Abstract
Zoonoses as causes of human infections have been increasingly reported, and many of these are viruses that cause central nervous system infections. This paper focuses on the henipaviruses (family Paramyxoviridae, genus henipavirus) that have recently emerged to cause severe encephalitis and systemic infection in humans and animals in the Asia-Pacific region. The pathological features in the human infections comprise vasculopathy (vasculitis, endothelial multinucleated syncytia, thrombosis, etc.) and parenchymal cell infection in the central nervous system, lung, kidney, and other major organs. Most animals naturally or experimentally infected show more or less similar features confirming the dual pathogenetic mechanism of vasculopathy-associated microinfarction and direct extravascular parenchymal cell infection as causes of tissue injury. The most promising animal models include the hamster, ferret, squirrel monkey, and African green monkey. With increasing evidence of infection in the natural hosts, the pteropid bats and, hence, probable future outbreaks in many more countries, a greater awareness of henipavirus infection in both humans and animals is imperative.
1. Zoonotic Viruses Associated with Viral Encephalitis
Numerous emerging infections are zoonoses of known or newly discovered viruses that have jumped the species barrier to infect humans. These include the human immunodeficiency virus (HIV), arboviruses, lyssavirus, henipaviruses, avian, and swine influenza viruses [1–8]. Many of these zoonotic viruses cause severe encephalitis associated with significant mortality and morbidity.
Since its origin has been traced to African nonhuman primates, HIV has become established in human populations [1]. The prevalence of HIV encephalitis is unknown; perhaps hundreds of thousands suffer from this condition since millions of HIV-infected patients still do not have adequate antiretroviral therapy. Among the arboviruses, Japanese encephalitis virus (JEV) infections, transmitted by mosquitoes from birds, is probably the most important, with more than 50,000 patients from the Indian subcontinent and southeast Asia [5]. West Nile virus, another known, similarly transmitted arbovirus, recently emerged to cause human neuroinvasive disease in North America, a region not previously known to be affected [9, 10].
Henipavirus genus, a recently established group of paramyxoviruses [11] comprising the Hendra virus (HEV) and Nipah virus (NIV), has emerged to cause severe encephalitis in humans and animals. There are several previous reviews on NiV or henipavirus infections [12–17], but the present one focuses on the epidemiology, clinical features, and comparative pathology in infected humans and animals and also includes some previously unpublished data.
HeV was first isolated after an outbreak in horses and 2 humans in the town of Hendra, QLD, Australia in 1994. Since then several other small outbreaks involving horses only, or horses and their carers, have been reported only in Australia and mainly in Queensland. Scores of horses and 7 humans (4 fatalities) have been infected so far [17–24]. NiV was named after the Nipah River village in Malaysia, very soon after the first known outbreak occurred mainly around pig farms from 1989 to 1999. Although a prevalence of 265 Malaysian cases of acute NiV encephalitis with 105 fatalities has been reported [25], the subsequent spread of the virus to Singapore and its ability to cause mild infections [26] suggested that the total number infected was probably more than 350 cases [14]. After the outbreak was controlled in Malaysia and Singapore in1999, at the beginning of 2001, several recurrent NiV outbreaks were reported from Bangladesh and the adjacent Bengal area of India [27, 28] that have involved more than 120 people thus far.
2. Henipavirus Transmission
The natural host of henipaviruses is the fruit bat (Pteropus species or “flying foxes”) [29–31], and bat-to-human transmission may be direct or indirect via intermediate hosts. The horse is the main if not the only intermediate host for HeV transmission [18, 23, 24]. Numerous other domestic animals and wildlife investigated were negative for naturally acquired HeV infection [21]. Contact with virus in horse oronasal secretions and urine appears to be the most likely route of transmission [32, 33]. Although person-to-person HeV transmission has not been reported, involvement of the lung and kidney in acute infection and presence of virus in nasopharyngeal secretions strongly suggest this possibility. The natural mode of bat-to-horse transmission remains unclear and unproven experimentally [32]. It was suggested that ingestion of feed or pasture contaminated by bat-derived foetal tissues or urine may be responsible.
In the Malaysia/Singapore outbreak, the pig was the main intermediate host and human transmission was strongly linked to close contact with pigs or fresh pig products [25, 34–37]. Massive culling of sick pigs and banning of exports stopped the epidemic [36, 38]. Similar to HeV, demonstration of virus in oropharyngeal/respiratory secretions suggests spread by either direct contact or aerosols [39, 40]. In contrast, absence of virus in pig urine could indicate that spread via urine may be inefficient. It was suggested that bat-to-pig transmission could have resulted from ingestion of half-eaten contaminated fruits dropped by bats near farms [29].
Person-to-person transmission in the Malaysian hospital setting is probably very low, but a nurse could have been infected from patients' tracheal secretions or urine [41–44]. There is no documentation of such transmission among and between farm workers and their families, but this remained a distinct possibility. In contrast, in the Bangladesh/India outbreaks, there was a high incidence of person-to-person transmission involving health care workers or other people [28, 45, 46]. No animals have been positively identified as intermediate hosts although there were associations with sick cows, pigs, and goats [45, 47] Bat-contaminated, date palm sap drunk raw as a local delicacy has been implicated in some cases of bat-to human transmissions in Bangladesh [48].
3. Clinical Aspects of Henipavirus Infection
The incubation period ranges from a few days to 2 weeks [19, 23, 24, 49, 50]. Milder symptoms include fever, headache, influenza-like illness, and drowsiness. Severe HeV infection, may present either as a neurological or a pulmonary syndrome, but since there have been very few patients, the clinical features were not well characterized. Neurological signs include confusion, motor deficits and seizures while the pulmonary syndrome presents with an influenza-like illness, hypoxaemia, and diffuse alveolar shadowing in chest X-Rays [23, 24]
Severe NiV encephalitic syndrome presents mainly with fever, headache, dizziness, vomiting, and reduced consciousness [50]. Clinical signs such as areflexia, hypotonia, abnormal pupillary and doll's eye reflex, tachycardia, hypertension, myoclonus, meningism, and convulsions were observed. A pulmonary syndrome has been described in some patients who present with cough, atypical pneumonia, and abnormal chest X-Ray findings [49–51]. Brain MR scans in acute henipavirus encephalitis show typical, disseminated, small discrete hyperintense lesions in both grey and white matter [23, 52, 53].
