Abstract
Selenium is an antioxidant trace element linked to cardiovascular disease and cancer. Although diet is a major source, relatively little else is known about independent determinants of selenium levels in free-living humans. In this study, we aimed to investigate the independent demographic, lifestyle, and dietary determinants of selenium levels in 1,997 men and 1,905 women in two large prospective U.S. cohorts. Toenail selenium levels were quantified using neutron activation analysis. Diet, geographic residence, demographic, and environmental factors were assessed by validated self-administered questionnaires. Multivariate generalized linear models were conducted to assess the independent relations of these factors with toenail selenium levels, correcting for measurement error in the diet. In multivariable-adjusted analyses, independent predictors of higher selenium were male gender (6.3% higher levels); living in West and Northern-Midwest U.S. regions (8.9% and 7.4% higher than Southern-Midwest regions, respectively); consumption of beef and bread products (between 0.7 - 2.5% higher per daily serving); and selenium supplement use (6.9% higher than non-users); whereas cigarette smoking (5-10% lower than never smokers) , older age (0.6% lower per 5 years), and consumption of eggs, white rice, dairy products, coffee, and alcohol (between 0.1 to 2.0% lower per daily serving) were associated with lower selenium. Multiple dietary and non-dietary factors independently predicted selenium levels, suggesting that both consumption and non-dietary processes (e.g., related to oxidant status) may affect levels. Significant geographic variation in selenium levels exists in the US.
Keywords: Selenium, toenail, determinants
Introduction
Selenium is an essential trace dietary nutrient that may actively protect against damage from free radicals and reactive oxygen species produced during oxidative stress; for example, through its role as an essential component of numerous selenoproteins in critical antioxidant systems [1,2]. Despite its central role in antioxidant defense systems and putative involvement in the development of chronic diseases, the major determinants of selenium levels in free-living humans are not well-understood. Of course selenium is consumed in the diet, but non-dietary factors, such as gastrointestinal absorption, tissue uptake, protein incorporation, or tissue metabolism or depletion, could also affect concentrations in the body. For example, older age [3], alcohol intake [4], and cigarette smoking [3-6] have been associated with lower selenium levels, suggesting that non-dietary individual or environmental factors (e.g., related to oxidative stress) may alter selenium stores. However, these prior studies were generally small and did not fully adjust for dietary consumption or other potential confounding factors. Investigations through large studies have been limited by challenges in estimating selenium consumption from dietary questionnaires due to significant variation in selenium contents of similar appearing foods [3,7]. Additionally, measuring selenium contents of foods is limited by considerable geographic variability, and more importantly, only identifies potential selenium sources rather than what is actually consumed in the population. For example, a food that contains a high selenium level but is rarely consumed will contribute minimally to selenium exposure in the population. Advanced neutron activation analysis (NAA) measurements now allow precise measurement of selenium levels in toenails [8,9], providing an objective biomarker of long-term exposure in populations. Toenail selenium concentrations are well correlated with selenium intake as well as selenium levels in serum or whole blood [8,9]. In addition to individual behavioral determinants, geographic variation could play a strong role in selenium exposure. Selenium levels in foods vary directly with concentrations in the soil or water from which the food is derived [10,11]. Conversely, in modern industrialized nations, foods are grown, processed, and transported from around the country and indeed the globe, potentially eliminating any significant residual geographic variation in exposure compared to, for example, developing nations. Unfortunately, most previous studies of selenium levels in free-living populations have been conducted in geographically relatively limited cohorts [3,6,7,12] limiting ability to evaluate any independent contributions of geographic variation.
To elucidate each of these issues, we investigated the independent dietary, demographic, lifestyle, and geographic determinants of selenium levels, as assessed by objective biomarker levels in toenails, in 3,902 U.S. men and women in 2 large U.S. cohorts. Our main aims were to determine [1] the major independent dietary predictors of selenium levels [2]; whether lifestyle and demographic factors that might influence oxidative stress, such as age, smoking, adiposity, physical activity, and alcohol use, are independent predictors of selenium levels after adjusting for diet, and [3] whether significant geographic variation would remain after accounting for differences in diet, demographic, and lifestyle factors.
