Abstract
Background: In inflammatory bowel disease (IBD) number of thromboembolic events are increased due to hypercoagulupathy and platelet activation. Increases in mean platelet volume (MPV) can lead to platelet activation, this leads to thromboembolic events and can cause acute coronary syndromes. In IBD patients, QT-dispersion and P-wave dispersion are predictors of ventricular arrhythmias and atrial fibrilation; MPV is accepted as a risk factor for acute coronary syndromes, we aimed at evaluating the correlations of these with the duration of disease, its localization and activity.
Methods: The study group consisted of 69 IBD (Ulcerative colitis n: 54, Crohn's Disease n:15) patients and the control group included 38 healthy individuals. Disease activity was evaluated both endoscopically and clinically. Patients with existing cardiac conditions, those using QT prolonging medications and having systemic diseases, anemia and electrolyte imbalances were excluded from the study. QT-dispersion, P-wave dispersion and MPV values of both groups were compared with disease activity, its localization, duration of disease and the antibiotics used.
Results: The P-wave dispersion values of the study group were significantly higher than those of the control group. Duration of the disease was not associated with QT-dispersion, and MPV levels. QT-dispersion, P-wave dispersion, MPV and platelet count levels were similar between the active and in mild ulcerative colitis patients. QT-dispersion levels were similar between IBD patients and the control group. No difference was observed between P-wave dispersion, QT-dispersion and MPV values; with regards to disease duration, disease activity, and localization in the study group (p>0.05).
Conclusions: P-wave dispersion which is accepted as a risk factor for the development of atrial fibirilation was found to be high in our IBD patients. This demonstrates us that the risk of developing atrial fibrillation may be high in patients with IBD. No significant difference was found in the QT-dispersion, and in the MPV values when compared to the control group.
Keywords: Inflammatory bowel diseases, QT-dispersion, P-wave dispersion, mean platelet volume
Introduction
Crohn's disease and ulcerative colitis are the main entities of inflammatory bowel disease (IBD). They are systemic diseases, which frequently involve other organs with environmental, genetic, immune and microbial factors playing important roles in the etiology. More than 40% of patients with IBD have extraintestinal complications, involving almost every organ. Cardiac extraintestinal manifestations such as pericarditis, myocarditis, endocarditis, cardiomyopathy and complete heart block have been reported 1-4.
Corrected QT-interval and QT-dispersion indicate ventricular repolarization time and heterogeneity. Increased corrected QT and/or QT-dispersion are known to be the cause of ventricular arrhythmia in various systemic diseases and lead to increase in mortality and morbidity 5-7. Knorr et al. demonstrated ciprofloxacin-induced QT-interval prolongation in patients diagnosed with IBD 8. The reports regarding QT-interval and QT-dispersion in IBD with patients are inadequate.
P-wave dispersion is defined as the difference between the maximum and the minimum P-wave durations in 12-lead surface electrocardiograms. P-wave dispersion is considered to reflect the discontinuous and inhomogeneous propagation of sinus impulses, and the prolongation of atrial conduction time. Increased P-wave dispersion and maximum P-wave duration predict the development of atrial fibrillation in patients with various heart diseases 9,10. Sharma et al 11 reported that there was a significant decrease in parasympathetic function in patients with IBD. Coruzzi et al 12 demonstrated the presence of autonomic dysfunction in patients with IBD, and that there was a decrease in parasympathetic cardiac regulation particularly in cases with ulcerative colitis. But, there is inadequate data about P-wave dispersion. In IBD patients there is an increase in thromboembolic events due to increased hypercoagulopathy and thrombocyte activity. This is an important cause of mortality and morbidity. Incidence of the risk of thromboembolism ranges between 1.2% and 39%. Increased mean platelet volume (MPV) may cause platelet activation, which in turn leads to thromboembolic events and acute coronary syndrome 13,14.
Our aim in this study was to investigate the relationship of QT-interval, QT-dispersion; which are the predictors of ventricular arrhythmia; P-wave dispersion the predictor of atrial fibrillation and MPV accepted as a risk factor for acute coronary syndrome with respect to disease duration, type and activity, in IBD patients with undetermined cardiac and ischemic diseases.