Specific antihenipavirus antibodies that can be detected in the serum and cerebrospinal fluid (CSF) in most patients are critical to diagnosis. More is known about seroconversion after NiV infection than HeV infection. In NiV infection, IgM seroconversion by about 2 weeks was 100% and persisted for more than 3 months. IgG seroconversion was 100% by about 3 weeks and may persist for several years [54, 55]. Specific neutralizing IgM or IgG antibodies have been reported in HeV-infected patients [19, 20, 24].
CSF examination showed elevated protein levels and/or white cell counts in more than 75% of NiV patients, but glucose levels were normal [50, 56]. Electroencephalography most commonly showed continuous, diffuse, symmetrical slowing with or without focal discharges in acute NiV encephalitis [57].
Mortality in HeV infection is about 50%, while in severe NiV infection it ranges from about 40% (Malaysia) to 70% (Bangladesh/India) [25, 27, 28]. In acute NiV encephalitis, brainstem involvement, presence of virus in the CSF, and diabetes mellitus are poor prognostic indicators [50, 58, 59]. The majority of Malaysian patients apparently recovered with no serious sequelae. However, henipavirus infection may be complicated by relapsing encephalitis after initial recovery. One case of relapsing HeV encephalitis and more than 20 cases of relapsing NiV encephalitis (probably <10% of survivors) have been reported thus far [20, 26]. The single case of relapsing HeV encephalitis occurred about 13 months after exposure, while an average of 8 months elapsed before relapsing NiV encephalitis occurred. Some cases of relapsing NiV encephalitis only had fever and headache during the acute phase and have also been called “late-onset” encephalitis. Clinical, radiological, and pathological findings suggest that relapsing NiV encephalitis is distinct from acute NiV encephalitis and that relapsing henipavirus encephalitis is the result of viral recrudescence [13, 26, 52, 60].
4. Pathology of Acute Henipavirus Infection in Humans
Although published data on HeV infection consists of a single case and most of the information on human henipavirus infection is derived from NiV studies, we believe both viruses cause essentially the same pathology. Acute infection is characterized by disseminated small vessel vasculopathy comprising true vasculitis, endothelial ulceration, and intramural necrosis in the central nervous system (CNS), lung, kidney, and many other major organs (Figure 1) [60, 61]. Occasionally, endothelial multinucleated giant cells or syncytia may be detected (Figure 1(b)). Vascular occlusion by vasculitis-induced thrombosis (Figure 1(a)) and perivascular haemorrhage were observed. Viral antigens, RNA, and nucleocapsids could be detected in vascular endothelium, multinucleated giant cells, and smooth muscle [61, 62].
Figure 1.
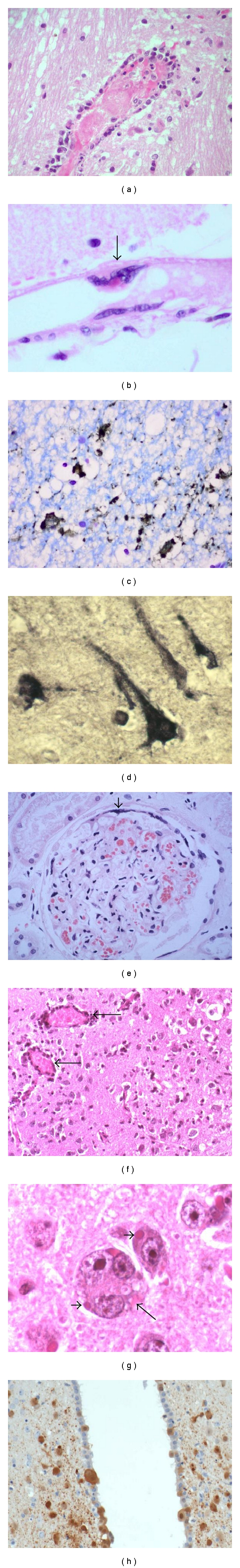
Pathology of human and hamster henipavirus infection. (a) Vasculitis and associated intravascular thrombosis in human brain. (b) In an uninflamed meningeal vessel, a multinucleated giant cell (arrow) with viral inclusion arises from the endothelial surface. (c) Neuronal viral antigens in human Nipah infection. (d) Neuronal viral RNA in human Hendra infection. (e) Glomerulus in human Nipah infection with thrombosis, necrosis, and peripheral multinucleated giant cell formation (arrowhead). (f) Mild vasculitis (arrows) and encephalitis in Nipah-infected hamster brain. (g) Viral inclusions in neurons (arrowheads) and the rare neuronal syncytia (arrow) in Nipah-infected hamster brain. (h) Nipah viral antigens in neurons and ependymal cells in infected hamster. (h and e) stains (a, b, e, f, g), immunoperoxidase stains (c, h), in situ hybridisation (d). Magnification, objective ×20 (a, c, f, h), ×40 (b, d, e, g).
In NiV infection, CNS vasculopathy was most severe compared to other organs. Vasculopathy was often associated with discrete necrotic or more subtle vacuolar plaque-like lesions that corresponded with lesions seen in the MR scans. These lesions were characterized by necrosis, oedema, and inflammation, and often viral antigens (Figure 1(c)) and RNA (Figure 1(d)) were demonstrable in adjacent neurons [60]. Hence, it is believed that both microinfarction and neuronal infection give rise to necrotic plaques. In some cases, focal neuronophagia, microglial nodule formation, clusters of foamy macrophages, perivascular cuffing, and meningitis can be found. A more extensive review of the CNS pathology has been published elsewhere [13]. In the lung, kidney (Figure 1(e)), lymphoid organs, and so forth, vasculopathy, parenchymal inflammation, and necrosis with occasional multinucleated giant cells were also observed [60, 61].
5. Pathology of Acute Henipavirus Infection in Animals
Consistent with in vitro experiments that showed extensive infectivity of henipaviruses in different cell lines [63], natural or in vivo experimental infections on a variety of mammalian species have been reported. The table summarises these findings and is organized on the assumption that henipaviruses as a group probably causes similar pathology in the same animal species. We are aware there may yet be differences between HeV and NiV infections in the same animal, but to date there is no published study that directly compares these viruses under identical experimental conditions.
