Abstract
The rat possesses a hemochorial form of placentation. Pronounced intrauterine trophoblast cell invasion and vascular remodeling characterize this type of placentation. Strain-specific patterns of placentation are evident in the rat. Some rat strains exhibit deep intrauterine trophoblast invasion and an expanded junctional zone [Holtzman Sprague-Dawley (HSD), Dahl salt sensitive (DSS)], whereas placentation sites of other rat strains are characterized by shallow invasion and a restricted junctional zone [Brown Norway (BN)]. In this report, we identified a quantitative trait that was used to distinguish strain-specific features of rat placentation. Junctional zone prolactin family 5, subfamily a, member 1 (Prl5a1) transcript levels were significantly greater in BN rats than in HSD or DSS rats. Prl5a1 transcript levels were used as a quantitative trait to screen placentation sites from chromosome-substituted rat strains (BN chromosomes introgressed into the DSS inbred strain; DSS-BN panel). Litter size, placental weights, and fetal weights were not significantly different among the chromosome-substituted strains. Regulation of the junctional zone Prl5a1 transcript-level quantitative trait was multifactoral. Chromosome-substituted strains possessing BN chromosomes 14 or 17 introgressed into the DSS inbred rat strain displayed Prl5a1 transcript levels that were significantly different from the DSS pattern and more closely resembled the BN pattern. The in situ placental distribution of Prl5a1 mRNA and the structure of the junctional zone of DSS-BN17 rats mimicked that observed for the BN rat. Prl5a1 gene expression was also assessed in BN vs. HSD trophoblast stem cells and following reciprocal BN and HSD embryo transfer. Strain differences intrinsic to trophoblast and maternal environment were identified. In summary, we have identified chromosomes 14 and 17 as possessing regulatory information controlling a quantitative trait associated with rat placentation.
Keywords: prolactin, Brown Norway rat, trophoblast
the placenta is a specialized extraembryonic tissue that acts as an interface between the mother and fetus ensuring nutrient delivery and providing signals requisite for viviparity. The rat possesses a hemochorial form of placentation, characterized by pronounced intrauterine trophoblast cell invasion and vascular remodeling (1, 7, 41). Rat placentation sites are organized into discrete compartments (2). The location of nutrient and waste exchange with the fetal vasculature is referred to as the labyrinth zone. The junctional zone of the rat placenta is situated between the labyrinth zone and the uterine decidua, representing the place where invasive trophoblast lineages arise. Invasive trophoblast cells enter the uterine mesometrial compartment (decidua and metrial gland) where they replace segments of the uterine spiral arterial endothelium (endovascular invasive trophoblast) and infiltrate into spaces between the uterine vasculature (interstitial invasive trophoblast) (1, 7, 19, 41). The maternal environment influences intrauterine trophoblast cell invasion. Maternal hypoxia and hypertension impact the depth of intrauterine trophoblast invasion and remodeling of the uterine spiral arteries (10, 11, 15, 32). Trophoblast-directed uterine vascular remodeling regulates placental perfusion (42).
Strain-specific patterns of placentation are evident in the rat (12, 18, 19). The Holtzman Sprague-Dawley (HSD) outbred and ACI, Fischer 344 (F344), and Dahl salt-sensitive (DSS) inbred strains exhibit robust placentation, including a well-developed junctional zone and extensive intrauterine trophoblast invasion (19). In contrast placentation in the Brown Norway (BN) rat is more restrictive (19). The BN rat junctional zone is reduced in size and intrauterine trophoblast invasion is shallow, resulting in a placental insufficiency that adversely affects fetal growth and the success of pregnancy (12, 18, 19). These observations suggest that a genetic approach for investigating the regulation of placentation may be possible.
Phenotypic analysis of chromosome-substituted strains of mice and rats is an effective strategy for ascribing function to the genome (8, 16, 28, 36). Chromosome-substituted strains of rats have proven to be particularly valuable tools for studying the genetics of hypertension and vascular disease (17, 20, 24–26, 31). These efforts have included generation of a panel of chromosome-substituted rat strains possessing each of the BN rat chromosomes introgressed into the DSS rat genome (20). DSS and BN rat strains exhibit differences in their cardiovascular and renal biology (22). The DSS strain exhibits significant vascular and renal pathologies when maintained on a high-salt diet that can be attenuated when DSS rats are maintained on a low-salt diet (22, 27). Vascular and renal pathologies are rare in the BN strain maintained on low- or high-salt diets (22).
Given that BN and DSS rat strains possess distinctive placentation phenotypes (18, 19), the purpose of this study was to use the DSS-BN chromosome-substituted panel to elucidate genetic insights about the regulation of placentation. We identified a trait associated with placentation [junctional zone expression of prolactin family 5 subfamily a, member 1 (Prl5a1)] showing quantitative differences between BN and DSS parent strains. We investigated 21 DSS-BN chromosome-substituted strains (for all 20 autosomes + the X chromosome) to identify chromosomes affecting the quantitative trait. Chromosomes 14 and 17 were identified as contributors to the placentation phenotype.
MATERIALS AND METHODS
Animals and tissue preparation.
DSS, BN, and F344 inbred rat strains were obtained from Charles River Laboratories (Wilmington, MA). HSD outbred rats were purchased from Harlan Sprague-Dawley (Indianapolis, IN). DSS-BN chromosome-substituted rat strains (20) were obtained from PhysioGenix (Milwaukee, WI). Animals were housed in an environmentally controlled facility, with a 14:10 light-dark photoperiod (lights on from 0600 to 2000) and were allowed ad libitum access to food and water. DSS parent and DSS-BN chromosome-substituted strains were maintained on low-salt diets obtained from Harlan Teklad (3075S; Madison, WI) or Purina (5010; Richmond, IN). HSD rats were provided a standard rodent laboratory diet (8604; Purina). BN rats were provided the low-salt diet or a standard rodent diet. The latter diet was used for comparisons with HSD rats. Pregnancy parameters are similar for BN rats fed either the low-salt or standard laboratory diets (18, 19).
Virgin female rats 8–10 wk of age for each strain were cohabited with adult males (>3 mo of age) of the same strain. Mating was assessed by daily inspection of vaginal lavages. The presence of sperm in the vaginal lavage was considered as day 0.5 of pregnancy. Female rats were made pseudopregnant by mating with vasectomized males. Presence of seminal plugs was designated day 0.5 of pseudopregnancy. Embryo transfer was accomplished through the collection of day 0.5 embryos and transferring them into oviducts of day 0.5 pseudopregnant recipients. Survival surgery was performed under isofluorothane anesthesia. On gestation day 18.5, females were killed and placental and fetal tissues collected and weighed. The number of viable vs. dead and/or resorbing conceptuses was determined. Placentation sites were collected and junctional zone tissues dissected as previously described (2). Tissues were snap-frozen in liquid nitrogen for RNA analyses. For in situ hybridization placentation sites were frozen in dry ice-cooled heptane. Nonpregnant adult female DSS, BN, and F344 rats were killed; their livers were dissected and used for the isolation of genomic DNA. All tissue samples were stored at −80°C until processed. Protocols for these methods have been previously described (2, 18). The University of Kansas Medical Center Animal Care and Use Committee approved all procedures for handling and experimentation with the rats.
DNA microarray analysis.
