Abstract
OBJECTIVES:
The inflammatory bowel diseases (IBDs) emerged after industrialization. We studied whether ambient air pollution levels were associated with the incidence of IBD.
METHODS:
The health improvement network (THIN) database in the United Kingdom was used to identify incident cases of Crohn's disease (n=367) or ulcerative colitis (n=591), and age- and sex-matched controls. Conditional logistic regression analyses assessed whether IBD patients were more likely to live in areas of higher ambient concentrations of nitrogen dioxide (NO2), sulfur dioxide (SO2), and particulate matter <10μm (PM10), as determined by using quintiles of concentrations, after adjusting for smoking, socioeconomic status, non-steroidal anti-inflammatory drugs (NSAIDs), and appendectomy. Stratified analyses investigated effects by age.
RESULTS:
Overall, NO2, SO2, and PM10 were not associated with the risk of IBD. However, individuals ≤23 years were more likely to be diagnosed with Crohn's disease if they lived in regions with NO2 concentrations within the upper three quintiles (odds ratio (OR)=2.31; 95% confidence interval (CI)=1.25–4.28), after adjusting for confounders. Among these Crohn's disease patients, the adjusted OR increased linearly across quintile levels for NO2 (P=0.02). Crohn's disease patients aged 44–57 years were less likely to live in regions of higher NO2 (OR=0.56; 95% CI=0.33–0.95) and PM10 (OR=0.48; 95% CI=0.29–0.80). Ulcerative colitis patients ≤25 years (OR=2.00; 95% CI=1.08–3.72) were more likely to live in regions of higher SO2; however, a dose–response effect was not observed.
CONCLUSIONS:
On the whole, air pollution exposure was not associated with the incidence of IBD. However, residential exposures to SO2 and NO2 may increase the risk of early-onset ulcerative colitis and Crohn's disease, respectively. Future studies are needed to explore the age-specific effects of air pollution exposure on IBD risk.
INTRODUCTION
Sir Samuel Wilks introduced ulcerative colitis into the medical vernacular in 1875 (1). The predominant air pollutant at that time was sulfurous gases arising from combustion of coal in industrial regions (2). Crohn's disease became readily recognizable in 1932 during the advent of the automobile era (1). Traffic-related pollutants such as nitrogen oxides and particulate matter that arise from the combustion of fossil fuels began to increase in urban societies during the 20th century (2).
Early in the 20th century, the incidence of ulcerative colitis was higher than Crohn's disease. However, although the incidence rates of ulcerative colitis have stabilized, the incidence of Crohn's disease began to steadily rise in industrialized nations in Europe (3) and North America (4). In developing nations, inflammatory bowel disease (IBD) rarely occurred; however, as these nations became industrialized, the incidence of ulcerative colitis and then Crohn's disease increased (5,6). Despite numerous studies, few environmental risk factors for IBD have been identified (4); however, they have not completely explained the processes that provoke IBD. Moreover, risk factors associated with industrialization have been incompletely investigated.
Air pollution directly affects pulmonary diseases including asthma and lung cancer (7). Air pollution exposure has also been shown to impair lung function development in children (8). In addition, air pollution has also been associated with a variety of non-pulmonary diseases including myocardial infarction (9), appendicitis (10), and rheumatoid arthritis (11). Air pollution-mediated inflammation has been implicated as the cause of several adverse health effects (12). Similar pro-inflammatory processes occur in IBD (13). Thus, we studied whether residential exposures to ambient air pollution concentrations were associated with the development of Crohn's disease and ulcerative colitis.
METHODS
Patient data source
The health improvement network (THIN) database consists of general practice electronic medical records for a sample of patients in the United Kingdom. Although the database is cleared of personal identifiers, it includes electronic information on patients at an individual level that can be tracked through time and includes demographics, physician defined diagnoses, prescription medications, cigarette smoking status, and death certificates. The THIN database also includes postcode linked area based socioeconomic and environmental indicators. Every 3 months, data from practices are exported to the THIN administrators and changes (e.g. new diagnosis) are recorded (14). Clinical diagnoses and prescribing data in the THIN database have been shown to be similar to the general practice research database (15).
Environmental data source
The National Environmental Technology Centre and Department of Environment, Food and Rural Affairs monitored ambient air quality in the United Kingdom using urban and rural fixed site monitors. Air pollutants that were monitored included particulate matter with an aerodynamic diameter of <10 μm (PM10), sulfur dioxide (SO2), and nitrogen dioxide (NO2). Nitrogen oxides were also recorded; however, this pollutant was excluded in this study because nitrogen oxides were highly correlated to NO2. Air pollutants were measured by fixed monitoring sites that include the Automatic Urban Network (103 sites), the Automatic Rural Network (24 sites), and the Automatic London Network (14 sites). Regional mean air pollution concentrations were extrapolated by creating air pollution concentration interpolation maps (16).
In 2001, the annual means for NO2, SO2, and PM10 were determined for each ward (∼2,000 residents). The wards were then stratified into quintiles based on the distribution of the pollution levels. The range of annual mean air pollutant concentrations for each quintile can be found in Supplementary Appendix online. On the basis of residential postcode, the participants in the THIN database were assigned a pollution score. The first linkage of air pollutant quintiles to postcodes occurred on 1 July 2006 and was updated if the case/control moved residence but remained registered within the practice. In total, 322 practices had air pollution data linked to 2,496,166 active members.