Materials and Methods
Patients and control group
A total of 107 cases, between the ages of 18 and 71 were included in the study, which was conducted between June 2008 and June 2009. Written informed consent was obtained from all participants following the decision of the Local Ethics Committee. The study group consisted of 69 IBD patients and the control group included 38 healthy individuals. The IBD patients were allocated into two groups for evaluation, the ulcerative colitis group (n=54) and the Crohn's disease group (n=15). Among the IBD patients, those with ulcerative colitis were subdivided as distal, left colon and pancolonic types, whereas in the Crohn's disease group, patients with involvement of the colon were referred to as colonic while those with only terminal ileum involvement were referred to as ileum 15. There were no patients with simultaneous involvement of terminal ileum and colon. Cardiovascular risk factors of the patients and drugs used were recorded. Clinical activity of the disease was evaluated using the Truelove-Witts criteria in ulcerative colitis patients 16, and the Crohn's activity index in Crohn's disease patients 17.
Patients who developed severe anemia (Htc<30) from IBD were excluded from the study. Moreover, the patients with ischemia or structural heart disease, those who used QT-interval prolonging drugs, with systemic diseases like hypertension and diabetes mellitus, pregnant women, alcoholics, obese (BMI>30) and those with electrolyte imbalance, and patients with any form of arrhythmia and conduction defect, were also excluded from the study. The control group consisted of healthy individuals who did not have a history of medication use due to any diseases, who did not have any systemic diseases, heart diseases, cardiac rhythm problems or IBD and who did not have any abnormalities in their biochemical tests.
QT and P wave dispersion measurement
12-lead electrocardiograms (ECG) of all the patients were obtained at amplitude of 20mm/mV and a velocity of 50mm/s. The patients were subjected to manual ECG analyses by two cardiologists who were blinded for the study. QT-intervals were manually measured in all possible leads. The QT-interval was defined as the interval from the onset of the QRS complex to the end of the T wave, which was defined as its return to the T-P baseline. The measurements were carried out with a precision of 0.01 mm (0.4 ms). If the U wave was present, the QT-interval was measured to the nadir of the curve between the T and U waves.
QT-intervals were corrected by the Bazett's Formula to compensate for its known dependence on heart rate: QTc =QT / √RR. Measurements on QT and RR intervals were carried out in 3 consecutive cardiac cycles in all leads, and average values were obtained 6. QT-dispersion was determined as the difference between the maximal and minimal corrected QT-interval in different leads. The beginning of the P-wave was defined as the point where the initial deflection of the P-wave crossed the isoelectric line, and the end of the P-wave was defined as the point where the final deflection of the P-wave crossed the isoelectric line. The difference between maximum and minimum P-wave durations was defined as P-wave dispersion 6.
Echocardiography and blood tests
Transthoracic echocardiography measurements (Vivid 3, General Electric Medical systems, USA) using a 3-MHz transducer were performed on patients in both the study and control groups. Left ventricular ejection fraction, left ventricular systolic and diastolic diameters, septal and posterior wall thickness, right ventricular and atrial diameters, the E/A ratio, and pulmonary arterial pressure measurements were obtained by using the M-mode, 2-D, Color and Pulse-Continous Doppler. Blood samples were collected from the patients after a 12-hr overnight fasting. All routine biochemical tests were carried out on an autoanalyser. For the analysis of MPV, blood samples with K3 EDTA were analyzed after one hour of venipuncture by the Beckman Coulter-LH 780 analyzer (Beckman Coulter, U.S.A). The corrected QT, QT-dispersion, Pmax, Pmin, P-wave dispersion and MPV values of both groups were compared with disease duration, activities, localization, types and antibiotics administered.
Statistical analyses
The NCSS 2007&PASS 2008 Statistical Software (Utah, USA) program was used for statistical analyses. Definitive statistical methods (mean, standard deviation, frequency) were used alongside the One-way Anova test, and the Kruskal Wallis test for the comparison of data and quantitative data, respectively. Comparison of parameters was performed using the Student t test, Mann Whitney U test and the Chi-square test. The Pearson's correlation test was used to analyze the relationship between parameters. Results were evaluated at a 95% confidential interval and p<0.05 was considered as significant.