Total RNAs were prepared from gestation day 18.5 HSD and BN rat junctional zone tissues (n=9 for each strain) using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. RNA extractions were pooled to form three groups of three for each rat strain in nuclease-free water at a concentration of 1.0 μg/μl. RNA was quantified by spectrophotometry, and quality was verified using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). RNA samples were hybridized to Affymetrix Rat 230 2.0 DNA microarray chips using the GeneChip Hybridization Oven 640 (Affymetrix, Santa Clara, CA). Washing and staining of the hybridized chips were conducted with the GeneChip Fluidics Station 450 (Affymetrix). Chips were scanned using the Affymetrix GeneChip Scanner 3000 (Affymetrix) with autoloader by the KUMC Biotechnology Support Facility (Kansas City, KS). Hybridization signals were normalized with internal controls. Expression data sets were analyzed using the R statistics software (http://www.r-project.org/) with BioConductor software (http://www.bioconductor.org/) packages. The MAS5 method from the BioConductor software was used for background correction, normalization, and summarization of the DNA microarray data. Transcripts showing more than a twofold change with analysis of variance (ANOVA, P < 0.05) were considered as differentially expressed genes and subjected to Gene Ontology (GO) analysis using GO-elite software (http://www.genmapp.org/go_elite/). A subset of genes exhibiting robust differences between the two genetic rat strains were selected for further validation by quantitative RT-PCR (qRT-PCR).
qRT-PCR.
Total RNAs were prepared from gestation day 18.5 HSD, DSS, BN, and DSS-BN chromosome-substituted strains (n=5 for each strain). cDNAs were synthesized from total RNA (1 μg) for each sample using Moloney murine leukemia virus reverse transcriptase (Invitrogen), diluted five times with water, and subjected to qRT-PCR to estimate mRNA levels. Primers were designed using Primer Express 2.0 (Applied Biosystems, Foster City, CA) with a melting temperature of 60°C. Primer sequences are provided in Table 1. Diluted cDNA was used as a template for quantitative real-time PCR amplification using Power SYBR Green PCR Master Mix and the ABI Prism 7500 Real Time PCR System (Applied Biosystems). To ensure quality of the amplification of real-time PCR products, a dissociation profile (melting curve) was analyzed for each gene product generated. Cycling conditions included an initial hold step (95°C for 10 min) and 40 cycles of a two-step PCR (92°C for 15 s, then 60°C for 1 min), followed by a dissociation step (95°C for 15 s, 60°C for 15 s, and then 95°C for 15 s). The comparative cycle threshold method (ΔΔCT) was used for relative quantification of the amount of mRNA for each sample normalized to 18S RNA.
Table 1.
Primer sequences used for quantitative RT-PCR
| Symbol | Accession No. | Forward Primer | Reverse Primer |
|---|---|---|---|
| RGD1309969 | AI059078 | CTTGTGGGCGAAGATTCATT | GGAAGCCTGAGACAGACAGG |
| LOC683295 | AI764437 | CCATCAGTTGGGCAGAAGTT | AACAGCCCAACACTGGAAAC |
| Prtfdc1 | AI407006 | ATGTCTTCGTGGTGGGCTAC | CACTGACGAGTTTTGCTCCA |
| Mmp10 | NM_133514 | CCACACTCTTGGCTTTCCTC | GTCTCGGGAAGCCTTTATCC |
| Dhrs8 | BE098506 | GTGATAGCGATTTCCCCAGA | CGGAGAGTGTGAATCGAACA |
| Prl3a1 | AB019791 | TGTGAGGTCTGCCAATGAAC | TAGGGCCTCAAGGTAAGTGG |
| Efemp1 | BF284634 | GGAAGTGGGCATTTAATCCA | ATTGCTTGGTCCCATTTTTG |
| Slpi | NM_053372 | GAATCCTGTTCCCATTCGTG | TTCCCACACATACCCTCACA |
| Echdc2 | AI172274 | GTGGGCAAGTAACCCAGCTA | CCATGCCAATCACACTGTTC |
| Spp1 | AB001382 | GATCGATAGTGCCGAGAAGC | TGAAACTCGTGGCTCTGATG |
| Pdia5 | AI045590 | TGGTGCCCACACTGTAAGAA | TCCTGCTGACACAGGTCTTG |
| Prl5a1 | AF226607 | TCCACACCAGACATTCCAGA | TTTCCAGGAAGCCAACATTC |
| Tpbpa | NM_172073 | GCAAGAGCAGAAGGGTAAAGAAGG | TTTCTATGTCGAGCTCCTCCTCCT |
| 18S rRNA | M11188 | GCAATTATTCCCCATGAACG | GGCCTCACTAAACCATCCAA |
In situ hybridization.
Transcript localization was performed as described previously (1). We prepared 10 μm cryosections of placentation sites and stored them at −80°C until processed. A plasmid containing a cDNA for rat Prl5a1 (3, 19) was used as a template for synthesizing sense and antisense digoxigenin-labeled riboprobes according to the manufacturer's instructions (Roche Molecular Biochemicals, Indianapolis, IN). Prl5a1 is also known as prolactin-like protein-L (38). Tissue sections were air dried and fixed in ice-cold 4% paraformaldehyde in phosphate-buffered saline. Prehybridization, hybridization, and detection of alkaline phosphatase-conjugated anti-digoxigenin were performed as previously reported (1).
Analysis of the 5′-region flanking the Prl5a1 gene.
Rat Prl5a1 5′-flanking DNA spanning −5,048 to +63 (relative to the transcription start site) was PCR amplified using genomic DNA templates isolated from BN, DSS, and F344 liver tissues. Primer sequences used for the Prl5a1 5′-flanking DNA are provided in Table 2. PCR amplified 5′-flanking regions were initially cloned into the TA cloning vector (Invitrogen) for sequence confirmation and then segments spanning −3,650 to +63 (referred to as 3.7 kb promoter) and −950 to +63 (referred to as 1.0 kb promoter) were subcloned into XhoI and NcoI sites of the pGL4.10 [luc2] luciferase vector (Promega, Madison, WI) for in vitro assays using Rcho-1 trophoblast stem (TS) cells (9, 33) and rat embryonic fibroblasts (REFs). Rcho-1 TS cells were derived from a rat trophoblast tumor and possess the capacity for self-renewal and differentiation into multiple trophoblast lineages (9, 33). REFs do not express Prl5a1 and were used to evaluate promoter specificity. Cells grown in 24-well plates were cotransfected with 100 ng of reporter vector and 15 ng of control Renilla vector using Lipofectamine 2000 (Invitrogen). Twelve hours after transfection, medium was changed and incubations continued for an additional 48 h before passive cell lysis and processing with a standard dual luciferase assay (Promega).
Table 2.
Primer sequences used for cloning the Prl5a1 5′-flanking region
| Primer | Amplicon | Primer Sequence |
|---|---|---|
| Prl5a1-953 forward (−5048) | −5048 to +63 | AGTGGAGACTCCCAGCTCAA |
| Prl5a1-6063 reverse (+63) | −5048 to +63 | AGGGATGTGGTTGAATCTGC |
| 2349XhoI-forward (−3650) | −3650 to +63 | GCTTGGTGGCACATCTCGAGACTT |
| 5050Xho-forward (−951) | −951 to +63 | GGGCTCTAAAGGCTCGAGGA |
| 6063NcoI-reverse (+63) | −3650 to +63 and −951 to +63 | CTTACAGGGCCATGGTTGAATCTG |
Establishment of BN rat TS cell lines.
Blastocysts were recovered from uteri of gestation day 4.5 BN rats and TS cell lines established as previously described (5). BN TS cells were compared with previously established HSD TS cell lines (5). TS cells were cultured in basal culture medium [RPMI 1640 (Cellgro, Herndon, VA); 20% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA); 100 μM 2-mercaptoethanol (Sigma); 1 mM sodium pyruvate (Cellgro); 50 μM penicillin and 50 U/ml streptomycin (Cellgro)] supplemented with 70% REF conditioned medium, FGF4 (25 ng/ml, Sigma), and heparin (1 μg/ml, Sigma). Differentiation of the TS cells was induced by removal of FGF4, heparin, and REF conditioned medium.
