Abstract
Custom assays for unique proteins are often limited to time consuming manual detection and quantitation techniques such as ELISA or Western blots due to the complexity of development on alternate platforms. BioScale's proprietary Acoustic Membrane MicroParticle (AMMP) technology allows sandwich immunoassays to be easily developed for use on the ViBE platform, providing better sensitivity, reproducibility, and automated operation. Provided as an example, this protocol outlines the procedure for developing a custom Chinese Hamster Ovary- Host Cell Protein (CHO-HCP) assay. The general principles outlined here can be followed for the development of a wide variety of immunoassays.
An AMMP assay measures antigen concentration by measuring changes in oscillation frequency caused by the binding of microparticles to the sensor surface to calculate. It consists of four major components: (1) a cartridge that contains a functionalized eight sensor chip (2) antibody labeled magnetic microparticles, (3) hapten tagged antibody that binds to the surface of the functionalized chip (4) samples containing the antigen of interest. BioScale's biosensor is a resonant device that contains eight individual membranes with separate fluidic paths. The membranes change oscillation frequency in response to mass accumulating on the surface and this frequency change is used to quantitate the amount of added mass.
To facilitate use in a wide variety of immunoassays the sensor is functionalized with an anti-hapten antibody. Assay specific antibodies are modified through the covalent conjugation of a hapten tag to one antibody and biotin to the other. The biotin label is used to bind the antibody to streptavidin coupled magnetic beads which, in combination with the hapten-tagged antibody, are used to capture the analyte in a sandwich. The complex binds to the chip through the anti-hapten/hapten interaction. At the end of each assay run the sensors are cleaned with a dilute acid enabling the sequential analysis of columns from a 96-well plate.
Here, we present the method for developing a custom CHO-HCP AMMP assay for bioprocess development. Developing AMMP assays or modifying existing assays into AMMP assays can provide better performance (reproducibility, sensitivity) in complex samples and reduced operator time. The protocol shows the steps for development and the discussion section reviews representative results. For a more in-depth explanation of assay optimization and customization parameters contact BioScale. This kit offers generic bioprocess development assays such as Residual Protein A, Product titer, and CHO-HCP.
Protocol
1. MicroParticle Preparation
Label Antibodies using Biotin NHS Chemistry
Determination of the Degree of Biotinylation
- Conjugate Streptavidin Beads with Biotinylated Antibody
- Resuspend the beads in the original vial by vortexing for 1-2 minutes
- Aliquot the desired volume of beads into a microcentrifuge tube
- Wash the beads 3 times with PBST:
- Place the tube containing the beads on the magnet for 2 minutes
- Remove the supernatant by aspiration with the tube on the magnet. Be careful not to disrupt the beads
- Remove the tube from the magnet and add 1 mL PBST
- Vortex for 20 seconds to resuspend the beads
- Centrifuge for 5 seconds to remove the beads from the cap
- Repeat 2.3.1-2.3.5 twice more
- After the third wash place the tube on the magnet and aspirate the PBST
- Add biotinylated antibody to the beads and resuspend
- If necessary bring the volume of the biotinylated antibody up to 300 μL with PBS. If the antibody volume is already greater than 300 μL do not add any PBS
- Incubate the bead/antibody suspension for 30 minutes at RT on the vortex shaker or tube rotator
- Wash 3 times with PBST as in step 3.
- Block beads with biotinylated BSA
- After the third wash add 10 μg/mg biotinylated BSA, adjust the volume to 300 μL with PBS if necessary, and incubate with agitation for 30 minutes
- Wash 3 times with PBST as in step 3
- Resuspend the beads in 100 μL of PBS/0.05% BSA. Sodium azide can be added for long term storage
Using standard NHS Fluorescein protocols, fluoresceinate the detector antibody
Determine the Degree of Fluorescination
2. HCP - Development
- Prepare working solutions:
- Fluorescein labeled Antibody (Fl-Ab): Most CHO-HCP assays use Fl-Ab at a final concentration of 400 ng/mL. The Fl-Ab is added as ¼ of the final assay volume, therefore the concentration of the working solution is 1.6 μg/mL. Prepare this solution by diluting FlAb stock with BioScale Bead/FlAb Diluent.