Subjects and Methods
Population and design
The population and design of the Health Professionals' Follow-Up Study (HPFS) and Nurses' Health Study (NHS) have been described [13,14]. Briefly, the HPFS is a prospective cohort study among 51,529 male U.S. health professionals aged 40-75 years at enrollment in 1986, and the NHS is a prospective cohort study among 121,700 female U.S. registered nurses aged 30-55 years at enrollment in 1976. The HPFS included participants from all 50 states and the District of Columbia, Puerto Rico, Guam, and the Virgin Islands; the NHS included participants from 11 major U.S. states. For the analysis of selenium levels, we included 1,997 HPFS participants and 1,905 NHS participants who were randomly selected as controls from prior or ongoing nested case-control studies of cancer or cardiovascular disease (CVD) outcomes, after excluding 278 individuals who did not complete the Food Frequency Questionnaire, left ≥ 10 food items blank, or had implausible energy intake (< 500 kcal/d or > 4,500 kcal/d), and 46 individuals with toenail selenium concentrations > 1.5 µg/g that could reflect exogenous contamination or considerable excess ingestion of selenium supplements.
Assessment of selenium levels
Between 1982-1984 for women subjects and in 1987 for the men, participants were asked to provide toenail clippings from all 10 toes and return them for storage and analysis. Overall, 68% of HPFS participants and 52% of NHS participants returned toenail samples. The baseline characteristics, including age, smoking status, alcohol intake, Body Mass Index, and total calorie intake, of those supplying toenail samples were very similar to those not providing clippings among both in men and women. Before analyzing the toenail clippings, samples were washed in sonicator with deionized water. Selenium levels were quantified using neutron activation analysis (NAA) at the University of Missouri Research Reactor by laboratory personnel blinded to all participant characteristics. The validity, reproducibility, and reliability over time of these biomarkers have been described [8,9,15-17]. Samples of toenail clippings from all toes were combined at the time of collection, providing a time-integrated measure of exposure over approximately the prior year. Toenail sample mass was adequate for NAA in all participants. Intra-assay variability was excellent, with CV = 3.0%.
Toenail selenium levels provide an excellent biomarker of long-term selenium levels [8,9]. Other long-term depots such as hair and fingernails can be contaminated by use of selenium-based anti-dandruff shampoos, and blood selenium levels provide a measure of more short-term status [8,9,18]. In prior analyses from these cohorts, we have shown that toenail selenium is an excellent biomarker of usual exposure. Toenail selenium concentrations respond to long-term changes in dietary intake, correlate with supplement use, and correlate with selenium levels in serum or whole blood [8,9]. Toenail selenium concentration at one time point is a reliable predictor of future exposure, with a Spearman correlation (r) of 0.48 (P < 0.001) for levels assessed in toenail samples obtained 6 years apart [15]. This is only modestly lower than correlations of 0.6-0.7 typically observed, over a similar time interval, for widely used epidemiological measures such as blood pressure [19].
Assessment of dietary and environmental determinants
In both cohorts, demographics, lifestyle habits, other health information, and disease status were assessed by validated self-administered questionnaires every 2 years since enrollment. For this analysis, we used the reports closest in time to the collection of toenail samples from each participant. Information on state of residence was collected from every participant based on mailing address. Smoking status was assessed as never, former, and current, with 1-14, 15-34, and 35 or more cigarettes per day. BMI was calculated using self- reported body weight and height as the ratio of weight in kilograms to the square of height in meters; self-reported weight has been validated against technician-measured weight (r = 0.96) among both men and women in these cohorts [20]. Because physical activity levels were assessed in different ways in the two cohorts, including only semi-quantitative measures in the NHS in this time period, we calculated sex-specific quintiles using a series of questions on the types and quantities of different activities, as described previously [21]. Usual dietary habits and alcohol consumption were assessed by means of validated food frequency questionnaires that inquired about usual intake of foods over the prior year, including open-ended questions, specific questions on types of breakfast cereals and oils and fats used for cooking, and the use of dietary supplements including multivitamins, selenium, and other dietary supplements. The reproducibility and validity of the food frequency questionnaires have been described in detail [22,23]. The NHS dietary questionnaires in 1980 and 1982 included limited numbers of foods, while the 1984 cycle included a full questionnaire but was administered in the year after the toenail sampling for most participants. To minimize misclassification yet still capture dietary intake at or before the time of toenail sampling, we used the average values of all food items reported in the 1980-1984 NHS questionnaires. For the HPFS, we utilized data from the full dietary questionnaire administered in 1986, the year before toenail collection in that study.
Statistical analysis
Multivariate linear regression was used to test associations of the demographic, lifestyle, and dietary factors with log-transformed toenail selenium levels as the dependent variable. To increase interpretability and minimize multiple comparisons of dietary factors, in preliminary analyses the 131 food items assessed in the food frequency questionnaires were grouped into 21 categories based on groupings of similar types of food and unadjusted Spearman correlations between individual food items and toenail selenium. U.S. states were initially evaluated separately, evaluating fully multivariable-adjusted means of toenail selenium for each state. The states were then grouped into 5 geographic regions based on similarities of toenail selenium levels and geographic locations. The 5 defined regions were West, Midwest-North, Midwest-South, South, and Northeast + Other (Alaska, Guam, and Puerto Rico, that had relatively few numbers of participants and similar selenium levels as Northeastern states).
