Abstract
Renin-expressing cells are crucial in the control of blood pressure and fluid-electrolyte homeostasis. Notch receptors convey cell-cell signals that may regulate the renin cell phenotype. Because the common downstream effector for all Notch receptors is the transcription factor RBP-J, we used a conditional knockout approach to delete RBP-J in cells of the renin lineage. The resultant RBP-J conditional knockout (cKO) mice displayed a severe reduction in the number of renin-positive juxtaglomerular apparatuses (JGA) and a reduction in the total number of renin positive cells per JGA and along the afferent arterioles. This reduction in renin protein was accompanied by a decrease in renin mRNA expression, decreased circulating renin, and low blood pressure. To investigate whether deletion of RBP-J altered the ability of mice to increase the number of renin cells normally elicited by a physiological threat, we treated RBP-J cKO mice with captopril and sodium depletion for 10 days. The resultant treated RBP-J cKO mice had a 65% reduction in renin mRNA levels (compared with treated controls) and were unable to increase circulating renin. Although these mice attempted to increase the number of renin cells, the cells were unusually thin and had few granules and barely detectable amounts of immunoreactive renin. As a consequence, the cells were incapable of fully adopting the endocrine phenotype of a renin cell. We conclude that RBP-J is required to maintain basal renin expression and the ability of smooth muscle cells along the kidney vasculature to regain the renin phenotype, a fundamental mechanism to preserve homeostasis.
Keywords: juxtaglomerular cells, cell identity, homeostasis, recruitment, conditional knockout, recombination signal binding protein for immunoglobulin kappa J region
in the adult mammalian kidney renin is synthesized, stored, and released by juxtaglomerular (JG) cells, a specialized group of myoepithelioid granulated cells located in the afferent arteriole at the entrance to the glomerulus (6, 34). We have shown that renin precursor cells originate from the metanephric mesenchyme and give rise to JG cells, arteriolar smooth muscle cells (SMCs), mesangial cells, and a subset of proximal tubular cells (30, 31). In adult animals, when homeostasis is threatened (such as by dehydration or hypotension) there is an increase in the number of renin-expressing cells along the renal arterioles and in the glomeruli and interstitium, resembling the fetal pattern, a phenomenon called recruitment (5, 7). The process does not involve cell replication and/or migration (2), but it occurs by retransformation of arteriolar SMCs and mesangial cells into renin-expressing cells (30). We suggested that the ability of adult cells to synthesize renin does not occur randomly in any cell type but depends instead on the cell's lineage. In other words, only cells that previously expressed renin have the memory and capability to re-express renin when homeostasis is challenged (30).
The events underlying the acquisition, maintenance, and reacquisition of the renin phenotype are intriguing. Different mechanisms seem to control the phenotype of renin-expressing cells (3, 14, 15, 17, 26, 29, 32). Interactions of renin cells with other cell types are likely to be crucial to promote, maintain, and regulate the expression of renin. In this regard, the Notch signaling pathway is very attractive to investigate because cell-cell signals conveyed by these receptors may be crucial in maintaining the renin cell phenotype. Interestingly, in vitro studies have shown that the DNA-binding protein RBP-J (Recombination signal Binding Protein for immunoglobulin kappa J region; CBF1 in mammals, Suppressor of Hairless in Drosophila, Lag-1 in Caenorhabditis elegans) within the renin promoter acts as a transcriptional repressor; however, the intracellular Notch 1 receptor can counteract this repressor function and activate the renin promoter in cooperation with the transcription factors HOXD10-PBX1b-PREP1 (19).
Canonical Notch cell-to-cell signaling plays a prominent role during development, imparting cell fate decisions in many tissues, and is involved in vasculogenesis and organogenesis in multicellular organisms (1a, 12, 28, 33). In vertebrates, Notch receptors belong to a receptor superfamily containing Notch 1 through 4. Notch signaling is activated by membrane anchored ligands on juxtaposed cells: the ligands are Delta-like and Serrate/Jagged family members. Upon interaction with one of these ligands, two consecutive proteolytic cleavages liberate the intracellular Notch receptor domain (NIC), which translocates to the nucleus (1, 16). Once in the nucleus, NIC binds to RBP-J. NIC association replaces co-repressors from RBP-J and upregulates transcription (1). Thus, RBP-J is the main transcriptional effector of all Notch signaling. The specific role of the Notch pathway in the acquisition, maintenance, and plasticity of the renin phenotype has not been explored. In the present study, we used a conditional knockout approach to delete RBP-J in cells of the renin lineage to determine its role in the acquisition of renin expression during development and its subsequent maintenance during adult life. Furthermore, we investigated whether RBP-J is crucial in the plasticity of cells to respond to a homeostatic threat with a re-enactment of the renin phenotype. The studies show that RBP-J is required to maintain renin expression during basal states in adult animals and that it is crucial in the plasticity of vascular SMCs to regain the renin phenotype.
METHODS
Generation of mice with conditional deletion of RBP-J in renin cells.