Study population
A nested case–control study was conducted by identifying incident cases of IBD and matched controls from within the THIN database. Subjects were eligible if they met the following criteria before 1 July 2005: between the ages of 5 and 84 years, enrolled in the THIN database for at least 3 years, and not previously diagnosed with IBD. The cohort members were followed from 1 July 2005 until the earliest of: first code of IBD, migration out of practice, death, or last data collection up to 31 October 2008.
During the study period, 2,063 THIN members were recorded with a new code for the IBDs. To minimize misclassification of IBD type, non-IBD patients, and prevalent cases, we excluded (i) 51 cases coded with both Crohn's disease and ulcerative colitis; (ii) 108 cases whose first code was defined as “exacerbation;” (iii) 732 patients coded for indeterminate or unexplained colitis; (iv) three cases without air pollution data; and (v) 211 subjects without a prescription for at least one of corticosteroids, mesalamine, sulfasalazine, azathioprine, 6-mercaptopurine, methotrexate, infliximab, and adalimumab. In total, 961 patients were newly diagnosed with Crohn's disease (n=367) or ulcerative colitis (n=591) between 1 July 2005 and 31 October 2008. The index date for an incident case was the date of a first code with IBD.
A pool of eligible controls was created by randomly sampling 100,000 members of the THIN database. From the pool of controls, five controls individually matched by age (within 1 year) and sex to each IBD incident cases were randomly selected. Controls were excluded if they were diagnosed with IBD before the index date of their matched case. The selection of controls was performed without replacement. Controls were subjected to the same inclusion/exclusion criteria as the cases. Age- and sex-matched controls were assigned the index date of their case for the purposes of the assignment of exposures. The study protocol was approved by the Conjoint Health Research Ethics Board at the University of Calgary.
Data analysis
The primary analysis involved stratifying individuals according to their air pollution levels based on quintiles grouped into high exposure (third, fourth, and fifth quintiles) and low exposure (first and second quintiles). Conditional logistic regression analysis was used to determine whether exposures to NO2, PM10, or SO2 were associated with an incident diagnosis of Crohn's disease and/or ulcerative colitis after adjusting for confounders.
Potential confounders that were controlled for included cigarette smoking status, socioeconomic status, prescription aspirin or non-steroidal anti-inflammatory drugs (NSAIDs), and appendectomy (4). Cigarette smoking status was defined as follows: (i) current smoker, coded for smoking within 3 months of the index date; (ii) ex-smoker, coded for smoking before, but not within 3 months of the index date; and (iii) never smoker, coded either as a lifetime non-smoker or not recorded as a smoker. Socioeconomic status was estimated using postcode level indicators generated from an index of deprivation based on the percentage of households: without access to a car, not in owner occupied accommodation, in overcrowded accommodation, and the percentage of the population aged 16–74 years who were unemployed (17). For prescription NSAIDs, the participant was classified as current user, prescription within 1 month of the index date; past user, prescription that ended before the month preceding the index date; or never user.
We separately analyzed models that were stratified by age quartiles for Crohn's disease (≤23, 24–43, 44–57, and ≥58 years) and ulcerative colitis (≤35, 36–48, 49–63, and ≥64 years). In a post hoc analysis, younger age groups (≤30, ≤25, and ≤20 years) were also evaluated for ulcerative colitis patients. All estimates were reported as adjusted odds ratios (ORs) with 95% confidence intervals (CIs). Linear trends in the adjusted ORs were evaluated across the quintiles to examine for a dose–response relationship. All analyses were performed using SAS software (SAS, version 9.2, SAS Institute, Cary, NC).
Sensitivity analysis
Additional sensitivity analyses were performed to explore the potential effects of misclassification biases with both exposure and health outcome measures. As air pollution scores were assigned based on residence after 1 July 2006, a sensitivity analysis was performed to restrict the analysis to patients diagnosed after 1 July 2006. To minimize the misclassification of prevalent cases, the analysis was restricted to at least a 5-year washout period without a code for IBD before the index date. A sensitivity analysis was performed to exclude potentially prevalent IBD patients prescribed immunosuppressant within 3 months of the index date. Finally, subjects living in wards classified in the third quintile were excluded allowing for comparisons between the highest (fifth and fourth) and lowest (first and second) exposures. Finally, we restricted our analysis to cases and controls living in urban wards at the index date. Urban areas were defined as settlements, with a population size exceeding 10,000 persons (18).
RESULTS
We identified 591 individuals newly diagnosed with UC and 367 with Crohn's disease. The demographics at diagnosis of ulcerative colitis and Crohn's disease patients, as well as their matched controls at the index date, are shown in Table 1.