Linear spline regressions were used to assess potential non-linear relations between toenail selenium and covariates including age, physical activity, BMI, alcohol intake, and dietary factors. Multivariable stepwise regressions, both backward and forward methods (P = 0.05 for exit/entry), were used to identify the independent dietary determinants of selenium levels, adjusting for age, sex, smoking status, physical activity, multivitamin use, selenium supplement use, obesity, alcohol intake, and geographic region. Foods that were selected in both forward and backward stepwise regression models were considered to have independent relationships with selenium. Coefficients were corrected for measurement error [24,25] in the dietary questionnaires, compared with weighed diet records over 12 months [22,23]. Other demographic and lifestyle factors were evaluated after adjustments for all independent dietary predictors. Potential effect modification was assessed between smoking, sex, and supplement use separately using the Wald test for statistical significance of multiplicative interaction terms in the multivariable-adjusted linear regression model. Data analyses were conducted using SAS version 9.1 (SAS Institute Inc, Cary, NC), with two-tailed alpha = 0.05.
Results
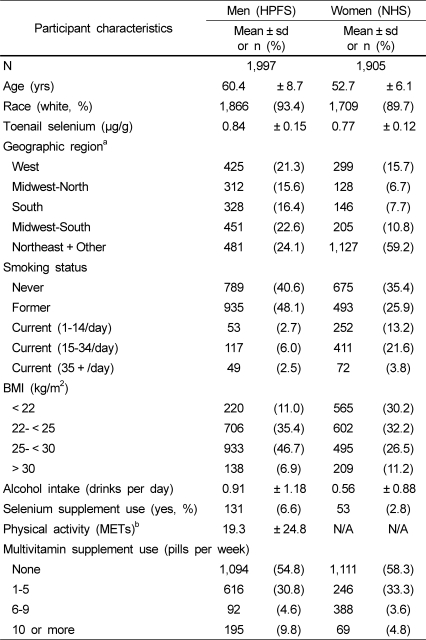
The average age of participants was 60 ± 9 y in men and 53 ± 6 y in women (Table 1). Approximately one-half of the study participants were overweight or obese, and 11% of men and 39% of women were current smokers. Selenium supplement use was uncommon, used by only 7% of men and 3% of women; multivitamin supplements were more common, taken by 45% of men and 42% of women. Linear spline models suggested linear relationships between toenail selenium and covariates; thus, these were evaluated as continuous variables in the multivariable models.
Table 1.
Demographic and lifestyle characteristics in the NHS (1980-1982) and HPFS (1986)
aGeographical regions were grouped based on selenium levels and locations.
West: AZ, CA, CO, ID, MT, NM, NV, OR, UT, WA, WY HI
Midwest/north: IA, KS, MI, ND, NE, SD, WI MN
South: AL, FL, LA, MS, TN, TX, AR, GA, OK
Midwest/south IL, IN, KY, MO, VA, SC, NC, OH, WV
Northeast and other: DE, MD, NJ, DC, AK, CT, GU, MA, ME, MI, NH, NY, PA, PR, PI, VI, VT
bMetabolic equivalents (METs) per week were calculated in men, whereas the average recreational physical activity levels were assessed qualitatively in women.
NHS, Nurses' Health Study: HPFS, Health Professionals Follow-up Study: BMI, Body Mass Index
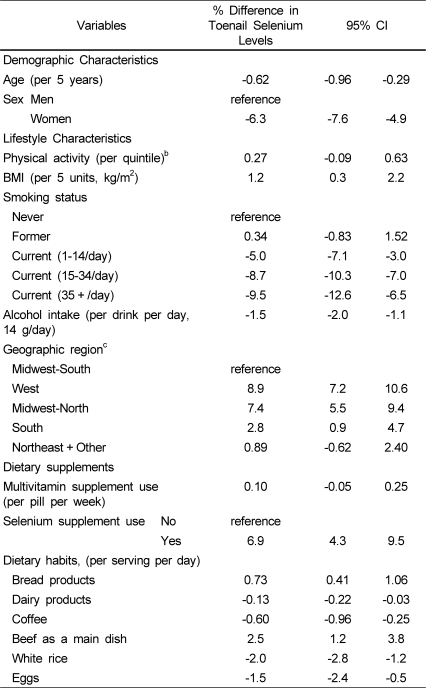
Among the 21 food groups evaluated, consumption of beef, breads (including cakes, muffins, and other breads), coffee, dairy products, eggs, and white rice were identified in multivariable stepwise regression models as independent determinants of selenium levels. Consumption of beef and bread products were each associated with higher levels, whereas consumption of coffee, dairy products, eggs and white rice were each associated with lower levels.