To study the role of RBP-J in renin cells we crossed Ren1dcre/+ mice (30) to RBP-J floxed (RBP-Jfl/fl) mice (kind gift of Dr. Tasuku Honjo) (10), which contain LoxP sites that flank exons 6–7 of the RBP-J gene. Cre-mediated recombination deletes exons 6 and 7 that code for the DNA binding domain of the RBP-J protein. To generate the conditional knockout (cKO) study mice (RBP-Jfl/fl;Ren1dcre/+), mice heterozygous for both RBP-J+/fl and Ren1dcre/+ were crossed to generate homozygous deletion of RBP-J. The control mice designated as RBP-J+/+ are heterozygous for Ren1dcre/+. All procedures were performed following the National Institutes of Health guide for care and use of laboratory animals and were approved by the Animal Care and Use Committee of the University of Virginia.
Generation of Ren1c-YFP mice with conditional deletion of RBP-J in renin cells.
To label renin cells with deletion of RBP-J we bred RBP-Jfl/fl mice with Ren1c-YFP transgenic mice previously described (26). To generate the study animals (Ren1c-YFP;RBP-J cKO) RBP-Jfl/fl;Ren1dcre/cre mice were bred with RBP-Jfl/fl;Ren1c-YFP mice.
Cell isolation.
Ren1c-YFP;RBP-J cKO and Ren1c-YFP;RBP-J+/+ mice were anesthetized with tribromoethanol as previously described (31). The kidneys were excised, decapsulated, cut longitudinally, and placed in a sterile beaker with chilled buffer 1 (130 mM NaCl, 5 mM KCl, 2 mM CaCl2, 10 mM glucose, 20 mM sucrose, 10 mM HEPES). The kidney cortices from each animal were dissected and placed into a glass Petri dish, minced to a smooth consistency, and transferred into a 50 ml Falcon tube with 5 ml of 37°C enzyme solution [0.1% collagenase A (Roche, Indianapolis, IN), 0.25% trypsin (Sigma-Aldrich, St. Louis, MO), and 0.0021% DNase I (Roche Applied Science)] for 15 min with gentle stirring. After the 15 min period the solution was pipetted up/down 10 times with a sterile transfer pipette and allowed to settle for 2 min, and the supernatant was collected with a plastic pipette and transferred to a 50 ml tube on ice. Enzyme solution was added to the 50 ml Falcon tube containing the remaining undigested cortices, and the digestion procedure was repeated two more times. The supernatants collected from the three digestions were pooled and centrifuged in a Sorvall RT 6000 Refrigerated Centrifuge at 200 g for 7 min at 4°C. The cell pellet was resuspended with 25 ml of fresh buffer 1, and the suspension was poured through a sterile 70 μm nylon mesh (BD Biosciences, Chicago, IL) and washed with an additional 3–5 ml of buffer 1. The flow-through was poured through a sterile 20 μm nylon mesh and washed with 3–5 ml of buffer 1. The flow-through was centrifuged at 200 g for 7 min at 4°C. The cell pellet was resuspended in 1 ml red blood cell lysis buffer (Sigma-Aldrich) at room temperature (RT). After 1 min incubation at RT, 2 ml of buffer 1 were added and the suspension centrifuged at 200 g for 7 min at 4°C. The red blood cell lysis procedure was repeated one time. After the second centrifugation the cell pellet was resuspended in ∼1 ml resuspension buffer (PBS, 1% FBS, 1 mM EDTA, 25 mM HEPES, pH 7) and analyzed using a fluorescent-activated cell sorter (Becton Dickinson FACSVantage SE Turbo Sorter with DIVA Option).
Genotyping.
Genotyping of mice was conducted by PCR of DNA from tails using primers designed to specifically identify the wild-type, floxed, and deleted RBP-J alleles as well as Ren1d and cre alleles as previously described (10, 30).
Histological and immunohistochemical analysis.
Animals were anesthetized with tribromoethanol (300 mg/kg). The kidneys were removed, weighed, and fixed overnight in Bouin's solution and embedded in paraffin. Serial sections (5 μm) were deparaffinized in xylenes and graded alcohols. Sections were processed for immunohistochemistry for renin and α-smooth muscle actin and stained with hematoxylin or with Masson's trichrome (to assess collagen deposition) as previously described (8, 32). To visualize renin granules, 2 μm kidney sections were deparaffinized through xylenes and graded alcohols followed by a wash with PBS for 5 min. The sections were incubated in 0.02% toluidine blue in water for 5–10 min (monitoring until granules were visible under the microscope), washed with running water for 2 min, and then dehydrated in graded alcohols and xylenes.
Morphometric measurement.
The juxtaglomerular apparatus (JGA) index was calculated as the number of renin-positive JGA/total number of glomeruli and expressed as a percentage. To determine the number of renin-expressing cells per section we counted the number of renin-positive cells in each JGA plus the number of renin-positive cells along the arterioles with visible glomeruli attached to them.