Statistical analyses.
Statistical analyses were performed using the R Statistical Package (http://www.r-project.org/). One-way or two-way ANOVA followed by Tukey tests were used depending upon the data structure. Nonparametric Wilcoxon rank sum tests or ANOVA with general linear model followed by Tukey contrast tests were applied for qRT-PCR data.
RESULTS
Identification of placenta-associated quantitative traits.
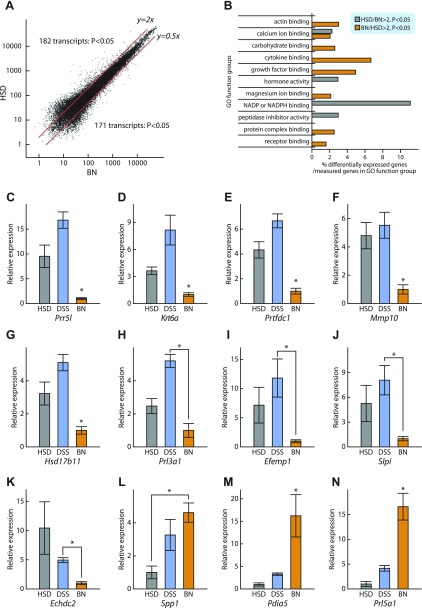
Structural differences within placentation sites of the BN strain vs. the HSD and DSS strains are striking but their quantification is not conducive to high-throughput analyses. These differences include the thickness of the junctional zone, which can be readily dissected and is more homogenous than other components of the placentation site (2). HSD and BN rat gestation day 18.5 junctional zone gene expression profiles were determined using DNA microarray analysis. All DNA microarray data presented in this report are deposited in the Gene Expression Omnibus (GEO) repository under the GSE29247 accession number. Among the 31,097 transcripts examined, 182 transcripts were more abundantly expressed in HSD junctional zone, whereas 171 transcripts were more abundant in BN junctional zone (>2-fold-change, P < 0.05; Fig. 1A, Supplemental Tables S1 and S2).1 Differentially expressed genes did not exhibit a chromosomal bias. Strain differences in gene expression associated with specific functional pathways were observed (Fig. 1B). Twelve representative genes reflecting the most prominent strain differences in gene expression were examined by qRT-PCR (Table 3; Fig. 1, C–N). Nine of these genes (Prr5l, Krt6a, Prtfdc1, Mmp10, Hsd17b11, Prol3a1, Efemp1, Slpi, Echdc2) showed higher expression in HSD and/or DSS rat junctional zone vs. BN rat junctional zone (Fig. 1, C–K), whereas three of the genes (Spp1, Pdia5, Prl5a1) exhibited the reverse relationship (Fig. 1, L–N). HSD and DSS rat junctional zone gene expression showed similar relationships to BN rat patterns except for Spp1 (Fig. 1L). In summary, gene expression profiling provides several reliable measures of strain-specific junctional zone attributes that can be adapted to high-throughput analysis.
Fig. 1.
Identification of genes differentially expressed in junctional zone tissue from Holtzman Sprague-Dawley (HSD), Dahl salt-sensitive (DSS), and Brown Norway (BN) rat strains. A: gene profiling of junctional zone tissue from HSD and BN rat strains using the Affymetrix Rat 230 2.0 chip. Among the transcripts expressed in junctional zone, 182 transcripts were highly expressed in HSD and 171 transcripts were highly expressed in BN (fold change >2, P < 0.05). B: Gene Ontology (GO) analysis of the DNA microarray results. C–N: selected genes exhibiting strain differences in their expression patterns based on DNA microarray analysis were verified by quantitative (q) RT-PCR for HSD, DSS, and BN rat strains. *Significant differences (P < 0.05).
Table 3.
Differentially expressed genes in gestation day 18.5 junctional zone
| Symbol | Accession No. | Description | Chromosome | HSD/BN* |
|---|---|---|---|---|
| Echdc2 | NM_001106675 | enoyl CoA hydratase domain containing 2, mitochondrial | 5 | 76.09 |
| Krt6a | NM_001101007 | keratin complex 2, basic, gene 6a | 7 | 47.03 |
| Prr5l | NM_001080150 | proline-rich 5-like | 3 | 13.53 |
| Slpi | NM_053372 | secretory leukocyte peptidase inhibitor | 3 | 6.97 |
| Efemp1 | NM_001012039 | EGF-containing fibulin-like extracellular matrix protein 1 | 14 | 6.75 |
| Prtfdc1 | NM_001106127 | phosphoribosyl transferase domain containing 1 | 17 | 5.20 |
| Mmp10 | NM_133514 | matrix metallopeptidase 10 (stomelysin-2) | 8 | 4.25 |
| Prl3a1 | NM_153736 | prolactin family 3, subfamily a, member 1 (prolactin-like protein-I) | 17 | 3.55 |
| Hsd17b11 | NM_001004209 | hydroxysteroid 17β dehydrogenase 11 | 14 | 3.02 |
| Spp1 | NM_012881 | secreted phosphoprotein 1 (osteopontin) | 14 | 0.32 |
| Prl5a1 | NM_138527 | prolactin family 5, subfamily a, member 1 (prolactin-like protein-L) | 17 | 0.24 |
| Pdia5 | NM_001014125 | protein disulfide isomerase A5 | 11 | 0.15 |
Ratio of Holtzman Sprague-Dawley to Brown Norway (HSD/BN) junctional zone expression as determined by the DNA microarray.
Prl5a1 transcript localization.
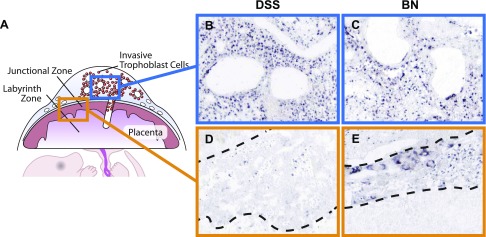
PRL5A1 is a member of the expanded prolactin family of cytokines (3, 37, 39) and a product of the placenta (1). In the HSD rat placentation site, Prl5a1 transcripts are confined to invasive trophoblast cells located within the uterine mesometrial compartment (1). Prl5a1 gene expression in the BN junctional zone contrasts with previous observations for the HSD rat (Fig. 1; Ref. 1). Consequently, we monitored Prl5a1 expression in gestation day 18.5 placentation sites of BN and DSS rats by in situ hybridization. DSS rat placentation sites exhibited a Prl5a1 expression pattern similar to that previously reported for the HSD rat (Fig. 2; Ref. 1). Expression of Prl5a1 in the BN uterine mesometrial compartment was conserved (Fig. 2C); however, in contrast to DSS and HSD placentation sites, the BN placentation site showed significant hybridization in trophoblast cells throughout the junctional zone (Fig. 2E). Prl5a1 expression inversely correlated with the thickness of the junctional zone. Thus the BN rat placentation site can be distinguished from DSS and HSD rat placentation sites through a fundamental difference in junctional zone expression of Prl5a1.
Fig. 2.
