This work examines the molecular and biochemical mechanisms underlying the functions of BOTRYTIS-INDUCED KINASE1 (BIK1). We demonstrate that BIK1 regulates responses to ethylene and PAMP-triggered immunity to distinct class of pathogens. The role of phosphorylation on the complex functions of BIK1 as well as genetic interactions with regulators of different immune response pathways are delineated.
Abstract
Arabidopsis thaliana BOTRYTIS-INDUCED KINASE1 (BIK1) regulates immune responses to a distinct class of pathogens. Here, mechanisms underlying BIK1 function and its interactions with other immune response regulators were determined. We describe BIK1 function as a component of ethylene (ET) signaling and PAMP-triggered immunity (PTI) to fungal pathogens. BIK1 in vivo kinase activity increases in response to flagellin peptide (flg22) and the ET precursor 1-aminocyclopropane-1-carboxylic acid (ACC) but is blocked by inhibition of ET perception. BIK1 induction by flg22, ACC, and pathogens is strictly dependent on EIN3, and the bik1 mutation results in altered expression of ET-regulated genes. BIK1 site-directed mutants were used to determine residues essential for phosphorylation and biological functions in planta, including PTI, ET signaling, and plant growth. Genetic analysis revealed flg22-induced PTI to Botrytis cinerea requires BIK1, EIN2, and HUB1 but not genes involved in salicylate (SA) functions. BIK1-mediated PTI to Pseudomonas syringae is modulated by SA, ET, and jasmonate signaling. The coi1 mutation suppressed several bik1 phenotypes, suggesting that COI1 may act as a repressor of BIK1 function. Thus, common and distinct mechanisms underlying BIK1 function in mediating responses to distinct pathogens are uncovered. In sum, the critical role of BIK1 in plant immune responses hinges upon phosphorylation, its function in ET signaling, and complex interactions with other immune response regulators.
INTRODUCTION
Mechanisms of plant defense against microbial infection have been studied extensively and the major immune response pathways identified along with many of their genetic components. Recognition of microbial effectors activates a highly specific and efficient form of plant defense known as effector-triggered immunity (ETI) (Jones and Dangl, 2006). Alternatively, pathogen-associated molecular patterns (PAMPs), evolutionarily conserved components of pathogens, can also elicit PAMP-triggered immunity (PTI), an attenuated but broad spectrum disease resistance. Bacterial flagellin and peptidoglycan, fungal chitin, and oligogalacturonides are some of the well-studied molecules that activate PTI in Arabidopsis thaliana (Boller and Felix, 2009). The idea that plant immune responses span a continuum between PTI and ETI rather than acting as distinct defense responses has recently been raised (Thomma et al., 2011). Although the recognition mechanisms and downstream responses in ETI and PTI are known, the intermediate genetic and biochemical events are less well understood. PTI and ETI converge on downstream immune responses, such as the oxidative burst, deposition of callose, and defense gene expression (Tsuda and Katagiri, 2010). Despite such convergence, the intensity, strength, and, thus, effectiveness of ETI and PTI responses are different albeit interaction dependent (Jones and Dangl, 2006).
Recognition of PAMPs or effectors is mediated by diverse plant proteins that confer specificity in the activation of immune responses. Effector recognition is mediated by R proteins, which have been widely studied and predominantly consist of nucleotide binding site–leucine-rich repeat proteins but also include receptor-like kinases (RLKs). Thus far, RLKs, such as the chitin receptor LysM/CERK1, the flagellin receptor FLS2, and the receptor for bacterial EF-TU EFR1, have been identified as critical early determinants of PTI (Gómez-Gómez and Boller, 2000; Zipfel et al., 2006; Miya et al., 2007). BAK1, previously known for its role in brassinolide signaling, is an RLK central to PTI responses initiated by various PAMPs likely by acting as a coreceptor (Chinchilla et al., 2007). Receptor-like cytoplasmic kinases (RLCKs) are a subclass of RLKs that lack extracellular domains but due to their homology are classified within the RLK superclade (Shiu and Bleecker, 2001). The Arabidopsis genome contains 610 RLKs and RLCKs that are predicted to function in plant response signaling to microbial infection, hormones, and other endogenous and environmental cues (Shiu and Bleecker, 2001; Becraft, 2002). RLCKs have also been implicated to function in ETI and PTI responses, acting in concert with surface-localized RLKs or indirectly as intracellular receptors of microbial effectors. The tomato (Solanum lycopersicum) Pto kinase serves as an intracellular receptor for the Pseudomonas syringae pv tomato (Pst) bacterial effector AvrPto (Tang et al., 1996). Arabidopsis RLCK PBS1 is a target of the P. syringae pv phaseolicola effector AvrPphB and a known component of ETI mediated by the nucleotide binding site–leucine-rich repeat R protein RPS5 (Shao et al., 2003). Tomato TPK1b and Arabidopsis BOTRYTIS-INDUCED KINASE1 (BIK1) are typical RLCKs localized to the plasma membrane that act early in the defense response pathways that contribute to defense against fungal necrotrophs (Veronese et al., 2006; Abuqamar et al., 2008). BIK1 and closely related kinases are also required for PTI to Pst downstream of FLS2 and BAK1 (Lu et al., 2010; Zhang et al., 2010). In addition, BIK1 regulates plant growth traits, demonstrating a dual function in plant immune and growth responses similar to BAK1 (Veronese et al., 2006). Both BAK1 and BIK1 appear to affect plant development and defense through their function in hormone biosynthesis and/or signaling.
Plant hormone synthesis and signaling modulates immune responses and development. Jasmonate (JA) and ethylene (ET) are generally regarded as the primary regulators of immune responses to necrotrophic pathogens (Glazebrook, 2005). By contrast, salicylate (SA) is central to resistance to biotrophic and hemibiotrophic pathogens (Dempsey et al., 1999), but its role in defense against necrotrophs is complex. In Arabidopsis, SA accumulation exceeding wild-type levels promotes susceptibility to necrotrophic fungi (Veronese et al., 2006), whereas deficiency in SA or SA signaling has no impact (Veronese et al., 2004) or only affects resistance at the site of inoculation (Ferrari et al., 2003). Biological or chemical activation of systemic acquired resistance, an SA-dependent immune response, has no effect on resistance to B. cinerea in Arabidopsis (Govrin and Levine, 2002). More recent data reveal that JA, SA, ET, gibberellin, and abscisic acid all contribute to plant immune responses to necrotrophic pathogens (Grant and Jones, 2009). The interaction between these pathways is a major factor in determining resistance, allowing plants to fine-tune immune responses depending on the invading pathogen. Mutual antagonism between SA and JA/ET-dependent defenses and its impact on immune responses to biotrophic and necrotrophic pathogens have been established (Spoel et al., 2007). Thus, hormone homeostasis is important for normal immune responses as well as typical growth and development.
Previously, we have shown that Arabidopsis BIK1 is required for resistance to Botrytis cinerea and Alternaria brassicicola but suppresses defense against Pst (Veronese et al., 2006). More recently, data showing BIK1 function in the integration of PTI responses downstream of the PAMP receptors FLS2, EFR1, and CERK1 was published (Zhang et al., 2010). Consistent with this, BIK1 is a component of the flagellin-receptor complex that is a key determinant of flagellin-mediated immune responses (Lu et al., 2010). However, neither the role of BIK1 in PTI to fungal pathogens nor the role of BIK1 phosphorylation in any disease resistance was examined. In this study, the genetic, molecular, and biochemical function of BIK1 in PTI to bacterial and fungal pathogens was studied in detail. New functions for BIK1 in ET-mediated plant immune responses and growth traits were determined. In addition, epistasis analysis of the interactions between BIK1 and other Arabidopsis immune response regulators uncovered the genetic requirements for BIK1 function in PTI to fungal necrotrophs as well as virulent and nonpathogenic strains of Pst, ET, and flagellin responses.
RESULTS
Kinase Assays on Recombinant BIK1 Identifies Residues Required for in Vitro Phosphorylation Activity
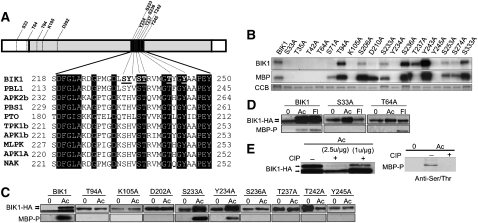
The BIK1 amino acid sequence was analyzed to identify regulatory regions and putative phosphorylation sites. BIK1 has a large conserved catalytic kinase domain (KD) and nonconserved flanking regions predicted to contribute to its biological functions (Figure 1A). Within the KD is the activation domain (AD), a regulatory region important for general kinase activity and biological function (Hanks and Hunter, 1995). Analysis of the BIK1 AD identified seven phosphorylatable residues, of which, four (Ser-236, Thr-237, Thr-242, and Tyr-245) are invariant in BIK1-related kinases from Arabidopsis and other species (Figure 1A). Preceding the AD is an ATP binding site, the conserved Arg and Asp residues in domain VIb that classify BIK1 as an RD-type kinase (Hanks and Quinn, 1991; Hanks and Hunter, 1995) as well as additional putative phosphorylation sites.
Figure 1.
BIK1 Conserved Residues and in Vitro and in Vivo Kinase Assays.
(A) Structure of the BIK1 protein and comparison of residues in the ADs of BIK1 and related kinases. Gray region denotes the KD and black the AD. Residues noted in the BIK1 protein indicate those substituted for in planta assays.
(B) Kinase activity of recombinant BIK1 and Ala substitution mutants produced in E. coli detected by autoradiogram. CCB, Coomassie blue staining.
(C) and (D) BIK1 substitution mutants in vivo detected by a mobility shift on an HA-immunoblot or by phosphoserine/Thr-specific antibody.
(E) BIK1 and MBP phosphorylation is abrogated by phosphatase treatment. Protein dephosphorylation was performed according to the manufacturer’s protocol (New England Biolabs) with ~1 to 2.5 units CIP/μg protein (left) or (~2.5 μ/μg) (right). −, Buffer; +, CIP.
In (C) and (D), plants were treated with ACC (Ac) or flg22 (Fl) for 3 h and assayed for changes in BIK1 and MBP phosphorylation activity. In (C) to (E), in vivo BIK1 phosphorylation was detected by ACC or flagellin-induced mobility shifts observable by HA-immunoblot. The top band corresponds to phosphorylated BIK1, which is migrating slower than the unphosphorylated form. MBP phosphorylation by BIK1 was detected by immunoblots with a phosphoserine/Thr-specific antibody.