RNA isolation and qRT-PCR analysis.
Whole kidneys were cut sagitally and stored in RNAlater (Ambion, Austin, TX) overnight at 4°C and then processed for RNA extraction or stored at −20°C until used. RNA extraction and cDNA generation by reverse transcription and quantitative real-time PCR using SYBR Green (Invitrogen, Eugene, OR) were performed as previously described (8). Renin mRNA expression was normalized to GAPDH expression. The changes in expression were determined by the ΔΔCt method and are reported as relative expression compared with control mice.
Analyses of plasma renin concentration and blood pressure measurement.
Plasma renin concentration was determined as previously described (24, 25). Blood pressure (BP) was measured in conscious mice by a computerized tail-cuff method (CODA; Kent Scientific, Torrington, CT). Mice were acclimated for 2 days, and the measurements taken on the third day were analyzed.
Recruitment study.
To stimulate expression of renin, 2.5 mo old mice were treated with low-sodium diet (0.05%, Harlan, Madison, WI) plus captopril added to the drinking water (0.5 g/l) for 10 days. At the end of the treatment period, kidneys were harvested and processed for immunohistochemistry and histological analysis, and blood was collected to assess plasma renin concentration.
Statistics.
Statistical significance was assessed by t-test and Mann-Whitney rank sum test using Sigma Plot 11.0.
RESULTS
Deletion of RBP-J results in a decreased number of renin cells.
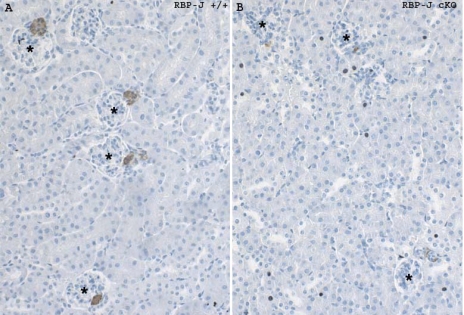
As illustrated in Table 1 and Fig. 1, RBP-J cKO mice at 1 and 4 mo of age have a significant decrease in the number of renin-positive JGAs when corrected for the total number of glomeruli (JGA index). At 4 mo of age, the percentage of glomeruli containing renin-positive JGAs in control mice was 44.2 ± 3.6, whereas in the RBP-J cKO mice the JGA index was decreased to 16.1 ± 2.1 (Table 1). In addition, RBP-J cKO mice had a severe decrease in the total number of renin-positive cells in the JGAs and along the afferent arterioles (control RBP-J+/+ mice: 221 ± 42.6 cells, RBP-J cKO mice: 54 ± 10.4 cells; Table 1). Similar findings were observed in 1 mo old mice (Table 1). The decrease in the number of cells expressing renin in the RBP-J cKO animals both at 4 and 1 mo of age was accompanied by a significant decrease in renin mRNA expression compared with controls. At 1 mo of age, kidneys from homozygous mutant mice had a relative renin mRNA expression of 34% with respect to control RBP-J+/+ mice (Table 1). At 4 mo of age, mutant mice showed a relative renin mRNA expression of 44% compared with controls (Table 1). Although the number of cells expressing renin was significantly diminished in 1 and 4 mo old RBP-J cKO mice, the number of renin-expressing cells was not affected early in ontogeny (not shown), suggesting that the decreased number of renin-expressing cells in the adult is not likely due to a decreased endowment of renin cell precursors. In addition, the renal size and morphology (including the renal vasculature) were not altered in the RBP-J cKO mice (not shown).
Table 1.
Deletion of RBP-J results in a significant decrease in the number of renin-expressing cells and renin mRNA levels in the kidney
| Denominations | Genotype | Age, mo | n | JGA index | Renin Cells in the JGA + aa/per Section | Relative Expression of Renin mRNA, % |
|---|---|---|---|---|---|---|
| RBP-J+/+ | RBP-J+/+;Ren1dcre/+ | 1 | 3 | 32.8 ± 0.9 | 264 ± 43 | 100 |
| RBP-J cKO | RBP-Jfl/fl;Ren1dcre/+ | 1 | 7 | 9.6 ± 2.6† | 53 ± 17.7† | 34.3 ± 6* |
| RBP-J+/+ | RBP-J+/+;Ren1dcre/+ | 4 | 5 | 44.2 ± 3.6 | 221 ± 42.6 | 100 |
| RBP-J cKO | RBP-Jf1/f1;Ren1dcre/+ | 4 | 11 | 16.1 ± 2.1† | 54 ± 10.4† | 44.2 ± 12* |
Values are means ± SE.
n, Number of mice. JGA, juxtaglomerular apparatus; aa, along the afferent arteriole.
P < 0.0005 and
P < 0.02 when compared with control RBP-J+/+ mice.
Fig. 1.