Results
Out of 107cases 54.2% were females and 45.8% were males. 21.7% of the IBD patients had Crohn's disease, whereas 78.3% had ulcerative colitis. Localization of the diseases was as follows: in the Crohn's disease group 66.6% were in the ileum while 33.3% were colonic. On the other hand, of the ulcerative colitis patients 42.5% had pancolitis, 33.3% had left colonic localization and 24% had distal colitis. Of all the IBD patients, 30.4% had severe disease, 33.3% were mild, while 36.2% were moderate. There was no difference between age, sex, cardiovascular risk factors, corrected QT, and QT-dispersion values between the IBD and the control group.
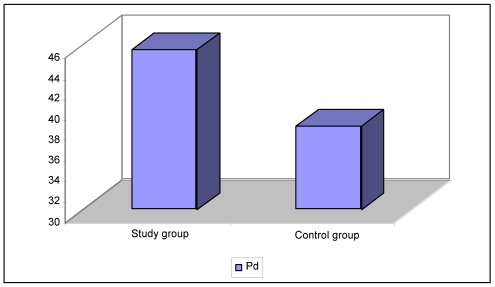
Comparison of the P-wave variability parameters of both groups demonstrated that there was no significant difference between the Pmax and Pmin values (p: 0.166, p: 0.472 respectively); The P-wave dispersion levels of IBD patients were higher than those of the control group (p: 0.027) (Table 1, Figure 1). The MPV, hematocrit, platelet count and leucocyte count were similar between the two groups (p>0.05) (Table 1). There was no difference between the IBD types, disease duration, localization, activity status, and the Pmax, Pmin, P-wave dispersion, QT-dispersion, corrected QT and MPV levels (p>0.05) (Table 2). Moreover, the parameters did not differ in correlation with the localizations of ulcerative colitis and Crohn disease groups among themselves (Table 3, 4). No relationship was found between the echocardiographic parameters ejection fraction, left ventricular, left atrium, right atrium and right ventricular diameters, and the E/A ratio and pulmonary arterial pressure of the study and control groups (p>0.05).
Table 1.
Group evaluation
| Study group (n=69) |
Control group (n=38) |
p | |
|---|---|---|---|
| Mean±SD | Mean±SD | ||
| Age LVEF (%) Pmax (ms) |
39.69±13.21 64.10±5 102.75±22.10 |
41.59±13.12 65.13±4 97.89±13.59 |
0.456 0.158 0.163 |
| Pmin (ms) | 56.95±20.88 | 59.73±15.15 | 0.472 |
| Pd (ms) | 45.50±16.98 | 38.15±14.86 | 0.027* |
| QTd (ms) | 76.68±31.99 | 79.92±28.31 | 0.603 |
| QTc (ms) | 412.01±35.97 | 413.60±21.83 | 0.777 |
| WBC | 7.65±2.32 | 7.47±1.93 | 0.235 |
| Hct | 39.88±4.56 | 39.60±4.09 | 0.702 |
| MPV | 8.51±1.34 | 8.56±0.83 | 0.584 |
| Plt | 291.67±78.73 | 266.39±55.23 | 0.085 |
The Student t test was used. * p<0.05; NS: None-significant
LVEF=Left ventricular ejection fraction; Pmax=Maximum P-wave duration; Pmin=Minimum P-wave duration; Pd=P-wave dispersion; QTd=corrected QT dispersion; QTc=corrected QT interval; WBC=White blood cells; Hct=hematocrit; MPV=mean platelet volume; Plt=platelet count
Figure 1.
Pd; P wave dispersion
Table 2.