In situ detection of Prl5a1 transcript distribution in gestation day 18.5 placentation sites from DSS and BN rat strains. In situ hybridization was performed on cryosections from gestation day 18.5 placentation sites of DSS and BN rats using antisense and sense Prl5a1 probes. Anti-sense hybridization indicated Prl5a1 expression in invasive trophoblast of both strains (blue boxed images) and the junctional zone of only the BN rat strain (orange boxed images). Sense probes served as controls and did not yield significant hybridization signals. The dashed lines on each image bracket the junctional zone.
DSS-BN chromosome-substituted panel screen.
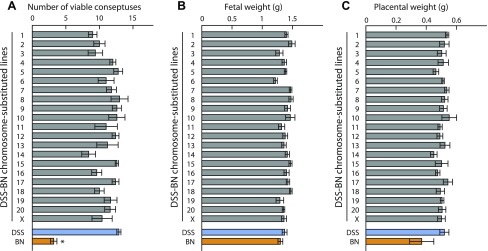
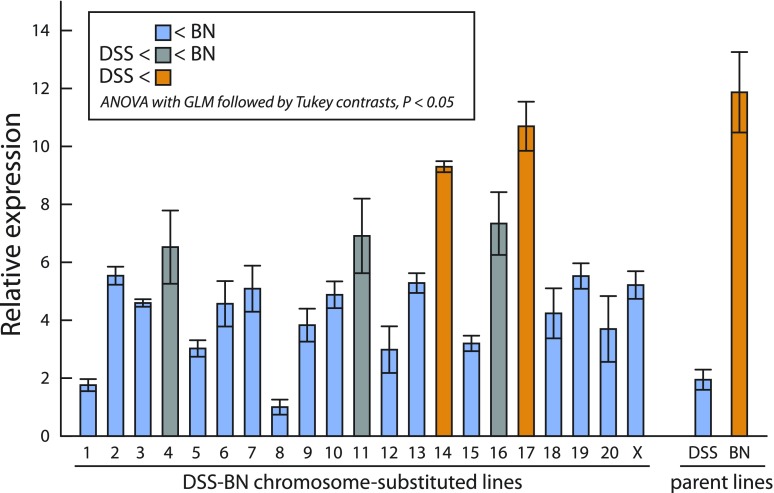
A DSS-BN chromosome-substituted panel was surveyed to gain additional insights about the genetics of placentation in the rat. Litter size was significantly greater in the DSS rat strain vs. the BN rat strain (Fig. 3A). However, no significant differences in the number of viable conceptuses, fetal weights, or placental weights were found within the DSS-BN chromosome-substituted panel (Fig. 3, A–C). We next screened the DSS-BN chromosome-substituted panel for junctional zone Prl5a1 transcript levels. Prl5a1 transcript level in the junctional zone of the placenta was selected for monitoring the chromosome-substituted panel because: 1) there is a robust difference in Prl5a1 transcript levels in the junctional zone of BN and DSS strains (Fig. 1); 2) Prl5a1 expression is restricted to trophoblast cells and more specifically to the invasive trophoblast lineage of the HSD rat, a cell population compromised in the BN rat (1, 19); 3) Prl5a1 expression in the BN junctional zone represents a “gain-of-function” phenotype. The results suggest a multifactoral regulation of the “junctional zone Prl5a1 transcript level” trait (Fig. 4). DSS-BN4, DSS-BN11, DSS-BN14, DSS-BN16, and DSS-BN17 junctional zone tissues each possessed Prl5a1 transcript levels that were significantly greater than the DSS parent tissue transcript level (Fig. 4). The DSS-BN4, DSS-BN11, and DSS-BN16 exhibited an intermediate phenotype. Although junctional zone Prl5a1 transcript levels were significantly greater than the DSS parent strain, they were significantly less than the parent BN phenotype, whereas the DSS-BN14 and DSS-BN17 phenotypes did not differ significantly from the BN parent phenotype (Fig. 4). Chromosome 17 is also where the Prl5a1 locus resides (3).
Fig. 3.
Analysis of the number of viable conceptuses and fetal and placental weights in DSS-BN chromosome-substituted rat strains on day 18.5 of pregnancy. A: number of viable conceptuses. *Significant difference between the parent lines (P < 0.05). B: fetal weights. C: placental weights. Sample size n=5 for each strain.
Fig. 4.
Expression of Prl5a1 mRNA in gestation day 18.5 junctional zone tissue of DSS-BN chromosome-substituted rat strains. Total RNA samples from gestation day 18.5 junctional zone tissue were isolated and Prl5a1 transcript levels measured by qRT-PCR (SYBR Green, ΔΔCt method). 18S rRNA served as an internal control. Data were analyzed by ANOVA with general linear model (GLM) followed by Tukey contrast tests. Sample size n=5 for each strain.
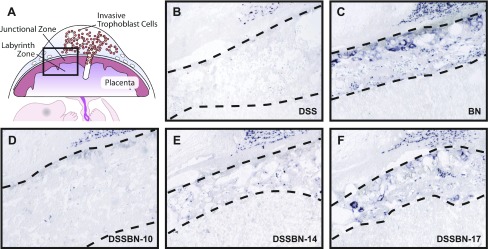
The distribution of Prl5a1 transcripts was determined in selected chromosome-substituted strains by in situ hybridization. Prl5a1 mRNA distribution within the junctional zone was similar in the DSS-BN17 chromosome-substituted strain and the BN parent strain (Fig. 5, C and F). DSS-BN10 chromosome-substituted strains lacked detectable junctional zone Prl5a1 similar to the DSS parent strain (Fig. 5, B and F). Prl5a transcript expression in the DSS-BN14 was intermediate between that observed in BN and DSS parent strains (Fig. 5, B, C, and E). Prl5a1 expression inversely correlated with the thickness of the junctional zone. The BN and DSS parent strains and three DSS-BN chromosome-substituted strains (DSS-BN 10, 14, and 17) each showed abundant Prl5a1 in invasive trophoblast cells of the uterine mesometrial compartment (Fig. 5, B–F).
Fig. 5.
In situ detection of Prl5a1 transcript distribution in gestation day 18.5 placentation sites from DSS, BN, DSS-BN10, DSS-BN14, and DSS-BN17 rat strains. In situ hybridization was performed on cryosections from gestation day 18.5 placentation sites of DSS, BN, DSS-BN10, DSS-BN14, and DSS-BN17 rats using antisense and sense Prl5a1 probes. Antisense hybridization indicated Prl5a1 expression in invasive trophoblast of all strains and the junctional zone of the BN and DSS-BN17 rat strains. The DSS-BN14 strain showed low levels of Prl5a1 expression. Sense probes served as controls and did not yield significant hybridization signals. The dashed lines on each image bracket the junctional zone.
In summary, the control of junctional zone Prl5a1 transcript levels is multigenic with major contributions associated with regulatory information located on chromosomes 14 and 17.
Placentation phenotype of chromosome-substituted strains.
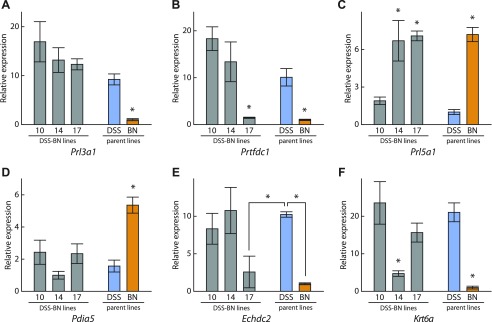
We next attempted to determine whether the placentation phenotype observed in DSS-BN14 and DSS-BN17 chromosome-substituted strains extended beyond Prl5a1. Transcript levels of a subset of genes differentially expressed in junctional zone tissue of DSS and BN parent strains (Fig. 1) were examined in DSS-BN10 (control), DSS-BN14, and DSS-BN17. In agreement with the results in Fig. 4, DSS-BN10 rats exhibited the DSS parent Prl5a1 expression phenotype, while DSS-BN14 and DSS-BN17 exhibited the BN parent Prl5a1 expression phenotype (Fig. 6A). Prl3a1 and Pdia5 transcript profiles were similar to the DSS parent phenotype for each of the three chromosome-substituted strains (Fig. 6, B and C), suggesting that the BN expression patterns for these genes was not regulated by genetic information on chromosomes 10, 14, or 17. In addition to the regulation of junctional zone Prl5a1 transcript levels, chromosome 17 also contributed to the regulation of Prtfdc1 and Echdc2 transcript levels (Fig. 6, D and E), whereas chromosome 14 contributed to the regulation of Krt6a transcript levels (Fig. 6F). These observations reinforce the multifactoral regulation of the BN placentation phenotype.