To determine the biochemical basis for the biological functions of BIK1, highly conserved residues and predicted phosphorylation sites were substituted with Ala. Recombinant BIK1 proteins carrying site-specific substitutions in these 18 selected residues were made in and purified from Escherichia coli and assayed for auto- and transphosphorylation activities. Recombinant BIK1 phosphorylates itself as well as the commonly used artificial kinase substrate myelin basis protein (MBP), as previously shown (Veronese et al., 2006). The BIK1S33A, BIK1T35A, BIK1T42A, BIK1T64A, BIK1K105A, BIK1Y234A, and BIK1Y245A substitutions eliminated all kinase activities (Figure 1B). Mutations at Thr-94, Ser-206, Ser-233, Ser-236, Ser-253, Ser-274, and Ser-333 did not alter BIK1 auto- or MBP phosphorylation. Autophosphorylation was lost in BIK1S71A and BIK1D210A, yet both proteins retained substrate kinase activity. By contrast, BIK1T237A activity was restricted to self-phosphorylation.
BIK1 Phosphorylation Residues Are Required for ET-Induced BIK1 Kinase Activity in Vivo
To determine the biological functions of BIK1 residues, selected BIK1 substitutions, based on results from the experiments above and known functions in related proteins, were tagged with the hemagglutinin-epitope and expressed in the bik1 mutant. The sites of the substituted residues used for in planta assays are indicated in Figure 1A. BIK1 phosphorylation is visible as a mobility shift after treatment with 1-aminocyclopropane-1-carboxylic acid (ACC) (Figures 1C to 1E). Phosphorylation of BIK1 and MBP increased after treatment with the ET precursor ACC (Figure 1C) or flagellin peptide flg22 (Figure 1D). Treatment with protein phosphatase restored mobility of BIK1-HA and eliminated MBP phosphorylation, indicating that these are phosphorylated forms (Figure 1E).
Generally, BIK1 residues required for in vitro auto and MBP phosphorylation activities were also required for BIK1 phosphorylation in vivo. Similar to in vitro activities, Lys-105 and Tyr-245 were required for all kinase activities as were Thr-94, Asp-202, Ser-236, and Thr-237. Alternatively, BIK1S233A and BIK1Y234A show BIK1 and MBP phosphorylation comparable to BIK1. Intriguingly, Ala substitution at Thr-242 leads to BIK1 phosphorylation prior to elicitation and a loss of trans-kinase activity. Due to the differential responses of the substitution plant lines (see next section), the contributions of Ser-33 and Thr-64 to BIK1 kinase activity were further analyzed for their effects on flg22-induced phosphorylation in addition to ACC (Figure 1D). BIK1T64A abolished BIK1 phosphorylation and ACC-induced MBP phosphorylation but did not affect MBP phosphorylation in response to flg22, indicating differential contributions to regulation of activity in response to different signals. BIK1S33A blocked all MBP phosphorylation activity as well as flg22-triggered BIK1 phosphorylation.
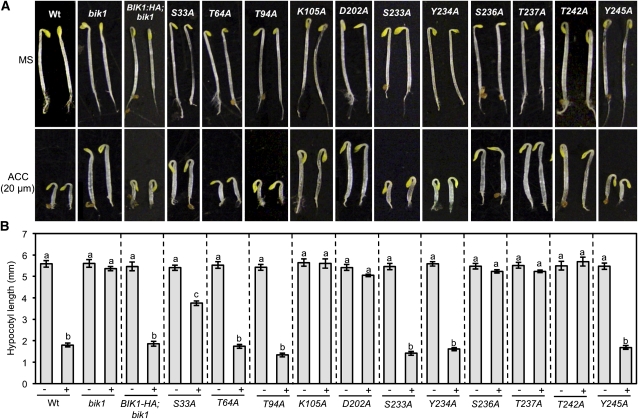
BIK1 Mediates flg22 and Wound-Induced Immunity to B. cinerea
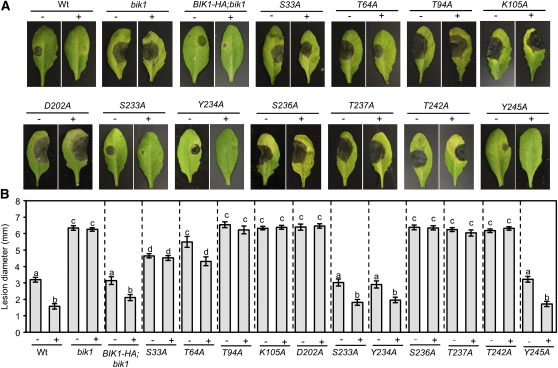
To determine the role of BIK1 regulatory residues and those required for phosphorylation in PTI responses, we assayed transgenic bik1 plants expressing BIK1 Ala substitution mutants for basal (water-treated) and flagellin-induced resistance (flg22-PTI) to B. cinerea. Treatment with flg22 prior to inoculation significantly reduced disease lesion size in wild-type plants but failed to confer protection in the bik1 background (Figures 2A and 2B). As wounding has also been found to confer strong resistance to B. cinerea in Arabidopsis, we examined this type of induced immunity in bik1 and found the mutant is only partially protected relative to wounded wild-type plants (see Supplemental Figure 1 online).
Figure 2.
BIK1 Is Required for PTI to B. cinerea.
Disease symptoms (A) and mean lesion size (B) of water (−) and flg22-treated (+) bik1 and BIK1 substitution mutants after drop inoculation with B. cinerea (2.5 × 105 spores/mL). Data in (B) represent mean ± se from a minimum of 30 disease lesions. The statistical significance of the mean lesion sizes was determined using analysis of variance and Tukey’s test. The mean values followed by different letters are significantly different from each other (P = 0.01) Experiments were repeated at least three times with similar results. Images were taken 3 d after inoculation. The BIK1 site-directed mutants and the wild-type (wt) BIK1-HA are expressed in the bik1 mutant background.
Expression of BIK1S233A, BIK1Y234A, and BIK1Y245A fully restored the basal and flg22-induced B. cinerea resistance of bik1 to wild-type levels comparable to 35S:BIK1-HA (Figures 2A and 2B). BIK1S33A partially restored basal resistance but completely abrogated flg22-induced resistance, whereas BIK1T64A partially restored both. BIK1T94A, the ATP binding site mutant, BIK1K105A, BIK1D202A, BIK1S236A, BIK1T237A, and BIK1T242A inhibited resistance both with and without flg22 treatment, suggesting their critical importance in the PTI function of BIK1. Among these, BIK1D202A disrupts the RD domain, and in RD kinases, the phosphorylated, negatively charged residues in the AD interact with the positively charged Arg of the RD domain, providing proper spatial arrangement for substrate access to the catalytic Asp (Johnson et al., 1996). Loss of BIK1 and MBP phosphorylation as well as the lack of complementation in bik1;BIK1D202A are consistent with the role of the RD domain for the biochemical and biological functions of protein kinases (Johnson et al., 1996). Most residues that abrogated kinase activity also failed to complement the disease resistance and loss of PTI of bik1, suggesting that BIK1 phosphorylation is important for its function in immune responses.
Resistance of the bik1 substitution lines to A. brassicicola was similar to that observed for B. cinerea with the exception of BIK1Y234A, which partially restored bik1 resistance but fully rescued bik1 susceptibility to B. cinerea (see Supplemental Figure 2 online). Induced resistance to A. brassicicola was not tested due to the high level of resistance in uninduced wild-type plants. In sum, BIK1 is a regulator of PTI required for flagellin-induced immunity to B. cinerea as well as full protection conferred by wounding.
BIK1 Is Required for Seedling Growth Responses to ET and Glc
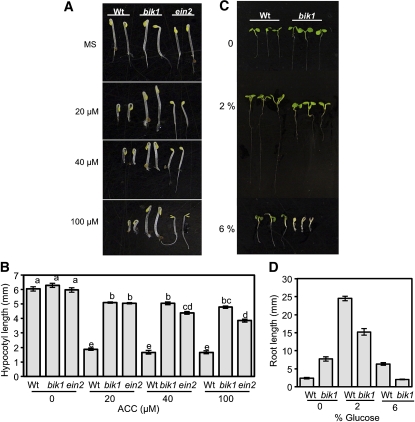
In assaying for hormone-related functions that may explain the role of BIK1 in plant immune and growth responses, we observed that bik1 is altered in the triple response (Figures 3A and 3B). The triple response is induced in the dark in response to ET or its precursor ACC, producing seedlings with exaggerated apical hooks and swollen hypocotyls that are inhibited in root/hypocotyl elongation (Guzmán and Ecker, 1990). bik1 shows a clear insensitivity to ACC evidenced by a lack of growth inhibition in the hypocotyl compared with wild-type seedlings. The ET response mutant ein2 shows typical impaired ET responses consistent with published data (Figures 3A and 3B). By contrast, bik1 roots appear responsive to ACC, whereas ein2 shows no significant changes in root length even at higher concentrations of ACC (40 and 100 μM). However, at these same concentrations, bik1 hypocotyls show greater growth insensitivity than ein2. This variation is consistent with ein2 being the only mutant that inhibits all aspects of seedling growth responses to ET and suggests BIK1 affects specific aspects of ET-mediated seedling growth responses (Guo and Ecker, 2004).
Figure 3.
BIK1 Is Required for Responses to ET and Glc.
(A) and (B) Triple response phenotype (A) and hypocotyl lengths (B) of bik1 seedlings on different concentrations of ACC (μM) and unsupplemented MS media. wt, wild type.
(C) and (D) Sensitive growth response (C) and root length (D) of bik1 on media containing increased levels of Glc.
Data in (B) and (D) represent mean ± se from a minimum of 60 seedlings. Experiments were repeated at least three times with similar results. Statistical analysis was performed as described in the legend of Figure 2.
Genetic and molecular studies in Arabidopsis suggest that Glc and ET interact antagonistically (Yanagisawa et al., 2003). For instance, the ET-insensitive etr1 and ein2 mutants are sensitive to Glc, but the constitutive ET signaling mutant ctr1 is insensitive (Zhou et al., 1998). In the presence of 2 or 6% Glc, bik1 seedlings showed clear growth hypersensitivity, further confirming altered ET responses and lending support to BIK1 function in ET signaling (Figures 3C and 3D).