To understand whether other demographic and lifestyle factors were associated with selenium after accounting for diet, we then performed multivariable-adjusted analyses including adjustments for each of the identified dietary factors, and multivariable-adjusted results were similar in men and women, so the combined results are shown, adjusted for gender (Table 2). Even adjusting for diet as well as other lifestyle and environmental factors, both age and gender were independent determinants of selenium levels. Selenium levels were 0.62% lower for every 5 years of older age (95% CI: 0.29, 0.96), and were 6.3% lower in women vs. men (95% CI: 4.9, 7.6). This sex difference was consistent in analyses excluding current smokers and stratified by geographic region (data not shown).
Table 2.
Independent demographic, lifestyle, and dietary determinants of toenail selenium levelsa
aMultivariable-adjusted findings including each of the variables in the Table. Independent dietary predictors were identified using stepwise regression (see Methods).
bMetabolic equivalents (METs) per week were calculated in men, whereas the average recreational physical activity levels were assessed qualitatively in women.
cWest: AZ, CA, CO, ID, MT, NM, NV, OR, UT, WA, WY HI
Midwest/north: IA, KS, MI, ND, NE, SD, WI MN
South: AL, FL, LA, MS, TN, TX, AR, GA, OK
Midwest/south IL, IN, KY, MO, VA, SC, NC, OH, WV
Northeast and other : DE, MD, NJ, DC, AK, CT, GU, MA, ME, MI, NH, NY, PA, PR, PI, VI, VT
Several key lifestyle factors also remained independently associated with selenium levels after adjustment for demographics and diet (Table 2). Higher BMI was associated with higher selenium levels, with 1.2% higher levels for each 5 kg/m2 (95% CI: 0.3, 2.2). Compared with never smokers, current smokers had significantly lower selenium levels, with an additional dose-response relationship for the number of cigarettes smoked: selenium levels were 5% lower in those smoking 1-14 cigarettes per day, 9% lower in those smoking 15-34 cigarettes per day, and 10% lower in those smoking 35 or more cigarettes per day. Interestingly, former smokers did not have significantly different selenium levels than never smokers. Alcohol consumption was also associated with selenium levels, with 1.5% lower levels for each drink per day (95% CI: 1.1, 2.0). As expected, the use of selenium supplements was associated with 6.9% higher selenium (95% CI: 4.3, 9.5). The use of multivitamin supplements was not associated with selenium levels.
Even after adjustment for each of these demographic, lifestyle, and dietary characteristics, significant geographic variation in selenium levels was identified (Table 2). Compared to participants residing in Southern Midwestern states, those living in the West, Midwest-North, and South had significantly higher toenail selenium levels (West: 8.9% higher, 95% CI: 7.2, 10.6; Northern Midwest: 7.4% higher, 95% CI: 5.5, 9.4; and South: 2.8% higher, 95% CI: 0.9, 4.7). There was little evidence that these relationships varied by sex, smoking, or supplement use. Results remained identical after additional adjustment for total energy intake.
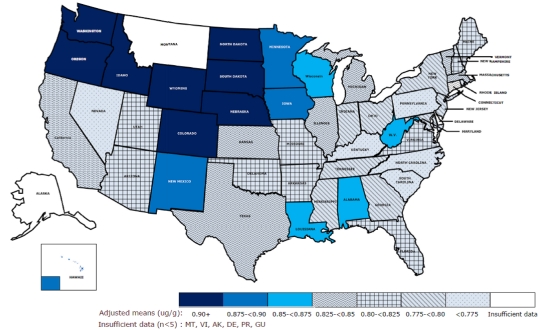
Adjusted mean selenium levels in men in individual U.S. states are shown in Fig. 1. Overall, men in the northwestern states generally had higher levels of selenium than those in the southeastern states. The highest levels of selenium were found in Wyoming, South and North Dakota, Nebraska, and Washington state; the adjusted means of toenail selenium in these states were 1.00, 0.97, 0.96, 0.94, and 0.94 µg/g, respectively. States with the lowest selenium levels were North Carolina, Kentucky, Pennsylvania, Nevada, and South Carolina, in which adjusted selenium means were only 0.77, 0.77, 0.77, 0.76, and 0.75 µg/g, respectively. Average levels were lower but geographic differences similar in women in 11 major U.S. states (data not shown). There were no significant effect modifications between smoking, sex, and supplement use.