Renin cell distribution in the kidney of control (RBP-J+/+) and mutant [RBP-J conditional knockout (cKO)] mice. A: kidney tissue section of an adult control mouse showing distribution of renin confined to the juxtaglomerular apparatus (JGA). B: deletion of RBP-J resulted in a severe decrease in the number of renin-positive JGAs. *Glomeruli, and renin expression appears in brown color. Values are means ± SE.
RBP-J is necessary to maintain the number of YFP-positive JG cells.
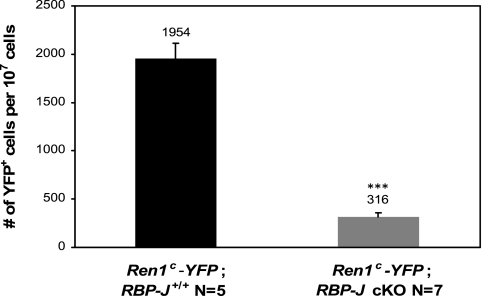
To further investigate whether the decrease in renin mRNA was due to a decrease in the number of cells expressing the renin gene, we crossed our RBP-J cKO mice with mice that express yellow fluorescent protein (YFP) under the control of the Ren1c promoter (Ren1c-YFP). Expression of YFP therefore indicates cells actually transcribing renin mRNA. Using fluorescent activated cell sorting we quantified the number of YFP-positive cells in Ren1c-YFP;RBP-J+/+ and Ren1c-YFP;RBP-J cKO mice. At 2 mo of age, deletion of RBP-J significantly reduced the number of YFP-positive cells (Fig. 2). Ren1c-YFP;RBP-J+/+ mice had 0.02% of YFP-positive cells per two kidneys, whereas the RBP-J cKO mice had 0.003% of YFP cells (P < 0.0001).
Fig. 2.
Deletion of RBP-J reduced the number of yellow fluorescent protein (YFP)-positive cells in Ren1c-YFP mice. The number of YFP-positive cells in Ren1c-YFP;RBP-J+/+ and Ren1c-YFP;RBP-J cKO mice was quantified by fluorescent activated cell sorting (FACS). The cKO kidneys had a reduced number (#) of YFP+ cells. The number of YFP-positive cells was corrected per 107 sorted cells from kidney cortices. Values are means ± SE, ***P < 0.0001.
Effect of RBP-J deletion on arterial BP and circulating renin.
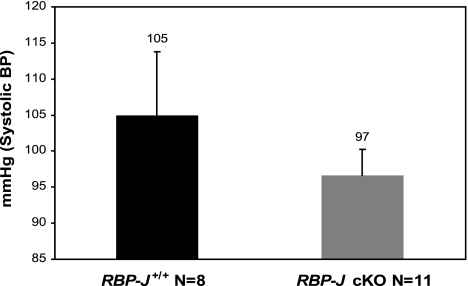
Systolic BP in control 2.5 mo old mice was 105 ± 8.8 mmHg (n = 8) and in RBP-J cKO mice was 97 ± 3.6 mmHg (n = 11) (Fig. 3). Although RBP-J cKO mice had a slight tendency to reduced BP, due to the inherent variability of BP measurements the difference compared with control mice was not statistically significant (P = 0.355). We also observed that control mice had wider variation in their systolic BP (ranging from 76 to 153 mmHg) when compared with RBP-J cKO animals. On the other hand, RBP-J cKO mice had a limited variation in their systolic BP values (range: 83 to 116 mmHg), indicating that deletion of RBP-J limited the physiological BP variability. Under basal conditions, plasma renin concentration (PRC, ANG I μg·ml−1·h−1) was 20% lower in the RBP-J cKO (2.0 ± 0.7, n = 24) mice compared with control mice (2.5 ± 0.8, n = 27). This difference was not statistically significant.
Fig. 3.
Systolic blood pressure (BP) measurement in control and RBP-J cKO mice. Values are means ± SE, P = 0.355.
Deletion of RBP-J in the renin cells impairs the recruitment response.