BIK1 Induction by ET and Flagellin Is EIN3 Dependent
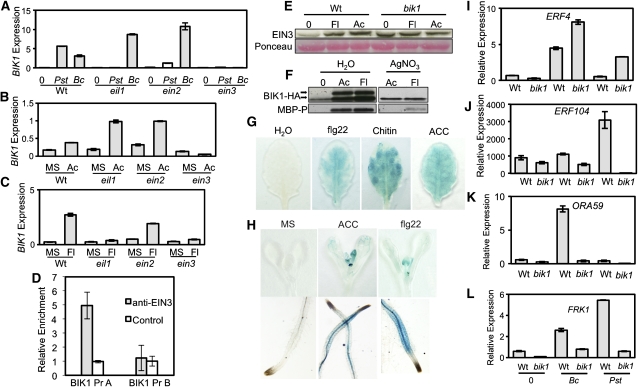
To gain better insight into how BIK1 functions in ET responses, we studied its expression in ein3 and other ET-signaling mutants. The transcription factor EIN3 is a member of a functionally redundant gene family involved in the regulation of ET and immune responses (Chao et al., 1997; Chen et al., 2009). In response to B. cinerea, BIK1 shows a significant induction in wild-type plants that was completely abolished in ein3 (Figure 4A). Similar to B. cinerea, ACC-induced BIK1 expression also showed strict dependence on EIN3 (Figure 4B). By contrast, BIK1 was expressed at a higher level than the wild type in both ein3-like (eil1) and ein2 plants in response to both treatments. Interestingly, BIK1 induction in response to PstDC3000 and flg22 requires EIN3 and EIL1 but shows only partial dependence on EIN2 (Figures 4A and 4C). The strict dependence of BIK1 expression on EIN3 is reinforced by the presence of EIN3 binding consensus sequences (Solano et al., 1998; Kosugi and Ohashi, 2000) in the BIK1 promoter (see Supplemental Figure 3 online). Next, we performed chromatin immunoprecipitation–quantitative PCR (ChIP-qPCR) experiments using EIN3 antiserum. We show that the EIN3 protein is associated with the BIK1 promoter, suggesting that it directly controls BIK1 gene expression (Figure 4D). Recently, both EIN3 and EIL1 have been demonstrated to contribute to ET-mediated PTI responses through the direct transcriptional regulation of FLS2 (Boutrot et al., 2010). Furthermore, bik1 plants accumulate elevated levels of EIN3 that do not show any significant increase in response to ACC or flg22-treatment relative to wild-type seedlings, raising the possibility of feedback control of EIN3 by BIK1 adding another dimension to their regulatory relationship (Figure 4E). Interestingly, ACC- and flg22-induced BIK1 phosphorylation is attenuated by silver nitrate, which blocks ET perception, suggesting that ET signaling or perception is required for BIK1 phosphorylation (Figure 4F). β-Glucuronidase (GUS) activity in transgenic plants harboring a GUS reporter gene under the transcriptional control of the BIK1 promoter showed a clear diffuse increase in response to flg22, ACC, and chitin in adult leaves (Figure 4G). In seedlings, ACC and flg22-induced GUS activity was also observed but largely localized to the vascular tissue (Figure 4H). Regulation of BIK1 expression with ACC, flg22, and chitin supports its function in ET signaling and PTI. In addition, the coregulation of FLS2 and BIK1 by ET, flagellin, and EIN3 is consistent with their interaction as part of the flg22 receptor complex (Lu et al., 2010; Zhang et al., 2010).
Figure 4.
BIK1 Functions in the ET Response Pathway.
(A) to (C) Expression of BIK1, as determined by quantitative RT-PCR and normalized to actin following pathogen inoculation (A) and during the triple response (B) and flg22 (Fl) treatment (C) in ET response mutants. wt, wild type.
(D) ChIP-PCR showing the association of EIN3 protein with the BIK1 promoter. Chromatin from wild-type seedlings was immunoprecipitated with an anti-EIN3 polyclonal antibody. Enrichment of BIK1 promoter region was verified by qPCR using BIK1 promoter-specific primers. BIK1 Pr region A refers to position −1221 to −774 and region B from −494 to −166 in the BIK1 promoter region. The BIK1 promoter sequence is shown in Supplemental Figure 3 online.
(E) EIN3 protein levels in bik1 and wild-type seedlings in response to flg22 (Fl) and ACC (Ac) treatment.
(F) BIK1 phosphorylation is inhibited by blocking ET perception with silver nitrate (AgNO3). Changes in BIK1 phosphorylation were visualized as a mobility shift observable by HA-immunoblot. The top band corresponds to the slowly migrating band caused by BIK1 phosphorylation. MBP phosphorylation was detected on an immunoblot with a phosphoserine/Thr-specific antibody.
(G) and (H) Histochemical assay showing GUS activity in transgenic BIK1pr:GUS plants (G) and seedlings (H) in response to flg22, chitin, and ACC.
(I) to (L) Quantitative RT-PCR determination of pathogen-induced expression of ERF4 (I), ERF104 (J), ORA59 (K), and FRK1 (L) in wild-type and bik1 plants. Phosphorylation detection was performed as described in the legend of Figure 1.
Experiments were performed as described in the Methods and repeated at least three times with similar results.
BIK1 Regulates Expression of Genes Involved in PTI- and ET-Mediated Immune Responses
The Arabidopsis ETHYLENE RESPONSE FACTOR (ERF) domain transcription factors function in JA/ET signaling and defense against fungal pathogens (Lorenzo et al., 2003). In bik1 plants, ERF4 expression was significantly upregulated in response to B. cinerea and Pst (Figure 4I). By contrast, ERF104 induction in bik1 shows a severe reduction in response to B. cinerea and is completely abolished in response to Pst (Figure 4J). ERF104 contributes to plant immune responses, flg22 growth responses, and resistance to B. cinerea (Bethke et al., 2009). Similarly, B. cinerea–induced ORA59 expression is significantly attenuated in bik1 (Figure 4K). ORA59 regulates JA- and ET-mediated expression of several defense genes and contributes to B. cinerea resistance through the integration of these two signaling pathways (Pré et al., 2008; Leon-Reyes et al., 2010; Zarei et al., 2011). Expression of FRK1, a marker for PTI, is also severely impaired in bik1 in response to B. cinerea and Pst (Figure 4L). These data further strengthen the role of BIK1 in ET-dependent PTI responses.
BIK1 Residues Required for Phosphorylation Are Also Required for BIK1 Function in Seedling Growth Responses to ET and flg22
The bik1;35S:BIK1-HA line complemented all growth phenotypes of the bik1 mutant as well as the seedling response to flg22 and ACC (Figures 5A and 5B). Among the substitution mutants, BIK1S33A partially restored growth sensitivity to ACC similar to its role in B. cinerea resistance, whereas BIK1T64A, BIK1T94A, BIK1S233A, BIK1Y234A, and BIK1Y245A led to full restoration, suggesting that these residues are dispensable for BIK1 function in ET responses. By contrast, BIK1K105A, BIK1D202A, BIK1S236A, BIK1T237A, and BIK1T242A failed to complement the bik1 triple response phenotype, correlating with their importance in other BIK1 functions. Interestingly, whereas Thr-94 is required for basal and flg22-PTI to B. cinerea, it is completely dispensable for ET responses at the concentration tested.
Figure 5.
Ala Substitution Mutants Reveal BIK1 Residues Required for the Arabidopsis Triple Response.
Triple response phenotypes (A) and hypocotyl lengths (B) of bik1 and BIK1 substitution seedlings on 20 μM ACC (+) and unsupplemented MS media (−). Data in (B) represent mean ± se from a minimum of 60 seedlings. Experiments were repeated at least three times with similar results. Statistical analysis was performed as described in the legend of Figure 2. All BIK1 site-directed mutants are expressed in the bik1 background. wt, wild type.
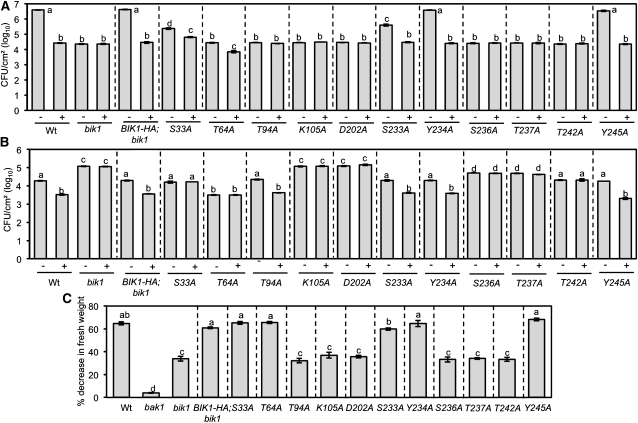
BIK1 Residues Required for Phosphoryation Regulate PTI Responses to Virulent and Nonpathogenic Strains of P. syringae
Although bik1 plants are resistant to PstDC3000, they are susceptible to the nonpathogenic strain PstDC3000 hrcC- (Pst hrcC-), which lacks Type III secretion but retains ability to activate PTI responses (Zhang et al., 2010) (Figures 6A and 6B). The bik1;BIK1-HA plants showed wild-type responses to both PstDC3000 and PsthrcC− strains. Interestingly, many of the substitution mutants retained full or partial resistance to PstDC3000 relative to bik1 (Figure 6A). BIK1S33A partially restored wild-type responses to PstDC3000 and flg22-PTI, whereas BIK1T64A maintained bik1 resistance yet partially complemented PTI in the mutant. Conversely, the BIK1 AD residue Ser-233 is partially required for basal resistance to PstDC3000 but not flg22-induced protection. Only BIK1Y245A and BIK1Y234A fully restored wild-type responses to PstDC3000 both before and after flg22-PTI, analogous to their rescue of bik1 resistance to necrotrophic infection. Since bik1 resistance to PstDC3000 is largely due to high endogenous SA (Veronese et al., 2006), it is likely that substitutions other than BIK1Y245A and BIK1Y234A failed to restore normal SA levels. This may also suggest residues Tyr-234 and Tyr-245 are sufficient for suppression of SA accumulation.
Figure 6.
BIK1 Mediates Plant Responses to Pst Strains and flg22.
(A) and (B) Bacterial growth ([cfu]/cm2 leaf area) in water- (−) and flg22-treated (+) plants 3 d after inoculation with PstDC3000 (A) or the nonpathogenic Pst hrcC− strain (B), deficient in Type III secretion. CFU, colony-forming units; wt, wild type.
(C) Percentage decrease in fresh weight of seedlings after growth in 10 nM flg22. Data represent mean values ± se from three experiments and a minimum of 120 seedlings for bacterial growth and fresh weights, respectively. Experiments were repeated at least three times with similar results. Statistical analysis was performed as described in the legend of Figure 2. All BIK1 site-directed mutants are in bik1.