Table 1. Characteristics of patients at diagnosis of Crohn's disease or ulcerative colitis, as well as matched controls at the index date.
| Crohn's disease, n=367 | Matched controlsa, n=1,833 | Ulcerative colitis, n=591 | Matched controlsa, n=2,962 | |
|---|---|---|---|---|
| Age, median (IQR) | 43 (23, 58) | 43 (23, 58) | 49 (35, 64) | 49 (35, 64) |
| Sex, % | ||||
| Male | 48.8 | 48.8 | 57.5 | 57.4 |
| Female | 51.2 | 51.2 | 42.5 | 42.6 |
| Smoking, % | ||||
| Neverb | 49.6 | 60.5 | 48.9 | 56.2 |
| Current | 22.6 | 19.5 | 9.3 | 18.3 |
| Ex-smoker | 27.8 | 20.0 | 41.8 | 25.5 |
| Socioeconomic statusc, % | ||||
| 1 (Least deprived) | 29.4 | 27.6 | 25.4 | 28.7 |
| 2 | 24.8 | 22.0 | 24.9 | 23.0 |
| 3 | 22.1 | 21.7 | 23.5 | 20.7 |
| 4 | 13.6 | 17.7 | 17.1 | 16.9 |
| 5 (Most deprived) | 10.1 | 11.0 | 9.1 | 10.7 |
| Prescription NSAID, % | ||||
| Never | 41.7 | 44.0 | 37.5 | 39.8 |
| Current | 9.0 | 6.8 | 9.5 | 9.0 |
| Past | 49.3 | 49.2 | 53.0 | 51.2 |
| Appendectomy, % | 9.5 | 5.2 | 2.7 | 6.7 |
| Diagnosis IBD medicationsd, % | ||||
| Corticosteroids | 38.4 | 1.4 | 46.4 | 1.8 |
| 5-ASA | 67.6 | 0.1 | 81.0 | 0.2 |
| Azathioprine or 6-mercaptopurine | 13.6 | 0.05 | 3.9 | 0.2 |
| Methotrexate | 1.1 | 0.1 | 0.3 | 0.3 |
| Infliximab or adalimumab | 0 | 0 | 0 | 0 |
5-ASA, 5-aminosalicylic acid; IBD, inflammatory bowel disease; IQR, interquartile range; NA, not applicable; NSAID, non-steroidal anti-inflammatory drug.
Controls matched on age (within 1 year) and sex.
Never smokers were defined as either no record of smoking in the database or coded as a ‘never smoker' before index date.
Socioeconomic status was derived from an index of deprivation by area based on percentage of households: without access to a car, not in owner occupied accommodation, in overcrowded accommodation, and percentage of the economically active population aged 16–74 years who were unemployed.
Prescription registered within 3 months of the index date for cases and controls. Patients may be prescribed one or more IBD-related medications.
Crohn's disease
Table 2 presents estimates of the risk of developing Crohn's disease after adjusting for NO2, smoking, socioeconomic status, NSAID use, and prior appendectomy. Compared with lifetime non-smokers, current smokers (OR=1.55; 95% CI=1.14–2.11) and ex-smokers (OR=1.86; 95% CI=1.38–2.52) were at increased risk of being diagnosed with Crohn's disease. Appendectomy (OR=1.94; 95% CI=1.28–2.95) increased the risk of developing Crohn's disease. The adjusted ORs reported in Table 2 did not significantly change when we modeled these variables with SO2 or PM10 rather than NO2.
Table 2. The risk of developing Crohn's disease and ulcerative colitis associated with smoking, socioeconomic status, NSAID use, and prior appendectomy.
| Crohn's disease odds ratio (95% CI)a n=367/1,833b | Ulcerative colitis odds ratio (95% CI)a n=591/2,962b | |
|---|---|---|
| Smoking | ||
| Neverc | 1.00 | 1.00 |
| Currentd | 1.55 (1.14–2.11) | 0.59 (0.43–0.81) |
| Ex-smoker | 1.86 (1.38–2.52) | 2.05 (1.67–2.53) |
| Socioeconomic status | ||
| 1 (Least deprived) | 1.00 | 1.00 |
| 2 | 1.01 (0.74–1.38) | 1.21 (0.94–1.56) |
| 3 | 0.89 (0.64–1.23) | 1.30 (1.00–1.68) |
| 4 | 0.68 (0.47–0.98) | 1.14 (0.86–1.51) |
| 5 (Most deprived) | 0.77 (0.51–1.16) | 0.97 (0.68–1.38) |
| NSAID | ||
| Never | 1.00 | 1.00 |
| Currentd | 1.32 (0.83–2.09) | 1.05 (0.74–1.49) |
| Past | 1.03 (0.80–1.33) | 1.06 (0.87–1.29) |
| Appendectomy | 1.94 (1.28–2.95) | 0.38 (0.23–0.64) |
CI, confidence interval; NSAID, non-steroidal anti-inflammatory drug.
Matched for age and sex and adjusted for exposure to nitrogen dioxide, smoking, socioeconomic status, prior appendectomy, and NSAID use.
n denotes the number of cases/controls.
Never smokers were defined as either no record of smoking in the database or coded as a ‘never smoker' before index date.
Current defined as exposure at index date.