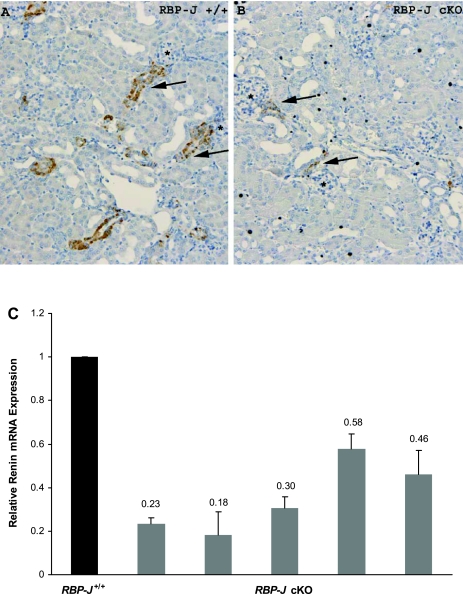
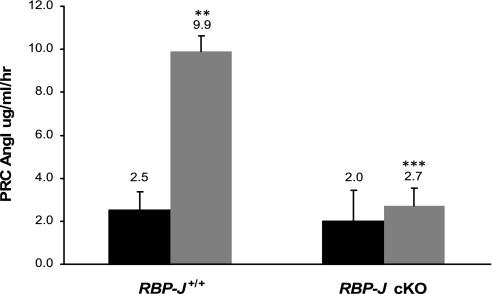
To investigate whether deletion of RBP-J altered the ability of mice to elicit a recruitment response (an increase in the number of renin cells under physiological threat), we treated RBP-J cKO mice and controls (RBP-J+/+) with captopril and sodium depletion for 10 days. This treatment is known to elicit recruitment of renin cells in intact animals. Treated RBP-J cKO mice displayed an average relative expression of renin mRNA of 35% (range: 18–58%) when compared with control mice (Fig. 4C). As illustrated in Fig. 4, A and B, immunostaining for renin shows that in contrast to control mice, RBP-J cKO mice were unable to increase the number of renin-positive cells and to adopt the renin cell phenotype to the same extent as in the controls after treatment (Fig. 4B). RBP-J cKO mice had fewer and thinner renin-positive cells along the afferent arterioles compared with controls. Furthermore, whereas control-treated mice increased PRC fourfold (P < 0.0001) after 10 days of treatment compared with untreated controls, RBP-J cKO mice were unable to increase PRC in response to a physiological challenge (Fig. 5). Circulating renin in treated control RBP-J+/+ mice was 9.9 ± 4.3, whereas in the RBP-J cKO mice renin values were significantly lower (2.7 ± 1.9, P = 0.006). Thus, under physiological stress, RBP-J cKO mice were incapable of increasing the number of cells expressing renin with the resultant lack of elevation in circulating renin.
Fig. 4.
Impaired recruitment response in captopril-treated RBP-J cKO mice. Control and RBP-J cKO mice were treated with low-sodium diet and captopril for 10 days. A: immunostaining for renin in control-treated mice showed renin expression along the afferent arteriole (arrows). B: RBP-J cKO mice were unable to increase the number of renin-positive cells. Renin cells along the afferent arterioles (arrows) are also thinner. *Glomeruli, and renin expression is shown in brown. C: renin mRNA in captopril-treated RBP-J cKO mice. Black bar, control (n = 5); gray bars, individual RBP-J cKO mice. The error bars indicate triplicate determinations of the same animal. Values are means ± SE, P = 0.0006.
Fig. 5.
RBP-J cKO mice are unable to increase circulating renin in response to a homeostatic threat. RBP-J+/+ and RBP-J cKO mice were maintained for 10 days on a low-sodium diet plus captopril in the drinking water. Plasma renin concentration (PRC) was determined in treated mice and compared with untreated animals. Black bars, untreated; gray bars, treated. **P < 0.0001 compared with untreated control RBP-J+/+; ***P = 0.006 compared with treated control mice, values are means ± SE. Number of animals: untreated RBP-J+/+, n = 27 and RBP-J cKO, n = 24, treated RBP-J+/+, n = 9 and RBP-J cKO, n = 6.
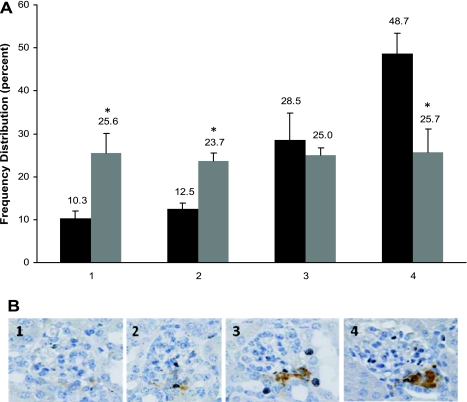
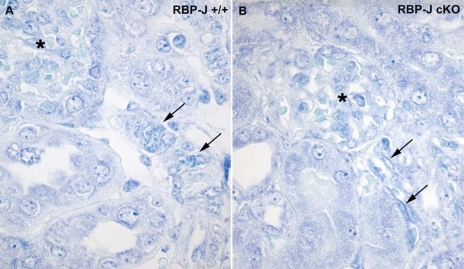
Interestingly, although the mean JGA index in treated RBP-J cKO mice was significantly reduced compared with control mice (RBP-J+/+: 55 ± 4, n = 5 and RBP-J cKO mice: 45 ± 2.2, n = 6; P < 0.02), the difference of only 10% indicated an attempt at compensation by the RBP-J cKO mice. However, the intensity of immunostaining was markedly different between the two genotypes. To illustrate this point, we quantified and scored the intensity of the staining of renin-positive JGAs from 1, low intensity to 4, high intensity (Fig. 6B). As shown in Fig. 6, treated RBP-J cKO mice had a significant decrease in the proportion of JGAs with the highest intensity scores (score 4) when compared with control mice (control RBP-J+/+ mice 49%, RBP-J cKO mice 26%; P < 0.02). This finding suggested that although the mutant renin cells attempt to compensate, most of the cells have a decreased amount of renin per cell, indicating that intracellular renin stores were depleted. To corroborate this, toluidine blue staining to detect renin granules showed that RBP-J cKO mice had a marked decrease in the number of granules per cell (Fig. 7). These results demonstrate the inability of individual renin cells to mount the appropriate physiological renin expression response when RBP-J is absent.
