Abstract
Despite an abundance of evidence to the contrary from animal studies, large clinical trials on humans have shown that estrogen administered to postmenopausal women increases the risk of cardiovascular disease. However, timing may be everything, as estrogen is often administered immediately after ovariectomy (Ovx) in animal studies, while estrogen administration in human studies occurred many years postmenopause. This study investigates the discrepancy by administering 17β-estradiol (E2) in a slow-release capsule to Norway Brown rats both immediately following Ovx and 9 wk post-Ovx (Late), and studying differences in gene expression between these two groups compared with age-matched Ovx and sham-operated animals. Two different types of microarray were used to analyze the left ventricles from these groups: an Affymetrix array (n = 3/group) and an inflammatory cytokines and receptors PCR array (n = 4/group). Key genes were analyzed by Western blotting. Ovx without replacement led to an increase in caspase 3, caspase 9, calpain 2, matrix metalloproteinase (MMP)9, and TNF-α. Caspase 6, STAT3, and CD11b increased in the Late group, while tissue inhibitor of metalloproteinase 2, MMP14, and collagen I α1 were decreased. MADD and fibronectin were increased in both Ovx and Late. TNF-α and inducible nitric oxide synthase (iNOS) protein levels increased with Late replacement. Many of these changes were prevented by early E2 replacement. These findings suggest that increased expression of inflammatory genes, such as TNF-α and iNOS, may be involved in some of the deleterious effects of delayed E2 administration seen in human studies.
Keywords: timing hypothesis, cardiovascular disease
the effect of estrogen loss vs. aging on cardiovascular disease is not well understood. However, there is an acceleration of atherosclerosis and a significantly increased incidence of myocardial infarction postmenopause (37). Observational studies had suggested that estrogen postmenopause was protective; however, randomized clinical trials using conjugated equine estrogen did not demonstrate a positive effect from estrogen replacement (3, 16). A key issue has been the timing of estrogen replacement (the timing hypothesis), as the average subject was 10 or more years past menopause; it has been suggested that earlier estrogen replacement might be beneficial (46).
In contrast to the human studies, several animal studies have shown that estrogen replacement is actually beneficial to the heart. Additionally, studies have shown that estrogen, given alone or in the presence of progesterone, lowers oxidative stress levels in blood vessels and tissues of young rats (49) and that estrogen is vasoprotective after vascular balloon injury in young female rats (29). Our lab has recently shown that immediate estrogen replacement in aged ovariectomized (Ovx) rats prevents deleterious changes in vascular function (40). In another study, Ovx young adult Sprague-Dawley rats (200–250 g) had increased serum levels of the inflammatory cytokines macrophage inflammatory protein (MIP1) and monocyte chemoattractant protein (MCP)-1, and this was prevented by low dose 17β-estradiol (E2) (1).
The purpose of this study was to investigate changes in gene expression in aged Ovx rats with and without E2 treatment. Both immediate E2 and late E2 treatment were used to model the late E2 replacement done in clinical trials.
MATERIALS AND METHODS
Animal Model
Aged Norway Brown rats (18–22 mo old) were obtained from the National Institutes on Aging (Bethesda, MD), housed in standard female-only conditions, and fed standard laboratory rat chow. Rats were divided into four groups [Sham, Ovx, and Ovx with immediate estrogen replacement (Early), or late estrogen replacement (“Late”: 9 wk post-Ovx, then 4 wk estrogen replacement)]. E2 replacement for all Early and Late was done with a subcutaneous sustained-release pellet (Innovative Research, Sarasota, FL). Uterine size and ovary absence were checked at time of tissue collection to verify treatment groups. Estrogen levels were measured by radioimmunoassay as previously described (21). All animal protocols were approved by the University of California, Davis Animal Research Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgical Methods
Rats were anesthetized with 5 mg/kg ketamine and 50 mg/kg xylazine injected intraperitoneally. The rats assigned to the Sham group were anesthetized and had their body cavities opened and then immediately closed with no tissue removal; the rats assigned to the Ovx group underwent ovariectomy. Those assigned to the Early group after ovariectomy had a 0.5 mg 17-β estradiol 90-day slow release pellet (Innovative Research of America, Sarasota, FL) implanted subcutaneously in the back of the neck at the time of surgery. Rats assigned to the Late group underwent Ovx and had a subcutaneous estrogen pellet placed, as above, at 9 wk post-Ovx. Treatment groups were assigned randomly at the time of surgery, and all rats were killed at the same age. The left ventricle containing cardiomyocytes and the associated vasculature was collected at the completion of the protocol, flushed with ice-cold PBS and flash-frozen in liquid nitrogen.
RNA Preparation
Target preparation was performed according to the instructions of the manufacturer (Affymetrix). RNA was isolated as previously described (14). For each group, good-quality total RNA from three rats were pooled together in equal amounts and used to synthesize double-strand cDNA using a one-cycle cDNA synthesis kit (Affymetrix, P/N 900431). This approach allows repetitive analysis of all groups by the array in a group representative and cost-effective manner, given the four groups and 24 RNA samples. This approach has been recognized as a practical approach to analysis of multiple groups and has been used effectively by other investigators (2,28,33,35). The double-strand cDNA was purified and served as a template in the subsequent in vitro transcription (IVT) reaction for complementary RNA (cRNA) amplification and biotin labeling (IVT Labeling Kit, Affymetrix, P/N 900449). The biotinylated cRNA targets were then cleaned (Sample cleanup module, Affymetrix, P/N 900371), checked for the absorbance at 260 and 280 nm to determine cRNA concentration and purity, and examined on an Agilent 2100 Bioanalyzer to estimate the yield and size distribution. Subsequently, the cRNA targets were fragmented, loaded onto a test chip to optimize the hybridization conditions, and then hybridized to a rat gene expression microarray. These data have been submitted to the National Center for Biotechnology Information Gene Expression Omnibus, in compliance with the Microarray Gene Expression Data Society.
Affymetrix Microarray Data Analysis
Data transformation and normalization.
A logarithmic (base 2) transformation was applied to the signal intensities before further analysis. Then, the log-transformed array data were normalized with a common method, median normalization, to eliminate the influence of nontreatment factors on the signal intensities.
Identifying differentially expressed genes.
As there were two arrays for each group in this study, fold change of gene expression was chosen to identify the differentially expressed genes between any two groups. Based on the logarithmically transformed data, the fold change of a gene (e.g., gene i) between two groups was calculated as the difference between the average log-intensities of the two groups for gene i.
Gene Ontology and pathway analysis.
The Gene Ontology (GO) analysis provides a list of GO categories that have more genes differentially expressed among groups than expected by chance (4). To identify the differentially expressed GO categories, a functional class scoring analysis was performed as described by Pavlidis et al. (32). Briefly, a P value was computed for each gene in a GO category, and then the set of P values for a GO category was summarized by the LS and KS summary statistics. For a set of n genes, the LS statistic was defined as
and the KS statistic was defined as
The statistical significance of a GO category containing n genes was evaluated by computing the empirical distribution of the LS and KS statistics in random samples of n genes.