When tested for basal resistance and flg22-PTI to PsthrcC−BIK1T94A, BIK1S233A, BIK1Y234A, and BIK1Y245A fully restored both responses, the phenotypes of the latter three being consistent with their dispensability in BIK1-mediated ET growth responses and defense against B. cinerea (Figure 6B). BIK1S33A and BIK1T64A are required for PTI, yet BIK1S33A restored wild-type level of resistance, whereas BIK1T64A actually conferred increased basal resistance. Thus, the already heightened resistance of BIK1T64A may account for its apparent unresponsiveness to flg22 as it may have already reached a threshold for resistance. This also suggests phosphorylation of Thr-64 may suppress plant responses to PsthrcC−. BIK1 Lys-105, Asp-202, Ser-236, Thr-237, and Thr-242 are required for full resistance as their Ala substitutions failed to rescue bik1 susceptibility (Figure 6B). BIK1K105A and BIK1D202A were the only substitutions that completely failed to rescue both basal and flg22-PTI consistent with their conserved and critical role in kinase catalytic activity. Surprisingly, BIK1T242A showed no difference from the wild type in basal defense against PsthrcC− but was unresponsive in flg22-PTI, suggesting that this residue is required for flg22-induced resistance.
The Role of BIK1 in flg22 Growth Responses Correlated with Immune Response Function and Kinase Activity
To determine whether the role of BIK1 in flg22-induced immunity correlated with its function in flg22-induced growth responses, we assayed the BIK1 substitution lines for growth sensitivity to the flg22 peptide. The average decrease in fresh weight of bik1 seedlings on flg22 was roughly half that observed for the wild type yet significantly higher than that of the insensitive bak1 mutant (Figure 6C). BIK1K105A, BIK1D202A, BIK1S236A, BIK1T237A, and BIK1T242A failed to restore wild-type sensitivity analogous to their failure to rescue the other bik1 mutant phenotypes. This confirms a direct correlation between the role of BIK1 in ET and flagellin perception and regulation of immune responses. BIK1T64A, BIK1T94A, BIK1Y234A, and BIK1Y245A led to full restoration of flg22 sensitivity, whereas it was only partially restored in BIK1S233A. The partial sensitivity of BIK1S233A correlates only with its PstDC3000 susceptibility as it fully rescued all other bik1 phenotypes. As the majority of bik1 resistance to PstDC3000 is based on elevated SA, this may suggest S233 is required for BIK1 function in integrating flagellin and SA responses.
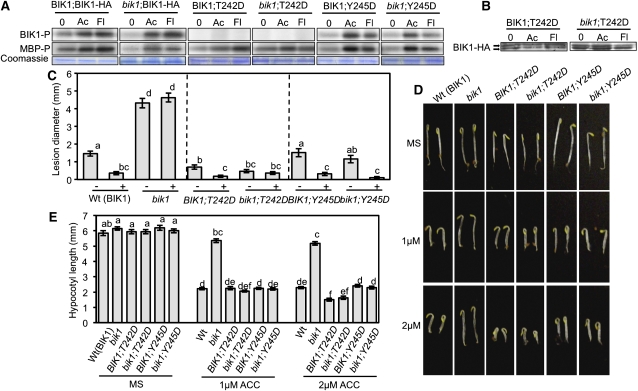
BIK1 Phosphomimic Mutation at Thr-242 Is Sufficient for Increased ET Sensitivity and Resistance to B. cinerea
The activation of protein kinases is regulated by phosphorylation events in the AD (Johnson et al., 1996). Among BIK1 AD phosphorylation sites, residues Thr-242 and Tyr-245 are invariant in BIK1-related kinases but also affect BIK1 kinase activity (Figures 1B and 1C). These two BIK1 residues were substituted with Asp to create phosphomimic BIK1 mutations and transformed into bik1 and wild-type plants. Acidic amino acids such as Asp can mimic the negative charge conferred by phosphorylation and lead to partial or constitutive activation of kinases (Johnson et al., 1996). In vivo kinase assays of BIK1T242D and BIK1Y245D suggest that they do not lead to constitutive activation but result in altered phosphorylation and plant phenotypes (Figure 7). Relative to bik1;BIK1-HA, the BIK1;BIK1-HA plants have higher basal BIK1 and MBP phosphorylation, which becomes further enhanced upon treatment with ACC or flg22 (Figure 7A). In both wild-type and bik1 plants, BIK1Y245D displayed ACC and flg22-induced kinase activity, with ACC eliciting a more robust increase in both BIK1 and MBP phosphorylation than flg22. BIK1;BIK1T242D displays MBP phosphorylation comparable to that of BIK1;BIK1-HA yet has no observable BIK1 phosphorylation with or without induction. Similarly, BIK1 phosphorylation was not detected for bik1;BIK1T242D despite clear phosphorylation of MBP. To further understand the role of T242 in BIK1 kinase activity and determine if the loss of BIK1T242D phosphorylation is a result of substitution or artifact due to its activity being below the sensitivity threshold of our assay, we performed immunoblots on precipitated BIK1T242D to compare basal and induced phosphorylation (Figure 7B). In wild-type and bik1 plants, without ACC or flg22, BIK1T242D shows phosphorylation that does not change after treatment, suggesting constitutive activation (Figure 7B). This may account for the lack of increase in BIK1 phosphorylation with [γ-32P]ATP as BIK1T242D phosphosites are already occupied with unlabeled phosphate; this also supports the ability of the protein to phosphorylate MBP as it is likely present in an activated state.
Figure 7.
Phosphomimic Substitution at Thr-242 in the AD of BIK1 Confers Enhanced ET Responses and PTI to B. cinerea.
(A) In vivo kinase activity of the BIK1T242D and BIK1Y245D substitution mutants in response to ACC (Ac) and flg22 (Fl). Kinase activity of the phosphomimic mutants visualized by audioradiograms of radiolabeled proteins following incubation with [γ-32P]ATP.
(B) Constitutive activation of BIK1 phosporylation by the BIK1T242D phosphomimic mutation. BIK1 phosphorylation was visualized as a mobility shift detected by HA-immunoblot. Coomassie blue was used as a loading control.
(C) Lesion diameter in water (−) and flg22 (+) pretreated plants following drop inoculation with B. cinerea (2.5 × 105 spores/mL). Wt, wild type.
(D) and (E) Triple response (D) and average hypocotyl length (E) of seedlings on different concentrations of ACC (μM) (+) or unsupplemented MS media (−).
In (A), protein staining with Coomassie blue was used as a loading control. Data in (C) and (E) represent mean ± se from a minimum of 30 disease lesions or 60 seedlings, respectively. Experiments were repeated at least three times with similar results. Statistical analysis was performed as described in the legend of Figure 2.
[See online article for color version of this figure.]
Subsequent phenotypic analyses revealed that BIK1;BIK1T242D and bik1;BIK1T242D plants have increased resistance to B. cinerea relative to their respective controls, with BIK1;BIK1T242D further enhanced for flg22-PTI (Figure 7C). BIK1Y245D had no effect on B. cinerea resistance in the presence of functional BIK1 but rescued susceptibility of the bik1 mutant. Expression of BIK1Y245D in both backgrounds also significantly enhanced the disease resistance conferred by flg22 treatment. Basal resistance to PstDC3000 was restored to wild-type levels in BIK1;BIK1T242D, BIK1;BIK1Y245D, and bik1;BIK1Y245D lines, whereas bik1;BIK1T242D susceptibility was only partially restored (see Supplemental Figure 4A online). All lines showed flg22-PTI to B. cinerea, with resistance in bik1;BIK1Y245D and BIK1;BIK1T242D lower and exceeding that observed for wild-type plants, respectively. Both phosphomimic mutations also restored bik1 PTI and susceptibility to Pst hrcC− with minimal gains of resistance observed for bik1;BIK1T242D and BIK1;BIK1Y245D plants (see Supplemental Figure 4B online). Growth responses to flg22 were restored to wild-type levels for all the Asp substitution lines other than bik1;BIK1Y245D, which maintained slight insensitivity (see Supplemental Figure 4C online). In contrast with BIK1T242A, which failed to complement bik1, the BIK1T242D and all of the phosphomimic transgenic lines displayed wild-type triple responses on 20 μM ACC (see Supplemental Figure 5 online). However, at 2 μM ACC, BIK1T242D conferred enhanced sensitivity observable as significantly shorter hypocotyls relative to both wild-type and bik1 seedlings (Figures 7D and 7E).
BIK1 Thr-242 and Tyr-245 Are Required for Negative Regulation of Plant Growth Similar to Their Function in Suppression of P. syringae Resistance
Loss of BIK1 function results in altered growth and development characterized by small statured early-flowering plants with exaggerated leaf serration and weak stems (Veronese et al., 2006). Overall, the growth patterns in bik1 plants expressing the BIK1 substitutions largely followed their contributions to other functions of BIK1, particularly ET sensitivity. Interestingly, both BIK1T242A and BIK1Y245A grew significantly bigger leaves and were larger in overall stature relative to wild-type plants (see Supplemental Figures 6A and 6B online). However, leaves of BIK1T242A are more narrow and serrated than the bik1 mutant, whereas those of BIK1Y245A display wild-type morphology. Thus, it is possible that phosphorylation of these residues negatively regulate plant growth, with Thr-242 also contributing to leaf development. BIK1T64A and BIK1T94A were generally smaller than bik1 plants.