Fig. 6.
Expression profiles of selected genes in DSS-BN10, DSS-BN14, and DSS-BN17 chromosome-substituted rat strains and DSS and BN parent rat strains. Total RNA samples from gestation day 18.5 junctional zone tissue were isolated and transcript levels of selected genes measured by qRT-PCR (SYBR Green, ΔΔCt method). 18S rRNA served as an internal control. Sample size n=5 for each strain. *Significant differences (P < 0.05).
Prl5a1 promoter analysis.
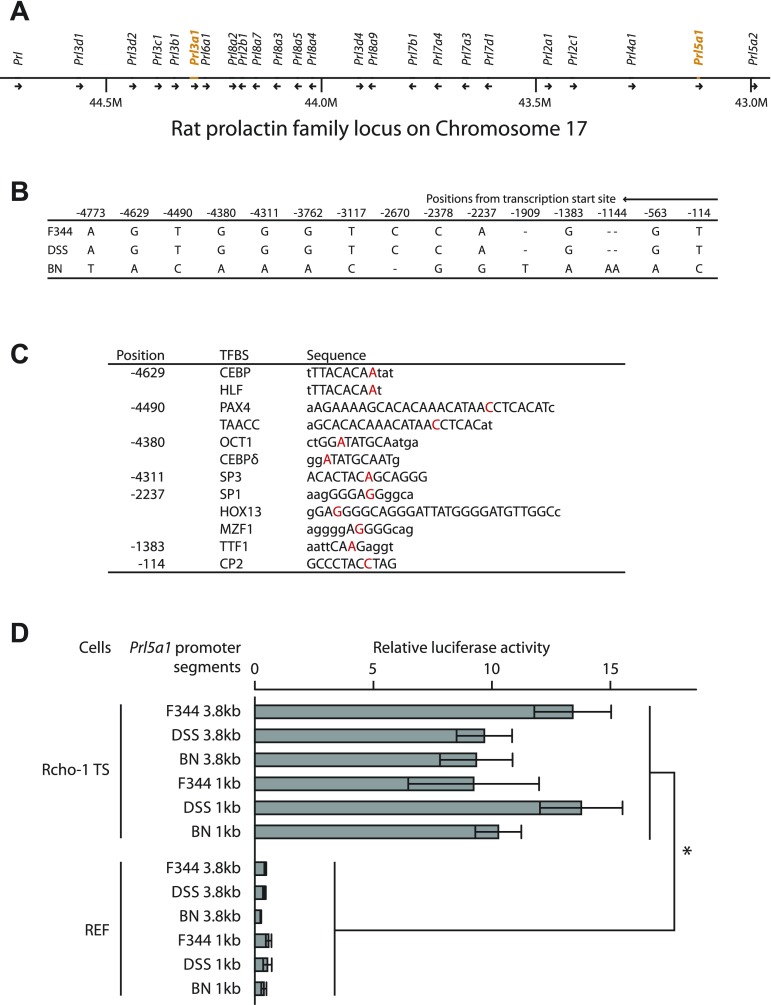
Prl3a1 and Prl5a1 are members of the expanded prolactin gene family, which is situated within a 1.7-megabase region on chromosome 17 (Fig. 7A; Refs. 3, 39). Since chromosome 17 contributed to the junctional zone Prl5a1 transcript BN parent phenotype, we next investigated 5′-flanking sequence associated with the Prl5a1 gene. Segments spanning over 5 kb upstream of the Prl5a1 transcription start site for F344, DSS, and BN rat genomic DNA were cloned and sequenced. F344 and DSS Prl5a1 5′-flanking nucleotide sequences were identical. The BN 5′-flanking sequence differed from the F344 and DSS shared sequence at 15 sites (Fig. 7B), resulting in nucleotide changes in several putative transcription factor-binding elements (based on in silico evaluation, Fig. 7C). F344, DSS, and BN upstream regions were subcloned into a luciferase reporter system and promoter activity tested in Rcho-1 TS cells and REFs. Each promoter-reporter construct exhibited similar luciferase activities in Rcho-1 TS cells and were inactive in REFs (Fig. 7D). Thus basal Prl5a1 promoter activity is not affected by the strain-associated nucleotide changes; instead these nucleotide changes may impact regulatory pathways controlling Prl5a1 transcription or alternatively they may be irrelevant to the junctional zone Prl5a1 transcript level trait.
Fig. 7.
Examination of Prl5a1 promoter regions from F344, DSS, and BN rat strains. A: rat prolactin family locus. B: summary of nucleotide differences within ∼5 kb of 5′-flanking DNA among F344, DSS, and BN rat strains. C: potential transcription factor binding sites (TFBS) determined by rVista 2.0 (Transfac) analysis. D: F344, DSS, and BN promoter-luciferase reporter analysis in Rcho-1 trophoblast stem cells (TS) and rat embryonic fibroblasts (REF). *Significant differences (P < 0.05).
Trophoblast vs. maternal environment impact on Prl5a1 expression.