Fig. 6.
Renin intensity scores in RBP-J cKO mice subjected to a homeostatic challenge. A: frequency distribution of renin intensity scores in kidney sections from treated RBP-J+/+ and RBP-J cKO mice. Black bars, RBP-J+/+; gray bars, RBP-J cKO. B: JGA intensity scoring standards (1–4). JGA staining was scored as an intensity of 1 through 4, in which 4 represents the highest intensity. The number of JGAs in each category was corrected by the total number of glomeruli and the percent in each category is shown above the bars in A. Treated RBP-J+/+ (n = 5) show a prominence of high intensity scores, 49% of JGA had scores of 4 compared with 26% in the treated RBP-J cKO (n = 6) mice. *P < 0.02 compared with control animals within the same score value. Values are means ± SE.
Fig. 7.
JGAs and arterioles from RBP-J cKO mice have reduced numbers of granules. A: treated control mice have multiple cells containing granules widely distributed along the arteries. B: RBP-J cKO mice have markedly diminished numbers of granules per cell. Arrows indicate cells with granules; *glomeruli.
DISCUSSION
Role of RBP-J in renin-expressing cells.
In the present study, we show that RBP-J is crucial for the maintenance and plasticity of the renin cell phenotype. The severe reduction in the number of JG cells in RBP-J cKO mice is accompanied by: 1) decreased renin mRNA levels, 2) decreased circulating renin, 3) lower blood pressure, and 4) marked impairment in the ability of SMCs to re-express renin in response to a homeostatic threat.
The severe decrease in the number of JG cells in RBP-J cKO mice indicates that RBP-J is indispensable for the maintenance of the renin phenotype in the basal state. Significant progress has been made regarding some of the pathways that control renin expression (4, 8, 9, 13, 14, 17, 18, 20–23, 26, 29, 32); however, the role of RBP-J in maintaining renin expression in vivo has not been previously reported. Here, we show in vivo that RBP-J is an important factor in the expression of renin in unstressed adult mice. In vitro studies have shown that RBP-J can function either as a repressor or activator of transcription of the rat renin promoter; mutation of the RBP-J binding site in the renin gene resulted in an increase in renin promoter activity in vitro identifying the site as a repressor, whereas the presence of the intracellular receptors (Notch 1 and Notch 3) enhanced renin activation elucidating its activator ability (19). The present study suggests that RBP-J functions in vivo to maintain the number of renin-expressing cells in the kidney. The molecular mechanism and associated partners responsible for this effect remain to be determined.
The reason for the decrease in the number of renin-expressing cells is intriguing. It is unlikely that the deletion of RBP-J resulted in a diminished endowment of renin precursor cells because in our studies newborn mice do not show the striking decrease in the number of JG cells clearly found at one mo of age (not shown). It is possible that an early mosaic deletion of RBP-J may explain the lack of a noticeable phenotype at an early age. As the physiological demands of life (and activity of the renin promoter) accumulate, successive deletions occur, eventually affecting the phenotype, that become clearly manifest in the adult. Given that RBP-J has a binding site in the renin promoter, it is likely that the decreased number of renin-expressing cells is due to a direct effect of RBP-J on the renin gene. However, in silico analysis of the genes most prominently expressed in the renin cell (unpublished) indicates that >100 genes have predicted RBP-J binding sites in their promoters and could have potentially contributed to the observed findings. It could be argued that renin cells may undergo apoptosis and/or necrosis, diminishing the number of JG cells in the adult kidney. However, this possibility is unlikely because the number of apoptotic cells in the newborn and adult RBP-J cKO mice were not different from control animals (not shown). The most likely possibility is that lack of RBP-J results in decreased renin gene expression due to diminished RBP-J action on the renin promoter. Alternatively, deficiency of RBP-J could have resulted in a phenotypic switch of the renin cell altering its fate to another cell type with the attendant downregulation of renin expression. To answer this question, we are currently performing lineage-tracing studies in RBP-J cKO animals.
One of the most striking effects of the RBP-J deletion is the inability of mutant mice to elicit an appropriate recruitment response when their homeostasis is threatened. In response to sodium depletion and captopril treatment RBP-J cKO mice were unable to increase circulating renin. The lack of increase in circulating renin was due to the combination of several factors: first, in response to the homeostatic threat, RBP-J cKO mice were unable to increase the number of cells expressing renin, which normally occurs by retransforming vascular SMCs along the afferent arterioles into renin-expressing cells (5, 30, 35, 36). Thus, RBP-J may play a role in the reacquisition of the endocrine phenotype of arteriolar SMCs.