Fig. 1.
Selenium levels in men. ug/g in toenails using multivariable generalized linear models adjusted for age, smoking, alcohol, Body Mass Index, physical activity, multivitamin/selenium supplement use, and diet. Average levels were lower but geographic differences similar in US women, not shown.
Discussion
In this well-characterized population of U.S. men and women, multiple independent determinants of toenail selenium levels were identified. It would be expected that selenium levels in the body should be affected by dietary factors and supplement use, and both were identified here as independent determinants. However, although the magnitude of these observed relationships is likely to be underestimated due to some misclassification in estimation of diet, even so these dietary factors explained only a modest relative proportion of overall variation in selenium levels. Notably, several other demographic and lifestyle factors were independent determinants of selenium levels, including smoking, alcohol intake, age, and sex. Furthermore, geographic residence remained a strong independent predictor, even after adjustment for types of foods consumed and each of the other independent determinants of toenail selenium.
Beef and bread products are recognized as good sources of selenium [26], and bioavailability of selenium from these foods is relatively high [27,28]. However, consumption of white rice was also negatively correlated with selenium levels in our study. This could be explained by findings that consumption of high glycemic index (GI) foods leads to higher oxidative stress [29,30]. Selenoproteins are important components of several critical antioxidant systems, such as glutathione peroxidase, that actively protect against damage from free radicals and reactive oxygen species [31,32]. Thus, it is possible that consuming foods that induce oxidative stress, such as high GI foods, could deplete selenium levels. It is also possible that foods such as white rice, which are typically refined and depleted of phytochemicals, could be replacing other higher-selenium foods in the diet. Interestingly, intakes of coffee, dairy products, and eggs were each inversely associated with selenium levels. No prior study has evaluated intakes of these foods in relation to selenium levels. Our findings support the need for further study of potential effects of these foods on selenium levels.
We observed a dose-response relationship between the number of cigarettes currently smoked and toenail selenium levels, consistent with findings from previous studies [3-5], even after multivariable adjustment for dietary habits, demographics, and other lifestyle factors. However, why cigarette smokers have lower levels of selenium has not been established. Swanson et al. [6] and van den Brandt et al. [5] reported that compared to nonsmokers, current smokers have lower consumption of selenium as estimated from dietary questionnaires, suggesting that lower selenium levels in smokers could at least partly reflect dietary differences. However, we identified current smoking as a significant predictor of lower selenium levels even after adjustment for other dietary and non-dietary determinants. Although residual confounding from unmeasured dietary factors and measurement error in the observed dietary factors cannot be excluded, the magnitude and observed dose-response support biological plausibility of a causal relationship. Cigarette smoking is known to increase oxidative stress [33-35], with a positive dose-response relationship per number of cigarettes smoked [36], and biomarkers of oxidative stress are reduced substantially within 2 weeks of smoking cessation [34]. These results support our findings of lower selenium levels in current but not former smokers, and the lowest levels among those who smoked the most cigarettes per day. Our findings indicate that factors which promote oxidative stress, such as current smoking, may directly affect tissue selenium levels, for example by increasing selenium utilization and causing depletion of tissue selenium stores. This concept of selenium depletion is further supported by the relationships we found between some other non-dietary characteristics and selenium levels. For example, older age was associated with lower toenail selenium levels, consistent with previous reports [3,37], which may be explained by increased oxidative damage as a function of aging [38,39]. In addition, we observed alcohol-related reductions in selenium levels, a finding supported by reports that alcohol consumption induces oxidative stress [40,41]. Thus, our findings together support a new hypothesis, in which factors that promote oxidative stress may deplete selenium levels in the body.
Prior studies of selenium levels have found inconsistent sex differences [4-6,42-44]. The sex differences we observed should be interpreted conservatively since these were two separately recruited cohorts. Interestingly, prior findings have also been inconsistent in terms of whether oxidative stress levels are higher in men or women [45]. The reasons for these discrepancies in relationships between sex and both selenium and oxidative stress status are unknown, and further investigation of these issues is warranted.