Evaluation of parameters according to CD and UC disease types
| Study group | CD(n=15) | UC(n=54) | p |
|---|---|---|---|
| Mean±SD | Mean±SD | ||
| Pmax (ms) | 111.00±31.40 | 100.46±18.48 | 0.232 |
| Pmin (ms) | 61.67±31.26 | 55.65±17.13 | 0.484 |
| Pd (ms) | 48.66±12.02 | 44.63±18.11 | 0.419 |
| QTd (ms) | 76.13±35.32 | 76.83±31.37 | 0.941 |
| QTc (ms) | 409.93±27.44 | 412.59±38.21 | 0.802 |
| MPV | 8.28±1.38 | 8.58±1.34 | 0.454 |
The Student t test was used.
UC;Ulcerative colitis; CD;Crohn's disease; Pmax=Maximum P-wave duration; Pmin=Minimum P-wave duration; Pd=P-wave dispersion; QTd=corrected QT dispersion; QTc=corrected QT interval; MPV=mean platelet volume
Table 3.
Involvement related evaluations in UC patients
| UC Patients | Pancolitis (n=23) | Left Colon (n=18) | Distal Colitis (n=13) | p |
|---|---|---|---|---|
| Mean±SD | Mean±SD | Mean±SD | ||
| Pmax | 97,60±21,20 | 107,22±15,64 | 96,15±15,29 | 0,161 |
| Pmin | 56,08±17,58 | 57,78±14,77 | 51,92±19,95 | 0,644 |
| Pd | 41,52±16,68 | 48,89±22,98 | 44,23±12,04 | 0,440 |
| QTd | 80,48±30,23 | 78,17±27,33 | 68,53±38,86 | 0,544 |
| QTc | 411,95±33,54 | 412,05±41,23 | 414,46±44,32 | 0,980 |
| MPV | 8,19±0,94 | 8,72±1,50 | 9,05±1,59 | 0,157 |
Oneway ANOVA test was used.
UC;Ulcerative colitis; Pmax=Maximum P-wave duration; Pmin=Minimum P-wave duration; Pd=P-wave dispersion; QTd=corrected QT dispersion; QTc=corrected QT interval; MPV=mean platelet volume
Table 4.
Involvement related evaluations in CD patients
| CD patients | Ileum (n=10) | Colonic (n=5) | p |
|---|---|---|---|
| Mean±SD (Median) | Mean±SD (Median) | ||
| Pmax | 111,50±34,32 (110) | 110,00±28,28 (100) | 0,901 |
| Pmin | 62,50±32,42 (50) | 60,00±32,40 (40) | 0,491 |
| Pd | 49,00±13,09 (50) | 48,00±10,95 (40) | 0,851 |
| QTd | 79,50±35,53 (70) | 69,40±37,96 (56) | 0,581 |
| QTc | 409,00±25,64 (410) | 411,80±33,89 (396) | 0,806 |
| MPV | 8,60±1,55 (8,17) | 7,63±0,70 (7,60) | 0,198 |
Mann Whitney U test was used
CD;Crohn's disease; Pmax=Maximum P-wave duration; Pmin=Minimum P-wave duration; Pd=P-wave dispersion; QTd=corrected QT dispersion; QTc=corrected QT interval; MPV=mean platelet volume
Discussion
IBD is a systemic disease associated with extraintestinal manifestations, complications and other autoimmune disorders 18,19. The incidence of extraintestinal manifestations ranges between 6%-47% 20-23. Although concomitance of IBDs with cardiac disorders is rare, sporadic cases of complication with pericardial effusion, pericarditis, myocarditis, endocarditis, cardiomyopathy, thromboembolic events, conduction defects and drug use have been reported 3,24. On the other hand, early atherosclerosis and thromboembolic events, and as a consequence cardiovascular mortality are known to increase in cases with IBD 25-28.
There is insufficient data concerning complications such as electrocardiographic abnormalities, sudden death, ventricular tachycardia, and atrial fibrillation in patients with IBD. Atrial fibrillation is a common type of arrhythmia; associated with complications such as thromboembolism, heart failure, and cardiomyopathy. Increase in P-wave dispersion is an indication of the heterogeneity of intra-atrial and inter-atrial conduction and of anisotropic propagation of sinus impulses. P-wave duration and P-wave dispersion are the most important ECG markers used to evaluate the risk of atrial arrhythmias 9, 29.