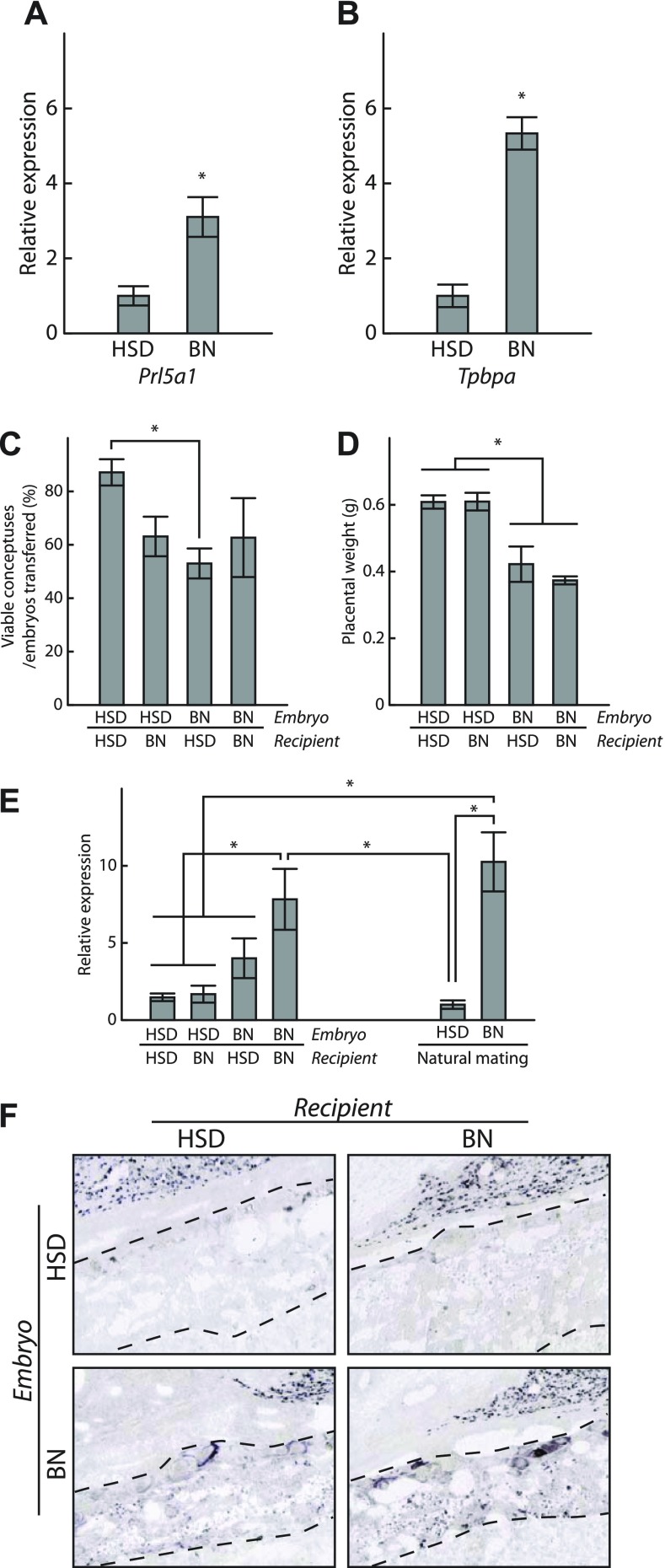
BN rat TS were generated from gestation day 4.5 blastocysts using methodologies as previously described (5). The initial BN blastocyst outgrowths were more difficult to establish and once formed were not disaggregated until sufficient cell expansion was evident. Derivation of BN TS cells was less efficient (2 - TS cell lines generated from 63 blastocysts; 3% efficiency) than the HSD rat strain (∼21% efficiency; Ref. 5). BN and HSD TS cells were evaluated following 12 days of differentiation for the expression of Prl5a1 and Tpbpa, a marker of commitment to junctional zone trophoblast cell lineages (5). Prl5a1 and Tpbpa transcript levels were significantly elevated in BN TS cells (Fig. 8, A and B).
Fig. 8.
Trophoblast and maternal impact on Prl5a1 expression. A and B: HSD and BN TS cells were compared for their expression of Prl5a1 and Tpbpa. TS cells were differentiated for 12 days and transcript levels were analyzed by qRT-PCR. C–F: analysis of placentation sites from HSD-BN reciprocal embryo transfers (HSD embryos transferred to HSD recipients, n=6; HSD embryos to BN recipients, n=9; BN embryos in HSD recipients, n=5; BN embryos in BN recipients, n=4). Viability of placentation sites (C), placental weights (D), qRT-PCR junctional zone Prl5a1 transcript levels (E), and distribution of Prl5a1 transcripts (F) were determined on gestation day 18.5. Data were analyzed initially by ANOVA followed by Tukey contrast tests. *Significant differences (P < 0.05).
The maternal environment has a significant contribution to the subfertility of the BN rat (18). BN rats exhibit defective uterine decidualization leading to impaired trophoblast development (18). Consequently, we evaluated maternal influences on HSD and BN rat junctional zone Prl5a1 transcript levels following reciprocal embryo transfer with HSD and BN rat strains. HSD and DSS strains possess a similar junctional zone Prl5a1 transcript level trait, which differs significantly from the BN trait (Fig. 1). Viability and placental weights were monitored at gestation day 18.5 (Fig. 8, C and D). BN-embryo derived placentas were significantly smaller (Fig. 8D), which may be attributed to strain differences in responses to experiential factors associated with the embryo transfer procedure. HSD junctional zone Prl5a1 transcript levels were uniformly low and independent of uterine environment. In contrast, the BN junctional zone phenotype was modified when developing in the HSD uterus; Prl5a1 transcript levels were significantly decreased but not equivalent to the HSD phenotype (Fig. 8E). These findings were supported by analysis of Prl5a1 mRNA distribution within the junctional zones of the experimental animals (Fig. 8F).
The results suggest that both intrinsic and extrinsic factors contribute to the regulation of the BN placentation phenotype.
DISCUSSION
Hemochorial placentation is an effective reproductive strategy facilitating fetal development within the female reproductive tract. This strategy is utilized by many mammalian species, including the rat. Genetic differences exist in the organization of the rat placenta (19). In contrast to some rat strains, the BN rat placentation site is characterized by a thin junctional zone and shallow intrauterine trophoblast invasion. Strain differences in quantitative traits have been successfully exploited to elucidate genetic mechanisms impacting cardiovascular and renal function (17, 20, 24–26, 31). In this report, we identified a quantitative trait associated with rat placentation (junctional zone Prl5a1 transcript levels) and utilized chromosome-substituted strains to fractionate the rat genome and ascribe the trait to specific chromosomes. Prl5a1 was selected as an index of placentation because it is expressed in the invasive trophoblast lineage, a cell population compromised in the BN placentation site (1, 19). Genetic regulation of the junctional zone Prl5a1 transcript level trait was multifactoral with chromosome 14 and especially chromosome 17 having significant roles in influencing elaboration of the phenotypic measure.
Several strain-specific differences in junctional zone gene expression were identified. These genes reside on various chromosomes throughout the genome. Junctional zone Prl5a1 transcript level was initially identified as a strain-specific quantitative trait using DNA microarray analysis and subsequently verified by qRT-PCR and in situ hybridization. Prl5a1 encodes a member of the expanded prolactin family of cytokines and was initially characterized based on its expression in invasive trophoblast (1, 40). In the BN rat, Prl5a1 is uniquely expressed in the junctional zone, thus representing a gain-of-function phenotype. The junctional zone serves as a reservoir of precursor cells capable of generating invasive trophoblast. BN progenitors residing in the junctional zone mature to a stage capable of expressing Prl5a1 but do not invade into the uterine mesometrial compartment or invade less efficiently. Regulatory factors controlling the invasive trophoblast lineage are unknown. The biological actions of PRL5A1 have not been determined and whether they contribute to an autocrine/paracrine signaling pathway regulating invasive trophoblast cells is unknown. At a minimum Prl5a1 transcript levels may represent no more than a robust measure of a junctional zone trait. Genetic information on chromosomes 14 and 17 contribute to the regulation of the junctional zone Prl5a1 transcript level trait, which may include control of the differentiation and/or migratory behavior of Prl5a1-positive trophoblast cells.
Control of the junctional zone Prl5a1 transcript level trait may be indirect. Preparation of the uterus for embryo implantation and development of uterine decidua are defective in the BN rat (18). The BN rat exhibits luteal insufficiency and progesterone resistance, which contribute to its subfertility. Maternal environment is a major factor governing placentation (4). In support of such a maternal contribution, reciprocal embryo transfer experiments, performed herein, indicate that the BN junctional zone Prl5a1 transcript level trait is, at least partially, sensitive to the uterine milieu. The HSD maternal environment was more dominant than was the BN maternal environment. This contrasts with the relative impact of the HSD and BN uterine milieu on early stages of placental morphogenesis (18). Intrinsic differences were also identified in differentiated TS cells from BN and HSD strains. Thus the multifactoral regulation of the junctional zone Prl5a1 transcript level trait may include both maternal and extraembryonic contributions.