The Pathway analysis was performed using the method similar to the GO analysis, except that the genes were grouped by KEGG and BioCarta pathways, rather than GO categories (18). The pathways chosen were apoptosis, inflammation, extracellular matrix/fibrosis, vascular function/disease, signaling, and heat shock protein (Hsp) genes.
Gene Expression Chip Hybridization
The fragmented biotinylated cRNA targets were hybridized to Rat Genome 230 2.0 Array (Affymetrix, P/N 900506), which is the first whole genome array to interrogate >30,000 transcripts and variants from the rat genome, including >28,000 well-substantiated rat genes, according to the company. Two target cRNAs for each group, each containing transcripts from three rats, were hybridized to array chips. All relevant procedures including hybridization, washing, staining, scanning, and data compilation regarding the hybridization signal strength were done at the UC Davis School of Medicine Microarray Core Facility. Hybridizations were repeated once to generate two sets of data.
Real-time RT-PCR
Two-step real-time RT-PCR was performed to verify the expression of the genes listed in Table 1. Total RNA extracted from the left ventricle was reverse-transcripted into cDNA using RT reagents (Applied Biosystems, 4310179) and processed by real-time quantitative PCR with SYBR Green as double-strand DNA binding dye (Applied Biosystems, 4309155). PCR reactions were carried out in a 7900HT Sequence Detection System (Applied Biosystems), and the standard curve method was used to calculate relative expression levels. The PCR primers are listed in Table 1. They were either purchased from SuperArray Bioscience or introduced from published references and RTPrimer database (http://medgen.ugent.be/rtprimerdb/index.php). β-Actin was employed as an internal control (Microarray showed no change in β-actin). Results were expressed as ratios relative to the amount of β-actin.
Table 1.
Affy array real-time PCR
| Gene | P < 0.05 | Sham | Ovx | Early | Late |
|---|---|---|---|---|---|
| Apoptosis | |||||
| APAF1 | sham, Ovx > early, late | 1.00 ± 0.22 | 0.67 ± 0.15 | 0.38 ± 0.09 | 0.16 ± 0.06 |
| Caspase 3 | Ovx > all | 0.29 ± 0.02 | 0.74 ± 0.14 | 0.28 ± 0.02 | 0.3 ± 0.01 |
| Caspase 6 | late > sham | 0.88 ± 0.08 | 1.10 ± 0.24 | 1.15 ± 0.11 | 1.68 ± 0.19 |
| Caspase 9 | Ovx > sham | 1.25 ± 0.08 | 1.85 ± 0.19 | 1.61 ± 0.13 | 1.50 ± 0.10 |
| Calpain2 | Ovx > sham, early | 1.30 ± 0.07 | 2.12 ± 0.42 | 1.27 ± 0.11 | 1.38 ± 0.09 |
| MADD | Ovx, late > sham | 1.44 ± 0.27 | 1.74 ± 0.25 | 1.15 ± 0.09 | 2.15 ± 0.18 |
| Inflammation | |||||
| IL-6R1 | Ovx > all | 0.25 ± 0.01 | 0.86 ± 0.06 | 0.55 ± 0.07 | 0.46 ± 0.11 |
| iNOS* | Ovx > all | 0.40 ± 0.07 | 1.20 ± 0.34 | 0.39 ± 0.14 | 0.59 ± 0.28 |
| MIP-1 | late > all | 0.73 ± 0.08 | 0.81 ± 0.10 | 0.98 ± 0.04 | 1.59 ± 0.23 |
| Soluble epoxide hydrolase* | ns | 1.23 ± 0.12 | 1.16 ± 0.07 | 1.08 ± 0.07 | 1.28 ± 0.14 |
| SOCS2* | ns | 0.97 ± 0.08 | 1.22 ± 0.31 | 0.88 ± 0.08 | 1.15 ± 0.19 |
| SOCS3* | ns | 1.28 ± 0.24 | 1.51 ± 0.26 | 2.69 ± 0.76 | 1.30 ± 0.40 |
| STAT3 | late > sham | 0.15 ± 0.05 | 0.17 ± 0.04 | 0.25 ± 0.10 | 0.63 ± 0.20 |
| TNF-α | Ovx > all | 3.26 ± 0.62 | 6.50 ± 0.68 | 3.58 ± 0.77 | 3.22 ± 0.65 |
| Extracellular Matrix | |||||
| Collagen I α1 | late < Ovx | 0.71 ± 0.13 | 1.26 ± 0.14 | 0.88 ± 0.21 | 0.47 ± 0.09 |
| Connexin 43 | Ovx > sham | 1.00 ± 0.08 | 1.35 ± 0.04 | 0.98 ± 0.08 | 0.95 ± 0.16 |
| Fibromodulin | ns | 0.56 ± 0.02 | 0.75 ± 0.14 | 0.56 ± 0.10 | 0.62 ± 0.12 |
| Fibronectin | late, Ovx > sham | 0.73 ± 0.03 | 0.92 ± 0.02 | 0.86 ± 0.05 | 0.93 ± 0.06 |
| MMP9 | Ovx > early | 9.05 ± 1.28 | 40.8 ± 14.7 | 5.68 ± 2.14 | 20.5 ± 2.29 |
| MMP14 | late < sham | 3.84 ± 0.56 | 4.10 ± 0.42 | 2.4 ± 0.42 | 1.58 ± 0.07 |
| TIMP1 | ns | 0.63 ± 0.09 | 0.82 ± 0.19 | 0.86 ± 0.26 | 0.49 ± 0.16 |
| TIMP2 | late < all | 1.21 ± 0.10 | 1.16 ± 0.18 | 1.05 ± 0.06 | 0.54 ± 0.06 |
| Vascular Function/Disease | |||||
| ACE | 0.059 | 0.15 ± 0.03 | 0.22 ± 0.04 | 0.33 ± 0.09 | 0.38 ± 0.05 |
| CD44 | ns | 0.25 ± 0.04 | 0.15 ± 0.04 | 0.70 ± 0.55 | 0.22 ± 0.05 |
| Integrin-α6 | ns | 0.84 ± 0.10 | 1.18 ± 0.23 | 1.20 ± 0.11 | 1.61 ± 0.46 |
| Integrin-αM (CD11b) | late > Ovx | 1.08 ± 0.32 | 0.25 ± 0.16 | 1.18 ± 0.66 | 69.6 ± 38.6 |
| sGuanylyl Cyclase-α* | Ovx < all | 1.01 ± 0.07 | 0.61 ± 0.07 | 0.97 ± 0.04 | 0.97 ± 0.09 |
| sGuanylyl Cyclase-β* | Ovx < all | 1.09 ± 0.05 | 0.76 ± 0.01 | 0.82 ± 0.05 | 0.91 ± 0.04 |
| iNOS* | Ovx > all | 0.40 ± 0.07 | 1.20 ± 0.34 | 0.39 ± 0.14 | 0.59 ± 0.28 |
Column 1, gene name and functional group to which the gene belongs; column 2, the groups that are statistically different based on a P value of <0.05; real-time PCR values shown in column 3 (Sham group), column 4 [ovariectomized (Ovx) group], column 5 (Early group: Ovx followed by immediate estrogen replacement), and column 6 (Late group: Ovx followed by delayed estrogen replacement).