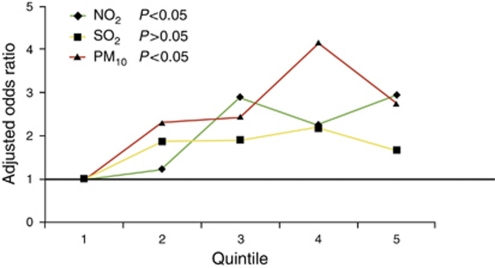
Overall, individuals who lived in regions of higher concentrations of NO2, SO2, and PM10 were not at risk of Crohn's disease ( Table 3). However, individuals ≤23 years were significantly more likely to be diagnosed with Crohn's disease if they lived in regions of higher concentrations of NO2 (OR=2.31; 95% CI=1.25–4.28) after adjusting for confounders. Crohn's patients aged 44–57 years were less likely to live in regions of higher NO2 (OR=0.56; 95% CI=0.33–0.95) and PM10 (OR=0.48; 95% CI=0.29–0.80) ( Table 3). Among patients ≤23 years, the adjusted OR increased linearly across quintile levels for NO2 (P=0.02) and PM10 (P=0.05), but not SO2 (Figure 1).
Table 3. The age-stratified adjusted risk of developing Crohn's disease among individuals living in wards with higher concentrations of NO2, SO2, and PM10.
|
Crohn's disease odds ratio (95% confidence interval)a |
|||||
|---|---|---|---|---|---|
| All agesc, n=367/1,833 | ≤23 Yearsc, n=93/465 | 24–43 Yearsc, n=95/474 | 44–57 Yearsc, n=84/420 | ≥58 Yearsc, n=95/474 | |
| NO2 | 1.02 (0.79–1.32) | 2.31 (1.25–4.28) | 0.68 (0.41–1.13) | 0.56 (0.33–0.95) | 1.28 (0.78–2.09) |
| SO2 | 0.95 (0.74–1.21) | 1.23 (0.73–2.05) | 0.88 (0.55–1.43) | 0.67 (0.40–1.11) | 1.09 (0.68–1.76) |
| PM10 | 0.91 (0.71–1.17) | 1.73 (0.98–3.03) | 0.76 (0.46–1.27) | 0.48 (0.29–0.80) | 1.10 (0.67–1.82) |
NO2, nitrogen dioxide; NSAID, non-steroidal anti-inflammatory drug; PM10, particulate matter <10μm; SO2, sulfur dioxide.
Matched for age and sex and adjusted for smoking, socioeconomic status, prior appendectomy, and NSAID use. Air pollution levels were stratified into high exposure (third, fourth, and fifth quintiles) and low exposure (referent).
n denotes the number of cases/controls.
Age cutoffs were defined by the distribution of ages (i.e. quartiles) at diagnosis of Crohn's disease.
Figure 1.
Dose–response relationship across quintiles of nitrogen dioxide (NO2), sulfur dioxide (SO2), and particulate matter <10 μm (PM10) exposures for the adjusted odds ratio of developing Crohn's disease ≤23 years.
Ulcerative colitis
Table 2 shows the risk of developing ulcerative colitis after adjusting for NO2, smoking, socioeconomic status, NSAIDs, and appendectomy. Compared with non-smokers, current smokers were protected from ulcerative colitis (OR=0.59; 95% CI=0.43–0.81), whereas ex-smokers were at increased risk (OR=2.05; 95% CI=1.67–2.53). Patients with an appendectomy were less likely (OR=0.38; 95% CI=0.23–0.64) to be diagnosed with ulcerative colitis. The adjusted ORs reported in Table 2 did not significantly change when we modeled these variables with SO2 or PM10.
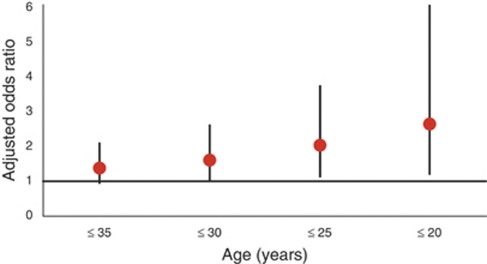
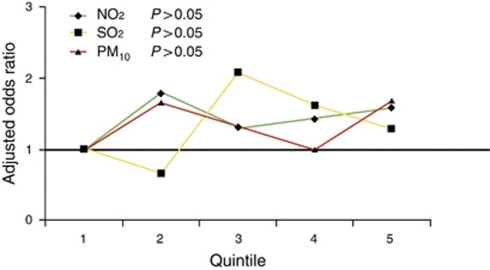
Overall, NO2, SO2, and PM10 were not associated with the diagnosis of ulcerative colitis at all ages and when the age groups were stratified by quartiles ( Table 4). However, patients diagnosed with ulcerative colitis ≤25 (OR=2.00; 95% CI=1.08–3.72) and ≤20 (OR=2.62; 95% CI=1.15–6.00) years were significantly more likely to live in regions of higher concentrations of SO2 (Figure 2). Reducing the age interval (i.e. ≤25 and ≤20 years) did not influence the risk estimates associated with NO2 or PM10. The ORs did not increase linearly across quintile levels (Figure 3).