Various studies have demonstrated that P-wave dispersion has a predictive value for atrial fibrillation, in cardiac and some noncardiac disorders. In the study by Dilaveris et al 30, the sensitivity and specificity for P-wave dispersion in paroxysmal idiopathic atrial fibrillation was found to be 83% and 85%, respectively. In a study by Dogan et al 31, on Behçet's disease and on rheumatoid arthritis by Guler et al 32 and Yavuzkır et al 33, increase in maximum P-wave duration and P-wave dispersion were reported. In this study, the mean values of the patient and control group were, Pmax: 102.75±22.10 vs 97.89±13.59, Pmin: 56.95±20.88 vs 59.73±15.15 and P-wave dispersion: 45.50±16.98 vs 38.15±14.86, respectively. Results of our study demonstrated that there was no increase in the maximum P-wave duration of patients with IBD when compared to the control group. However, increase in P-wave dispersion was reported, suggesting that there was no association with the type, duration, activity and extent of the disease. In our study, in the IBH group, as a predictor of atrial fibrillation predicted P-wave dispersion value was found to be high as was the case in other studies. Some studies have been published concerning the controversial association of autonomic dysfunction in patients with IBD. In their study, Sharma et al 11 and Coruzzi et al 12 reported that there was a significant decrease in parasympathetic function in patients with IBD.
Autonomic dysfunction has been suggested in various studies to be associated with local subclinical inflammation. Symptoms of cardiac autonomic dysfunction (like orthostatic hypotension, exercise intolerance, silent myocardial ischemia, etc.) were not reported in our study group. There was no organic cardiac involvement (myocarditis, pericarditis, cardiomyopathy, etc.) in our patients, and there was no reported use of medication, which could lead to arrhythmia. Notwithstanding, we are of the opinion that decrease in parasympathetic activity; result of the effect of autonomic dysfunction on local subclinical inflammation may play a role in the P-wave dispersion. These results demonstrate that there is a risk of atrial fibrillation in patients with IBD. Various studies have demonstrated that increased QT-interval and QT-dispersion may increase the incidence of sudden death and ventricular tachycardia in many cardiac and noncardiac diseases (diabetes mellitus, systemic lupus erythematosus, sarcoidosis, Behçet's disease, hypertension, hypertrophic cardiomyopathy), indication that it plays an important prognostic and clinical role 34-40. In our study, corrected QT-interval and QT-dispersion were not found to be significant in the study group and the control group with respect to disease duration, type, and activity (p=0.777 and p=0.603, respectively). Curione et al 41 found corrected QT-interval and QT-dispersion to be high in the IBD group. They reported the necessity to follow-up these QT changes in instances of electrolyte imbalances and in patients using cardiotoxic medications like infliximab and they stated that these should not go unreported. By taking these instances into consideration, the patients in our study group were selected from among those patients who did not have a history of using cardiotoxic and QT -interval modifying drugs and who did not have any electrolyte imbalances. Knorr et al 8 demonstrated ciprofloxacin-induced QT-interval prolongation in patients diagnosed with IBD. Patients in our study group didn't use ciprofloxacin and the other medications which could induce QT-interval prolongation. No electrolyte imbalance, myocarditis, pericarditis and cardiomyopathy were observed. No studies have been documented on the association of autonomic dysfunction with corrected QT and QT-dispersion, in patients with IBD. Results of our study demonstrated that there is no heterogeneity of cardiac involvement and ventricular repolarization in patients with IBD.
Kapsoritakis et al 13 reported an increase in platelet count and a decrease in the MPV in patients with IBD. Platelet activation, increases in MPV and thromboembolic events have been reported to play important roles in the development and prognosis of acute coronary syndrome. Although the MPV values of our study group were lower than those in the control group, no significant relationship was demonstrated with regards to the type, duration, activity and the extend of the disease. There are also studies showing that the decreases in MPV demonstrated an increase in IBD activity 42 and have not found this parameter useful in differentiating disease activity 43 as has been the case in our study. Unlike acute coronary syndrome in IBD patients, the low MPV levels in our study as well as in other studies, when compared to the control group, suggests that the MPV, which is recognized as a risk factor for acute coronary syndrome in IBD patients may not be a risk factor on its own.