PCR performed on genes not on original Affymetrix array.
PCR Array
To further investigate changes in inflammatory genes that might have been missed by the Affy array, the Inflammatory Cytokines and Receptors PCR array (SABiosciences) was used, along with the cDNA synthesis kit and the SYBR green double-stranded DNA detection kit. This PCR-based array is performed precisely like real-time PCR, except that the primers are preloaded into the PCR plate wells. The PCR was performed on an ABI Prism Sequence Detection System (Applied Biosystems), and the data were analyzed using the RT2 Profiler PCR Array Data Analysis (SABiosciences, available online at http://www.sabiosciences.com). Gene expression levels were normalized to ribosomal protein, large, P1 (Rplp1) expression levels, which did not change between groups and are reported as fold of sham. Only those genes whose expression levels showed a 1.5-fold or greater expression difference between groups and had a P value of <0.20 were considered significant changes between groups. To select candidate gene changes, a P value of 0.20 was chosen due to the variation on the arrays resulting from a low sample size number (25, 29). Since only three arrays were performed for each group, it was necessary to increase the P value over the traditional 0.05 to have a representative sampling of genes whose expression was altered on the array and eliminate the sampling errors that come from a small sample size.
Western Blot Analysis
Analysis was performed as previously described (13), except for fibronectin Westerns, where a special approach was used to solubilize this large protein, as previously described (20). Antibodies were used in the following dilutions: TNF-α, 1:500 (Santa Cruz Biotechnology, Santa Cruz, CA); MIP-1β 1:1,000 (PeproTech, Rocky Hill, NJ); inducible nitric oxide synthase (iNOS), 1:500 (BD Biosciences, San Jose, CA); endothelial (e)NOS, 1:500 (Cell Signaling, Danvers, MA); fibronectin, 1:5,000 (Rockland Immunochemicals, Gilbertsville, PA); caspase 9, 1:2,000 (Abcam, Cambridge, MA); tissue inhibitor of metalloproteinase (TIMP)2, 1:1,000 (Millipore, Billerica, CA); heme oxygenase-1, 1:2,000 (Cell Signaling); chemokine (C-x3-C motif) ligand 1 (CX3CR1), 1:5,000 (Abcam); MCP-1, 1:5,000 (Abcam); ABCF1, 1:1,000, (Abcam); endothelial monocyte activating polypeptide II (EMAP II) 1:1,000 (Abcam); GAPDH, 1:50,000 (Fitzgerald, Concord, MA), and the appropriate horseradish peroxidase-conjugated secondary antibodies of anti-mouse or anti-rabbit (GE Healthcare UK), were used at 1:1,000 dilutions and developed using the West Pico enhanced chemiluminescence (Thermo Scientific, Waltham, MA). Proteins were normalized to GAPDH, which did not vary among groups.
Data Analysis
Changes in gene expression levels seen with real-time PCR and Western blot analyses were statistically analyzed using an ANOVA on Ranks, followed by a Student-Newman-Keuls test or Dunn's test, where appropriate, with P < 0.05 considered significant. See Supplementary Methods for full details of Affymetrix Array analysis.1
RESULTS
Model
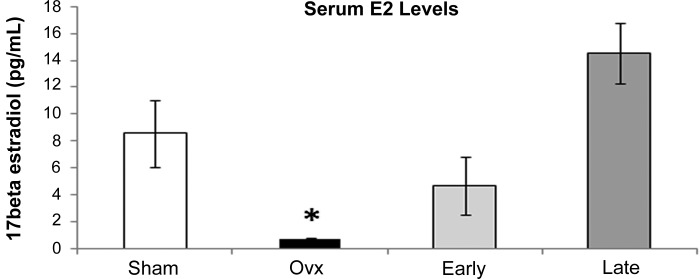
Norway Brown rats were chosen for this work because they are one of several strains, including Fischer rats, that are considered to be ideal aging models. The Norway Brown and the Fischer rat both age without concomitant obesity. Thus one can study aging without a key confounding factor, obesity. The timing of treatments was based on previous work, where we found that post-Ovx cardiac HSP 72 levels took 9 wk to drop to the levels found in males (48). Likely this represents a cascade of changes that occur postloss of estrogen. Therefore, we waited 9 wk post-Ovx before collecting samples. To investigate the effect of late replacement added to the gene expression defined at 9 wk post-Ovx vs. post-Ovx with immediate replacement, we added an additional group of rats with delayed estrogen replacement group for 4 wk. Tissues were collected from these rats at 13 wk. The results of the Women's Health Initiative (WHI) and other studies suggest that late replacement of estrogen is deleterious. To further understand this distinction, we incorporated both immediate estrogen replacement (Early) and delayed estrogen replacement (Late) into our study design. The serum E2 was measured to verify treatment groups (Fig. 1) and showed no statistically significant difference between the E2 levels in the Early and Late replacement groups, but the expected significant decrease in serum E2 levels upon Ovx.
Fig. 1.
Serum estrogen levels. Serum estrogen (E2) levels are shown for the Sham (white bar), ovariectomized (Ovx, black bar), Early (Ovx and immediate E2 replacement, light gray bar), and Late (Ovx and delayed E2 replacement, dark gray bar) with the units given in pg/ml. *P < 0.05 vs. Early and Late.
Affymetrix Array and Apoptosis Pathway
Genes changing in the left ventricle will reflect not only changes in cardiac myocytes but also changes in the coronary arterial cells, in fibroblasts, and in leukocytes and other cell types that may be present in the heart at time of tissue collection. Using an Affymetrix array containing 10,000 genes, we found a number of genes whose expression levels differed among the groups twofold or more. As discussed in materials and methods, a subset of KEGG/BioCarta pathways was chosen for further analysis by real-time PCR. These included apoptosis, inflammation, extracellular matrix/fibrosis, and vascular function/disease genes. For the apoptosis pathway, a number of changes in apoptosis genes were identified and confirmed by real time PCR (Table 1). Apoptosis-associated factor (APAF) 1 was greater in Sham and Ovx groups than the two E2 replacement groups. Caspase 3 was nearly tripled in Ovx vs. all (P < 0.05). Similarly, caspase 9 and calpain 2 were increased in the Ovx group (P < 0.05). MADD was highest in the Late group and in both Ovx and Late was significantly higher than sham (P < 0.05).
Inflammation Pathway
IL-6R1 and TNF-α were increased in Ovx (P < 0.05). iNOS was not detected by the Affymetrix array, but given its importance in cardiovascular disease, its expression was examined by real-time PCR. iNOS was found to be significantly increased in Ovx (Table 1, P < 0.05). MIP-1 and STAT3 were increased in the Ovx group on the array but, by real-time PCR, were significantly higher in the Late group (Table 1, P < 0.05). SOCS2, SOCS3, and soluble epoxide hydrolase were investigated as we have previously observed these genes to change in young rats, but there were no differences for any of these genes (14).