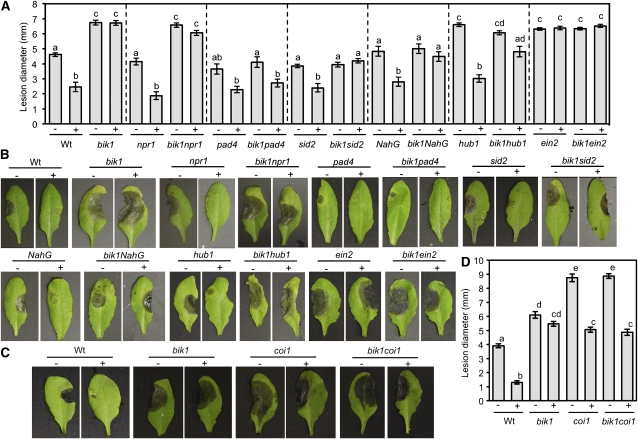
flg22-Induced PTI to B. cinerea Requires BIK1, EIN2, and HUB1 but Is Antagonized by COI1
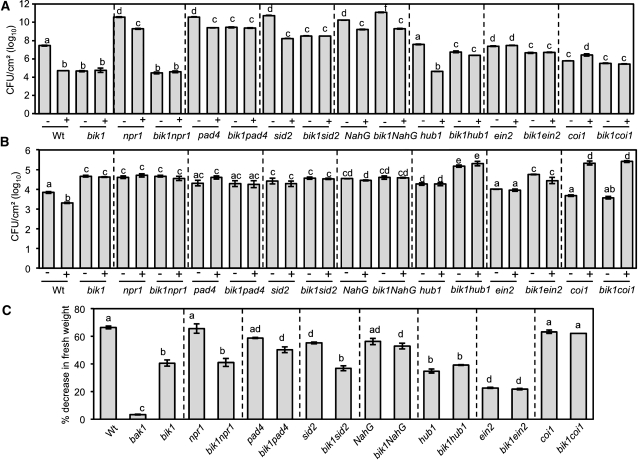
To determine the genetic interaction between BIK1 and other regulators of Arabidopsis immune responses, double mutants between bik1 and npr1 (Cao et al., 1994), sid2 (Dewdney et al., 2000), NahG (Delaney et al., 1994), pad4 (Glazebrook et al., 1997), ein2 (Guzmán and Ecker, 1990), coi1 (Xie et al., 1998), and histone monoubiquitination1 (hub1) (Liu et al., 2007; Dhawan et al., 2009) were generated and assayed for their effects on BIK1 mediated basal and flg22-PTI to fungal and bacterial pathogens. The npr1, pad4, and sid2 mutants and NahG transgenic plants showed wild-type responses to B. cinerea and were fully responsive to flg22-induced resistance, suggesting SA is dispensable for flg22-PTI to this pathogen (Figures 8A and 8B). The bik1 sid2 plants have wild-type basal resistance but show no flg22-PTI, suggesting that the loss of induced resistance in bik1 is independent of SA. These data were further confirmed by loss of flg22 PTI in bik1 NahG plants. Interestingly, loss of flg22-PTI in bik1 requires the functions of PAD4. Basal and flg22 PTI of bik1 npr1 plants was comparable to bik1, indicating that BIK1 function is independent or upstream of NPR1-dependent SA signaling during PTI.
Figure 8.
Epistasis Analysis of the Interaction between BIK1 and Other Immune Response Genes in flg22-Induced PTI to B. cinerea.
Lesion diameter ([A] and [D]) and disease symptoms ([B] and [C]) in water (−) and flg22 (+) pretreated single and double mutants after drop inoculation with B. cinerea (2.5 × 105 spores/mL). Data in (A) and (D) represent mean ± se from a minimum of 30 disease lesions. Experiments were repeated at least three times with similar results. Statistical analysis was performed as described in the legend of Figure 2. Images were taken 3 d after inoculation. Wt, wild type.
As previously reported the bik1, coi1, hub1, and ein2 mutants have increased susceptibility to B. cinerea (Thomma et al., 1998; Veronese et al., 2006; Dhawan et al., 2009) (Figure 8). The coi1 and hub1 mutants were competent in flg22-induced resistance to B. cinerea, whereas similar to bik1, flg22 PTI was blocked in ein2 (Figures 8A to 8D). The bik1 hub1 double mutant showed susceptibility similar to both parental lines but displayed a partial loss of flg22-induced resistance, suggesting that histone H2B monoubiquitination contributes to PTI. The disease susceptibility of bik1 coi1 and bik1 ein2 plants was comparable to coi1 and ein2, respectively. The loss of flg22-PTI in ein2 bik1 was comparable to that of its parental mutants. Despite the extreme susceptibility of the untreated coi1 mutant, it was significantly protected by flg22, indicating that COI1 is not required for flg22-PTI. Intriguingly, the highly susceptible bik1 coi1 double mutant was also significantly protected by flg22. Thus, it appears that loss of flg22-PTI in bik1 plants is dependent on intact COI1-mediated JA responses, raising the intriguing possibility that COI1 may function as a repressor of BIK1. In sum, flg22-induced resistance to B. cinerea is independent of SA accumulation, but EIN2 and BIK1 are critical positive regulators of both basal and flg22-PTI and COI1 is either dispensable for PTI or suppresses the loss of PTI in bik1.
bik1 Susceptibility to A. brassicicola Is Dependent on SA Signaling and Synthesis
The role of the various defense genes on BIK1-mediated resistance to A. brassicicola was determined by comparing the level of in planta fungal DNA accumulation (as a measure of fungal growth) and the size of disease lesions (see Supplemental Figures 7A and 7B online). Similar to their responses to B. cinerea, bik1 sid2 and bik1 NahG have wild-type resistance to A. brassicicola. Disease lesion size in bik1 ein2 plants was comparable to that observed for bik1 but fungal growth decreased relative to bik1. Resistance in bik1 hub1 was comparable to both parental lines, indicating these two genes likely affect the same defense pathway during A. brassicicola infection. Based on lesion diameter, no significant difference was observed between coi1 and bik1 coi1 plants, yet the double mutant did support significantly lower fungal growth compared with coi1. The difference in fungal accumulation may be a result of nutrient depletion due to the extreme necrosis in bik1 coi1. Overall, the genetic requirements for BIK1-mediated resistance to A. brassicicola and B. cinerea were similar with the exceptions of PAD4 and NPR1, which have different contributions.
Basal and Induced PTI Responses to Virulent and Nonpathogenic Pst Strains Are Modulated by SA, ET, and JA Responses
We assayed the bik1 double mutants for basal and flg22-induced resistance to virulent and nonpathogenic Pst strains to compare the genetic requirements for responses to those during necrotrophic infection. In noninduced plants, bik1 resistance is independent of NPR1 but requires PAD4, EIN2, HUB1, and SA accumulation (Figure 9A). Though the level of resistance varied, hub1, npr1, pad4, sid2, and NahG were responsive to flg22-induced PTI, supporting less bacterial growth than their respective water-treated controls. Flg22-PTI was fully lost in the bik1 npr1, bik1 pad4, bik1 sid2, and bik1 hub1 double mutants. While bik1 npr1 maintained resistance comparable to bik1, pad4 bik1 and sid2 bik1 were significantly more susceptible than bik1 but less than pad4 and sid2, respectively, suggesting that bik1 resistance is partially dependent on SA signaling. Similarly, bik1 responses to PstDC3000 are partially dependent on HUB1 as the double mutant shows a loss of resistance relative to bik1, with the disease phenotype of bik1 hub1 plants being intermediate to both parental lines. ein2 plants showed increased basal resistance relative to the wild type and remained unresponsive to flg22, mirroring the responses of bik1. bik1 ein2 maintained resistance intermediate to both single mutants, while both the double mutant and ein2 were unresponsive to flg22. Intriguingly, the coi1 mutant showed enhanced susceptibility after flg22 treatment, suggesting that COI1 suppresses flg22-PTI to PstDC3000 although the coi1 mutant was responsive to flg22-PTI to B. cinerea, revealing a distinct role for COI1 in PTI to bacterial and fungal pathogens. The bik1 coi1 plants maintained coi1 levels of susceptibility, showing a loss of resistance relative to bik1 but lost flg22-PTI to PstDC3000.
Figure 9.
The Role of Arabidopsis Immune Response Genes on the Functions of BIK1 in flg22-Induced PTI to Virulent and Nonpathogenic Strains of Pst.
(A) and (B) Bacterial growth ([cfu]/cm2 leaf area) in water (−) and flg22 (+) pretreated single and double mutants 3 d after inoculation with Pst DC3000 (A) or the nonpathogenic Pst hrcC− strain (B), deficient in Type III secretion. CFU, colony-forming units; Wt, wild type.
(C) Percentage decrease in fresh weight of seedlings after growth in 10 nM flg22.
Data in (A) to (C) represent mean values ± se from three experiments and a minimum of 120 seedlings, respectively. Experiments were repeated at least three times with similar results. Statistical analysis was performed as described in the legend of Figure 2.
Inoculation with Pst hrcC− revealed a loss of basal resistance for all the mutants except ein2, coi1, and bik1 coi1 (Figure 9B). This suggests that basal resistance to Pst hrcC− requires SA synthesis and signaling as well as HUB1 function. The hub1 mutant, impaired in chromatin modifications, shows no altered responses to PstDC3000 but has enhanced susceptibility to Pst hrcC− that is unresponsive to flg22-PTI. Interestingly, susceptibility of bik1 hub1 is higher than that of its parental lines showing an additive action of HUB1 and BIK1 in resistance to Pst hrcC−. Similar to PstDC3000, flg22 treatment dramatically increased coi1 susceptibility. However, whereas PstDC3000-inoculated bik1coi1 plants appeared unresponsive to flg22-induced PTI, flg22 increased susceptibility to Pst hrcC− in the double mutant to the same degree as observed for coi1.
The coi1 Mutation Suppresses bik1 Seedling Growth Insensitivity to flg22
The roles of SA, JA, and ET in BIK1-mediated seedling growth responses to flg22 were tested to determine the relationship between flg22-induced disease resistance and seedling sensitivity to the peptide (Figure 9C). npr1, pad4, NahG, and coi1 seedlings showed no altered growth responses to flg22, whereas sid2 was slightly but significantly insensitive to flg22. COI1 is dispensable for flg22 growth responses, but the coi1 mutation restores bik1 sensitivity to flg22 to wild-type level. This suggests insensitivity of bik1 to flg22 is dependent on functional JA responses. The insensitivity of bik1 npr1 and bik1 sid2 plants mirrored bik1. By contrast and consistent with recent reports (Boutrot et al., 2010), ein2 insensitivity exceeded that of bik1, with bik1 ein2 seedlings showing ein2 levels of flg22 growth inhibition. Interestingly, both hub1 and bik1 hub1 have insensitivity comparable to bik1. In sum, similar to PTI to B. cinerea, bik1 growth responses to flg22 appear to be dependent on PAD4 functions and COI1-mediated JA responses. The growth responses to flg22 correlated with the susceptibility to B. cinerea and Psthrcc− in bik1, hub1, and ein2. Furthermore, the insensitivity of hub1 and bik1 hub1 is consistent with HUB1 functioning in the same pathway as BIK1 in PTI to PsthrcC− as well as defense against necrotrophic infection.
BIK1 Function in Seedling Growth Responses to ET Is Independent of SA and HUB1-Mediated Responses
All the double mutants with the exception of bik1 ein2 are comparable to bik1 in their insensitivity to ACC, suggesting that bik1 growth responses to ET are independent of SA-related functions or ET is upstream of SA and HUB1 related functions (see Supplemental Figures 8A and 8B online). At higher concentrations of ACC, bik1 ein2 actually has increased insensitivity compared with both bik1 and ein2 based on hypocotyl length (see Supplemental Figures 8C and 8D online). The susceptibility of the double mutants to B. cinerea correlated with seedling growth responses to ET only in npr1 bik1 (Figures 8A and 8B). SA accumulation has a significant impact on bik1 responses to B. cinerea, whereas seedling ET responses are independent of SA.