Although the extensive transport system of food products in the United States is thought to reduce variability in the selenium contents of foods in different regions, significant geographic heterogeneity in selenium levels was observed, even after accounting for dietary habits, demographics, and other lifestyle factors. This could be due to significant variation in the selenium contents of foods in different U.S. regions, or to residual confounding from unmeasured differences in other dietary habits or lifestyle characteristics between regions. Whereas our findings cannot definitively distinguish between these two possibilities, our evaluation and adjustment for multiple dietary habits, demographic characteristics, and lifestyle factors suggests that the former explanation may be more plausible. Previous studies have shown inconsistent geographic distributions of selenium in crops and human levels in the U.S. [3,42,46,47], and most prior studies in humans [3,42] have not reported the independent effect of geographic variation adjusted for other potential selenium determinants, including diet and lifestyle factors. Our study provides unique geographic information about the distribution of selenium levels, adjusted for other major determinants, in two large U.S. cohorts.
This analysis has several strengths. Rather than measuring selenium content in foods and estimating exposure, we assessed actual tissue biomarkers of selenium levels in 3,902 free-living men and women from across the U.S., providing a better estimate of actual exposure to selenium in the population. Selenium levels were evaluated in toenails using well-established methods, providing a valid and reproducible biomarker of long-term levels. The cohort size and standardized assessment of a wide variety of participant characteristics allowed multivariable-adjustment for several demographic, lifestyle, and dietary factors, including multiple potential dietary sources of selenium, minimizing residual confounding. We concurrently evaluated multiple potential determinants, providing the best evidence to-date of how each of these demographic, lifestyle, and dietary factor may independently influence long-term selenium levels.
Limitations should also be considered. The participants were Americans of higher socioeconomic status and mostly white, and our findings may not be generalizable to other socioeconomic groups or nations. On the other hand, there is little reason to expect that the biological effects on selenium levels of some factors, such as smoking, might vary substantially in other socioeconomic groups, races, or countries. These cohorts do not represent random samples of the U.S. population, and thus the geographic findings should be viewed qualitatively rather than as definitive for the U.S. population. Although extensive covariate information was collected using validated instruments, some residual confounding due to unmeasured or imperfectly measured factors may remain.
In summary, we identified several independent dietary, and interestingly, non-dietary predictors of selenium levels in the U.S. population. For many of the non-dietary factors, the findings were consistent with expected effects of oxidative stress, suggesting that tissue selenium, and potentially selenium-related anti-oxidant defenses, may depend on the balance between dietary consumption vs. depletion by oxidant stressors. Geographic variation was also an independent determinant, suggesting that even in the U.S. substantial regional differences in the selenium contents of foods may be present. Finally, although we identified a number of independent determinants of selenium-providing critical information for future studies examining selenium levels and chronic disease - a large proportion of the total variance was left unexplained. Future studies, including the examination of genetic factors, are needed to better understand the biological pathways through which environmental and genetic determinants affect selenium concentrations in the human population.
Footnotes
This study was supported by NIH grant R01 ES 014433 (NIEHS and NHLBI) and the Harvard School of Public Health Genes and Environment Initiative (GENI), with additional support from NIH grants CA55075 and HL35464 and the Yeungnam University research grant in 2011.
References
- 1.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 2.Chu FF, Doroshow JH, Esworthy RS. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J Biol Chem. 1993;268:2571–2576. [PubMed] [Google Scholar]
- 3.Hunter DJ, Morris JS, Chute CG, Kushner E, Colditz GA, Stampfer MJ, Speizer FE, Willett WC. Predictors of selenium concentration in human toenails. Am J Epidemiol. 1990;132:114–122. doi: 10.1093/oxfordjournals.aje.a115623. [DOI] [PubMed] [Google Scholar]