Animals naturally infected by henipaviruses and whose tissues have been examined for pathological changes are few and include the dog, cat, horse, and pig [32, 40, 66, 84]. Hooper et al. described pulmonary inflammation and glomerular and tubular necrosis associated with syncytia formation in NiV-infected dogs [67]. We examined two naturally infected dogs and found pulmonary vasculitis (Figure 2(a)), alveolar oedema, and inflammation (unpublished data). In the kidney, many glomeruli and adjacent tubules were thrombosed or necrotic with varying degrees of inflammation (Figure 2(b)). Viral antigens and RNA were demonstrated (Figure 2(c)). Serological studies confirmed that the dog is susceptible to NiV infection [71], but susceptibility to HeV was inconclusive [21, 68]. Nonsuppurative meningitis, cerebral ischaemia and vasculopathy have been described, but there is no published data on direct neuronal infection [67].
Figure 2.
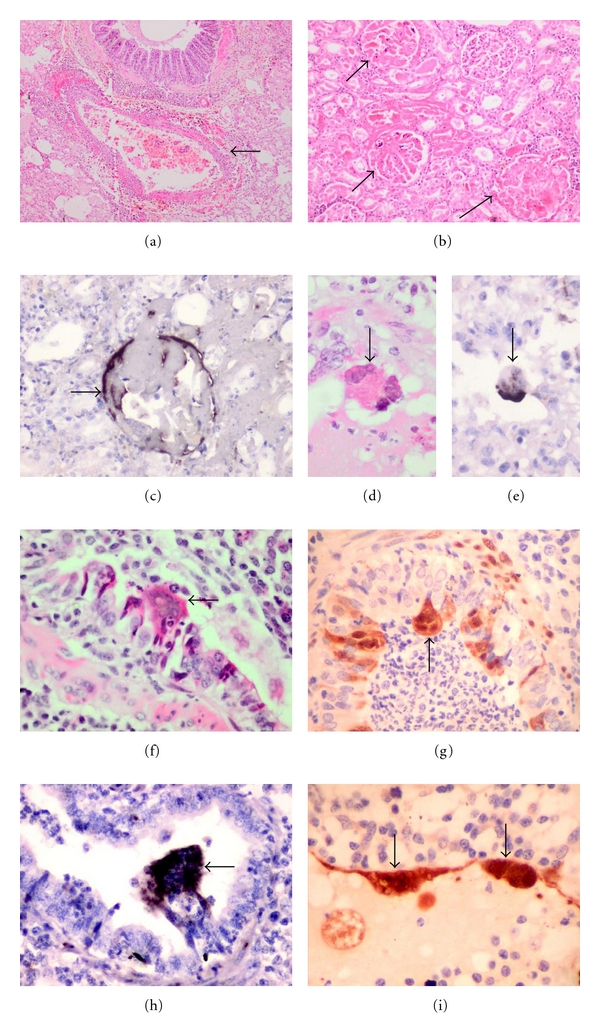
Pathology of dog and pig henipavirus infection. (a) Pulmonary vasculitis (arrow) and oedema in the Nipah-infected dog lung. (b) Glomerular (arrow) and tubular necrosis in the dog kidney. (c) Nipah viral RNA in dog glomerulus (arrow). Intra-alveolar multinucleated giant cell containing Nipah viral inclusions (d) (arrow) and viral RNA (e) (arrow). Bronchiolar syncytia (f) (arrow), viral antigens (g) (arrow) and RNA (h) (arrow) in Nipah-infected pig lung. Endothelial giant cell in pig pulmonary vessel (i) (arrows). (h and e) stains (a, b, d, f), immunoperoxidase stains (g, i), in situ hybridisation (c, e, h). Magnification, objective ×4 (a), ×10 (b), ×20 (c), ×40 (d-i).
The cat is very susceptible to henipavirus infection under natural or experimental conditions. Vasculopathy consisting of vasculitis, endothelial syncytia, and viral immunolocalisation in endothelium and vascular smooth muscle was observed in many organs, except perhaps in the brain parenchyma, but meninges were involved (Table 1). There is severe pulmonary inflammation, and bronchial epithelium involvement may be prominent [40, 66, 67, 69]. Lymphoid tissues, such as the spleen, lymph nodes, thymus, and Peyer's patches, and kidney parenchymal tissues including glomeruli were often involved.
Table 1.
Summary of animal susceptibility to henipavirus infection and range of pathologies reported in the literature.
| Animal | Susceptibility to Hendra virus | Susceptibility to Nipah virus | CNS pathology of henipavirus infection | Non-CNS pathology of henipavirus infection | Remarks | Refs | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Natural infection | Experimental infection | Natural infection | Experimental infection | Vasculopathy*** | Parenchymal lesions | Vasculopathy | Parenchymal lesions | |||
| Bat | Yes | Yes | Yes | Yes | Yes Mainly meninges |
NR | Yes Gastrointestinal tract, kidney, spleen, placenta, lung |
Yes Kidney, heart, liver, salivary gland, testis, lung, trigeminal ganglion, intestine, urinary bladder, prostate |
Neuronal infection not reported so far | [29, 31, 32, 64, 65] |
| Cat | NR* | Yes | Yes | Yes | Yes Meninges mainly |
Yes Mainly meningitis |
Yes Lung, gastrointestinal tract, kidney, urinary bladder, heart, liver, lymphoid organs |
Yes Lung, urinary bladder, kidney, lymphoid organs, gastrointestinal tract |
Bronchial epithelium infection prominent. Encephalitis /neuronal infection rare |
[21, 40, 66–69] |
| Chicken embryo/ adult |
NR | NA** | NA | Yes | Yes | Yes | Yes Heart, lung, liver, kidney, spleen, proventriculus, skin, peripheral ganglion, yolk sac |
Yes Heart, lung, kidney, spleen, skin, feather, allantochorion, proventriculus, peripheral ganglion |
[21, 70] | |
| Dog | NR | NR | Yes | NA | Yes | Yes | Yes Lung, kidney, liver |
Yes Lung, kidney |
[21, 67, 68, 71], authors' unpublished data | |
| Ferret | NA | NA | NA | Yes | Yes | Yes | Yes Lung, kidney |
Yes Lung, kidney, lymphoid organs, urinary bladder, adrenal cortex, fallopian tube, thyroid |
[72, 73] | |
| Guinea pig | NA | Yes | NA | Yes | Yes | Yes | Yes Lymphoid organs, urinary bladder, female genital tract, gastrointestinal tract, skeletal muscles, placenta, adrenal gland, thymus, thyroid, heart, lung, kidney |
Yes Lymphoid organs, urinary bladder, female genital tract, lung, kidney, gastrointestinal tract, adrenal gland, thymus, thyroid, heart |
Encephalitis and neuronal infection more prominent with higher Hendra virus doses. Lung mainly mild inflammation |
[64–66, 74–76] |
| Hamster | NA | Yes | NA | Yes | Yes | Yes | Yes Lung, kidney, liver, heart |
Yes Lung, kidney, spleen, heart |
[76, 77] | |
| Horse | Yes | Yes | Yes | NA | Yes | Yes | Yes Lung, lymphoid organs, kidney, heart, gastrointestinal tract, urinary bladder, skeletal muscle |
Yes Lung, kidney, lymphoid organs, gastrointestinal tract |
Neuronal infection not reported so far | [18, 32, 66, 67] |
| Mouse/ Rat |
NR | NA | NA | No | NA | NA | NA | NA | [21, 76] | |
| Non human primates (Squirrel monkey, African green monkey) | NA | Yes | NA | Yes | Yes | Yes | Yes Lung, gastrointestinal tract, tongue, salivary gland, larynx, heart, gall bladder, sex organs, endocrine glands, skeletal muscle |
Yes Lung, kidney, spleen, urinary bladder |
The African green monkey may be more susceptible to infection than squirrel monkey | [78–80] |
| Pig | NR | Yes | Yes | Yes | Yes Meninges mainly |
Yes Mainly meningitis |
Yes Lung, nasal turbinate, heart, kidney, lymphoid organs, gastrointestinal tract |
Yes Lung, kidney, lymphoid organs, larynx, peripheral nerves, tonsil, nasal turbinate |
Bronchial epithelium infection very prominent. Encephalitis /neuronal infection rare |
[16, 21, 40, 67, 81–83], authors' unpublished data |
*NR= not reported; studies done.