Table 4. The age-stratified adjusted risk of developing ulcerative colitis among individuals living in wards with higher concentrations of NO2, SO2, and PM10.
|
Ulcerative colitis odds ratio (95% confidence interval)a |
|||||
|---|---|---|---|---|---|
| All ages, n=591/2,962b | ≤35 Years, n=152/763c | 36–48 Years, n=140/696c | 49–63 Years, n=148/749c | ≥64 Years, n=151/754c | |
| NO2 | 1.00 (0.82–1.22) | 0.76 (0.51–1.12) | 1.14 (0.74–1.77) | 1.07 (0.72–1.59) | 1.08 (0.73–1.62) |
| SO2 | 1.16 (0.95–1.41) | 1.34 (0.89–2.02) | 1.01 (0.67–1.54) | 1.07 (0.72–1.57) | 1.20 (0.81–1.78) |
| PM10 | 1.10 (0.90–1.34) | 1.11 (0.74–1.67) | 1.11 (0.73–1.70) | 1.12 (0.75–1.66) | 1.08 (0.74–1.58) |
NO2, nitrogen dioxide; NSAID, non-steroidal anti-inflammatory drug; PM10, particulate matter <10μm; SO2, sulfur dioxide.
Matched for age and sex and adjusted for smoking, socioeconomic status, prior appendectomy, and NSAID use. Air pollution levels were stratified into high exposure (third, fourth, and fifth quintiles) and low exposure (referent).
n denotes the number of cases/controls.
Age cutoffs were defined by the distribution of ages (i.e. quartiles) at diagnosis of ulcerative colitis.
Figure 2.
Adjusted risk of developing ulcerative colitis among individuals living in wards with higher concentrations of sulfur dioxide (SO2) stratified by decreasing age at diagnosis.
Figure 3.
Dose–response relationship across quintiles of nitrogen dioxide (NO2), sulfur dioxide (SO2), and particulate matter <10 μm (PM10) exposures for the adjusted odds ratio of developing ulcerative colitis ≤25 years.
Sensitivity analysis
We performed sensitivity analyses on each pollutant among the patients diagnosed with early onset Crohn's disease (≤23 years) and ulcerative colitis (≤25 years). When the washout period was extended from 3 to 5 years, the risk estimates marginally increased for both NO2 in Crohn's disease (OR=2.46; 95% CI=1.29–4.69) and for SO2 in ulcerative colitis (OR=2.12; 95% CI=1.14–3.94); in addition, young incident cases of Crohn's disease were significantly more likely to live in regions of higher PM10. Restricting analysis to IBD cases diagnosed after 1 July 2006 also increased the risk estimates for NO2 in Crohn's disease and for SO2 in ulcerative colitis; in addition, Crohn's patients were more likely to live in wards with higher concentrations of PM10 (2.08; 95% CI=1.04–4.14) exposure. Exclusion of cases/controls living third quintile wards strengthened the association for NO2 in Crohn's disease, whereas the effects of SO2 were no longer significant in ulcerative colitis. Excluding potentially prevalent cases/controls prescribed an immunosuppressant within 3 months of the index date reduced the risk estimates, but the associations were still significant. Restricting the analysis to only cases and controls living in urban areas strengthened the association between NO2 (3.27; 95% CI=1.32–8.06) and Crohn's disease ( Table 5).
Table 5. Sensitivity analyses.
|
Crohn's disease (≤23 years) odds ratio (95% confidence interval)a |
Ulcerative colitis (≤25 years) odds ratio (95% confidence interval)a |
|||||
|---|---|---|---|---|---|---|
| NO2 | SO2 | PM10 | NO2 | SO2 | PM10 | |
| Minimum registry in the THIN database of 5 years before diagnosis | 2.46 (1.29–4.69) | 1.21 (072–2.05) | 1.82 (1.02–3.26) | 1.00 (0.58–1.73) | 2.12 (1.14–3.94) | 0.97 (0.56–1.67) |
| IBD diagnosis after 1 July 2006 | 2.45 (1.18–5.10) | 1.72 (0.91–3.26) | 2.08 (1.04–4.14) | 1.24 (0.61–2.50) | 2.69 (1.20–6.04) | 1.13 (0.56–2.28) |
| Fifth/fourth vs. first/second quintileb | 2.48 (1.29–4.75) | 1.12 (0.64–1.97) | 1.71 (0.95–3.08) | 1.08 (0.56–1.96) | 1.47 (0.75–2.88) | 1.06 (0.59–1.88) |
| No immunosuppressant/biologic within 3 months of case/control index datec | 2.09 (1.05–4.17) | 1.16 (0.65–2.07) | 1.57 (0.83–2.97) | 0.93 (0.52–1.64) | 1.91 (1.02–3.60) | 0.93 (0.52–1.66) |
| Restricted to cases and controls living in urban centersd | 3.27 (1.32–8.06) | 1.20 (0.68–2.12) | 1.41 (0.72–2.78) | 0.94 (0.48–1.83) | 1.97 (0.94–4.10) | 0.89 (0.44–1.78) |
IBD, inflammatory bowel disease; NO2, nitrogen dioxide; NSAID, non-steroidal anti-inflammatory drug; PM10, particulate matter <10 μm; SO2, sulfur dioxide; THIN, the health improvement network.