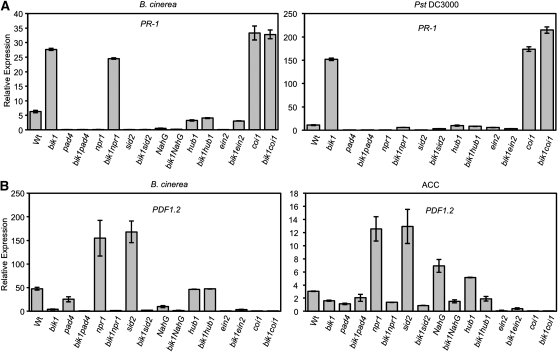
BIK1 Regulates SA- and JA/ET-Regulated Defense Gene Expression
Previously, we showed that the increased B. cinerea–induced PR-1 expression in bik1 can be suppressed with removal of SA (Veronese et al., 2006). Interestingly, high PR-1 expression is maintained in bik1 npr1 plants but completely abolished in bik1 sid2 and bik1 pad4 (Figure 10A). This reaffirms the requirement of SA for BIK1-dependent PR-1 expression and suggests that, in response to B. cinerea, BIK1 can bypass NPR1 regulation of PR-1. By contrast, combination with the npr1 mutation abolished bik1 PR-1 expression in response to Pst, as did sid2, pad4, hub1, and ein2 (Figure 10A). The high level of PR-1 expression in B. cinerea–infected bik1 coi1 plants showed no significant difference from that of bikl and coi1; however, Pst-induced PR-1 expression in the double mutant is higher than both parental lines. The ein2 single and bik1 ein2 double mutants have attenuated PR-1 expression relative to that of bik1 regardless of the pathogen, suggesting that EIN2 may positively function in the regulation of some SA responses during defense.
Figure 10.
Expression of Defense Marker Genes in bik1 and the Double Mutants in Response to Pathogen Infection and ACC.
(A) B. cinerea and PstDC3000-induced expression of PR-1. Wt, wild type.
(B) B. cinerea and ACC-induced expression of PDF1.2.
Expression was determined using quantitative RT-PCR 24 h after inoculation/treatment as described in Methods. Experiments were repeated at least two times with similar results.
Arabidopsis defensin PDF1.2 expression, a molecular marker for JA/ET responses, is induced in response to B. cinerea, ET, and JA in wild-type plants but reduced in bik1. In response to B. cinerea and ACC, npr1 and sid2 have significantly higher levels of PDF1.2 expression than wild-type and bik1 plants, indicating the suppressive role of SA on PDF1.2 (Figure 10B). Although the B. cinerea susceptibility of the bik1 mutant was rescued through removal of SA function by genetic crosses to pad4, sid2, or NahG, they did not restore PDF1.2 expression. The decrease in PDF1.2 expression correlates to B. cinerea susceptibility only for bik1npr1 plants, suggesting that B. cinerea resistance can be uncoupled from PDF1.2 expression. The expression of PDF1.2 in response to ACC was comparable to that observed in response to B. cinerea in the single and double mutants.
BIK1 Is Unique among Closely Related Kinases for Its Role in Defense against Necrotrophic Infection and Responses to ET
Arabidopsis BIK1 shares high sequence similarity to many Arabidopsis RLCKs (Veronese et al., 2006). BIK-like protein (At3g55450; PBL1) shows the highest sequence identity followed by APK1b (At2g28930), APK1a (At1g07570), and APK2b (At2g02800) to BIK1 (Veronese et al., 2006). Most of these also harbor conserved residues targeted for cleavage by the bacterial effector protease AvrPphB, with PBS1 and BIK1 both suggested virulence targets (Zhang et al., 2010). However, mutation in the BIK1-related kinases resulted neither in increased susceptibility to B. cinerea or A. brassicicola nor ET insensitivity (see Supplemental Figure 9 online). Thus, BIK1 is distinct or has evolved an important regulatory role in plant growth and defense. Additionally, though seedlings of pbs1 mutant alleles did not show enhanced insensitivity to ACC, some seedlings displayed altered growth featuring constitutive triple response in the dark on Murashige and Skoog (MS) media (see Supplemental Figure 9D online).
DISCUSSION
Here, we demonstrate the biochemical, molecular, and genetic bases of BIK1 function in PTI to the necrotrophic fungus B. cinerea and the hemibiotrophic bacterial pathogen Pst, extending our previous observations into the mechanisms underlying the role of BIK1 as an immune response regulator. In vitro and in vivo biochemical assays using BIK1 site-directed mutants identified BIK1 residues contributing to BIK1 and transphosphorylation as well as their biological roles in ET and PTI responses. The AD residues Ser-236, Thr-237, and Tyr-245 are critical for BIK1 and MBP phosphorylation and BIK1 biological function. BIK1T242A abrogated induced kinase activity and BIK1 function, whereas BIK1T242D was sufficient for enhanced PTI. Three KD residues had a differential role in seedling growth responses to ET and flg22, indicating the differential contributions of BIK1 phosphorylation sites. Mutation of the conserved Asp, which is part of the VIb domain in RD kinases, abrogated all functions of BIK1. Plants expressing BIK1Y245A grew significantly larger than the wild type, identifying Tyr-245 as important for negative regulation of plant growth. The effects of the site-directed mutants on phosphorylation and plant immune responses and growth phenotypes are summarized in Table 1. flg22-triggered immune response assays determined the genetic and biochemical requirements for BIK1-mediated PTI responses to B. cinerea and Pst. BIK1 is required for ET signaling that is important for its immune response as well as plant growth functions. Conversely, ET perception regulates BIK1 phosphorylation in response to ACC and flg22, further reinforcing the action of BIK1 in ET signal transduction. ET also regulates BIK1 transcription through EIN3, which is found associated with the BIK1 promoter. Epistasis analysis defined interactions between BIK1 and other plant immune response regulators. Histone monoubiquitination was found to potentiate PTI to bacterial and fungal pathogens as well as seedling growth responses to flg22 synergistically with BIK1. COI1 antagonizes flg22-induced immunity, while EIN2 and BIK1 are central positive regulators of PTI and ET signaling. Elevated SA accumulation in the bik1 mutant suppresses resistance to B. cinerea, but SA deficiency had no impact on flg22-PTI to this pathogen. Alternatively, SA is required for flg22-induced PTI to Pst hrcC−. In sum, BIK1 is a regulatory component of immune responses to P. syringae and necrotrophic fungi, with its function in immune responses dependent on its kinase activities and ET signaling.
Table 1.
Summary of Phenotypes and Kinase Activities of BIK1 Ala Substitution Mutants
| Phenotype/Activity | BIK1-HA | S33A | T64A | T94A | K105A | D202A | S233A | Y234A | S236A | T237A | T242A | Y245A |
| B. cinerea (basal/flg22 PTI) | +/+ | IN/- | -/IN | −/− | −/− | −/− | +/+ | +/+ | −/− | −/− | −/− | +/+ |
| A. brassicicola | + | IN | IN | - | - | - | + | IN | - | - | - | + |
| PstDC3000 (basal/flg22 PTI) | +/+ | IN/IN | -/D | −/− | −/− | −/− | IN/+ | +/+ | −/− | −/− | −/− | +/+ |
| Pst hrcC− (basal/flg22 PTI) | +/+ | +/− | D/+ | +/+ | −/− | −/− | +/+ | +/+ | IN/IN | IN/IN | +/− | +/+ |
| Growth sensitivity to flg22 | + | + | + | - | - | - | IN | + | - | - | - | + |
| Triple response | + | D | + | + | - | - | + | + | - | - | - | + |
| Plant growth | + | D | D | - | - | - | + | + | - | - | D | D |
| In vivo kinase activity (BIK1/MBP) | +/+ | IN/- | -/IN | −/− | −/− | −/− | +/+ | +/+ | −/− | −/− | D/- | −/− |
+, The wild type, complementation; −, bik1, no complementation; IN, intermediate; D, differential responses relative to the wild type and bik1. For kinase activity, (+) retains activity, while (−) loss of activity
Multiple lines of evidence support BIK1 involvement in ET signaling and the ensuing function in plant immune responses: (1) bik1 is impaired in typical seedling growth responses to ET, which is further confirmed by its hypersensitivity to Glc; (2) expression of several ET response genes, including PDF1.2 and ERF104, is dependent on functional BIK1; (3) pathogen, ACC, and flg22-induced BIK1 expression is strictly dependent on EIN3, a transcription factor central to ET signaling, suggesting that BIK1 is an EIN3 target; and (4) BIK1 kinase activity increases in response to ACC and flg22, with inhibition of ET perception blocking induction. In addition, BIK1 site-directed mutants that abrogate the triple response also abrogate immune responses and seedling flg22 growth, so (5) BIK1 kinase activity correlated with ET responses such that mutations that attenuate ET responses also eliminate plant immune responses. Consistent with this, ein2 was recently isolated as a flagellin-insensitive mutant and shown to have defects in PTI to Pst (Boutrot et al., 2010). In addition, expression of FLS2, encoding the flagellin receptor, is directly regulated by EIN3. Thus, ET regulates the different components of the flg22-receptor complex.
Plant RLKs function in diverse physiological processes but show remarkable sequence conservation. Comparisons of RLK sequences revealed 80% or greater identity in phosphorylatable residues in the AD- of RD-type RLKs (Wang et al., 2005). Variation in biological functions is thus attributed to differences in posttranslational regulation, including phosphorylation and interactions with up- or downstream partners. Both general and specific regulatory mechanisms have been associated with the control of physiological processes by RLKs. For instance, autophosphorylation of less conserved N- and C-terminal KD regions is suggested to create docking sites for kinase substrates to achieve specificity (Johnson et al., 1996). Accordingly, mutations in the KD phosphorylation sites Ser-33, Thr-64, and Thr-94 have differential roles in BIK1-mediated PTI, flg22, and ET growth responses. The differential impact of S33A and T64A on BIK1 phosphorylation in vitro as well as BIK1 phosphorylation in response to flg22 or ACC in vivo is also consistent with the role of this region in conferring specificity to BIK1 function. The phenotypes of the BIK1S33A and BIK1T64A lines also suggest that BIK1 phosphorylation sites have differential functions. BIK1T64A eliminated disease resistance but was dispensable for phosphorylation activities. BIK1 Thr-94 is required for basal and induced resistance to B. cinerea and growth responses to flg22 but not the triple response. It is not clear why BIK1 T64A impairs PTI as it maintains kinase activity. The equivalent residue in Pto (Pto Thr-38) is the main site of autophosphorylation and required for hypersensitive response (Sessa et al., 2000).