**NA= not available; no studies done.
***Vasculopathy and parenchymal lesions, respectively, includes morphological changes and/or immunolocalisation of viral antigens.
The horse as the intermediate host of HeV develops both a pulmonary and an encephalitic syndrome [18, 33], the latter being recognized only more recently. As in other infected animals, systemic vasculopathy is a prominent feature in the lungs, CNS, kidney, and other organs (Table 1). Apart from vasculopathy, observed encephalitis, necrosis and neuronal changes in the CNS suggest direct neuronal infection, but surprisingly so far there are no published reports to confirm this [67].
Naturally NiV-infected pigs develop a distinctive clinical syndrome called “porcine respiratory and encephalitis syndrome” or “barking pig syndrome” [85]. As the latter name suggests, pigs can develope a characteristic loud barking cough, which differs from other known porcine respiratory diseases. Respiratory distress was also observed in pigs experimentally infected with henipaviruses [39, 81]. Neurological signs included paralysis and abnormal movement and gait. Many pigs however may remain asymptomatic or, having developed clinical signs and symptoms, recover to a large extent [85].
In studies of both natural and experimental pig infections that we (unpublished data) and others have done, the most severe pathology appears to be found in the respiratory system [39, 40, 81–83]. There was evidence of tracheitis, bronchial inflammation, and pneumonia. Numerous macrophages, neutrophils, and multinucleated cells can be found within alveoli (Figures 2(d) and 2(e)) and bronchioles. Epithelial syncytia arising from the bronchial epithelium are prominent, and viral antigens and RNA (unpublished data) could be demonstrated (Figures 2(f) and 2(h)). Vasculitis and multinucleated syncytial cells were seen in small blood vessels (Figure 2(i)). Meningitis was characterised by vasculitis, inflammation, and viral antigens localised to the arachnoid membrane [40, 67]. Overall, encephalitis was thought to be rare, but neuronal and peripheral nerve infections have been demonstrated [39, 82]. Peripheral nerves may play a role in viral transmission into the CNS, a phenomenon suggested so far only in the pig.
Several other animals that have been experimentally infected successfully include the guinea pig, hamster, ferret, nonhuman primates (squirrel monkey and African green monkey), and chick embryo (Table 1). The infected guinea pig shows extensive vasculopathy (Table 1) in the urinary bladder, female reproductive tract, lymphoid organs, gastrointestinal tract, brain, and so forth. [64, 65, 74, 75, 84]. Notably, although pulmonary vasculopathy was described [67], the lung generally showed mild inflammation. Viral antigens and inclusions could be localised to neurons [74], but higher viral doses may be needed to produce encephalitis and/or neuronal infection [75]. Hamster tissues infected by henipaviruses generally showed systemic vasculopathy and parenchymal lesions in most major CNS and non-CNS organs examined (Table 1) [76, 77]. In the CNS, there was encephalitis, and there were viral inclusions, antigens and RNA in the neurons (Figures 1(f) and 1(h)). Very rarely, neuronal syncytia were observed (Figure 1(g)) (unpublished data). In addition to vasculopathy, pneumonia, glomerulitis and tubular lesions have been described. The squirrel monkey and African green monkey are susceptible by henipaviruses, and results suggest that they are good nonhuman primate animal models. As in the human infection, systemic vasculopathy and involvement of a broad range of organs were detected (Table 1) [78–80]. More detailed analysis of the pathological features in these models should enable the pathogenesis of henipavirus infection to be further investigated. Pathological data from the infected ferret shows systemic vasculopathy and parenchymal lesions in the CNS and non-CNS organs (Table 1) [72, 73]. The chick embryo also shows evidence of extensive CNS and non-CNS involvement suggesting that adult birds may also be susceptible to henipaviruses, but so far there is no data available [70].
As the natural reservoir host of henipaviruses, it is not surprising that experimentally infected bats did not develop severe disease nor severe pathological changes (Table 1) [64, 65]. Interestingly, mouse and rat do not apparently develop clinical disease for reasons yet to be investigated [76].
In general, the pathology described in various animal species reflects the pathological features seen in the human disease, namely, extensive vasculopathy, parenchymal lesions in multiple organs, and evidence of viral infection. However, there may be some significant differences among animals. In the pig and cat, respiratory tract involvement, notably of the bronchial epithelium, stands out as a prominent feature. In contrast, the guinea pig shows mild lung parenchymal inflammation. Encephalitis and/or neuronal infection may be more subtle in the pig and cat in contrast to human infection.
The pathological findings in the respiratory tracts of the horse and pig, particularly the latter, are of course consistent with the postulated modes of viral transmission to humans via oropharyngeal/respiratory fluids and aerosols. Interestingly, negative virus isolation from pig urine suggests inefficient viral spread by this means [39, 81] though rare involvement of the glomerulus still suggests this possibility [40]. We were unable to demonstrate glomerular or tubular pathology in the 2 pigs that we have examined (unpublished data). Thus, respiratory tract secretions may be the main mode of pig-to-human NiV transmission. On the other hand, extensive kidney involvement in dogs and cats, implicated as minor intermediate hosts, may be via contaminated urine and in cats via respiratory secretions as well [25, 67, 71, 86].