Earlier research efforts using recombinant inbred strains of rats (29) sought to identify genetic contributions to litter size and placental and fetal weight regulation (6, 47). These recombinant rat strains were originally established to elucidate the genetics of hypertension and involved analyses of BN and spontaneous hypertensive rat strain recombinants (29). Litter size traits were mapped to chromosome 8 (47), whereas placental and fetal size traits were linked to chromosomes 15 and 1, respectively (6). Although a prominent difference exists between litter size of DSS and BN rat strains (19), analysis of chromosome-substituted DSS-BN rat strains was not informative in identifying a rat chromosome(s) responsible for the litter size quantitative trait and thus did not support the earlier assignment of chromosome 8. Some trends suggested the potential involvement of chromosome 14 in a regulatory role controlling the litter size trait. However, substantial increases in sample size would be required to elucidate contributions of chromosome 14 or any other rat chromosome to this trait. Similarly, chromosome-substituted strains were not useful in ascribing the contributions of a chromosome(s) to the regulation of placental and fetal weight. Additionally, the junctional zone Prl5a1 transcript level for DSS-BN1, DSS-BN8, and DSS-BN15 did not deviate significantly from the parent DSS strain phenotype. Consequently, our efforts did not support the participation of chromosomes 1, 8, or 15 in regulating a pregnancy or placentation phenotype. Parent rat strains used to generate the chromosome-substituted and recombinant inbred strains differ, as does the mixing of the rat genome in these two experimental models, which could contribute to the disparate findings.
Quantitative trait loci (QTL) for a variety of physiological and pathological responses have been mapped to rat chromosomes 14 and 17 (Ref. 23; rat QTLs can be found at http://rgd.mcw.edu/). Although none of these QTLs directly reflect parameters associated with placentation, several impact growth, vascular function, and metabolism, which could indirectly impact placental development.
Experimentation using interspecific crosses between Mus species or Peromyscus species represents another strategy that has yielded information on the genetic regulation of placentation (30, 46). Interspecific crosses affect the organization of the placentation site, especially the size and structure of the junctional zone (21, 30, 46). This placental trait was mapped to the X chromosome in the mouse (13, 14, 45, 46) and is associated with disruptions in genomic imprinting (34, 35, 43, 44). In the present analysis, we did not identify any significant relationships between any of the parameters measured (placental weight, fetal weight, and junctional zone Prl5a1 transcript levels), and the X chromosome.
Identification of DSS-BN chromosome-substituted strains impacting the junctional zone Prl5a1 transcript level trait is an important first step toward identifying genetic information regulating aspects of placentation. Generation of DSS-BN congenic strains is the next logical step, a process that is markedly hastened by the availability of DSS-BN chromosome-substituted strains (8).
In conclusion, we have identified chromosomes possessing regulatory information controlling aspects of placentation, demonstrating the value of chromosome-substituted strains to elucidating genetic mechanisms associated with pregnancy and placentation and a physiology-driven approach for gene discovery.
GRANTS
The research was supported by National Institute of Child Health and Human Development Grants HD-020676, HD-049503, and HD-06115. S. J. Renaud is the recipient of a postdoctoral fellowship from the Lalor Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Howard J. Jacob, Melinda R. Dwinell, and Allen W. Cowley, Jr. from the Medical College of Wisconsin (Milwaukee, WI) for facilitating our acquisition of the chromosome-substituted rat strains and for providing advice during the course of this research. We also thank Dr. Melissa Larson for assistance with the embryo transfer experiments.
Present addresses: T. Konno, Laboratory of Animal Breeding, Graduate School of Agricultural and Life Science, The University of Tokyo, Japan; L. A. Rempel, US Dept. of Agriculture, Agricultural Research Serv., US Meat Animal Research Center, Clay Center, NE 68933; K. Asanoma, Dept. of Obstetrics and Gynecology, Graduate School of Medical Sciences, Kyushu University, Maidashi 3-1-1, Higashi-ku, Fukuoka 812-8582, Japan.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol 260: 176–190, 2003. [DOI] [PubMed] [Google Scholar]
- 2. Ain R, Konno T, Canham LN, Soares MJ. Phenotypic analysis of the rat placenta. Methods Mol Med 121: 295–313, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Alam SM, Ain R, Konno T, Ho-Chen JK, Soares MJ. The rat prolactin gene family locus: species-specific gene family expansion. Mamm Genome 17: 858–877, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Aplin J. Maternal influences on placental development. Semin Cell Dev Biol 11: 115–125, 2000. [DOI] [PubMed] [Google Scholar]
- 5. Asanoma K, Rumi MAK, Kent LN, Chakraborty D, Renaud SJ, Wake N, Lee DS, Kubota K, Soares MJ. FGF4-dependent stem cells derived from rat blastocysts differentiate along the trophoblast lineage. Dev Biol 351: 110–119, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buresova M, Zidek V, Musilova A, Simakova M, Fucikova A, Bila V, Kren V, Kazdova L, Di Nicolantonio R, Pravenec M. Gene relationship between placental and fetal weights and markers of the metabolic syndrome in rat recombinant inbred strains. Physiol Genomics 26: 226–231, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Caluwaerts S, Vercruysse L, Luyten C, Pijnenborg R. Endovascular trophoblast invasion and associated structural changes in uterine spiral arteries of the pregnant rat. Placenta 26: 574–584, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Cowley AW Jr, Roman RJ, Jacob HJ. Application of chromosomal substitution techniques in gene-function discovery. J Physiol 554: 46–55, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faria TN, Soares MJ. Trophoblast cell differentiation: establishment, characterization, and modulation of a rat trophoblast cell line expressing members of the placental prolactin family. Endocrinology 129: 2895–2906, 1991. [DOI] [PubMed] [Google Scholar]
- 10. Geusens N, Hering L, Verlohren S, Luyten C, Drijkoningen K, Taube M, Vercruysse L, Hanssens M, Dechend R, Pijnenborg R. Changes in endovascular invasion and spiral artery remodeling at term in a transgenic preeclamptic rat model. Placenta 31: 320–326, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Geusens N, Verlohren S, Luyten C, Taube M, Hering L, Vercruysse L, Hanssens M, Dudenhausen JW, Dechend R, Pijnenborg R. Endovascular trophoblast invasion, spiral artery remodeling and uteroplacental haemodynamics in a transgenic rat model of pre-eclampsia. Placenta 29: 614–623, 2008. [DOI] [PubMed] [Google Scholar]
- 12. Goyal R, Yellon SM, Longo LD, Mata-Greenwood E. Placental gene expression in a rat ‘model’ of placental insufficiency. Placenta 31: 568–575, 2010. [DOI] [PubMed] [Google Scholar]