The AD of protein kinases occupies the catalytic cleft harboring the regulatory elements, including the T-loop, where activating phosphorylation events occur, and the C-terminal P+1 loop, which plays a role in recognition and binding of protein substrates (Johnson et al., 1996). bik1 substitution mutants reveal the importance of the conserved AD residues Ser-236, Thr-237, and Thr-242 for kinase activity and BIK1 function in planta. In other protein kinases, phosphorylation of two to three AD residues is required for kinase activation. BIK1 Thr-242 falls in the T-loop, suggesting its phosphorylation may activate BIK1. T242A abolished induced kinase activity in vivo, PTI responses to all pathogens tested, and seedling sensitivity to flg22 and ACC, suggesting its critical contributions to the function of BIK1. The increased phosphorylation of BIK1T242A without elicitation suggests it may negatively regulate phosphorylation. Pto Thr-204 corresponds to BIK1 Thr-242 and is similarly important to Pto function, suggesting that the biological relevance of these residues is conserved. Phosphomimic mutation at BIK1 Thr-242 was sufficient for increased basal and flg22-induced resistance to B. cinerea. BIK1Y245A and BIK1Y234A were the only substitutions that completely rescued resistance to PstDC3000. This suggests these residues are sufficient for BIK1 function in the negative regulation of resistance to PstDC3000 and may possibly act by affecting SA synthesis. The phosphomimic Pto mutants Y207D (BIK1 Y245) and T204D (BIK1 T242) activate hypersensitive response in the absence of AvrPto (Sessa et al., 2000), whereas the Pto Tyr-207 and Thr-204 Ala substitution mutants failed to interact with AvrPto (Frederick et al., 1998; Rathjen et al., 1999). Thus, BIK1 Tyr-245 may influence substrate binding, whereas Thr-242 is likely required for substrate recognition and specificity. These observations suggest the C-terminal region of the AD is an important regulatory region in RLCKs. Overall, the loss of biochemical activity in BIK1 correlated with loss of basal and flg22-PTI as well as ET responses in the site-directed mutants. Finally, the phosphorylation results from BIK1-HA plants may have been influenced by proteins that coprecipitate with BIK1. Since BIK1 interacts or is likely to interact with many RLKs, including FLS2, BAK1, CERK1, and ERF1 (Lu et al., 2010; Zhang et al., 2010), it will be difficult to completely exclude this possibility. However, most of our results from the in vitro and in vivo experiments correlated, and the genetic data from the different genotypes support our conclusions.
Our genetic studies uncovered interactions between BIK1 and other components of the immune response pathways. The blunted PTI in the bik1 mutant is primarily due to inhibition of ET signaling. In support of this, the immune response functions of BIK1 mirror that of EIN2, a protein that regulates ET signaling and plays a central role in plant immune responses (Thomma et al., 1999; Boutrot et al., 2010). The responses of the ein2 bik1 double mutant to B. cinerea, PstDC3000, PsthrcC−flg22, and ACC are consistent with BIK1 and EIN2 functioning in the same pathway. Furthermore, the defects in ET signaling function of BIK1 may be the underlying cause of upregulated SA synthesis in the mutant. EIN3 and EIL proteins were recently shown to suppress SA synthesis through regulation of SID2 expression (Chen et al., 2009). Interestingly, EIN3 also directly regulates FLS2 transcription, thereby modulating PTI (Boutrot et al., 2010). Thus, components of ET signaling suppress SA and promote FLS2-mediated responses similar to BIK1. BIK1 expression is also EIN3 dependent through the direct transcriptional control by the later. The interaction between SA and ET in mediating PTI is complex, similar to the role of SA in BIK1-mediated immune responses. On one hand, BIK1 is a negative regulator of SA, with high SA accumulation in the bik1 mutant linked, at least partially, to its resistance to PstDC3000 and susceptibility to B. cinerea. On the other hand, SA is dispensable for flg22-PTI to B. cinerea but required for induced resistance to Pst hrcC−. The bik1 mutant, the single mutants in SA synthesis and signaling, and the double mutants with bik1 are susceptible to Pst hrcC−and the flg22-induced resistance is also completely abrogated. Overall, SA is important for flg22-PTI to Pst hrcC−but the basal susceptibility of bik1 to this strain is not linked to SA levels. NPR1-mediated SA signaling was completely dispensable for BIK1-mediated PTI responses. Alternatively, SA responses mediated by PAD4 contribute to the loss of basal and induced resistance to B. cinerea in the bik1 mutant. These observations are consistent with the role of SID2 and PAD4 in resistance to Pst hrcC− (Tsuda et al., 2008).
The growth response of bik1 seedlings to ACC was not affected by SA, suggesting that ET acts upstream of SA in the function of BIK1. Thus, the increased SA accumulation in bik1 may have resulted from defects in ET signaling. The expression of PDF1.2 was also not restored in bik1 by removal of SA, further supporting this conclusion. By contrast, bik1 seedling growth insensitivity to flg22 was modulated by SA. The susceptibility of bik1, hub1, ein2, ein2 bik1, and bik1 hub1 to Pst hrcC− correlated with their insensitivity to flg22. Overall, defects in ET signaling and histone H2B monoubiquitination are accompanied by flg22 insensitivity and impaired PTI responses.
The most unexpected result from the genetic analysis was the interaction between COI1 and BIK1. COI1 was dispensable for flg22-PTI to B. cinerea but essential for resistance in uninduced plants. Intriguingly, bik1 coi1 plants were responsive to flg22-mediated protection, implying that coi1 mutation suppresses the loss of flg22-PTI in bik1. Both coi1 and bik1coi1 showed resistance comparable to the wild type for Pst hrcC−again suggesting that coi1 mutation suppresses bik1 susceptibility. In addition, flg22 actually enhanced the susceptibility of coi1 and bik1 coi1 plants to Pst hrcC−. Furthermore, the flg22-insensitivity of bik1 seedlings was rescued by the coi1 mutation, consistent with flg22 enhancing susceptibility in coi1. Taken together, these data reveal that COI1 is either dispensable or suppresses PTI but also negatively regulates BIK1-mediated PTI. The higher basal resistance of nonelicited bik1 plants to B. cinerea relative to coi1 may indicate the increased contributions of COI1 to basal resistance in the absence of BIK1 and points to the role of BIK1 in limiting the contributions of COI1. The molecular mechanisms through which BIK1 suppresses COI1 function need to be investigated in the future. In the case of PstDC3000, coi1 failed to rescue bik1 responses to flg22 protection likely due to effector-mediated suppression of PTI. The E3 ligase HUB1, required for Histone H2B monoubiquitination, contributes to resistance to necrotrophic fungi but is dispensable for flg22-PTI to B. cinerea. By contrast, HUB1 contributes immensely to flg22-PTI to Pst hcC−with bik1 hub1 significantly more susceptible than any other double mutant, revealing that the role of HUB1 in flg22-induced PTI is additive to BIK1. It is likely that HUB1 is required for activation of PTI responses at the transcriptional level.
In conclusion, the central role of BIK1 in immune responses and plant growth is tightly linked to its signaling function in ET responses. Flagellin, a bacterial PAMP, induces PTI responses to distinct class of pathogens, suggesting a convergence of downstream immune responses regardless of the trigger consistent with recent reports that show BIK1 integrates immune responses from different upstream regulators (Zhang et al., 2010). Remarkably, our results reveal the complexity of PTI as well as the common and distinct genetic requirements underlying plant immune responses to pathogens with distinct pathogenesis strategies. Based on the documented biochemical data, activation of BIK1 may be linked to promotion or suppression of disease as well as PTI.
METHODS
Plant Growth Conditions, Diseases Assays, and flg22 Growth Responses
Plant growth conditions, fungal growth media, and fungal disease assays were done as previously described (Dhawan et al., 2009). Plants were grown in soil in growth chamber (Percival AR75L) with light intensity (140 to 150 μE m−2 s−1), temperature (24°C), relative humidity (70%), and a 12-h-light/12-h-dark cycle. flg22- and wound-induced resistance to Botrytis cinerea was performed as described (Ferrari et al., 2007; Chassot et al., 2008). Bacterial cultures and disease assays were performed as described by infiltrating 4-week-old plants with 106 colony-forming units (cfu)/mL of Pseudomonas syringae DC3000 or the hrcC− mutant derived from DC3000 1 d after infiltration with 1 μM flg22 or double deionized water (Zheng et al., 2006; Zhang et al., 2010). Assays for seedling growth sensitivity to flg22 were performed as previously described using the bak1 mutant as a positive control for insensitivity (Chinchilla et al., 2007). Silver nitrate treatment to inhibit ET signaling was performed on 4-week-old plants as described (Colville and Smirnoff, 2008). Seeds for mutants were obtained from the ABRC. ein3 and ein2 seeds were obtained from Joe Ecker’s lab at the SALK institute.
Generation of Recombinant Proteins and Transgenic Lines
For purification of recombinant BIK1 and BIK1 substitution mutants, the open reading frame of BIK1 was cloned into glutathione S-transferase fusion protein expression vector pGEX4T-1 (Pharmacia). To generate transgenic lines expressing BIK1 and BIK1 substitution mutants, the full-length BIK1 cDNA (GeneBank: AEC09703) was cloned after the cauliflower mosaic virus 35S promoter into a modified version of binary vector pCAMBIA 99-1. Binary vectors were transferred into Agrobacterium tumefaciens strain GV3101 and transformed into Arabidopsis thaliana wild-type or bik1 plants (Clough and Bent, 1998). Transgenic plants were selected on medium containing hygromycin and lines selected based on protein expression following immunoblot analysis using an anti-HA antibody (Covance). Primers used are listed in Supplemental Table 1 online.
Construction of Double Mutants and Mutant Alleles
Generation of the bik1 NahG double mutant was previously described (Veronese et al., 2006). Generation and selection of all other bik1 double mutants were performed as previously described (Dhawan et al., 2009). In addition, DNA from ACC-selected bik1ein2 plants was sequenced to ensure plants were homozygous for the ein2 point mutation. The ein3-1 and eil1-3 (SALK_049679C) mutant alleles were obtained from the ABRC.