If one considers as a prerequisite for a good animal model encephalitis and neuronal involvement in the CNS, in addition to systemic vasculopathy and severe inflammation in the lung, kidney, and other major organs, then perhaps the hamster, ferret, and monkey represent the best available small animal models of henipavirus infection. Although it is difficult to directly compare the relative susceptibility of these animals to henipaviruses as the viral sources and doses, inoculation routes, and animal and environmental characteristics may be different, perhaps among the nonhuman primates, the African green monkey could be more susceptible than the squirrel monkey. Nonetheless, all these models could be useful models for pathogenesis, therapeutic and vaccine studies as have already been done [77, 79, 87]. Overall, all the animal models confirm the dual pathogenetic mechanisms postulated for tissue injury in henipavirus infection, namely, vasculopathy-associated microinfarction and direct viral infection of extravascular parenchymal cells [61].
It is perhaps not surprising that henipaviruses cause similar infectious disease pathology in both humans and animals as it has now been shown that they share the same virus entry receptor. The main receptor has been identified as ephrin B2 [88, 89], and the alternative receptor is ephrin B3 [90]. These receptors are ubiquitous on plasma membranes of many mammalian cells, particularly in the blood vessels and CNS, thus accounting for the prominent clinic pathological features of vasculitis and CNS involvement.
The emergence of henipaviruses over a short period of a few years underscores the growing importance of this group of viruses as causative agents of previously unknown zoonoses. Because pteropid bats as natural hosts are found in many parts of the world, future henipavirus outbreaks should be anticipated [29, 30, 91–95].
6. Addendum
A very recent comparative study of NiV and HeV in the hamster model (Rockx et al. [96]) showed that while the type of pathological lesions was essentially similar between the two, there were differences in respiratory tract replication sites and the onset and severity of pathological lesions. HeV-induced lesions were found to appear earlier and were more severe.
Acknowledgment
The authors gratefully acknowledge Dr. Peter Daniels, CSIRO, Australia, for making available to us the dog and pig tissues for histopathological analysis.
References
- 1.Holmes EC. On the origin and evolution of the human immunodeficiency virus (HIV) Biological Reviews of the Cambridge Philosophical Society. 2001;76(2):239–254. doi: 10.1017/s1464793101005668. [DOI] [PubMed] [Google Scholar]
- 2.Peiris JSM, Yu WC, Leung CW, et al. Re-emergence of fatal human influenza A subtype H5N1 disease. The Lancet. 2004;363(9409):617–619. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Isolation of avian influenza (H5N1) viruses from humans—Hong Kong, May-December 1997. Morbidity and Mortality Weekly Report. 1997;46(50):1204–1207. [PubMed] [Google Scholar]
- 4.CDC. Outbreak of West Nile-like viral encephalitis-New York, 1999. Morbidity and Mortality Weekly Report. 1999;48:845–849. [PubMed] [Google Scholar]
- 5.Kabilan L, Rajendran R, Arunachalam N, et al. Japanese encephalitis in India: an overview. Indian Journal of Pediatrics. 2004;71(7):609–615. doi: 10.1007/BF02724120. [DOI] [PubMed] [Google Scholar]
- 6.Bautista E, Chotpitayasunondh T, Gao Z, et al. Clinical aspects of pandemic 2009 influenza a (H1N1) virus infection. New England Journal of Medicine. 2010;362(18):1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 7.Rabies, Asia. Weekly Epidemiological Record. 2001;76(41):320–323. [Google Scholar]
- 8.Schneider MC, Romijn PC, Uieda W, et al. Rabies transmitted by vampire bats to humans: an emerging zoonotic disease in Latin America? Revista Panamericana de Salud Publica. 2009;25(3):260–269. doi: 10.1590/s1020-49892009000300010. [DOI] [PubMed] [Google Scholar]
- 9.Lanciotti RS, Roehrig JT, Deubel V, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the Northeastern United States. Science. 1999;286(5448):2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 10.Lindsey NP, Staples JE, Lehman JA, Fischer M. Surveillance for human west Nile virus disease-United States, 1999-2008. Morbidity and Mortality Weekly Report. 2010;59(2):1–17. [PubMed] [Google Scholar]
- 11.Wang LF, Yu M, Hansson E, et al. The exceptionally large genome of Hendra virus: support for creation of a new genus within the family Paramyxoviridae. Journal of Virology. 2000;74(21):9972–9979. doi: 10.1128/jvi.74.21.9972-9979.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong KT. Emerging and re-emerging epidemic encephalitis: a tale of two viruses. Neuropathology and Applied Neurobiology. 2000;26(4):313–318. doi: 10.1046/j.1365-2990.2000.00256.x. [DOI] [PubMed] [Google Scholar]
- 13.Wong KT. Emerging epidemic viral encephalitides with a special focus on henipaviruses. Acta Neuropathologica. 2010;120(3):317–325. doi: 10.1007/s00401-010-0720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong KT, Shieh WJ, Zaki SR, Tan CT. Nipah virus infection, an emerging paramyxoviral zoonosis. Springer Seminars in Immunopathology. 2002;24(2):215–228. doi: 10.1007/s00281-002-0106-y. [DOI] [PubMed] [Google Scholar]
- 15.Tan CT, Wong KT. Nipah encephalitis outbreak in Malaysia. Annals of the Academy of Medicine Singapore. 2003;32(1):112–117. [PubMed] [Google Scholar]
- 16.Weingartl HM, Berhane Y, Czub M. Animal models of henipavirus infection: a review. Veterinary Journal. 2009;181(3):211–220. doi: 10.1016/j.tvjl.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Williamson MM, Torres-Velez FJ. Henipavirus: a review of laboratory animal pathology. Veterinary Pathology. 2010;47(5):871–880. doi: 10.1177/0300985810378648. [DOI] [PubMed] [Google Scholar]
