the bands. Each bar represents the mean ± SE of three independent experiments. Different letters indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.
ERL reduces protein and mRNA expression of Bax, and increases protein and mRNA expression of Bcl2 in MDA-MB-231 cells
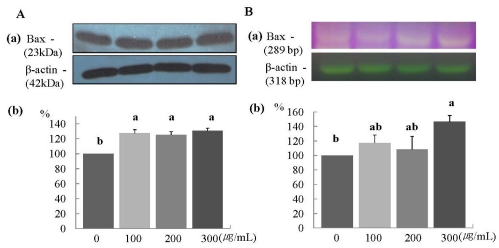
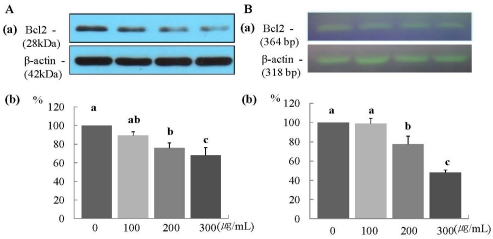
The protein and mRNA expression of Bax was increased significantly in a dose-dependent manner (P < 0.05) (Fig. 5A and 5B). Furthermore, the protein expression of Bcl2 was increased significantly at ERL concentrations of 100 µg/mL ERL and higher (P < 0.05) (Fig. 6A), and mRNA expression of Bcl2 was increased significantly at ERL concentrations of 300 µg/mL and higher (P < 0.05) (Fig. 6B).
Fig. 5.
ERL increases protein and mRNA expression of Bax in MDA-MB-231 cells. For Bax protein expression, MDA-MB-231 cells were seeded in a 100 mm dish at a density of 1×105 cells/dish with DMEM/F12 supplemented with 10% FBS for 48 h. The cells were then incubated in serum free medium for 24 h, after which they were incubated in the presence of ERL at concentrations of 0, 100, 200, or 300 µg/mL for 48 h. Equal amounts of cell lysates (30 µg) were then resolved by SDS-PAGE, transferred to a membrane, and probed with Bax (A). For Bax mRNA expression, the cells were cultured in serum-free medium with ERL at concentrations of 0, 100, 200, or 300 µg/mL for 48 h. Total RNA was isolated and RT-PCR was performed (B). a) Photographs of the bands, which are representative of three independent experiments. b) Quantitative analysis of the bands. Each bar represents the mean ± S.E. of three independent experiments. Different letters indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.
Fig. 6.
ERL increases protein and mRNA expression of Bcl2 in MDA-MB-231 cells. For Bcl2 protein expression, MDA-MB-231 cells were seeded in a 100 mm dish at a density of 1×105 cells/dish with DMEM/F12 supplemented with 10% FBS for 48 h. The cells were then incubated in serum free medium for 24 h, after which they were incubated in the presence of ERL at concentrations of 0, 100, 200, or 300 µg/mL for 48 h. Equal amounts of cell lysates (30 µg) were then resolved by SDS-PAGE, transferred to a membrane, and probed with Bcl2 (A). For mRNA expression, the cells were cultured in serum-free medium with ERL at concentrations of 0, 100, 200, or 300 µg/mL for 48 h. Total RNA was isolated and RT-PCR was performed (B). a) Photographs of the bands, which are representative of three independent experiments. b) Quantitative analysis of the bands. Each bar represents the mean ± S.E. of three independent experiments. Different letters indicate significant differences among groups at α = 0.05 as determined by Duncan's multiple range test.