Extracellular Matrix Pathway
For genes involved in the extracellular matrix pathway, connexin 43 and MMP9 were increased in the Ovx group (Table 1, P < 0.05). Collagen1 α1 was decreased in the Late group. Fibronectin was higher in both the Ovx and Late group, while fibromodulin was unchanged (Table 1). Both MMP14 and TIMP2 were decreased in the Late group (P < 0.05).
Vascular Function and Disease Pathway
Examination of the vascular function/disease pathway showed that ACE tended to be higher in the Late group, but this did not reach significance. Integrin-αM (also known as CD11b) was increased in the Late group (Table 1, P < 0.05). Soluble guanylyl cyclase (sGC)-α and -β were decreased with Ovx, in contrast to our findings in the young adult rat, where these did not differ from sham (14).
Although we found a number of changes with the rat Affymetrix array, we were concerned because there were a number of gene sequences for which the nomenclature exists as Caenorhabditis elegans, Drosophila, or other organisms that may show very little similarity to the rat gene. In addition, the rat array contained quite a few estimated sequence tags (ESTs), for which the gene they encode is as yet uncharacterized. For example, the Affymetrix array contains six sequences that code for fibronectin, but only two have been confirmed for fibronectin, while the other four are ESTs that are considered weakly or moderately similar to fibronectin. We were concerned that given this “noise” we might miss important changes. Therefore, it was decided to use an additional approach to investigate changes in proinflammatory genes.
Inflammatory Gene PCR Array
Inflammation is a major issue in aging and estrogen loss. As such, we chose to perform a more in-depth analysis of the inflammatory pathway using the SABiosciences Inflammatory Cytokines and Receptors PCR array. This array system has the advantage that there are only 89 genes total and all of the sequences are derived from the rat sequences. There are no ESTs, and every primer set corresponds to a verified rat gene. Software supplied by the company allowed analysis of each group compared with the rest.
Late E2 replacement affected the expression of a limited number of genes on the PCR array (Table 2 and Supplementary Table S2). MCP-1 expression by the PCR array was 49-fold lower vs. Sham, while MIP-1β was 3.9-fold higher vs. Ovx (Table 2), similar to findings on the Affymetrix array. EMAP II, also known as Scye 1, was increased 84-fold in the Late group compared with sham and slightly less in the Ovx group.
Table 2.
PCR array: all groups vs. Late
| Sham | Ovx | Early | ||
|---|---|---|---|---|
| Mcp1 | 49.86 | induces cell death through MCPIP and plays role in ischemic heart disease (46); release decreased by estradiol (33) | ||
| Mip1β | −3.87 | role in liver inflammation and transplant rejection (45, 34) | ||
| Emap II | −84.12 | 1.22 | also known as SCYE1: proinflammatory and antiangiogenic, recruits mononuclear cells and enhances expression of CSF-1 and MCP-1 in teeth (20) |
PCR array compared with Late. Column 1, gene name; column 2, fold-change of Sham compared with Late; column 3, fold-change of Ovx compared with Late; column 4, fold-change of Early compared with Late. Numbers are significantly different (P < 0.2) as compared with Late. The complete list of genes with altered expression is in Supplementary Table S2.
Surprisingly, the expression levels of several inflammatory genes in the Ovx group were much lower than those in the Sham group (Table 3 and Supplementary Table S3). Cx3cr1, also known as fractaline, which promotes leukocyte transmigration into the arterial wall and thus atherosclerosis (24), was decreased 13.6-fold vs. the Sham group and 2.1-fold vs. the Early group. Cx3cr1 was decreased even more in the Early group vs. sham (29-fold). Thus, Ovx decreased expression of Cx3cr1 regardless of immediate E2 replacement. MIP-1β, a proinflammatory gene (50), was less in the Ovx group than either the Early or Late group. Lastly, MCP-1, a monocyte adhesion protein (52), was 51.5-fold lower in Ovx than Sham.
Table 3.
PCR Array: all groups vs. Ovx
| Sham | Early | Late | ||
|---|---|---|---|---|
| Mcp1 | 51.46 | induces cell death through MCPIP and plays role in ischemic heart disease (46); release decreased by estradiol (33) | ||
| Mip1β | 2.72 | 3.87 | role in liver inflammation and transplant rejection (45, 34) | |
| Cx3cr1 | 13.62 | −2.13 | Cx3cl1 receptor, probably involved in heart failure (14); thought to increase atherosclerosis by promoting leukocyte transmigration (21) | |
| Emap II | −1.22 | also known as SCYE1; proinflammatory and antiangiogenic, recruits mononuclear cells and enhances expression of CSF-1 and MCP-1 in teeth (20) | ||
| Tnf-α | 2.97 | inflammatory cytokine; RNA levels increase with Ovx (18) |
PCR array compared with Ovx. Column 1, gene name; column 2, fold-change of Sham compared with Ovx; column 3, fold-change of Early compared with Ovx; column 4, fold-change of Late compared with Ovx. Numbers are significantly different (P < 0.2) as compared to Ovx. The complete list of genes with altered expression is in Supplementary Table S3.
A key question in our study was whether immediate E2 replacement could prevent deleterious changes seen with Ovx. Genes on the PCR array with altered expression by immediate E2 replacement (Early) are listed in Table 4 and Supplementary Table S3. MIP-1β was 2.7-fold higher in the Early vs. Ovx, but 3.9-fold lower than the Late group (Table 4). Cx3cr1 levels were 29-fold less in the Early vs. the Sham and 2.1-fold less in the Early vs. the Ovx (Table 4). Similarly, MCP-1 levels were decreased 17-fold vs. Sham.
Table 4.
PCR Array: all groups vs. Early
| Sham | Ovx | Late | ||
|---|---|---|---|---|
| Mcp1 | 16.97 | induces cell death through MCPIP and plays role in ischemic heart disease (46); release decreased by estradiol (33) | ||
| Mip1β | −2.72 | 3.87 | role in liver inflammation and transplant rejection (45, 34) | |
| Cx3cr1 | 29.00 | 2.13 | Cx3cl1 receptor, probably involved in heart failure38; thought to increase atherosclerosis by promoting leukocyte transmigration (21) | |
| Emap II | −35.51 | also known as SCYE1; proinflammatory and antiangiogenic, recruits mononuclear cells and enhances expression of CSF-1 and MCP-1 in teeth (20) |
PCR array compared with Early. Column 1, gene name; column 2, fold-change of Sham compared with Early; column 3, fold-change of Ovx compared with Early; column 4, fold-change of Late compared with Early. Numbers are significantly different (P < 0.2) as compared with Early. The complete list of genes with altered expression is in Supplementary Table S4.