Matched for age and sex and adjusted for smoking, socioeconomic status, prior appendectomy, and NSAID use. Air pollution levels were stratified into high exposure (third, fourth, and fifth quintiles) and low exposure (referent).
Cases and controls living in wards within the third quintile level were excluded.
Immunosuppressant or biologic agent defined as either azathioprine, 6-mercaptopurine, methotrexate, infliximab, or adalimumab.
Urban areas were defined as settlements with a population size exceeding 10,000 persons.
DISCUSSION
Overall, air pollution exposure was not associated with the risk of developing IBD. However, children and young adults living in areas with higher levels of SO2 were more likely to develop ulcerative colitis. Crohn's disease was more commonly diagnosed in young patients living in regions of higher concentrations of NO2. The effect of NO2 on early onset Crohn's disease persisted after adjusting for confounders and across sensitivity analyses. The association demonstrated a dose–response effect and was strengthened when the analysis was restricted to urban areas. Finally, middle-aged adults diagnosed with Crohn's disease demonstrated a paradoxical negative association to NO2 and PM10. Collectively, these findings suggest that traffic-related pollutants (i.e. NO2) and industrial-based pollutants (i.e. SO2) may have age-specific effects on the development of Crohn's disease and ulcerative colitis, respectively.
Early onset IBD differs from late-onset IBD by incidence patterns, having a more aggressive prognosis, and a different phenotypic expression (19,20). Likewise, children are more susceptible to the toxic effects of pollutants compared with adults (21). This may be explained by the fact that children and young adults tend to be more active and spend more time outdoors leading to greater exposure to air pollutants (21). Gastrointestinal absorption of pollutants (e.g. lead (22)) has been shown to be greater in children, and children metabolize pollutants less efficiently than adults (21). Furthermore, adverse health effects of pollutants may be greater in children because of dynamic development and growth (21).
Adults aged 44–57 years were less likely to develop Crohn's disease in regions of higher concentrations of NO2 and PM10. Differential effects observed across ages may have been due to exposure misclassification. Air pollution exposure was defined by residential measurements. Children and young adults spend more time outside their home and often attend schools nearby, whereas adults tend to work in areas with different air pollution exposures than their homes. Thus, residential air pollution measurements may more accurately reflect personal exposures in the young compared with older adults. Alternatively, environmental risk factors have been shown to have polarizing effects when comparing early onset IBD to late-onset IBD. For example, living with pets before the age of 5 years reduced the risk of developing adult-onset Crohn's disease, whereas living with pets increased the risk of pediatric-onset Crohn's disease (23). Future studies will be needed to determine whether this paradoxical finding was due to methodological considerations or because of the complex interaction between the environment and the age of onset of IBD.
Air pollution has been associated with other chronic inflammatory diseases that, like IBD, occur in genetically predisposed individuals who are exposed to environmental triggers. Elevated levels of SO2 and NO2 were associated with an 11-fold increased risk for a relapse of multiple sclerosis (24). Moreover, women exposed to traffic pollutants were at increased risk of rheumatoid arthritis (11). IBD patients are at higher risk of developing rheumatoid arthritis and multiple sclerosis (25), which suggest that these diseases may share genetic susceptibility and environmental risk factors. In addition, acute exposure to elevated levels of NO2 and ozone have been shown to increase the risk of appendicitis (26); likewise, appendicitis increased the risk of Crohn's disease in our study, as with others (27). These studies suggest a complex interaction between common environmental exposures such as air pollution and immune-mediated diseases.
The mechanisms by which air pollution may influence the development of IBD are speculative. The adverse health effects associated with air pollution are in part due to inflammation. The inhalation of diesel exhaust in healthy volunteers led to a rise in T-helper-1-associated cytokines such as tumor necrosis factor (12). Animals that fed air pollutants experienced oxidative damage of colonic mucosa (28). Thus, exposure of air pollutants, either through inhalation or ingestion, may incite inflammatory pathways that have been postulated to be central in the pathogenesis of IBD (13). Alternatively, diesel exhaust exposure increases susceptibility to bacterial (29) pulmonary infections through impairment of microbial defense (30). If air pollution similarly affects gastrointestinal innate immunity, then pollutants may predispose to IBD through altering interactions between the epithelial barrier and the intestinal microbiome. Future studies will be needed to elucidate the mechanistic relationship between air pollution and IBD.