- 4.Arnaud J, Bertrais S, Roussel AM, Arnault N, Ruffieux D, Favier A, Berthelin S, Estaquio C, Galan P, Czernichow S, Hercberg S. Serum selenium determinants in French adults: the SU.VI.M.AX study. Br J Nutr. 2006;95:313–320. doi: 10.1079/bjn20051528. [DOI] [PubMed] [Google Scholar]
- 5.van den Brandt PA, Goldbohm RA, van't Veer P, Bode P, Hermus RJ, Sturmans F. Predictors of toenail selenium levels in men and women. Cancer Epidemiol Biomarkers Prev. 1993;2:107–112. [PubMed] [Google Scholar]
- 6.Swanson CA, Longnecker MP, Veillon C, Howe M, Levander OA, Taylor PR, McAdam PA, Brown CC, Stampfer MJ, Willett WC. Selenium intake, age, gender, and smoking in relation to indices of selenium status of adults residing in a seleniferous area. Am J Clin Nutr. 1990;52:858–862. doi: 10.1093/ajcn/52.5.858. [DOI] [PubMed] [Google Scholar]
- 7.Satia JA, King IB, Morris JS, Stratton K, White E. Toenail and plasma levels as biomarkers of selenium exposure. Ann Epidemiol. 2006;16:53–58. doi: 10.1016/j.annepidem.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Longnecker MP, Stampfer MJ, Morris JS, Spate V, Baskett C, Mason M, Willett WC. A 1-y trial of the effect of high-selenium bread on selenium concentrations in blood and toenails. Am J Clin Nutr. 1993;57:408–413. doi: 10.1093/ajcn/57.3.408. [DOI] [PubMed] [Google Scholar]
- 9.Longnecker MP, Stram DO, Taylor PR, Levander OA, Howe M, Veillon C, McAdam PA, Patterson KY, Holden JM, Morris JS, Swanson CA, Willett WC. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology. 1996;7:384–390. doi: 10.1097/00001648-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Foster LH, Sumar S. Selenium in health and disease: a review. Crit Rev Food Sci Nutr. 1997;37:211–228. doi: 10.1080/10408399709527773. [DOI] [PubMed] [Google Scholar]
- 11.Mayne ST. Antioxidant nutrients and chronic disease: use of biomarkers of exposure and oxidative stress status in epidemiologic research. J Nutr. 2003;133:933S–940S. doi: 10.1093/jn/133.3.933S. [DOI] [PubMed] [Google Scholar]
- 12.Ghayour-Mobarhan M, Taylor A, New SA, Lamb DJ, Ferns GA. Determinants of serum copper, zinc and selenium in healthy subjects. Ann Clin Biochem. 2005;42:364–375. doi: 10.1258/0004563054889990. [DOI] [PubMed] [Google Scholar]
- 13.Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, Hunter D, Manson JE. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 14.Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, Rimm EB. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005;111:157–164. doi: 10.1161/01.CIR.0000152099.87287.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garland M, Morris JS, Rosner BA, Stampfer MJ, Spate VL, Baskett CJ, Willett WC, Hunter DJ. Toenail trace element levels as biomarkers: reproducibility over a 6-year period. Cancer Epidemiol Biomarkers Prev. 1993;2:493–497. [PubMed] [Google Scholar]
- 16.MacIntosh DL, Williams PL, Hunter DJ, Sampson LA, Morris SC, Willett WC, Rimm EB. Evaluation of a food frequency questionnaire-food composition approach for estimating dietary intake of inorganic arsenic and methylmercury. Cancer Epidemiol Biomarkers Prev. 1997;6:1043–1050. [PubMed] [Google Scholar]
- 17.Joshi A, Douglass CW, Kim HD, Joshipura KJ, Park MC, Rimm EB, Carino MJ, Garcia RI, Morris JS, Willett WC. The relationship between amalgam restorations and mercury levels in male dentists and nondental health professionals. J Public Health Dent. 2003;63:52–60. doi: 10.1111/j.1752-7325.2003.tb03474.x. [DOI] [PubMed] [Google Scholar]
- 18.Morris JS, Stampfer MJ, Willett W. Dietary selenium in humans toenails as an indicator. Biol Trace Elem Res. 1983;5:529–537. doi: 10.1007/BF02988944. [DOI] [PubMed] [Google Scholar]
- 19.Rosner B, Hennekens CH, Kass EH, Miall WE. Age-specific correlation analysis of longitudinal blood pressure data. Am J Epidemiol. 1977;106:306–313. doi: 10.1093/oxfordjournals.aje.a112466. [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Hu FB, Stampfer MJ, Colditz GA, Ascherio A, Rexrode KM, Willett WC, Manson JE. Physical activity and risk of stroke in women. JAMA. 2000;283:2961–2967. doi: 10.1001/jama.283.22.2961. [DOI] [PubMed] [Google Scholar]
- 22.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 23.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 24.Willett WC. Nutritional Epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 25.Beaton GH, Milner J, Corey P, McGuire V, Cousins M, Stewart E, de Ramos M, Hewitt D, Grambsch PV, Kassim N, Little JA. Sources of variance in 24-hour dietary recall data: implications for nutrition study design and interpretation. Am J Clin Nutr. 1979;32:2546–2559. doi: 10.1093/ajcn/32.12.2546. [DOI] [PubMed] [Google Scholar]