- 18.Murray K, Selleck P, Hooper P, et al. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268(5207):94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- 19.Hanna JN, McBride WJ, Brookes DL, et al. Hendra virus infection in a veterinarian. Medical Journal of Australia. 2006;185(10):562–564. doi: 10.5694/j.1326-5377.2006.tb00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Sullivan JD, Allworth AM, Paterson DL, et al. Fatal encephalitis due to novel paramyxovirus transmitted from horses. The Lancet. 1997;349(9045):93–95. doi: 10.1016/s0140-6736(96)06162-4. [DOI] [PubMed] [Google Scholar]
- 21.Rogers RJ, Douglas IC, Baldock FC, et al. Investigation of a second focus of equine morbillivirus infection in coastal Queensland. Australian Veterinary Journal. 1996;74(3):243–244. doi: 10.1111/j.1751-0813.1996.tb15413.x. [DOI] [PubMed] [Google Scholar]
- 22.Field HE, Barratt PC, Hughes RJ, Shield J, Sullivan ND. A fatal case of Hendra virus infection in a horse in north Queensland: clinical and epidemiological features. Australian Veterinary Journal. 2000;78(4):279–280. doi: 10.1111/j.1751-0813.2000.tb11758.x. [DOI] [PubMed] [Google Scholar]
- 23.Playford EG, McCall B, Smith G, et al. Human Hendra virus encephalitis associated with equine outbreak, Australia, 2008. Emerging Infectious Diseases. 2010;16(2):219–223. doi: 10.3201/eid1602.090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvey LA, Wells RM, McCormack JG, et al. Infection of humans and horses by a newly described morbillivirus. Medical Journal of Australia. 1995;162(12):642–645. doi: 10.5694/j.1326-5377.1995.tb126050.x. [DOI] [PubMed] [Google Scholar]
- 25.Parashar UD, Sunn LM, Ong F, et al. Case-control study of risk factors for human infection with a new zoonotic paramyxovirus, Nipah virus, during a 1998-1999 outbreak of severe encephalitis in Malaysia. Journal of Infectious Diseases. 2000;181(5):1755–1759. doi: 10.1086/315457. [DOI] [PubMed] [Google Scholar]
- 26.Tan CT, Goh KJ, Wong KT, et al. Relapsed and late-onset Nipah encephalitis. Annals of Neurology. 2002;51(6):703–708. doi: 10.1002/ana.10212. [DOI] [PubMed] [Google Scholar]
- 27.Hossain MJ, Gurley ES, Montgomery JM, et al. Clinical presentation of Nipah virus infection in Bangladesh. Clinical Infectious Diseases. 2008;46(7):977–984. doi: 10.1086/529147. [DOI] [PubMed] [Google Scholar]
- 28.Harit AK, Ichhpujani RL, Gupta S, et al. Nipah/Hendra virus outbreak in Siliguri, West Bengal, India in 2001. Indian Journal of Medical Research. 2006;123(4):553–560. [PubMed] [Google Scholar]
- 29.Chua KB, Koh CL, Hooi PS, et al. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes and Infection. 2002;4(2):145–151. doi: 10.1016/s1286-4579(01)01522-2. [DOI] [PubMed] [Google Scholar]
- 30.Halpin K, Young PL, Field HE, Mackenzie JS. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. Journal of General Virology. 2000;81(8):1927–1932. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- 31.Yob JM, Field H, Rashdi AM, et al. Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emerging Infectious Diseases. 2001;7(3):439–441. doi: 10.3201/eid0703.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williamson MM, Hooper PT, Selleck PW, et al. Transmission studies of Hendra virus (equine morbillivirus) in fruit bats, horses and cats. Australian Veterinary Journal. 1998;76(12):813–818. doi: 10.1111/j.1751-0813.1998.tb12335.x. [DOI] [PubMed] [Google Scholar]
- 33.Field H, Schaaf K, Kung N, et al. Hendra virus outbreak with novel clinical features, Australia. Emerging Infectious Diseases. 2010;16(2):338–340. doi: 10.3201/eid1602.090780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahani M, Parashar UD, Ali R, et al. Nipah virus infection among abattoir workers in Malaysia, 1998-1999. International Journal of Epidemiology. 2001;30(5):1017–1020. doi: 10.1093/ije/30.5.1017. [DOI] [PubMed] [Google Scholar]
- 35.Premalatha GD, Lye MS, Ariokasamy J, et al. Assessment of Nipah virus transmission among pork sellers in Seremban, Malaysia. Southeast Asian Journal of Tropical Medicine and Public Health. 2000;31(2):307–309. [PubMed] [Google Scholar]
- 36.Chew MHL, Arguin PM, Shay DK, et al. Risk factors for Nipah virus infection among abattoir workers in Singapore. Journal of Infectious Diseases. 2000;181(5):1760–1763. doi: 10.1086/315443. [DOI] [PubMed] [Google Scholar]
- 37.Ali R, Mounts AW, Parashar UD, et al. Nipah virus infection among military personnel involved in pig culling during an outbreak of encephalitis in Malaysia, 1998-1999. Emerging Infectious Diseases. 2001;7(4):759–761. doi: 10.3201/eid0704.010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CDC. Update: outbreak of Nipah virus—Malaysia and Singapore, 1999. Morbidity and Mortality Weekly Report. 1999;48:335–337. [PubMed] [Google Scholar]
- 39.Weingartl H, Czub S, Copps J, et al. Invasion of the central nervous system in a porcine host by Nipah virus. Journal of Virology. 2005;79(12):7528–7534. doi: 10.1128/JVI.79.12.7528-7534.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Middleton DJ, Westbury HA, Morrissy CJ, et al. Experimental Nipah virus infection in pigs and cats. Journal of Comparative Pathology. 2002;126(2-3):124–136. doi: 10.1053/jcpa.2001.0532. [DOI] [PubMed] [Google Scholar]
- 41.Chua KB, Lam SK, Goh KJ, et al. The presence of nipah virus in respiratory secretions and urine of patients during an outbreak of nipah virus encephalitis in Malaysia. Journal of Infection. 2001;42(1):40–43. doi: 10.1053/jinf.2000.0782. [DOI] [PubMed] [Google Scholar]
- 42.Mounts AW, Kaur H, Parashar UD, et al. A cohort study of health care workers to assess nosocomial transmissibility of Nipah virus, Malaysia, 1999. Journal of Infectious Diseases. 2001;183(5):810–813. doi: 10.1086/318822. [DOI] [PubMed] [Google Scholar]
- 43.Tan K-S, Sarji SA, Tan C-T, et al. Patients with asymptomatic Nipah virus infection may have abnormal cerebral MR imaging. Neurological Journal of South East Asia. 2000;5:69–73. [Google Scholar]
- 44.Tan CT, Tan KS. Nosocomial transmissibility of Nipah virus. Journal of Infectious Diseases. 2001;184(10):p. 1367. doi: 10.1086/323996. [DOI] [PubMed] [Google Scholar]