Limitations of our study were P-wave and QT-interval measurements were performed using a magnifying lens instead of by computer-assisted calculations. However, thermal, digital, and signal-averaging ECG systems were used to evaluate P-wave dispersion, which was measured manually either on paper or on a high-resolution computer screen. Our study had a small patient population; we suggest that extensive studies may be conducted with a large study population, holter recordings and autonomic dysfunction tests.
In conclusion, P-wave dispersion was not found to be associated with the duration, activity and extent of the disease, and was found to be significantly high compared to the control group. This demonstrates that the risk of developing atrial fibrillation may be high in patients with IBD. However, no significant difference was found in the corrected QT and QT-dispersion intervals, and in the MPV values when compared to the control group, indicating that the probability of ventricular arrhythmia or thromboembolism and/or acute coronary syndrome may be low. It should be noted that simple, noninvasive, easy to use, easily accessible ECG can be used to determine the risk of atrial fibrillation in patients with IBD.
Abbreviations
- IBD
Inflammatory bowel disease, MPV: Mean platelet volume, ECG: Electrocardiograms.
References
- 1.Rothfusss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol. 2006;12:4819–31. doi: 10.3748/wjg.v12.i30.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams H, Walker D, Orchard TR. Extraintestinal manifestations of inflamatory bowel diseases. Curr Gastroenterol. 2008;10:597–605. doi: 10.1007/s11894-008-0108-6. [DOI] [PubMed] [Google Scholar]
- 3.Stasinopoulou P, Kaziani A, Mantzaris G. et al. Paralel manifestation of Crohn's disease and acute pericarditis: a report of two cases. Int J Colorectal Dis. 2007;22:1123–25. doi: 10.1007/s00384-007-0327-6. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein CN, Blanchard JF, Rawsthorne P. et al. The prevalance of extraintestinal diseases in inflammatory bowel diseaese; a population-based study. Am J Gastroenterol. 2001;96:1116–22. doi: 10.1111/j.1572-0241.2001.03756.x. [DOI] [PubMed] [Google Scholar]
- 5.Dogru MT, Gunerı M, Tırelı E. et al. QT interval and dispersion differences between normal and prehypertensive patients: effects of autonomic and left ventricular functional and structural changes-Original Investigation. The Anatolian Journal of Cardiology. 2009;9:15–22. [PubMed] [Google Scholar]
- 6.Guntekın U, Gunes Y, Tuncer M. et al. The Effect of Altitude on P-Wave and QT Duration and Dispersion. Pacing Clin Electrophysiol. 2008;31:889–892. doi: 10.1111/j.1540-8159.2008.01104.x. [DOI] [PubMed] [Google Scholar]
- 7.Straus SM, Kors JA, De Bruin ML. et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–67. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 8.Knorr JP, Moshfeghi M, Sokoloski MC. Ciprofloxacin-induced Q-T interval prolongation. Am J Health Syst Pharm. 2008;65:547–51. doi: 10.2146/ajhp070081. [DOI] [PubMed] [Google Scholar]
- 9.Kosar F, Aksoy Y, Arı F. et al. P-Wave Duration and Dispersion in Obese Subjects. Ann of Noninvasive Electrocardiol. 2008;13:3–7. doi: 10.1111/j.1542-474X.2007.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozer N, Aytemır K, Atalar E. et al. P wave dispersion in hypertensive patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2000;23:1859–62. doi: 10.1111/j.1540-8159.2000.tb07038.x. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Makharia GK, Ahuja V. et al. Autonomic Dysfunctions in Patients with Inflammatory Bowel Disease in Clinical Remission. Dig Dis Sci. 2009;54:853–61. doi: 10.1007/s10620-008-0424-6. [DOI] [PubMed] [Google Scholar]