- Beads: Most CHO-HCP assays use beads at a final concentration of 2x105 beads/mL. The bead reagent constitutes ¼ of the final assay volume; therefore the concentration of the working solution is 8x105 beads/mL. Prepare this solution by diluting bead stock with BioScale Bead/FlAb Diluent. Vortex the bead stock well to make sure all the beads are in suspension prior to removing an aliquot.
- Running Buffer: The composition of the running buffer may have to be optimized for the antibodies used to develop the assay, however several CHO-HCP assays have been developed using 10 mM HEPES buffer, 1% Tween, pH 7.5. BioScale has developed an additive for the running buffer (supplied with the development kit) which is added to the buffer to a concentration of 1% prior to use.
Dilute calibrators and samples with BioScale Sample Diluent.
3. ViBE System Set Up
Turn the ViBE Workstation on
Connect the system supply and waste
Prime the system
Insert a ViBE Cartridge
Route the tubes
Prepare assay template by filling out the sheet labeled "Sample Setup". BioScale provides the capability to have one assay template for each ViBE Workstation assay. This allows the user to edit and save specific notes in the "Notes" sheet of the template for different assays keeping a complete record of the experiment.
Initial assay template set up: Begin by filling out the table (top left corner) of the concentration range of the standards in the assay in the "Sample Setup" sheet.
Next, enter additional information in the table to the right of the standards (Simple Column Read Setup, One Reagent Add and Two Reagent Add setups) to instruct the ViBE Workstation how long samples need to incubate, how much volume of reagent is to be transferred, how long the sample accumulation needs to be, and whether sensor conditioning and decontamination of probes and cartridge are necessary, etc
Fill out the plate layout.
Load sample/reagents into plates. The ViBE will provide the required volumes for the reagent plate. Sample volume can vary, but in half of a total assay volume of 120 μL is a good starting place.
Apply plate covers. Load plates onto workstation deck.
Run the assay start up wizard, go through wizard and start assay.
4. Representative Results
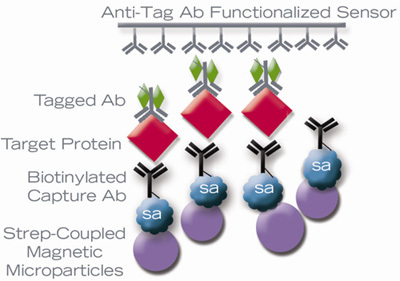 Figure 1: AMMP Sandwich Immunoassay
Figure 1: AMMP Sandwich Immunoassay
On a ViBE Workstation, an AMMP assay measures antigen concentration. Signal is measured when the bead-antigen-hapten antibody binds to the sensor through the hapten/anti-hapten interaction. Regeneration brings the sensor back to the beginning state by removing the immune complexes.
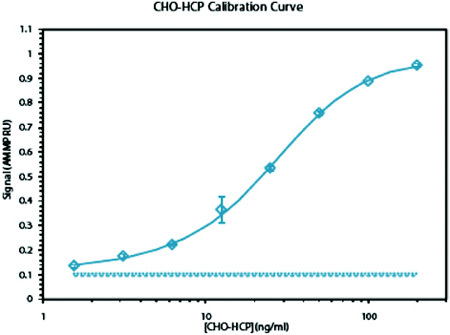 Figure 2: CHO-HCP AMMP Assay calibration curve
Figure 2: CHO-HCP AMMP Assay calibration curve
Figure 2 above is an example of a CHO HCP AMMP Assay calibration curve. Analysis can be performed in samples from across the purification stream including the reactor with high precision and reproducibility.