- 26.Chimonas S, Rozario NM, Rothman DJ. Show us the money: lessons in transparency from state pharmaceutical marketing disclosure laws. Health Serv Res. 2010;45:98–114. doi: 10.1111/j.1475-6773.2009.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi B, Spallholz JE. Bioavailability of selenium from raw and cooked ground beef assessed in selenium-deficient Fischer rats. J Am Coll Nutr. 1994;13:95–101. doi: 10.1080/07315724.1994.10718378. [DOI] [PubMed] [Google Scholar]
- 28.Djujić IS, Jozanov-Stankov ON, Milovac M, Janković V, Djermanović V. Bioavailability and possible benefits of wheat intake naturally enriched with selenium and its products. Biol Trace Elem Res. 2000;77:273–285. doi: 10.1385/BTER:77:3:273. [DOI] [PubMed] [Google Scholar]
- 29.Ceriello A. Acute hyperglycaemia and oxidative stress generation. Diabet Med. 1997;14:S45–S49. doi: 10.1002/(sici)1096-9136(199708)14:3+<s45::aid-dia444>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y, Block G, Norkus EP, Morrow JD, Dietrich M, Hudes M. Relations of glycemic index and glycemic load with plasma oxidative stress markers. Am J Clin Nutr. 2006;84:70–76. doi: 10.1093/ajcn/84.1.70. quiz 266-7. [DOI] [PubMed] [Google Scholar]
- 31.Holben DH, Smith AM. The diverse role of selenium within selenoproteins: a review. J Am Diet Assoc. 1999;99:836–843. doi: 10.1016/S0002-8223(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 32.Brown KM, Arthur JR. Selenium, selenoproteins and human health: a review. Public Health Nutr. 2001;4:593–599. doi: 10.1079/phn2001143. [DOI] [PubMed] [Google Scholar]
- 33.Frei B, Forte TM, Ames BN, Cross CE. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Protective effects of ascorbic acid. Biochem J. 1991;277:133–138. doi: 10.1042/bj2770133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., 2nd Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 35.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ Framingham Study. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 36.Reilly M, Delanty N, Lawson JA, FitzGerald GA. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996;94:19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- 37.Lloyd-Jones DM, Liu K, Colangelo LA, Yan LL, Klein L, Loria CM, Lewis CE, Savage P. Consistently stable or decreased body mass index in young adulthood and longitudinal changes in metabolic syndrome components: the Coronary Artery Risk Development in Young Adults Study. Circulation. 2007;115:1004–1011. doi: 10.1161/CIRCULATIONAHA.106.648642. [DOI] [PubMed] [Google Scholar]
- 38.Erden-Inal M, Sunal E, Kanbak G. Age-related changes in the glutathione redox system. Cell Biochem Funct. 2002;20:61–66. doi: 10.1002/cbf.937. [DOI] [PubMed] [Google Scholar]
- 39.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 40.Meagher EA, Barry OP, Burke A, Lucey MR, Lawson JA, Rokach J, FitzGerald GA. Alcohol-induced generation of lipid peroxidation products in humans. J Clin Invest. 1999;104:805–813. doi: 10.1172/JCI5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barden A, Zilkens RR, Croft K, Mori T, Burke V, Beilin LJ, Puddey IB. A reduction in alcohol consumption is associated with reduced plasma F2-isoprostanes and urinary 20-HETE excretion in men. Free Radic Biol Med. 2007;42:1730–1735. doi: 10.1016/j.freeradbiomed.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Niskar AS, Paschal DC, Kieszak SM, Flegal KM, Bowman B, Gunter EW, Pirkle JL, Rubin C, Sampson EJ, McGeehin M. Serum selenium levels in the US population: Third National Health and Nutrition Examination Survey, 1988-1994. Biol Trace Elem Res. 2003;91:1–10. doi: 10.1385/BTER:91:1:1. [DOI] [PubMed] [Google Scholar]
- 43.Morris JS, Spate VL, Ngwenyama RA. Determinants of selenium in the toenail biomonitor. J Radioanal Nucl Chem. 2006;269:283–290. [Google Scholar]
- 44.Morris JS, Rohan T, Soskolne CL, Jain M, Horsman TL, Spate VL, Baskett CK, Mason MM, Nichols TA. Selenium status and cancer mortality in subjects residing in four Canadian provinces. J Radioanal Nucl Chem. 2001;249:421–427. [Google Scholar]
- 45.Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on blood pressure control and cardiovascular disease. Clin Exp Pharmacol Physiol. 2007;34:938–945. doi: 10.1111/j.1440-1681.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- 46.Kubota J, Allaway WH, Carter DL, Gary EE, Lazar VA. Selenium in crops in the United States in relation to selenium-responsive diseases of animals. J Agric Food Chem. 1967;15:448–453. [Google Scholar]
- 47.Spallholz JE, Martin JL, Ganther HE. Selenium in Biology and Medicine. Westport, CT: AVI Publishing Company; 1981. [Google Scholar]