- 12.Coruzzi P, Castiglioni P, Parati G. et al. Autonomic cardiovascular regulation in quiescent ulcerative colitis and Crohn's disease. Eur J Clin Invest. 2007;37:964–70. doi: 10.1111/j.1365-2362.2007.01887.x. [DOI] [PubMed] [Google Scholar]
- 13.Kapsoritakis AN, Koukourakis MI, Sfiridaki A. et al. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol. 2001;96:776–81. doi: 10.1111/j.1572-0241.2001.03621.x. [DOI] [PubMed] [Google Scholar]
- 14.Danese S, Motte Cd Cde L, Fiocchi C. Platelets in inflammatory bowel disease: clinical, pathogenic, and therapeutic implications. Am J Gastroenterol. 2004;99:938–45. doi: 10.1111/j.1572-0241.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- 15.Ricart E, Panaccione R, Loftus EV Jr. et al. Autoimmune disorders and extraintestinal manifestations in first-degree familial and sporadic inflammatory bowel disease: a case-control study. Inflamm Bowel Dis. 2004;10:207–14. doi: 10.1097/00054725-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Truelove SC, Witts LJ. Cortisone in ulcerative colitis: Final report on a therapeutic trial. BMJ. 1955;2:1041. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Best WR, Becktel JM, Singleton JM. et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439. [PubMed] [Google Scholar]
- 18.Danese S, Semeraro S, Papa A. et al. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005;11:7227–36. doi: 10.3748/wjg.v11.i46.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novacek G, Haumer M, Schima W. et al. Aortic mural thrombi in patients with inflammatory bowel disease: report of two cases and review of the literature. Inflamm Bowel Dis. 2004;10:430–35. doi: 10.1097/00054725-200407000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol. 1996;23:29–34. doi: 10.1097/00004836-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Das KM. Relationship of extraintestinal involvements in inflammatory bowel disease: new insights into autoimmune pathogenesis. Dig Dis Sci. 1999;44:1–13. doi: 10.1023/a:1026629528233. [DOI] [PubMed] [Google Scholar]
- 22.Oshitani N, Watanabe K, Nakamura S. et al. Extraintestinal complications in patients with ulcerative colitis. Nippon Rinsho. 2005;63:874–78. [PubMed] [Google Scholar]
- 23.Mendoza JL, Lana R, Taxonera C. et al. Extraintestinal manifestations in inflammatory bowel disease: differences between Crohn's disease and ulcerative colitis. Med Clin (Barc) 2005;125:297–300. doi: 10.1157/13078423. [DOI] [PubMed] [Google Scholar]
- 24.Bansal D, Chahoud G, Ison K. et al. Pleuropericarditis and pericardial tamponade associated with inflammatory bowel disease. J Ark Med Soc. 2005;102:16–9. [PubMed] [Google Scholar]
- 25.Dorn SD, Sandler RS. Inflammatory Bowel Disease ıs not a rısk factor for cardiovascular disease mortality: Results from a Systematic Review and Meta-Analysis. Am J Gastroenterology. 2007;102:662–67. doi: 10.1111/j.1572-0241.2006.01018.x. [DOI] [PubMed] [Google Scholar]
- 26.Papa A, Santoliquido A, Danese S. et al. Increased carotid intima-media thickness in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2005;22:839–46. doi: 10.1111/j.1365-2036.2005.02657.x. [DOI] [PubMed] [Google Scholar]
- 27.Efremidis M, Prappa E, Kardaras F. Acute myocardial infarction in a young patient during an exacerbation of ulcerative colitis. Int J Cardiol. 1999;70:211–12. doi: 10.1016/s0167-5273(99)00084-4. [DOI] [PubMed] [Google Scholar]