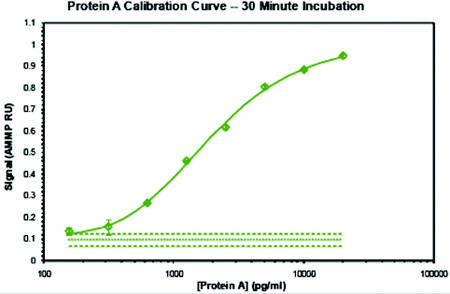 Figure 3: Protein A AMMP Assay calibration curve
Figure 3: Protein A AMMP Assay calibration curve
Figure 3 is a calibration curve for a Residual Protein A AMMP Assay, performed with a VIBE Residual Protein A Assay Kit. The same kit can be used for a faster assay with a 30 minute incubation period, or for more sensitive results and wider dynamic range with a longer incubation period without loss of precision or accuracy, both of which are typically better than equivalent ELISA's in users' hands
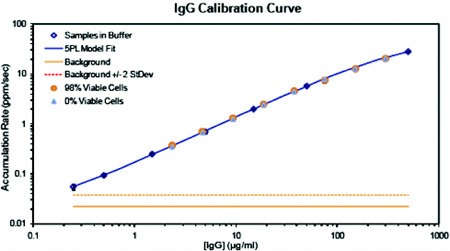 Figure 4: Product Titer AMMP Assay
Figure 4: Product Titer AMMP Assay
The ViBE Workstation can also be used for a Product Titer assay, results shown in figure 4. Performed differently than other AMMP Assays, this assay does not require sample treatment other than simple dilution, or beads, and quantitation has been verified in "no spin" samples containing over 10 million cells over a wide range of viabilities.
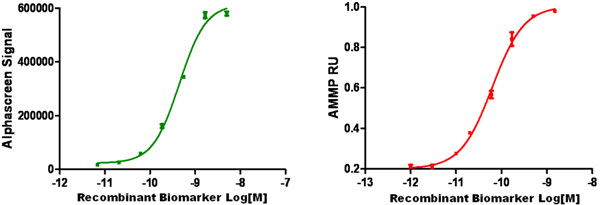 Figure 5: AMMP Assay standard curve for GST-Gadd34 biomarker assay
Figure 5: AMMP Assay standard curve for GST-Gadd34 biomarker assay
Shown in Figure 5 is a standard curve of signal obtained by the ViBE BioAnalyzer (Panel A) or AlphaScreen Technology using recombinant GST-Gadd34. Results on the ViBE platform were approximately a log more sensitive using the same antibodies. AMMP leverages the measurement of fewer larger particles yet still benefits from solution phase capture and detection complex formation.
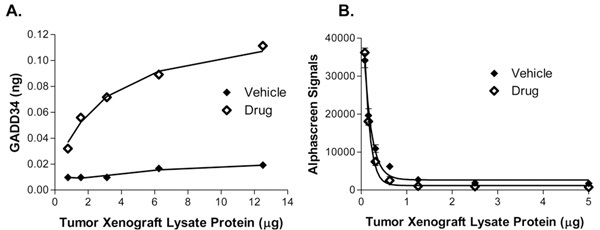 Figure 6: AMMP Assay standard curve for GST-Gadd34 in tumor sample
Figure 6: AMMP Assay standard curve for GST-Gadd34 in tumor sample
In Figure 6, the induction of Gadd34 from CWR22 xenografts was tested in the presence or absence of a boronate proteasome inhibitor as analyzed by either the ViBE BioAnalyzer (Panel A) or AlphaScreen (Panel B). The ViBE platform was able to produce sensitive, reproducible results in the in vivo tumor xenografts whereas the AlphaScreen signal was quenched with increased μg of protein.
This example shows the power of AMMP technology in highly complex samples. Due to the sensitive detection chip and high efficiency of the magnet near the chips, low numbers of magnetic microparticles can efficiently and reproducibly scavenge for low abundance target amidst the complexity of lysed cellular (including lysis reagents) and blood debris.
Discussion
The foregoing examples demonstrate AMMP's highly sensitive quantitative results in complex samples. This performance, when delivered with the ease and control of formatting reagents into the system is enabling for a variety of applications. Those applications may be specific for a target, as in Gadd34 in tumor, or generic for a spectrum of targets, as in host cell protein detection and quantitation in samples that cross the purification stream. The low overhead ViBE platform combined with highly flexible AMMP provides a powerful tool for delivering critical assays.
Disclosures
The authors of this article are employed by Bioscale, Inc that produces some of the instruments and reagents used in this article.