- 45.Hsu VP, Hossain MJ, Parashar UD, et al. Nipah virus encephalitis reemergence, Bangladesh. Emerging Infectious Diseases. 2004;10(12):2082–2087. doi: 10.3201/eid1012.040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurley ES, Montgomery JM, Hossain MJ, et al. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerging Infectious Diseases. 2007;13(7):1031–1037. doi: 10.3201/eid1307.061128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luby SP, Gurley ES, Hossain MJ. Transmission of human infection with nipah virus. Clinical Infectious Diseases. 2009;49(11):1743–1748. doi: 10.1086/647951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luby SP, Rahman M, Hossain MJ, et al. Foodborne transmission of Nipah virus, Bangladesh. Emerging Infectious Diseases. 2006;12(12):1888–1894. doi: 10.3201/eid1212.060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chong HT, Kunjapan SR, Thayaparan T, et al. Nipah encephalitis outbreak in Malaysia, clinical features in patients from Seremban. Neurological Journal of South East Asia. 2000;5:61–67. doi: 10.1017/s0317167100001785. [DOI] [PubMed] [Google Scholar]
- 50.Goh KJ, Tan CT, Chew NK, et al. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. New England Journal of Medicine. 2000;342(17):1229–1235. doi: 10.1056/NEJM200004273421701. [DOI] [PubMed] [Google Scholar]
- 51.Paton NI, Leo YS, Zaki SR, et al. Outbreak of Nipah-virus infection among abattoir workers in Singapore. The Lancet. 1999;354(9186):1253–1256. doi: 10.1016/S0140-6736(99)04379-2. [DOI] [PubMed] [Google Scholar]
- 52.Sarji SA, et al. Magnetic resonance imaging features of Nipah encephalitis. American Journal of Roentgenology. 2000;175:437–442. doi: 10.2214/ajr.175.2.1750437. [DOI] [PubMed] [Google Scholar]
- 53.Lim CCT, Lee KE, Lee WL, et al. Nipah virus encephalitis: serial MR study of an emerging disease. Radiology. 2002;222(1):219–226. doi: 10.1148/radiol.2221010499. [DOI] [PubMed] [Google Scholar]
- 54.Chong HT, Tan CT. Relapsed and late-onset Nipah encephalitis, a report of three cases. Neurological Journal of South East Asia. 2003;8:109–112. [Google Scholar]
- 55.Ramasundram V, et al. Kinetics of IgM and IgG seroconversion in Nipah virus infection. Neurological Journal of South East Asia. 2000;5:23–28. [Google Scholar]
- 56.Lee KE, Umapathi T, Tan CB, et al. The neurological manifestations of Nipah virus encephalitis, a novel paramyxovirus. Annals of Neurology. 1999;46(3):428–432. [PubMed] [Google Scholar]
- 57.Chew NK, et al. Electroencephalography in acute Nipah encephalitis. Neurological Journal of South East Asia. 1999;4:45–51. [Google Scholar]
- 58.Chong HT, et al. Occupational exposure, age, diabetes mellitus and outcome of acute Nipah encephalitis. Neurological Journal of South East Asia. 2001;6:7–11. [Google Scholar]
- 59.Chua KB, Lam SK, Tan CT, et al. High mortality in Nipah encephalitis is associated with presence of virus in cerebrospinal fluid. Annals of Neurology. 2000;48(5):802–805. [PubMed] [Google Scholar]
- 60.Wong KT, Robertson T, Ong BB, et al. Human Hendra virus infection causes acute and relapsing encephalitis. Neuropathology and Applied Neurobiology. 2009;35(3):296–305. doi: 10.1111/j.1365-2990.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- 61.Wong KT, Shieh WJ, Kumar S, et al. Nipah virus infection: pathology and pathogenesis of an emerging paramyxoviral zoonosis. American Journal of Pathology. 2002;161(6):2153–2167. doi: 10.1016/S0002-9440(10)64493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldsmith CS, Whistler T, Rollin PE, et al. Elucidation of Nipah virus morphogenesis and replication using ultrastructural and molecular approaches. Virus Research. 2003;92(1):89–98. doi: 10.1016/s0168-1702(02)00323-4. [DOI] [PubMed] [Google Scholar]
- 63.Bossart KN, Wang LF, Flora MN, et al. Membrane fusion tropism and heterotypic functional activities of the Nipah virus and Hendra virus envelope glycoproteins. Journal of Virology. 2002;76(22):11186–11198. doi: 10.1128/JVI.76.22.11186-11198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Middleton DJ, Morrissy CJ, van der Heide BM, et al. Experimental Nipah virus infection in pteropid bats (Pteropus poliocephalus) Journal of Comparative Pathology. 2007;136(4):266–272. doi: 10.1016/j.jcpa.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Williamson MM, Hooper PT, Selleck PW, Westbury HA, Slocombe RF. Experimental Hendra virus infection in pregnant guinea-pigs and fruit bats (Pteropus poliocephalus) Journal of Comparative Pathology. 2000;122(2-3):201–207. doi: 10.1053/jcpa.1999.0364. [DOI] [PubMed] [Google Scholar]
- 66.Hooper PT, Ketterer PJ, Hyatt AD, Russell GM. Lesions of experimental equine morbillivirus pneumonia in horses. Veterinary Pathology. 1997;34(4):312–322. doi: 10.1177/030098589703400407. [DOI] [PubMed] [Google Scholar]
- 67.Hooper P, Zaki S, Daniels P, Middleton D. Comparative pathology of the diseases caused by Hendra and Nipah viruses. Microbes and Infection. 2001;3(4):315–322. doi: 10.1016/s1286-4579(01)01385-5. [DOI] [PubMed] [Google Scholar]
- 68.Westbury HA, Hooper PT, Selleck PW, Murray PK. Equine morbillivirus pneumonia: susceptibility of laboratory animals to the virus. Australian Veterinary Journal. 1995;72(7):278–279. doi: 10.1111/j.1751-0813.1995.tb03549.x. [DOI] [PubMed] [Google Scholar]