Although the JGA index for the treated RBP-J cKO mice was significantly different compared with treated control mice, this difference was only 10%. This, however, does not represent how markedly different the mutant mice were compared with the treated control mice. In fact, we observed that the remaining cells that did attempt to re-express renin along the kidney vasculature displayed striking morphological features: not only were they fewer but they were also very thin and contained barely detectable amounts of renin protein and very few granules. Although RBP-J cKO mice showed an inability to mount a satisfactory physiological recruitment response the fact that the JGA index increased (although not to control levels) suggests that the remaining renin cells attempted such compensation. However, the individual cells attempting such response had minimal amounts of renin and fewer granules indicating that the synthetic capacity of these cells was depleted. Thus, the inability to increase circulating renin was not only due to a limited number of renin-expressing cells but also due to the inability of individual cells to produce enough renin when attempting a compensatory homeostatic response. We suspect that lack of RBP-J in renin cells impairs the synthetic machinery of renin cells, a defect that becomes more evident under physiological stress. As a consequence, the mutant cells are incapable of fully adopting the renin phenotype and are limited to the phenotype of a smooth muscle cell. Interestingly, when renin cells are placed in culture and are deprived of their normal intercellular interactions with other cell types, they stop synthesizing renin and adopt the phenotypic characteristics of smooth muscle and/or fibroblast cells (11). Thus, lack of the transducing mechanism conveying cell-cell interactions normally provided by RBP-J impairs the plasticity of cells to switch phenotype when confronted with a homeostatic challenge. Further studies will be conducted to determine the intracellular events whereby RBP-J controls the phenotypic endocrine switch of the renin cell.
In summary, we generated mice with deletion of RBP-J in cells of the renin lineage and showed in vivo that RBP-J is required to maintain renin expression and perhaps more importantly, RBP-J is necessary to maintain the memory of SMCs along the kidney vasculature to regain the renin phenotype, a fundamental mechanism to preserve homeostasis.
GRANTS
This work was supported by National Institutes of Health Grants R37HL-066242 Diversity Supplement Graduate Research Assistant and R01HL-096735 (to R. A. Gomez) and DK-075481 (to M. L. S. Sequeira-Lopez). We acknowledge the Roswell Park Cancer Institute's Gene Targeting and Transgenic Resource partially subsidized by the National Cancer Institute-funded Cancer Center Support Grant CA-016056.
DISCLOSURES
No conflicts of interest (financial or otherwise) are declared by the author(s).
ACKNOWLEDGMENTS
We thank Kimberly Hilsen-Durette for technical assistance with the mouse work, Dr. Tasuku Honjo for the RBP-Jfl/fl mice, Dr. Raphael Kopan for providing Dr. Honjo's RBP-Jfl/fl mice and help with the genotyping, and Dr. Tino Piscione for his genotyping protocol. We acknowledge Aimee Stablewski for helpful discussions.
Part of this work was presented at the High Blood Pressure Research Conference, October 13–16, 2010 in Washington, DC, and published in abstract form.
REFERENCES
- 1a.Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science 268: 225–232, 1995 [DOI] [PubMed] [Google Scholar]
- 1.Bailey AM, Posakony JW. Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev 9: 2609–2622, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Cantin M, Desormeaux Y, Benchimol S. On the lysosomal function of juxtaglomerular granules. Beitr Pathol 161: 310–327, 1977 [DOI] [PubMed] [Google Scholar]
- 3.Everett AD, Carey RM, Chevalier RL, Peach MJ, Gomez RA. Renin release and gene expression in intact rat kidney microvessels and single cells. J Clin Invest 86: 169–175, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glenn ST, Jones CA, Pan L, Gross KW. In vivo analysis of key elements within the renin regulatory region. Physiol Genomics 35: 243–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM. Recruitment of renin gene-expressing cells in adult rat kidneys. Am J Physiol Renal Fluid Electrolyte Physiol 259: F660–F665, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Gomez RA, Chevalier RL, Sturgill BC, Johns DW, Peach MJ, Carey RM. Maturation of the intrarenal renin distribution in Wistar-Kyoto rats. J Hypertens 4: s31–s33, 1986 [Google Scholar]