Inflammatory Gene Corresponding Protein Changes
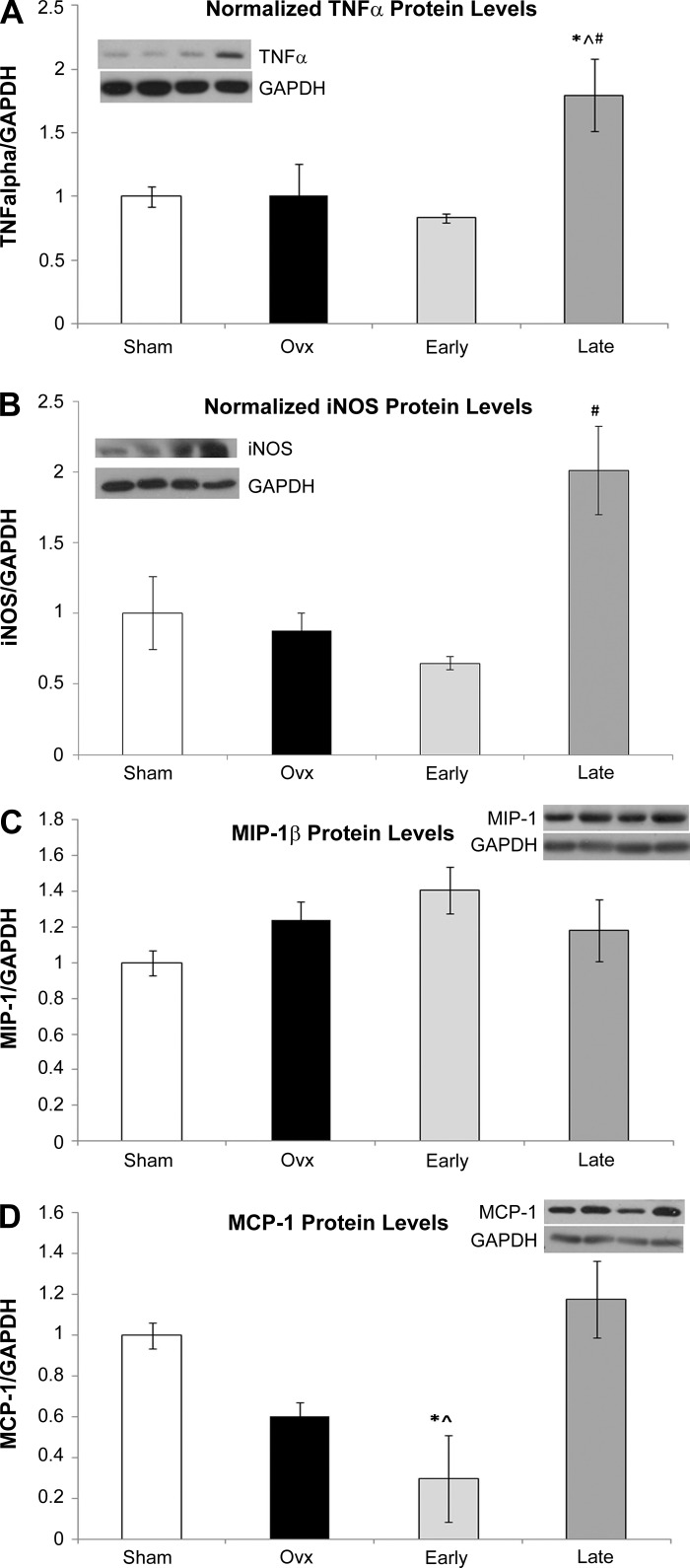
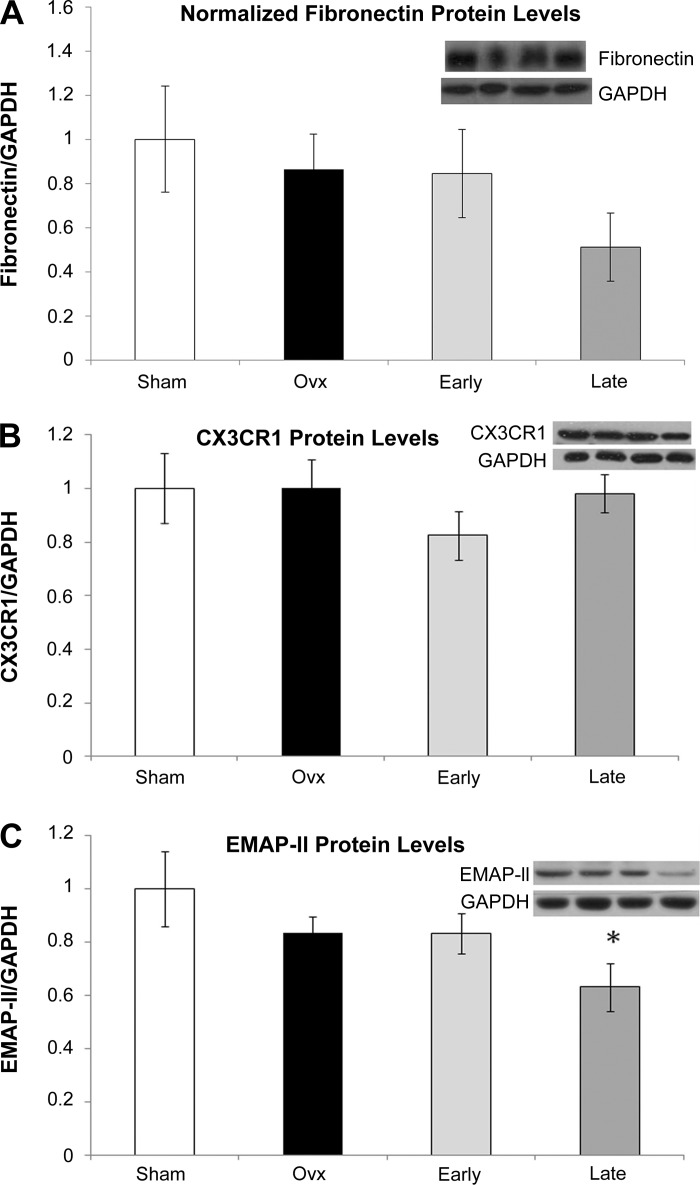
Western blotting was done for selective key inflammatory proteins to determine if the protein changed as well as the mRNA. TNF-α levels were doubled in the Late group, while there was no change in the Ovx group vs. Sham (P < 0.05, Fig. 2A). iNOS, which was not detected on the Affymetrix array, but found to be increased in the Ovx group by real-time PCR (Table 1), was also increased at the protein level, but only in the Late group (Fig. 2B). Although MIP-1β was less in the Ovx group than either the Early and Late group on the PCR array (Table 3), MIP-1β protein levels tended to be higher in Ovx and Early (Fig. 2C), but this was not significant. mRNA for MCP-1 was 51.5-fold lower (Table 3) in Ovx and 17-fold lower in Early (Table 4) compared with Sham; however, by Western blot only the Ovx MCP-1 protein levels were decreased significantly (P < 0.05) compared with Sham (Fig. 2D). Fibronectin, a key extracellular matrix protein that is thought to be proinflammatory, was increased in Late and Ovx groups by real-time PCR, but Western showed no significant difference in fibronectin expression among the groups (Fig. 3A). Cx3cr1 was decreased in Ovx and in the Early groups compared with sham by PCR array (Table 3), but the protein levels were unchanged (Fig. 3B). EMAP II, a proapoptotic cytokine, was significantly decreased in the Late group by Western (Fig. 3C), a difference from the increase in mRNA seen with this group. The Affymetrix array also showed increases in caspase 6, STAT3, and CD11b in the Late group.