- 28.Mutlu B, Ermeydan CM. et al. Acute myocardial infarction in a young woman with severe ulcerative colitis. Int J Cardiol. 2002;83:183–85. doi: 10.1016/s0167-5273(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 29.Turgut O, Tandogan I, Yilmaz MB. et al. Association of P wave duration and dispersion with the risk for atrial fibrillation: Practical considerations in the setting of coronary artery disease. Int J Cardiol. 2010;144:322–24. doi: 10.1016/j.ijcard.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Dilaveris PE, Gialafos EJ, Sideris SK. et al. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J. 1998;135:733–38. doi: 10.1016/s0002-8703(98)70030-4. [DOI] [PubMed] [Google Scholar]
- 31.Dogan SM, Aydın M, Gursurer M. et al. The increase in P-wave dispersion is associated with the duration of disease in patients with Behçet's disease. Int J Cardiol. 2008;124:407–10. doi: 10.1016/j.ijcard.2006.12.087. [DOI] [PubMed] [Google Scholar]
- 32.Guler H, Seyfelı E, Sahın G. et al. P wave dispersion in patients with rheumatoid arthritis: its relation with clinical and echocardiographic parameters. Rheumatol Int. 2007;27:813–18. doi: 10.1007/s00296-007-0307-8. [DOI] [PubMed] [Google Scholar]
- 33.Yavuzkır M, Ozturk A, Daglı N. et al. Effect of ongoing inflammation in rheumatoid arthritis on P-wave dispersion. J Int Med Res. 2007;35:796–802. doi: 10.1177/147323000703500608. [DOI] [PubMed] [Google Scholar]
- 34.Uchiyama K, Hayashi K, Fujino N. et al. Impact of QT Variables on Clinical Outcome of Genotyped Hypertrophic Cardiomyopathy. Ann Noninvasive Electrocardiol. 2009;14:65–71. doi: 10.1111/j.1542-474X.2008.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurgun C, Ercan E, Ceyhan C. et al. Cardiovascular involvement in Behçet's disease. Jpn Heart J. 2002;43:389–98. doi: 10.1536/jhj.43.389. [DOI] [PubMed] [Google Scholar]
- 36.Ziegler D, Zentai CP, Perz S. et al. KORA Study Group. Prediction of mortality using measures of cardiac autonomic dysfunction in the diabetic and nondiabetic population: the MONICA/KORA Augsburg Cohort Study. Diabetes Care. 2008;31:556–61. doi: 10.2337/dc07-1615. [DOI] [PubMed] [Google Scholar]
- 37.Psallas M, Tentolouris N, Cokkinos A. et al. QT dispersion: comparison between diabetic and non-diabetic individuals and correlation with cardiac autonomic neuropathy. Hellenic J Cardiol. 2006;47:255–62. [PubMed] [Google Scholar]
- 38.Yavuz B, Atalar E, Karadag O. et al. QT dispersion increases in patients with systemic lupus erythematosus. Clin Rheumatol. 2007;26:376–79. doi: 10.1007/s10067-006-0364-5. [DOI] [PubMed] [Google Scholar]
- 39.Uyarel H, Uslu N, Okmen E. et al. QT Dispersion in Sarcoidosis. Chest. 2005;128:2619–25. doi: 10.1378/chest.128.4.2619. [DOI] [PubMed] [Google Scholar]
- 40.Dimopoulos S, Nicosia F, Donati P. et al. QT Dispersion and Left Ventricular Hypertrophy in Elderly Hypertensive and Normotensive Patients. Angiology. 2008;59:605–12. doi: 10.1177/0003319707310276. [DOI] [PubMed] [Google Scholar]
- 41.Curione M, Aratari A, Amato S. et al. A study on QT interval in patients affected with inflammatory bowel disease without cardiac involvement. Intern Emerg Med. 2010;5:307–10. doi: 10.1007/s11739-010-0382-9. [DOI] [PubMed] [Google Scholar]
- 42.Yüksel O, Helvaci K, Başar O. et al. An overlooked indicator of disease activity in ulcerative colitis: mean platelet volume. Platelets. 2009;20:277–81. doi: 10.1080/09537100902856781. [DOI] [PubMed] [Google Scholar]
- 43.Zubcevic N, Mesihovic R, Zubcevic S. Usefulness of laboratory data in estimation of Crohn's disease activity. Med Arh. 2010;64:33–6. [PubMed] [Google Scholar]