- 13. Hemberger M, Kurz H, Orth A, Otto S, Lüttges A, Elliott R, Nagy A, Tan SS, Tam P, Zechner U, Fundele RH. Genetic and developmental analysis of X-inactivation in interspecific hybrid mice suggests a role for the Y chromosome in placental dysplasia. Genetics 157: 341–348, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hemberger MC, Pearsall RS, Zechner U, Orth A, Otto S, Ruschendorf F, Fundele R, Elliott R. Genetic dissection of X-linked interspecific hybrid placental dysplasia in congenic mouse strains. Genetics 153: 383–390, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hering L, Herse F, Verlohren S, Park JK, Wellner M, Qadri F, Pijnenborg R, Staff AC, Huppertz B, Muller DN, Luft FC, Dechend R. Trophoblasts reduce the vascular smooth muscle cell proatherogenic response. Hypertension 51: 554–559, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Hill AE, Lander ES, Nadeau JH. Chromosome substitution strains: a new way to study genetically complex traits. Methods Mol Med 128: 153–172, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Jacob HJ, Kwitek AE. Rat genetics: attaching physiology and pharmacology to the genome. Nat Rev Genet 3: 33–42, 2002. [DOI] [PubMed] [Google Scholar]
- 18. Konno T, Graham AR, Rempel LA, Ho-Chen JK, Alam SMK, Bu P, Rumi MAK, Soares MJ. Subfertility linked to combined luteal insufficiency and uterine progesterone resistance. Endocrinology 151: 4537–4550, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Konno T, Rempel LA, Arroyo JA, Soares MJ. Pregnancy in the Brown Norway rat: a model for investigating the genetics of placentation. Biol Reprod 76: 709–718, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Kunert MP, Drenjancevic-Peric I, Dwinell MR, Lombard JH, Cowley AW Jr, Greene AS, Kwitek AE, Jacob HJ. Consomic strategies to localize genomic regions related to vascular reactivity in the Dahl salt-sensitive rat. Physiol Genomics 26: 218–225, 2006. [DOI] [PubMed] [Google Scholar]
- 21. Kurz H, Zechner U, Orth A, Fundele R. Lack of correlation between placenta and offspring size in mouse interspecific crosses. Anat Embryol 200: 335–343, 1999. [DOI] [PubMed] [Google Scholar]
- 22. Kwitek AE, Jacob HJ, Baker JE, Dwinell MR, Forster HV, Greene AS, Kunert MP, Lombard JH, Mattson DL, Pritchard KA Jr, Roman RJ, Tonellato PJ, Cowley AW Jr. BN phenome: detailed characterization of the cardiovascular, renal, and pulmonary systems of the sequenced rat. Physiol Genomics 25: 303–313, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Lazar J, Moreno C, Jacob HJ, Kwitek AE. Impact of genomics on research in the rat. Genome Res 15: 1717–1728, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Liang M, Lee NH, Wang H, Greene AS, Kwitek AE, Kaldunski ML, Luu TV, Frank BC, Bugenhagen S, Jacob HJ, Cowley AW Jr. Molecular networks in Dahl salt-sensitive hypertension based on transcriptome analysis of a panel of consomic rats. Physiol Genomics 34: 54–64, 2008. [DOI] [PubMed] [Google Scholar]
- 25. Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Cowley AW Jr, Jacob HJ. Chromosomal mapping of the genetic basis of hypertension and renal disease in FHH rats. Am J Physiol Renal Physiol 293: F1905–F1914, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, Jacob HJ, Cowley AW Jr. Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am J Physiol Renal Physiol 295: F837–F842, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, Cowley AW Jr. Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiol Genomics 16: 194–203, 2004. [DOI] [PubMed] [Google Scholar]
- 28. Nadeau JH, Singer JB, Matin A, Lander ES. Analysing complex genetic traits with chromosome substitution strains. Nat Genet 24: 221–225, 2000. [DOI] [PubMed] [Google Scholar]
- 29. Printz MP, Jirout M, Jaworski R, Alemayehu A, Kren V. HXB/BXH rat recombinant inbred strain platform: a newly enhanced tool for cardiovascular, behavioral, and developmental genetics and genomics. J Appl Physiol 94: 2510–2522, 2003. [DOI] [PubMed] [Google Scholar]
- 30. Rogers JF, Dawson WD. Foetal and placental size in a Peromyscus species cross. J Reprod Fert 21: 255–262, 1970. [DOI] [PubMed] [Google Scholar]
- 31. Roman RJ, Cowley AW Jr, Greene A, Kwitek AE, Tonellato PJ, Jacob HJ. Consomic rats for the identification of genes and pathways underlying cardiovascular disease. Cold Spring Harb Symp Quant Biol 67: 309–315, 2002. [DOI] [PubMed] [Google Scholar]
- 32. Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol 314: 362–375, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sahgal N, Canham LN, Canham B, Soares MJ. Rcho-1 trophoblast stem cells: a model system for studying trophoblast cell differentiation. Methods Mol Med 121: 159–178, 2006. [PubMed] [Google Scholar]
- 34. Schütt S, Florl AR, Shi W, Hemberger M, Orth A, Otto S, Schulz WA, Fundele RH. DNA methylation in placentas of interspecies mouse hybrids. Genetics 165: 223–228, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shi W, Krella A, Orth A, Yu Y, Fundele R. Widespread disruption of genomic imprinting in adult interspecies mouse (Mus) hybrids. Genesis 43: 100–108, 2005. [DOI] [PubMed] [Google Scholar]
- 36. Singer JB, Hill AE, Burrage LC, Olszens KR, Song J, Justice M, O'Brien WE, Conti DV, Witte JS, Lander ES, Nadeau JH. Genetic dissection of complex traits with chromosome substitution strains of mice. Science 304: 445–448, 2004. [DOI] [PubMed] [Google Scholar]
- 37. Soares MJ. The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol 2: 51, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soares MJ, Alam SM, Duckworth ML, Horseman ND, Konno T, Linzer DI, Maltais LJ, Nilsen-Hamilton M, Shiota K, Smith JR, Wallis M. A standardized nomenclature for the mouse and rat prolactin superfamilies. Mamm Genome 18: 154–156, 2007. [DOI] [PubMed] [Google Scholar]
- 39. Soares MJ, Konno T, Alam SM. The prolactin family: effectors of pregnancy-dependent adaptations. Trends Endocrinol Metab 18: 114–121, 2007. [DOI] [PubMed] [Google Scholar]
- 40. Toft DJ, Linzer DI. Identification of three prolactin-related hormones as markers of invasive trophoblasts in the rat. Biol Reprod 63: 519–525, 2000. [DOI] [PubMed] [Google Scholar]
- 41. Vercruysse L, Caluwaerts S, Luyten C, Pijnenborg R. Interstitial trophoblast invasion in the decidua and mesometrial triangle during the last third of pregnancy in the rat. Placenta 27: 22–33, 2006. [DOI] [PubMed] [Google Scholar]
- 42. Verlohren S, Geusens N, Morton J, Verhaegen I, Hering L, Herse F, Dudenhausen JW, Muller DN, Luft FC, Cartwright JE, Davidge ST, Pijnenborg R, Dechend R. Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy. Hypertension 56: 304–310, 2010. [DOI] [PubMed] [Google Scholar]
- 43. Vrana PB, Fossella JA, Matterson P, de Rio T, O'Neill MJ, Tilghman SM. Genetic and epigenetic incompatibilities underlie hybrid dysgenesis in Peromyscus. Nat Genet 25: 120–124, 2000. [DOI] [PubMed] [Google Scholar]
- 44. Vrana PB, Guan XJ, Ingram RS, Tilghman SM. Genomic imprinting is disrupted in interspecific Peromyscus hybrids. Nat Genet 20: 362–365, 1998. [DOI] [PubMed] [Google Scholar]
- 45. Zechner U, Reule M, Burgoyne PS, Schubert A, Orth A, Hameister H, Fundele R. Paternal transmission of X-linked placental dysplasia in mouse interspecific hybrids. Genetics 146: 1399–1405, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zechner U, Reule M, Orth A, Bonhomme F, Strack B, Guenet JL, Hameister H, Fundele R. An X-chromosome linked locus contributes to abnormal placental development in the mouse interspecific hybrids. Nat Genet 12: 398–403, 1996. [DOI] [PubMed] [Google Scholar]
- 47. Zídek V, Pintír J, Musilová A, Bílá V, Kren V, Pravenec M. Mapping of quantitative trait loci for seminal vesicle mass and litter size to rat chromosome 8. J Reprod Fertil 116: 329–333, 1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.