Fig. 2.
Western blot analysis of selected array genes, normalized to GAPDH. Sham, white bars; Ovx, black bars; Early (immediate E2 replacement), light gray bars; Late E2 replacement, dark gray bars. A representative Western blot showing bands from each of the 4 groups in the order they are given on the graph plus a representative GAPDH blot are shown for each graph. A: TNF-α. B: inducible nitric oxide synthase (iNOS). C: macrophage inflammatory protein (MIP)-1β. D: monocyte chemoattractant protein (MCP). *P < 0.05 compared with Sham, #P < 0.05 compared with Ovx, †P < 0.05 compared with Late, P < 0.05 compared with Early; n = 4–7/group.
Fig. 3.
Western blot analysis of selected array genes, normalized to GAPDH. Sham, white bars; Ovx, black bars; Early, light gray bars; Late, dark gray bars. A representative Western blot showing bands from each of the 4 groups in the order they are given on the graph plus a representative GAPDH blot are shown for each graph. A: fibronectin. B: chemokine (C-x3-C motif) ligand 1 (CX3CR1). C: endothelial monocyte activating polypeptide II (EMAP II). *P < 0.05 compared with Sham; n = 4–7/group.
Additional real-time PCR studies demonstrated that iNOS was increased at the mRNA level in the Ovx group, but at the protein level, iNOS was markedly increased in the Late group only. In contrast TIMP2, MMP14, and collagen I α1 were decreased in the Late group, which may have an adverse effect on ventricular remodeling. Ovx without replacement led to an increase in caspase 3, caspase 9, calpain 2, MMP9, and TNF-α. MADD and fibronectin were increased in both Ovx and Late group, but this did not translate into a change in fibronectin protein levels. Many of these changes were prevented by early E2 replacement.
DISCUSSION
The left ventricle, which was the focus of the current study, includes not only cardiac myocytes, which constitute most of cardiac mass, but also endothelial cells, smooth muscle cells, fibroblasts and leukocytes. Late replacement of estrogen was characterized by increased expression of CD11b, MIP-1β, STAT3, EMAP II, fibronectin, caspase 6, and MADD. TNF-α and iNOS protein levels increased with Late replacement even though the RNA levels were not increased for these groups. These changes involve predominantly proinflammatory and proatherosclerotic proteins. TNF-α's proinflammatory effects include upregulation of the adhesion molecules ICAM-1, VCAM-1, and E-selectin in endothelial cells, enhancing the recruitment of leukocytes (11, 25, 45). TNF-α also increases oxidative stress by activation of NADPH oxidase (9). iNOS, another proinflammatory gene, was increased markedly at the protein level only in the Late group. CD11b has a central role in leukocyte adhesion and atherosclerosis (37). MIP-1β promotes leukocyte adhesion and atherosclerosis (43) and has been found to have a negative role in postinfarction remodeling (22). STAT3 has a complex role in the heart and can be either protective or proinflammatory (15). Fibronectin, an extracellular matrix protein found in the plasma, is expressed in atherosclerotic plaques and in the fibrous cap (5). Fibronectin is considered to have proinflammatory properties but also has key functions in wound repair and scar formation (11, 20). Additionally, increased fibrosis in the heart, caused in part by an increase in the expression of fibronectin, leads to left ventricular stiffness and diastolic dysfunction, a predominant cause of heart attacks in the aging population (19). Recently it has been reported that estrogen blocks cardiac fibrosis via estrogen receptor-β, inhibiting the increase in fibronectin, vimentin, and collagen that occurs with angiotensin II (34). EMAP II is considered a proinflammatory cytokine (44), as discussed below, while MADD and caspase 6 promote apoptosis. Thus, these changes in gene expression with late E2 replacement help to explain why in human studies E2 replacement many years postmenopause was associated with an increase in cardiovascular events.
TNF-α
TNF-α is a potent proinflammatory cytokine whose circulating levels increase with age and heart disease. Our lab has previously shown that TNF-α RNA levels increase with Ovx in young adult rats (14). Suzuki et al. (42) reported that after Ovx cerebral levels of TNF-α after middle cerebral artery occlusion (MCAO) were lower in mice with immediate E2 replacement. There was no difference in cerebral TNF-α levels after MCAO in mice with late E2 replacement vs. no replacement; however, basal TNF-α levels were not measured (42). In postmenopausal women, estrogen treatment inhibited the release of TNF-α from monocytes (31). Primary human bone marrow cultures collected within 5 yr postmenopause showed no change in TNF-α expression levels, whereas those from women recently discontinuing hormone replacement had elevated TNF-α (36). Thus, overall estrogen replacement decreases TNF-α levels and estrogen loss increases TNF-α levels, and the current study supports a marked increase in TNF-α with late estrogen replacement.
iNOS
iNOS expression was markedly increased at the protein level only in the Late replacement group. Increased iNOS occurs with aging in general; however, among the four aged groups, iNOS protein levels were clearly increased only in the Late group (10). iNOS is proinflammatory and can lead to an over production of NO, which then combines with superoxide ion (O2−) to generate peroxynitrite. Peroxynitrite is a potent oxidizing and nitrating agent that has been shown to contribute to myocardial and vascular dysfunction in many diseases (30). Peroxynitrite has been implicated in the inactivation of a plethora of enzymes, including antioxidants. Increased NO can also lead to protein s-nitrosylation (47). S-nitrosylation may result in protein dysfunction but at times can also be protective, as recently reviewed (12). Lastly, increased iNOS also leads to the production of inflammatory cytokines and apoptosis.
Ovx and Cardiac Gene Changes
Ovx without estrogen replacement was associated with increased expression of caspase 3, caspase 9, calpain 2, MADD, IL-6R1, iNOS, and fibronectin. These genes are primarily proapoptotic, proinflammatory, and profibrotic. MCP-1 is one of the circulating chemokines that recruits monocytes, macrophages, and T-cells to areas of inflammatory injury (8). Similar to other chemokines discussed below, MCP-1 is found in areas of atherosclerotic lesions and contributes to the recruitment of inflammatory cells into the arterial wall (26). In the current study, MCP-1 surprisingly decreased in all groups compared with sham by PCR array, but Western showed a decrease only in the Early group (P < 0.05). Others have shown that in 19 wk old mice MCP-1 protein levels decreased in areas of cerebral ischemia with immediate E2 replacement, but delayed E2 treatment did not alter the protein levels (6), a finding consistent with the current study.
Soluble Guanylyl Cyclase and Aging
sGC, both the α- and β-subunits, decreased by both the Affymetrix array and real-time PCR only with Ovx. This is consistent with our previous finding that sGC mRNA and protein decreased in the aorta only with the combination of aging and Ovx, and this could be prevented by E2 replacement (40). Loss of sGC results in impaired vascular relaxation, which is critical for normal vascular function. In contrast, in the young adult Sprague-Dawley rat, sGC was unchanged post-Ovx (14). Early E2 replacement prevented many of the changes associated with Ovx, including the down regulation of sGC-α and -β.