- 69.Mungall BA, Middleton D, Crameri G, et al. Feline model of acute Nipah virus infection and protection with a soluble glycoprotein-based subunit vaccine. Journal of Virology. 2006;80(24):12293–12302. doi: 10.1128/JVI.01619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanimura N, Imada T, Kashiwazaki Y, Sharifah SH. Distribution of viral antigens and development of lesions in chicken embryos inoculated with Nipah virus. Journal of Comparative Pathology. 2006;135(2-3):74–82. doi: 10.1016/j.jcpa.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Mills JN, Alim ANM, Bunning ML, et al. Nipah virus infection in dogs, Malaysia, 1999. Emerging Infectious Diseases. 2009;15(6):950–952. doi: 10.3201/eid1506.080453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bossart KN, Zhu Z, Middleton D, et al. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute Nipah virus infection. PLoS Pathogens. 2009;5(10) doi: 10.1371/journal.ppat.1000642. Article ID e1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pallister J, Middleton D, Crameri G, et al. Chloroquine administration does not prevent nipah virus infection and disease in ferrets. Journal of Virology. 2009;83(22):11979–11982. doi: 10.1128/JVI.01847-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torres-Velez FJ, Shieh WJ, Rollin PE, et al. Histopathologic and immunohistochemical characterization of nipah virus infection in the guinea pig. Veterinary Pathology. 2008;45(4):576–585. doi: 10.1354/vp.45-4-576. [DOI] [PubMed] [Google Scholar]
- 75.Williamson MM, Hooper PT, Selleck PW, Westbury HA, Slocombe RFS. A guinea-pig model of hendra virus encephalitis. Journal of Comparative Pathology. 2001;124(4):273–279. doi: 10.1053/jcpa.2001.0464. [DOI] [PubMed] [Google Scholar]
- 76.Wong KT, Grosjean I, Brisson C, et al. A golden hamster model for human acute Nipah virus infection. American Journal of Pathology. 2003;163(5):2127–2137. doi: 10.1016/S0002-9440(10)63569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guillaume V, Wong KT, Looi RY, et al. Acute Hendra virus infection: analysis of the pathogenesis and passive antibody protection in the hamster model. Virology. 2009;387(2):459–465. doi: 10.1016/j.virol.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 78.Marianneau P, Guillaume V, Wong KT, et al. Experimental infection of squirrel monkeys with Nipah virus. Emerging Infectious Diseases. 2010;16(3):507–510. doi: 10.3201/eid1603.091346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rockx B, Bossart KN, Feldmann F, et al. A novel model of lethal Hendra virus infection in African green monkeys and the effectiveness of ribavirin treatment. Journal of Virology. 2010;84(19):9831–9839. doi: 10.1128/JVI.01163-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geisbert TW, Daddario-Dicaprio KM, Hickey AC, et al. Development of an acute and highly pathogenic nonhuman primate model of nipah virus infection. PLoS ONE. 2010;5(5) doi: 10.1371/journal.pone.0010690. Article ID e10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li M, Embury-Hyatt C, Weingartl HM. Experimental inoculation study indicates swine as a potential host for Hendra virus. Veterinary Research. 2010;41(3):p. 33. doi: 10.1051/vetres/2010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tanimura N, Imada T, Kashiwazaki Y, Shahirudin S, Sharifah SH, Aziz AJ. Monoclonal antibody-based immunohistochemical diagnosis of Malaysian Nipah virus infection in pigs. Journal of Comparative Pathology. 2004;131(2-3):199–206. doi: 10.1016/j.jcpa.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 83.Tanimura N, Imada T, Kashiwazaki Y, et al. Reactivity of anti-Nipah virus monoclonal antibodies to formalin-fixed, paraffin-embedded lung tissues from experimental Nipah and Hendra virus infections. Journal of Veterinary Medical Science. 2004;66(10):1263–1266. doi: 10.1292/jvms.66.1263. [DOI] [PubMed] [Google Scholar]
- 84.Hooper PT, Westbury HA, Russell GM. The lesions of experimental equine morbillivirus disease in cats and Guinea pigs. Veterinary Pathology. 1997;34(4):323–329. doi: 10.1177/030098589703400408. [DOI] [PubMed] [Google Scholar]
- 85.Nor MNM, Gan CH, Ong BL. Nipah virus infection of pigs in peninsular Malaysia. OIE Revue Scientifique et Technique. 2000;19(1):160–165. doi: 10.20506/rst.19.1.1202. [DOI] [PubMed] [Google Scholar]
- 86.Tan KS, Tan CT, Goh KJ. Epidemiological aspects of Nipah virus infection. Neurological Journal of South East Asia. 1999;4:77–81. [Google Scholar]
- 87.Guillaume V, Contamin H, Loth P, et al. Nipah virus: vaccination and passive protection studies in a Hamster model. Journal of Virology. 2004;78(2):834–840. doi: 10.1128/JVI.78.2.834-840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Negrete OA, Levroney EL, Aguilar HC, et al. EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature. 2005;436(7049):401–405. doi: 10.1038/nature03838. [DOI] [PubMed] [Google Scholar]
- 89.Bonaparte MI, Dimitrov AS, Bossart KN, et al. Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(30):10652–10657. doi: 10.1073/pnas.0504887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Negrete OA, Wolf MC, Aguilar HC, et al. Two key residues in EphrinB3 are critical for its use as an alternative receptor for Nipah virus. PLoS Pathogens. 2006;2(2, article e7) doi: 10.1371/journal.ppat.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reynes JM, Counor D, Ong S, et al. Nipah virus in lyle’s flying foxes, Cambodia. Emerging Infectious Diseases. 2005;11(7):1042–1047. doi: 10.3201/eid1107.041350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Epstein JH, Prakash V, Smith CS, et al. Henipavirus infection in fruit bats (Pteropus giganteus), India. Emerging Infectious Diseases. 2008;14(8):1309–1311. doi: 10.3201/eid1408.071492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wacharapluesadee S, Lumlertdacha B, Boongird K, et al. Bat Nipah virus, Thailand. Emerging Infectious Diseases. 2005;11(12):1949–1951. doi: 10.3201/eid1112.050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y, Wang J, Hickey AC, et al. Antibodies to Nipah or Nipah-like viruses in bats, China. Emerging Infectious Diseases. 2008;14(12):1974–1976. doi: 10.3201/eid1412.080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hayman DTS, Suu-Ire R, Breed AC, et al. Evidence of henipavirus infection in West African fruit bats. PLoS ONE. 2008;3(7) doi: 10.1371/journal.pone.0002739. Article ID e2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rockx B, Brining D, Kramer J, et al. Clinical outcome of Henipavirus infection in hamsters is determined by the route and dose of infection. Journal of Virology. 2011;85(15):7658–7671. doi: 10.1128/JVI.00473-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