- 7.Gomez RA, Lynch KR, Chevalier RL, Everett AD, Johns DW, Wilfong N, Peach MJ, Carey RM. Renin and angiotensinogen gene expression and intrarenal renin distribution during ACE inhibition. Am J Physiol Renal Fluid Electrolyte Physiol 254: F900–F906, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Gomez RA, Pentz ES, Jin X, Cordaillat M, Sequeira Lopez ML. CBP and p300 are essential for renin cell identity and morphological integrity of the kidney. Am J Physiol Heart Circ Physiol 296: H1255–H1262, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez RA, Sequeira Lopez ML. Who and where is the renal baroreceptor?: the connexin hypothesis. Kidney Int 75: 460–462, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol 14: 637–645, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Karginova EA, Pentz ES, Kazakova IG, Norwood VF, Carey RM, Gomez RA. Zis: a developmentally regulated gene expressed in juxtaglomerular cells. Am J Physiol Renal Physiol 273: F731–F738, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Kato H, Sakai T, Tamura K, Minoguchi S, Shirayoshi Y, Hamada Y, Tsujimoto Y, Honjo T. Functional conservation of mouse Notch receptor family members. FEBS Lett 395: 221–224, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Kurtz L, Schweda F, de WC, Kriz W, Witzgall R, Warth R, Sauter A, Kurtz A, Wagner C. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol 18: 1103–1111, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Lopez ML, Gomez RA. The renin phenotype: roles and regulation in the kidney. Curr Opin Nephrol Hypertens 19: 366–371, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markus MA, Goy C, Adams DJ, Lovicu FJ, Morris BJ. Renin enhancer is crucial for full response in Renin expression to an in vivo stimulus. Hypertension 50: 933–938, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Morrow D, Scheller A, Birney YA, Sweeney C, Guha S, Cummins PM, Murphy R, Walls D, Redmond EM, Cahill PA. Notch-mediated CBF-1/RBP-Jκ-dependent regulation of human vascular smooth muscle cell phenotype in vitro. Am J Physiol Cell Physiol 289: C1188–C1196, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Pan L, Black TA, Shi Q, Jones CA, Petrovic N, Loudon J, Kane C, Sigmund CD, Gross KW. Critical roles of a cyclic AMP responsive element and an E-box in regulation of mouse renin gene expression. J Biol Chem 276: 45530–45538, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Pan L, Glenn ST, Jones CA, Gronostajski RM, Gross KW. Regulation of renin enhancer activity by nuclear factor I and Sp1/Sp3. Biochim Biophys Acta 1625: 280–290, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Pan L, Glenn ST, Jones CA, Gross KW. Activation of the rat renin promoter by HOXD10. PBX1b PREP1, Ets-1, and the intracellular domain of notch. J Biol Chem 280: 20860–20866, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Pan L, Gross KW. Transcriptional regulation of renin: an update. Hypertension 45: 3–8, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Pan L, Jones CA, Glenn ST, Gross KW. Identification of a novel region in the proximal promoter of the mouse renin gene critical for expression. Am J Physiol Renal Physiol 286: F1107–F1115, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Pan L, Wang Y, Jones CA, Glenn ST, Baumann H, Gross KW. Enhancer-dependent inhibition of mouse renin transcription by inflammatory cytokines. Am J Physiol Renal Physiol 288: F117–F124, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Pan L, Xie Y, Black TA, Jones CA, Pruitt SC, Gross KW. An Abd-B class HOX. PBX recognition sequence is required for expression from the mouse Ren-1c gene. J Biol Chem 276: 32489–32494, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Pentz ES, Lopez ML, Kim HS, Carretero O, Smithies O, Gomez RA. Ren1d and Ren2 cooperate to preserve homeostasis: evidence from mice expressing GFP in place of Ren1d. Physiol Genomics 6: 45–55, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Pentz ES, Moyano MA, Thornhill BA, Sequeira Lopez ML, Gomez RA. Ablation of renin-expressing juxtaglomerular cells results in a distinct kidney phenotype. Am J Physiol Regul Integr Comp Physiol 286: R474–R483, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Pentz ES, Sequeira Lopez ML, Cordaillat M, Gomez RA. Identity of the renin cell is mediated by cAMP and chromatin remodeling: an in vitro model for studying cell recruitment and plasticity. Am J Physiol Heart Circ Physiol 294: H699–H707, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393: 382–386, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Sequeira Lopez ML, Gomez RA. Novel mechanisms for the control of renin synthesis and release. Curr Hypertens Rep 12: 26–32, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA. Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 281: F345–F356, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Sequeira-Lopez ML, Weatherford ET, Borges GR, Monteagudo MC, Pentz ES, Harfe BD, Carretero O, Sigmund CD, Gomez RA. The microRNA-processing enzyme dicer maintains juxtaglomerular cells. J Am Soc Nephrol 21: 460–467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Struhl G, Adachi A. Nuclear access and action of notch in vivo. Cell 93: 649–660, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Taugner R, Hackenthal E. The Juxtaglomerular Apparatus: Structure and Function. Heidelberg: Springer Verlag, 1989 [Google Scholar]
- 35.Tufro-McReddie A, Arrizurieta EE, Brocca S, Gomez RA. Dietary protein modulates intrarenal distribution of renin and its mRNA during development. Am J Physiol Renal Fluid Electrolyte Physiol 263: F427–F435, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Tufro-McReddie A, Chevalier RL, Everett AD, Gomez RA. Decreased perfusion pressure modulates renin and ANG II type 1 receptor gene expression in the rat kidney. Am J Physiol Regul Integr Comp Physiol 264: R696–R702, 1993 [DOI] [PubMed] [Google Scholar]