Cx3cr1 and Inflammatory Signaling
Cx3cr1 is an inflammatory signaling receptor important in monocyte recruitment. Cx3cr1-mediated signaling leads to increased expression of ICAM-1 and neutrophil adhesion on endothelial cells (50). Cx3cr1 was decreased in both the Ovx and Early groups vs. sham, but not in the Late group. Thus, Ovx decreased expression of Cx3cr1 independently of E2 replacement. Late E2 reversed this change.
EMAP II
EMAP II is a proinflammatory cytokine that is released in response to stress. In the vasculature, EMAP II is increased after percutaneous coronary intervention (PCI), and EMAP II enhances the recruitment of inflammatory cells (44). Treatment with rapamycin prevents neointima formation and the increase in EMAP II expression post-PCI (27). EMAP II also inhibits angiogenesis, which can be beneficial in tumors but can be deleterious when manifest as inhibition of endothelial cell proliferation. EMAP II binds to VEGF receptor 1 and 2, blocking downstream signaling from VEGF (6). The effect of estrogen on EMAP II has not been studied. Our data indicate that treatment with E2 Early or Late after Ovx causes an increase in EMAP II mRNA, but the protein levels of EMAP II are decreased with Late E2 treatment and unchanged with Early E2 replacement.
Affymetrix Array vs. PCR Array
There was a disparity between the Affymetrix Array, real-time PCR done for the Affymetrix Array and the PCR Array. Both the Affymetrix Array and real-time PCR done at that time showed an increase in TNF-α mRNA. Previously we have found that TNF-α increased in young adult Sprague-Dawley rats 9 wk post-Ovx (14). In contrast to the Affymetrix array and real-time PCR where an increase in TNF-α was seen with Ovx, TNF-α RNA levels were unchanged on the PCR array (not shown). For our first real-time PCR we used primers selected from RTPrimerDB (http://medgen.ugent.be/rtprimerdb/index.php), a database of confirmed primers including specific primers for the Norway Brown rat. The PCR array uses proprietary primers, so the sequence is unknown. Differences in primers may account for the differences.
mRNA vs. Protein
There was a divergence between RNA findings and protein expression, which is not unexpected given the complexity of regulation of gene expression and translation. Only for MCP-1 do changes in RNA levels coincide with the protein levels. This lack of correlation between mRNA and protein is not surprising as real-time PCR is exquisitely sensitive compared with Western blotting, where it is challenging to convincingly show a 40% change. Also, many of the changes were seen in cytokines, which often have short half-lives as proteins. Finally, the turnover of both RNA and proteins is differentially regulated. Gene expression is controlled at multiple levels including transcription, posttranscription stability, translation, and posttranslation degradation. These conditions likely contributed to the differences between mRNA and protein expression level findings, as PCR is definitively more sensitive than protein analysis by Western blot.
This is the first study to our knowledge that investigates the effect of late estrogen replacement in aged rats post-Ovx as a model of menopause. A significant number of gene changes were found, many of these characterized as proinflammatory. A consistent finding was changes in genes that promote leukocyte attraction and adhesion, early steps in atherosclerosis. The most important finding is the increase in proinflammatory and proapoptotic proteins with late estrogen replacement, exemplified by the increase in TNF-α and iNOS protein levels with late replacement. As TNF-α increases the expression of adhesion molecules along with other proinflammatory effects, these findings suggest a mechanism for the increase in cardiovascular disease seen after delayed conjugated equine estrogen replacement in clinical trials such as the WHI. Further work will be needed to fully understand the effects of estrogen and its absence on the aging heart.
GRANTS
Supported by National Institutes of Health Grants AG-19327 (A. A. Knowlton) and T32 86350 (A. S. Pechenino), a Merit Award from the Department of Veterans Affairs (A. A. Knowlton), and HHMI-MIG to A. R. Lee.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Lorie Bischel and Dr. Le Chen for technical assistance.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Abu-Taha M, Rius C, Hermenegildo C, Noguera I, Cerda-Nicolas JM, Issekutz AC, Jose PJ, Cortijo J, Morcillo EJ, Sanz MJ. Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol 183: 1393–1402, 2009. [DOI] [PubMed] [Google Scholar]
- 2. Allison DB, Cui X, Page GP, Sabripour M. Microarray data analysis: from disarray to consolidation and consensus. Nat Rev Gen 7: 55–65, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Horn LV, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S, Women's Health Initiative Committee Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291: 1701–1712, 2004. [DOI] [PubMed] [Google Scholar]
- 4. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Swight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Gen 25: 25–29, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Astrof S, Hynes RO. Fibronectins in vascular morphogenesis. Angiogen 12: 165–175, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Awasthi N, Schwarz MA, Verma V, Cappiello C, Schwarz RE. Endothelial monocyte activating polypeptide II interferes with VEGF-induced proangiogenic signaling. Lab Invest 89: 38–46, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Barnett G, Jakobson AM, Tas M, Rice K, Carmichael J, Murray JC. Prostate adenocarcinoma cells release the novel proinflammatory polypeptide EMAP-II in response to stress. Cancer Res 60: 2850–2857, 2000. [PubMed] [Google Scholar]
- 8. Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA 26: 3652–3656, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chakrabarti S, Lekontseva O, Davidge ST. Estrogen is a modulator of vascular inflammation. IUBMB Life 60: 376–382, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Chung HY, Cesari M, Anton S, Marzetti E, Giovanni S, Seo AY, Carter C, Yu BP, Leeuwenburg C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 8: 18–30, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res 107: 418–428, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med 15: 391–404, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamilton KL, Gupta S, Knowlton AA. Estrogen and regulation of heat shock protein expression in female cardiomyocytes: cross-talk with NF kappa B signaling. J Mol Cell Cardiol 36: 577–584, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Hamilton KL, Lin L, Wang Y, Knowlton AA. Effect of ovariectomy on cardiac gene expression: inflammation and changes in SOCS gene expression. Physiol Genomics 32: 254–263, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Hilfiker-Kleiner D, Shukla P, Klein G, Schaefer A, Stapel B, Hoch M, Muller W, Scherr M, Theilmeier G, Ernst M, Hifiker A, Drexler H. Continuous glycoprotein-120-mediated signal transducer and activator of transcription-3 activation promotes inflammation, left ventricular rupture, and adverse outcome in subacute myocardial infarction. Circulation 122: 145–155, 2010. [DOI] [PubMed] [Google Scholar]
- 16. Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 280: 605–613, 1998. [DOI] [PubMed] [Google Scholar]