Discussion
Epidermal growth factor receptors (EGFR) are composed of family members including ErbB1, ErbB2, ErbB3, and ErbB4. The binding of EGFR to EGFR family ligands initiates the formation of heterodimers, which activate intercellular kinase domains and result in the autophosphorylation of tyrosine residues of intracellular domains of the receptors. The phosphorylated forms of these receptors then serve as docking sites for the cytoplasmic proteins involved in transducing signaling cascades for extracellular stimulation. ErbB3 may act as a physiological substrate for the tyrosine kinase activities of ErbB2, as this phosphorlylation is dependent on the formation of heterodimers with ErbB2 protein [15-17]. Slamon et al. [21] observed a significant increase in the incidence of ErbB2 gene amplification in node-positive breast cancer patients. Cooke et al. [22] reported that the overexpression of ErbB2 protein in hyperplastic ductal epithelium from node negative breast cancer patients had a significant correlation with disease recurrence and decreased survival. EGFR has been reported to promote cell proliferation and survival in a variety of malignancies, including breast, lung, and pancreatic cancers [23,24]. These studies suggest that the downregulation of overexpression or amplification of EGFR appears to be related to the reduced proliferation of cancer cells [22-25]. Our results demonstrate that treatment with ERL inhibited the proliferation of MDA-MB-231 human breast cancer cells by decreasing the expressions of ErbB2 and ErbB3.
The binding of extracellular ligands to EGFR initiates downstream signaling by activating the PI3K/Akt pathway [26]. Alterations of Akt were previously observed in late stage and high grade tumors, suggesting that Akt plays an important role in tumorogenesis [27,28]. Akt activation induces cell survival and suppresses apoptotic death [20]. Caspase 9, Bad, and proapoptotic transcription factors such as FKHR have been reported to be phosphorylated by Akt, resulting in the inhibition of their anti-apoptotic activities and contributing to cell survival [29-31].
To determine the mechanism that mediates the effects of ERL in MDA-MB-231 cells, we investigated its involvement with the Akt pathway. Akt has been reported to be an important downstream component of PI3K-mediated oncogenic signaling [29-31]. Chun et al. [32] suggested that Akt is activated in the early stage of carcinogenesis after they found that Akt activity was higher in an immortalized HBE cell line than in a carcinogen exposed HBE cell line or malignant HBE cell line. They suggested that increased Akt protein resulting from Akt overexpression is a common feature during the early stage of carcinogenessis and that inhibition of Akt might be a potential target for chemopreventive therapy. In the view of these finding, our results indicate that ERL inhibits the proliferation of MDA-MD-231 cells by suppressing the EGFR-Akt pathway.
This expands our understanding of the antitumorogenic effects of ERL treatment. The role of apoptosis in tumorogenesis has been extensively studied and it appears that inhibition of apoptosis leads to premalignant clonal expansion [33]. Bcl2 or Bcl-xL overexpression has been shown to suppress apoptosis [34,35], whereas the induction of Bax expression has been shown to increase apoptosis in transformed cells [36]. Binder et al. [34] showed that the upregulation of Bax was coupled with negative tumor grades, C-ErbB2, and proliferation activities, especially when Bcl2 expression was downregulated concomitantly. The results of this study were consistent with their findings.
Wolter et al. [37] demonstrated that in cells undergoing apoptosis, a change occurs in the intracellular localization of Bax that may form channels or pores allowing the release of factors such as cytochrome C from the mitochondria to propagate the apoptotic pathway. A substantial loss of cytochrome C can lead to cell death. The Bcl2 family controls the release of apoptotic inducing factors from mitochondria during apoptosis. The over expression of Bcl2 has been reported to block mitochondrial release of cytochrome C, preventing apoptosis [33]. Tsuruta et al. [38] suggested that the expression of Akt suppressed apoptotic stimulus-induced Bax translocation. After translocation to the mitochondria, Bax induces the release of cytochrome C, and Akt inhibits apoptosis by altering mitochondrial membrane potentials, thus inhibiting the release of cytochrome C [39-40]. Our study confirms that ERL is capable of inducing apoptosis in MDA-MB-231 human breast cancer cells. This agrees with previous results from Papi et al. [12] such that 4 methylthio-3 butenyl-isothiocyante from Rahapnus sativus L. sprouts had cytotoxic/apoptotic activities in human colon carcinoma cell lines.