Several limitations should be considered. First, IBD cases may have been misclassified and prevalent cases may have been mislabeled as incident. To minimize misclassification, IBD patients were only included if they also had a prescription for an IBD-related medication (i.e. mesalamine, corticosteroid, immunosuppressant, or biologic). We also performed sensitivity analyses to minimize misclassification of prevalent cases by increasing the washout period from 3 to 5 years and excluding patients prescribed an immunosuppressant within 3 months of the index date. In both sensitivity analyses, the primary results remained unchanged. Second, misclassification of air pollution exposures was possible. Air pollution scores were assigned based on regional estimates (i.e. at the ward level rather than the patient level) derived in 2001 and assigned to patients based on residence after 1 July 2006. Patients diagnosed after 1 July 2005 and who moved residence before 1 July 2006 may have been assigned an incorrect pollution score. However, this misclassification was presumably non-differential, which has the effect of biasing estimates toward the null. In fact, when the analysis was restricted to patients diagnosed after 1 July 2006, the risk estimates were strengthened. Third, the investigators cannot access postcodes in the THIN database and thus, more refined exposure assessments (e.g. using land use regression models) could not be performed. Fourth, other important air pollutants (e.g. ozone) were not available in the THIN database to study. Ozone exposure has been shown to increase the risk of appendicitis (26) and thus, future studies are necessary to evaluate the effects of other pollutants on IBD development. Fifth, the effect of SO2 on ulcerative colitis was not as robust as those observed between NO2 and Crohn's disease. SO2 did not follow a dose–response relationship and when extremes were compared (i.e. fourth and fifth vs. first and second quintiles), the effect of SO2 lost significance. Thus, one should cautiously interpret the effects of SO2 on ulcerative colitis. Sixth, residual confounding may have introduced bias into our risk estimates. Although important confounders (e.g. smoking) were controlled, others (e.g. occupational exposures) were missing. The lack of information on occupational exposures limited our assessment of adult-onset IBD. For example, adults employed in “driving” occupations, which have greater exposure to traffic-related pollutants such as NO2, have been shown to be at increased risk for developing Crohn's disease (31). Seventh, multiple comparisons were analyzed including three pollutants and several age groups. Furthermore, significant findings were only observed after subgroup analysis. Consequently, significant findings may have occurred by chance and these findings need to be replicated in other IBD populations. Eighth, countries such as China and India have significant air pollution, but have reported lower incidence rates of IBD. However, the incidence of IBD has rapidly increased in the past generation in developing nations as they have become industrialized (5,6) Also, genetic susceptibility to IBD differs across races (32); gene–environment interactions may explain differences between countries. Thus, future studies will be needed to corroborate our findings, to explore late-onset IBD in the context of air pollution exposures at home and at work, and to evaluate gene–pollutant interactions.
When all age groups were combined, air pollution did not increase the risk of developing IBD. However, exposure to NO2 and SO2 may increase the risk of developing early onset Crohn's disease and ulcerative colitis, respectively. In contrast, exposure of NO2 and PM10 in middle-aged adults was negatively associated with the development of Crohn's disease. If these findings are confirmed, then this work provides novel insight into the complex pathogenesis of IBD.
STUDY HIGHLIGHTS

Acknowledgments
We acknowledge Dr Mary Thompson and Harshvinder Bhullar from EPIC for providing data from the THIN database. The corresponding author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group and its licensees to permit this article (if accepted) to be published in Gut editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our license (http://group.bmj.com/products/journals/ instructions-for-authors/licence-forms).
Guarantor of the article: Gilaad G. Kaplan, MD, MPH.
Specific author contributions: Conceiving the study idea, developing the study design, preparing the administrative data, performing the analysis, interpreting results, writing of the manuscript, has full access to all the data in the study and has final responsibility for the decision to submit for publication, and has seen and approved the final version of the manuscript: Gilaad G. Kaplan; preparing the administrative data, analyzing the data, editing the manuscript, and has seen and approved the final version of the manuscript: James Hubbard; contributing to the study design, interpreting results, editing the manuscript, and has seen and approved the final version of the manuscript: Remo Panaccione; contributing to the study design, interpreting results, editing the manuscript, and has seen and approved the final version of the manuscript: Subrata Ghosh; guiding the analysis of the data set, interpreting results, editing of the manuscript, and has seen and approved the final version of the manuscript: Amanda J. Wheeler; contributing to the study design, interpreting results, editing the manuscript, and has seen and approved the final version of the manuscript: Bruce E. Sands; contributing to the study design, interpreting results, editing the manuscript, and has seen and approved the final version of the manuscript: Joshua Korzenik; developing the study design, performing the analysis, interpreting the results, writing of the manuscript, and has seen and approved the final version of the manuscript: Paul J. Villeneuve.
Financial support: This work was supported by an operating grant from the Broad Medical Research Program of the Broad Foundation and from the CCFC Chair in IBD Research. G.G.K. is supported through a New Investigator Award from the Canadian Institute of Health Research and a Population Health Investigator Award from the Alberta Heritage Foundation for Medical Research.