- 17. Husberg C, Nygard S, Finsen AV, Damas JK, Frigessi A, Oie E, Waehre A, Gullestad L, Aukrust P, Yndestad A, Christensen G. Cytokine expression profiling of the myocardium reveals a role for CX3CL1 (fractalkine) in heart failure. J Mol Cell Cardiol 45: 261–269, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Kanehisa M, Goto S, Kawashima S, Nakaya A. The KEGG databases at GenomeNet. Nucleic Acids Res 30: 42–46, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS research group Cardiovascular health study. Am J Cardiol 87: 413–419, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Knowlton AA, Connelly CM, Romo GM, Mamuya W, Apstein CS, Brecher P. Rapid expression of fibronectin in the rabbit heart after myocardial infarction with and without reperfusion. J Clin Invest 89: 1060–1068, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li M, Stallone JN. Estrogen potentiates vasopressin-induced contraction of female rat aorta by enhancing cyclooxygenase-2 and thromboxane function. Am J Physiol Heart Circ Physiol 289: H1542–H1550, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Liehn EA, Merx MW, Postea O, Becher S, Djalali-Talab Y, Shagdarsuren E, Kelm M, Zernecke A, Weber C. Ccr1 deficiency reduces inflammatory remodeling and preserves left ventricular function after myocardial infarction. J Cell Mol Med 12: 496–506, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu D, Wise GE. Expression of endothelia monocyte-activating polypeptide II in the rat dental follicle and its potential role in tooth eruption. Eur J Oral Surg 116: 334–340, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDermott DH, Fong AM, Yang Q, Sechler JM, Cupples LA, Merrell MN, Wilson PW, K'Agostino RB, O'Donnell CJ, Patel DD, Murphy PM. Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J Clin Invest 111: 1241–1250, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardio 6: 410–417, 2009. [DOI] [PubMed] [Google Scholar]
- 26. Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest 88: 1121–1127, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nuhrenberg TG, Langweiser N, Schwarz JB, Hou Y, Frank P, Sorge F, Matschurat S, Seidl S, Kastrati A, Schomig A, Clauss MA, Zohlnhofer D. EMAP-II downregulation contributes to the beneficial effects of rapamycin after vascular injury. Cardiovasc Res 77: 580–589, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Oommen S, Vasu VT, Leonard SW, Traber MG, Cross CE, Gohil K. Genome wide responses of murine lungs to dietary alpha-tocopherol. Free Radic Biol Med 41: 98–109, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oparil S, Levine RL, Chen SJ, Durand J, Chen YF. Sexually dimorphic response of the balloon-injured rat carotid artery to hormone therapy. Circulation 95: 1301–1307, 1997. [DOI] [PubMed] [Google Scholar]
- 30. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pacifici R, Rifas L, McCracken R, Vered I, McMurtry C, Avioli LV, Peck WA. Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci USA 86: 2398–2402, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pavlidis P, Qin J, Arango V, Mann JJ, Sibille E. Using the gene ontology for microarray data mining: a comparison of methods and application to age effects in human prefrontal cortex. Neurochem Res 29: 1213–1222, 2004. [DOI] [PubMed] [Google Scholar]
- 33. Pechenino AS, Frick KM. The effects of acute 17beta-estradiol treatment on gene expression in the young female mouse hippocampus. Neurobiol Learn Mem 91: 315–322, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pedram A, Razandi M, O'Mahony F, Lubahn D, Levin ER. Estrogen receptor-beta prevents cardiac fibrosis. Mol Endocrinol 24: 2152–2165, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peng X, Wood CL, Blalock EM, Chen KC, Landfield PW, Stromberg AJ. Statistical implications of pooling RNA samples for microarray experiments. BMC Bioinformatics 4: 26, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocrine Rev 23: 90–119, 2002. [DOI] [PubMed] [Google Scholar]
- 37. Regitz-Zagrosek V. Cardiovascular disease in postmenopausal women. Climacteric 6: 13–20, 2003. [PubMed] [Google Scholar]
- 38. Rodriguez E, Lopez R, Paez A, Masso F, Montano LF. 17beta-estradiol inhibits the adhesion of leukocytes in TNF-alpha stimulated human endothelial cells by blocking IL-8 and MCP-1 secretion, but not its transcription. Life Sci 71: 2181–2193, 2002. [DOI] [PubMed] [Google Scholar]
- 39. Roussoulieres AL, Raisky O, Chalabreysse L, Dureau G, Cerutti C, Thieblemont C, Boissonnat P, Sebbag L, Obadia JF, Ninet J, Bastien O, Thivolet-Bejui F, McGregor JL. Identification and characterization of two genes (MIP-1beta, VE_CADHERIN) implicated in acute rejection in human heart transplantation: use of murine models in tandem with cDNA arrays. Circulation 111: 2636–2644, 2005. [DOI] [PubMed] [Google Scholar]
- 40. Stice JP, Eiserich JP, Knowlton AA. Role of aging versus the loss of estrogens in the reduction in vascular function in female rats. Endocrinology 150: 212–219, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res 100: 884–893, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suzuki S, Brown CM, Cruz CDD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and anti-inflammatory actions. Proc Natl Acad Sci USA 104: 6013–6018, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tatara Y, Ohishi M, Yamamoto K, Shiota A, Hayashi N, Iwamoto Y, Takeda M, Takagi T, Katsuya T, Ogihara T, Rakugi H. Macrophage inflammatory protein-1beta induced cell adhesion with increased intracellular reactive oxygen species. J Mol Cell Cardiol 41: 104–111, 2009. [DOI] [PubMed] [Google Scholar]
- 44. Tsai BM, Wang M, Clauss M, Sun P, Meldrum DR. Endothelial monocyte-activating polypeptide II causes NOS-dependent pulmonary artery vasodilation: a novel effect for a proinflammatory cytokine. Am J Physiol Regul Integr Comp Physiol 287: R767–R771, 2004. [DOI] [PubMed] [Google Scholar]
- 45. Tsou JK, Gower RM, Ting HJ, Schaff UY, Insana MF, Passerini AG, Simon SI. Spatial regulation of inflammation by human aortic endothelial cells in a linear gradient of shear stress. Microcirculation 15: 311–323, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Turgeon JL, McDonnell DP, Martin KA, Wise PM. Hormone therapy: physiological complexity belies therapeutic simplicity. Science 304: 1269–1273, 2004. [DOI] [PubMed] [Google Scholar]
- 47. Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci 65: 1028–1041, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Voss MR, Stallone JN, Li M, Cornelussen RN, Knuefermann P, Knowlton AA. Gender differences in the expression of heat shock proteins: the effect of estrogen. Am J Physiol Heart Circ Physiol 285: H687–H692, 2003. [DOI] [PubMed] [Google Scholar]
- 49. Walsh BA, Busch BL, Mullick AE, Reiser KM, Rutledge JC. 17 beta-estradiol reduces glycoxidative damage in the artery wall. Arterioscler Thromb Vasc Biol 19: 840–846, 1999. [DOI] [PubMed] [Google Scholar]
- 50. Zernecke A, Weber C. Chemokines in the vascular inflammatory response of atherosclerosis. Cardiovasc Res 86: 192–201, 2010. [DOI] [PubMed] [Google Scholar]
- 51. Zhang T, Guo CJ, Li Y, Douglas SD, Qi XX, Song L, Ho WZ. Interleukin-1beta induces macrophage inflammatory protein-1beta expression in human hepatocytes. Cell Immunol 226: 45–53, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, Younce C, Binkley PF, Klattukudy PE. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res 98: 1177–1185, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.