In conclusion, our results suggest that radish leaf may be a clinically useful antitumor agent because it directly inhibits the growth of tumor cells and induces apoptosis.
Footnotes
This research was supported by 15 agenda research project from Rural Development Administration (project No. PJ0067062010).
References
- 1.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin D. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer; 2010. [Google Scholar]
- 2.Annual report of cancer statistics in Korea in 2008. National Cancer Center [Internet] [cited 2011 March 2]. Available from: http://ncc.re.kr/common/downloadByNTC.jsp?attnum=232&code=999_101.
- 3.Korea Health Statistics 2009, Korea National Health and Nutrition Examination Survey (KNHANES IV-3) Korea Institute of Health and Social Affairs, Ministry of Health and Welfare Affairs [Internet] [cited 2011 May 27]. Available from: http://knhanes.cdc.go.kr/
- 4.Kim BR, Park JH, Kim SH, Cho KJ, Chang MJ. Antihypertensive properties of dried radish leaves powder in spontaneously hypertensive rats. Korean J Nutr. 2010;43:561–569. [Google Scholar]
- 5.Beevi SS, Narasu ML, Gowda BB. Polyphenolics profile, antioxidant and radical scavenging activity of leaves and stem of Raphanus sativus L. Plant Foods Hum Nutr. 2010;65:8–17. doi: 10.1007/s11130-009-0148-6. [DOI] [PubMed] [Google Scholar]
- 6.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 7.Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993;85:1038–1049. doi: 10.1093/jnci/85.13.1038. [DOI] [PubMed] [Google Scholar]
- 8.Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, Heber D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J Agric Food Chem. 2006;54:9329–9339. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- 9.Pianetti S, Guo S, Kavanagh KT, Sonenshein GE. Green tea polyphenol epigallocatechin-3 gallate inhibits Her-2/neu signaling, proliferation, and transformed phenotype of breast cancer cells. Cancer Res. 2002;62:652–655. [PubMed] [Google Scholar]
- 10.Kameoka S, Leavitt P, Chang C, Kuo SM. Expression of antioxidant proteins in human intestinal Caco-2 cells treated with dietary flavonoids. Cancer Lett. 1999;146:161–167. doi: 10.1016/s0304-3835(99)00253-0. [DOI] [PubMed] [Google Scholar]
- 11.Seshadri S, Nambiar VS. Kanjero (Digera arvensis) and drumstick leaves (Moringa oleifera): nutrient profile and potential for human consumption. In: Simopoulos AP, Gopalan C, editors. Plants in Human Health and Nutrition Policy. 2003. pp. 41–59. [DOI] [PubMed] [Google Scholar]
- 12.Papi A, Orlandi M, Bartolini G, Barillari J, Iori R, Paolini M, Ferroni F, Grazia Fumo M, Pedulli GF, Valgimigli L. Cytotoxic and antioxidant activity of 4-methylthio-3-butenyl isothiocyanate from Raphanus sativus L. (Kaiware Daikon) sprouts. J Agric Food Chem. 2008;56:875–883. doi: 10.1021/jf073123c. [DOI] [PubMed] [Google Scholar]
- 13.Callahan R. Genetic alterations in primary breast cancer. Breast Cancer Res Treat. 1989;13:191–203. doi: 10.1007/BF02106570. [DOI] [PubMed] [Google Scholar]
- 14.Slamon DJ, Clark GM. Amplification of c-erbB-2 and aggressive human breast tumors? Science. 1988;240:1795–1798. doi: 10.1126/science.3289120. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi K, Ito F. EGF receptor in relation to tumor development: molecular basis of responsiveness of cancer cells to EGFR-targeting tyrosine kinase inhibitors. FEBS J. 2010;277:316–326. doi: 10.1111/j.1742-4658.2009.07450.x. [DOI] [PubMed] [Google Scholar]