Potential competing interests: None.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Supplementary Material
References
- Kirsner JB. Historical origins of current IBD concepts. World J Gastroenterol. 2001;7:175–184. doi: 10.3748/wjg.v7.i2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TM, Shofer S, Gokhale J, et al. Outdoor air pollution: overview and historical perspective. Am J Med Sci. 2007;333:230–234. doi: 10.1097/MAJ.0b013e31803b8c91. [DOI] [PubMed] [Google Scholar]
- Logan RF. Inflammatory bowel disease incidence: up, down or unchanged. Gut. 1998;42:309–311. doi: 10.1136/gut.42.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- Desai HG, Gupte PA. Increasing incidence of Crohn's disease in India: is it related to improved sanitation. Indian J Gastroenterol. 2005;24:23–24. [PubMed] [Google Scholar]
- Zheng JJ, Zhu XS, Huangfu Z, et al. Crohn's disease in mainland China: a systematic analysis of 50 years of research. Chin J Dig Dis. 2005;6:175–181. doi: 10.1111/j.1443-9573.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- Grigg J. Particulate matter exposure in children: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:564–569. doi: 10.1513/pats.200905-026RM. [DOI] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE, et al. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Kaplan GG, Dixon E, Panaccione R, et al. Air pollution and appendicitis: a Novel association. Am J Gastroenterol. 2008;103 (S1:P820. [Google Scholar]
- Hart JE, Laden F, Puett RC, et al. Exposure to traffic pollution and increased risk of rheumatoid arthritis. Environ Health Perspect. 2009;117:1065–1069. doi: 10.1289/ehp.0800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornqvist H, Mills NL, Gonzalez M, et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Lawrance IC, Wu F, Leite AZ, et al. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003;125:1750–1761. doi: 10.1053/j.gastro.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Schinnar R, Bilker WB, et al. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16:393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- Rogoveanu I, Saftoiu A, Cazacu S, et al. Color Doppler transabdominal ultrasonography for the assessment of the patients with inflammatory bowel disease during treatment. Rom J Gastroenterol. 2003;12:277–281. [PubMed] [Google Scholar]
- Morris R, Carstairs V. Which deprivation? A comparison of selected deprivation indexes. J Public Health Med. 1991;13:318–326. [PubMed] [Google Scholar]
- Baumgart DC, Wiedenmann B, Dignass AU. Biologic therapy of inflammatory bowel disease. Z Gastroenterol. 2003;41:1017–1032. doi: 10.1055/s-2003-42924. [DOI] [PubMed] [Google Scholar]
- Armitage E, Drummond H, Ghosh S, et al. Incidence of juvenile-onset Crohn's disease in Scotland. Lancet. 1999;353:1496–1497. doi: 10.1016/S0140-6736(99)00333-5. [DOI] [PubMed] [Google Scholar]
- Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135:1114–1122. doi: 10.1053/j.gastro.2008.06.081. [DOI] [PubMed] [Google Scholar]
- Sly PD, Flack F. Susceptibility of children to environmental pollutants. Ann N Y Acad Sci. 2008;1140:163–183. doi: 10.1196/annals.1454.017. [DOI] [PubMed] [Google Scholar]
- Ahamed M, Siddiqui MK. Environmental lead toxicity and nutritional factors. Clin Nutr. 2007;26:400–408. doi: 10.1016/j.clnu.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Lashner BA, Loftus EV., Jr True or false? The hygiene hypothesis for Crohn's disease. Am J Gastroenterol. 2006;101:1003–1004. doi: 10.1111/j.1572-0241.2006.00563.x. [DOI] [PubMed] [Google Scholar]
- Oikonen M, Laaksonen M, Laippala P, et al. Ambient air quality and occurrence of multiple sclerosis relapse. Neuroepidemiology. 2003;22:95–99. doi: 10.1159/000067108. [DOI] [PubMed] [Google Scholar]
- Cohen R, Robinson D, Jr, Paramore C, et al. Autoimmune disease concomitance among inflammatory bowel disease patients in the United States, 2001–2002. Inflamm Bowel Dis. 2008;14:738–743. doi: 10.1002/ibd.20406. [DOI] [PubMed] [Google Scholar]
- Kaplan GG, Dixon E, Panaccione R, et al. Effect of ambient air pollution on the incidence of appendicitis. CMAJ. 2009;181:591–597. doi: 10.1503/cmaj.082068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GG, Jackson T, Sands BE, et al. The risk of developing Crohn's disease after an appendectomy: a meta-analysis. Am J Gastroenterol. 2008;103:2925–2931. doi: 10.1111/j.1572-0241.2008.02118.x. [DOI] [PubMed] [Google Scholar]
- Dybdahl M, Risom L, Moller P, et al. DNA adduct formation and oxidative stress in colon and liver of Big Blue rats after dietary exposure to diesel particles. Carcinogenesis. 2003;24:1759–1766. doi: 10.1093/carcin/bgg147. [DOI] [PubMed] [Google Scholar]
- Sigaud S, Goldsmith CA, Zhou H, et al. Air pollution particles diminish bacterial clearance in the primed lungs of mice. Toxicol Appl Pharmacol. 2007;223:1–9. doi: 10.1016/j.taap.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowdy K, Krantz QT, Daniels M, et al. Modulation of pulmonary inflammatory responses and antimicrobial defenses in mice exposed to diesel exhaust. Toxicol Appl Pharmacol. 2008;229:310–319. doi: 10.1016/j.taap.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Li X, Sundquist J, Sundquist K. Educational level and occupation as risk factors for inflammatory bowel diseases: a nationwide study based on hospitalizations in Sweden. Inflamm Bowel Dis. 2009;15:608–615. doi: 10.1002/ibd.20815. [DOI] [PubMed] [Google Scholar]
- Gaya DR, Russell RK, Nimmo ER, et al. New genes in inflammatory bowel disease: lessons for complex diseases. Lancet. 2006;367:1271–1284. doi: 10.1016/S0140-6736(06)68345-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.