- 16.Burgess AW, Cho HS, Eigenbrot C, Ferguson KM, Garrett TP, Leahy DJ, Lemmon MA, Sliwkowski MX, Ward CW, Yokoyama S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 17.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 18.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 19.Bold RJ, Termuhlen PM, McConkey DJ. Apoptosis, cancer and cancer therapy. Surg Oncol. 1997;6:133–142. doi: 10.1016/s0960-7404(97)00015-7. [DOI] [PubMed] [Google Scholar]
- 20.Sen P, Mukherjee S, Ray D, Raha S. Involvement of the Akt/PKB signaling pathway with disease processes. Mol Cell Biochem. 2003;253:241–246. doi: 10.1023/a:1026020101379. [DOI] [PubMed] [Google Scholar]
- 21.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 22.Cooke T, Reeves J, Lannigan A, Stanton P. The value of the human epidermal growth factor receptor-2 (HER2) as a prognostic marker. Eur J Cancer. 2001;37:3–10. [PubMed] [Google Scholar]
- 23.Lewis S, Locker A, Todd JH, Bell JA, Nicholson R, Elston CW, Blamey RW, Ellis IO. Expression of epidermal growth factor receptor in breast carcinoma. J Clin Pathol. 1990;43:385–389. doi: 10.1136/jcp.43.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–2799. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 25.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janmaat ML, Kruyt FA, Rodriguez JA, Giaccone G. Response to epidermal growth factor receptor inhibitors in non-small cell lung cancer cells: limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin Cancer Res. 2003;9:2316–2326. [PubMed] [Google Scholar]
- 27.Yuan ZQ, Sun M, Feldman RI, Wang G, Ma X, Jiang C, Coppola D, Nicosia SV, Cheng JQ. Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene. 2000;19:2324–2330. doi: 10.1038/sj.onc.1203598. [DOI] [PubMed] [Google Scholar]
- 28.Bellacosa A, de Feo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, Wan M, Dubeau L, Scambia G, Masciullo V, Ferrandina G, Benedetti Panici P, Mancuso S, Neri G, Testa JR. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 29.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 30.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 31.Tang ED, Nuñez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 32.Chun KH, Kosmeder JW, 2nd, Sun S, Pezzuto JM, Lotan R, Hong WK, Lee HY. Effects of deguelin on the phosphatidylinositol 3-kinase/Akt pathway and apoptosis in premalignant human bronchial epithelial cells. J Natl Cancer Inst. 2003;95:291–302. doi: 10.1093/jnci/95.4.291. [DOI] [PubMed] [Google Scholar]
- 33.Ow YP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol. 2008;9:532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- 34.Binder C, Marx D, Binder L, Schauer A, Hiddemann W. Expression of Bax in relation to Bcl-2 and other predictive parameters in breast cancer. Ann Oncol. 1996;7:129–133. doi: 10.1093/oxfordjournals.annonc.a010538. [DOI] [PubMed] [Google Scholar]
- 35.Lee HS, Kim EJ, Kim SH. Chestnut extract induces apoptosis in AGS human gastric cancer cells. Nutr Res Pract. 2011;5:185–191. doi: 10.4162/nrp.2011.5.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakakura C, Sweeney EA, Shirahama T, Igarashi Y, Hakomori S, Nakatani H, Tsujimoto H, Imanishi T, Ohgaki M, Ohyama T, Yamazaki J, Hagiwara A, Yamaguchi T, Sawai K, Takahashi T. Overexpression of bax sensitizes human breast cancer MCF-7 cells to radiation-induced apoptosis. Int J Cancer. 1996;67:101–105. doi: 10.1002/(SICI)1097-0215(19960703)67:1<101::AID-IJC17>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 37.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuruta F, Masuyama N, Gotoh Y. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J Biol Chem. 2002;277:14040–14047. doi: 10.1074/jbc.M108975200. [DOI] [PubMed] [Google Scholar]
- 39.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]