Immunoprecipitation, Kinase Assays, and Immunoblot Analysis
Tissue from 4-week-old plants 3 h after treatment with double deionized water, 5 μM flg22, or 20 μM ACC by homogenization was frozen and homogenized in cold immunoprecipitation buffer with added protease inhibitor cocktail (Sigma-Aldrich) (50 mM HEPES, 5 mM EDTA, 5 mM EGTA, 25 mM NaF, 150 mM NaCl, 0.5% Nonidet P-40, 0.5% Brij 58, 50 mM β-glycerol phosphate disodium salt pentahydrate, 2 mM DTT, and 10% glycerol at pH 7.5). Subsequent immunoprecipitation steps were performed as previously described using an anti-HA affinity matrix (Roche) with the exception of overnight incubation of the supernatant (Schulze et al., 2010). In vivo kinase assays were performed on immunoprecipitated proteins as previously described with or without the addition of 2 μCi of [γ-32P]ATP (Lu et al., 2010). Phosphorylation of MBP was analyzed by immunoblot using an antiphosphoserine/Thr antibody (ECM Biosciences) or autoradiography after separation on 12% SDS-PAGE. Autophosphorylation of BIK1 and mutated BIK1 proteins was analyzed by immunoblot using an anti-HA antibody (Covance) or autoradiography after separation on 12% SDS-PAGE. Expression, purification, and in vitro kinase assays for the recombinant BIK1 proteins was performed as previously described (Abuqamar et al., 2008). Total protein extracted from 4-week soil-grown Arabidopsis or 10-d-old seedlings treated for 24 or 3 h, respectively, with 5 μM flg22 or 20 μM ACC as described (Zhang et al., 2010) was analyzed via immunoblots using anti-EIN3, WRKY33 (Qiu et al., 2008), or MPK4 antibodies. Total protein staining with Ponceau S (Sigma-Aldrich) or Coomassie Brilliant Blue R 250 (Thermo Scientific) was used as a loading control. Protein dephosphorylation was performed using calf intestinal alkaline phosphatase (CIP) according to the manufacturer’s protocol (New England Biolabs) with ~1 to 2.5 units of CIP (as indicated)/μg protein.
RNA Extraction and Expression Analysis
Total RNA was isolated with Trizol reagent according to the manufacturer’s instructions (Invitrogen). DNase treatment, cDNA synthesis, and quantitative RT-PCR were performed according to the manufacturer’s instructions using gene-specific primers with Arabidopsis Actin2 as an endogenous reference for normalization (Promega, Biotium). A minimum of three technical replicates of the quantitative RT-PCR assay was used for each sample with a minimum of two biological replicates. Expression levels were calculated by the comparative cycle threshold method (Applied Biosystems). Primers used are listed in Supplemental Table 1 online.
ChIP-PCR
ChIP was performed essentially as described (Saleh et al., 2008) using EIN3 antibody. Chromatin was immunoprecipitated from 3-week-old wild-type seedlings treated with 20 μM ACC for 4 h. ChIP-qPCR was performed using specific primers to the predicted EIN3 binding regions on the BIK1 promoter. A control ChIP lacking the EIN3 antibody was run in parallel. The EIN3 antibody used for the immunoprecipitation of EIN3-associated DNA was from Joe Ecker (SALK Institute). Actin was used as internal control for normalization. The primers used for the ChIP-qPCR are listed in Supplemental Table 1 online.
GUS Staining
Histochemical staining for GUS was done as previously described (Liu et al., 2007) on 4-week soil-grown Arabidopsis or 10-d-old seedlings treated for 24 or 3 h, respectively, with double deionized water, 5 μM flg22, 20 μM ACC, or 100 mg/L chitin.
Accession Numbers
Sequence data for the genes described in this article or used in the phylogenetic analysis can be found in the GenBank/EMBL data libraries under the following accession numbers: TPK1b (GenBank accession number EU555286), CAO21648, BIK1 (At2g39660), NAK (At5g02290), PBS1 (At5g13160), APK1b (At2g28930), APK1a (At1g07570), APK2b (At2g02800), MPLKe (BAD12263), PTO (A49332), and At3g55450.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Wound-Induced Immunity to B. cinerea Requires BIK1.
Supplemental Figure 2. Response of the BIK1 Substitution Mutants to A. brassicicola Infection.
Supplemental Figure 3. The BIK1 Promoter Sequence and Location of Putative EIN3 Binding Sequences in the BIK1 Promoter.
Supplemental Figure 4. Bacterial and Flagellin Responses of the BIK1 Phosphomimic Mutants.
Supplemental Figure 5. The Triple Response of BIK1 Thr-242 and Tyr-245 Phosphomimic Mutations at 20 μM ACC.
Supplemental Figure 6. Growth Morphology of the BIK1 Substitution Mutants.
Supplemental Figure 7. Disease Responses of the bik1 Double Mutants to A. brassicicola.
Supplemental Figure 8. The BIK1-Regulated ET Growth Response Is Independent of SA.
Supplemental Figure 9. BIK1 Function in Necrotrophic Defense and ET Responses Is Unique among Related Protein Kinases.
Supplemental Table 1. Primers Used in the BIK1 Study.
Supplementary Material
Acknowledgments
This research was funded by a grant (IOS-0749865) from the National Science Foundation to T.M. We thank Joe Ecker and Katherine Chang (The SALK institute) for the EIN3 antibodies and ein3-1 mutant seeds.
AUTHOR CONTRIBUTIONS
K.L. performed research and wrote the article. H.L., M.C., R.D., and Z.L. performed research. T.M. designed the research, analyzed data, and wrote the article.
References
- Abuqamar S., Chai M.F., Luo H., Song F., Mengiste T. (2008). Tomato protein kinase 1b mediates signaling of plant responses to necrotrophic fungi and insect herbivory. Plant Cell 20: 1964–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becraft P.W. (2002). Receptor kinase signaling in plant development. Annu. Rev. Cell Dev. Biol. 18: 163–192 [DOI] [PubMed] [Google Scholar]
- Bethke G., Unthan T., Uhrig J.F., Pöschl Y., Gust A.A., Scheel D., Lee J. (2009). Flg22 regulates the release of an ethylene response factor substrate from MAP kinase 6 in Arabidopsis thaliana via ethylene signaling. Proc. Natl. Acad. Sci. USA 106: 8067–8072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Felix G. (2009). A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Boutrot F., Segonzac C., Chang K.N., Qiao H., Ecker J.R., Zipfel C., Rathjen J.P. (2010). Direct transcriptional control of the Arabidopsis immune receptor FLS2 by the ethylene-dependent transcription factors EIN3 and EIL1. Proc. Natl. Acad. Sci. USA 107: 14502–14507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Bowling S.A., Gordon A.S., Dong X. (1994). Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6: 1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Q., Rothenberg M., Solano R., Roman G., Terzaghi W., Ecker J.R. (1997). Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Chassot C., Buchala A., Schoonbeek H.J., Métraux J.P., Lamotte O. (2008). Wounding of Arabidopsis leaves causes a powerful but transient protection against Botrytis infection. Plant J. 55: 555–567 [DOI] [PubMed] [Google Scholar]
- Chen H., Xue L., Chintamanani S., Germain H., Lin H., Cui H., Cai R., Zuo J., Tang X., Li X., Guo H., Zhou J.M. (2009). ETHYLENE INSENSITIVE3 and ETHYLENE INSENSITIVE3-LIKE1 repress SALICYLIC ACID INDUCTION DEFICIENT2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell 21: 2527–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D., Zipfel C., Robatzek S., Kemmerling B., Nürnberger T., Jones J.D., Felix G., Boller T. (2007). A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Colville L., Smirnoff N. (2008). Antioxidant status, peroxidase activity, and PR protein transcript levels in ascorbate-deficient Arabidopsis thaliana vtc mutants. J. Exp. Bot. 59: 3857–3868 [DOI] [PubMed] [Google Scholar]
- Delaney T.P., Uknes S., Vernooij B., Friedrich L., Weymann K., Negrotto D., Gaffney T., Gut-Rella M., Kessmann H., Ward E., Ryals J. (1994). A central role of salicylic acid in plant disease resistance. Science 266: 1247–1250 [DOI] [PubMed] [Google Scholar]
- Dempsey D.A., Shah J., Klessig D.F. (1999). Salicylic acid and disease resistance in plants. Crit. Rev. Plant Sci. 18: 547–575 [Google Scholar]
- Dewdney J., Reuber T.L., Wildermuth M.C., Devoto A., Cui J., Stutius L.M., Drummond E.P., Ausubel F.M. (2000). Three unique mutants of Arabidopsis identify eds loci required for limiting growth of a biotrophic fungal pathogen. Plant J. 24: 205–218 [DOI] [PubMed] [Google Scholar]
- Dhawan R., Luo H., Foerster A.M., Abuqamar S., Du H.N., Briggs S.D., Mittelsten Scheid O., Mengiste T. (2009). HISTONE MONOUBIQUITINATION1 interacts with a subunit of the mediator complex and regulates defense against necrotrophic fungal pathogens in Arabidopsis. Plant Cell 21: 1000–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Galletti R., Denoux C., De Lorenzo G., Ausubel F.M., Dewdney J. (2007). Resistance to Botrytis cinerea induced in Arabidopsis by elicitors is independent of salicylic acid, ethylene, or jasmonate signaling but requires PHYTOALEXIN DEFICIENT3. Plant Physiol. 144: 367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Plotnikova J.M., De Lorenzo G., Ausubel F.M. (2003). Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J. 35: 193–205 [DOI] [PubMed] [Google Scholar]
- Frederick R.D., Thilmony R.L., Sessa G., Martin G.B. (1998). Recognition specificity for the bacterial avirulence protein AvrPto is determined by Thr-204 in the activation loop of the tomato Pto kinase. Mol. Cell 2: 241–245 [DOI] [PubMed] [Google Scholar]
- Glazebrook J. (2005). Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43: 205–227 [DOI] [PubMed] [Google Scholar]
- Glazebrook J., Zook M., Mert F., Kagan I., Rogers E.E., Crute I.R., Holub E.B., Hammerschmidt R., Ausubel F.M. (1997). Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics 146: 381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L., Boller T. (2000). FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Govrin E.M., Levine A. (2002). Infection of Arabidopsis with a necrotrophic pathogen, Botrytis cinerea, elicits various defense responses but does not induce systemic acquired resistance (SAR). Plant Mol. Biol. 48: 267–276 [DOI] [PubMed] [Google Scholar]
- Grant M.R., Jones J.D. (2009). Hormone (dis)harmony moulds plant health and disease. Science 324: 750–752 [DOI] [PubMed] [Google Scholar]
- Guo H., Ecker J.R. (2004). The ethylene signaling pathway: New insights. Curr. Opin. Plant Biol. 7: 40–49 [DOI] [PubMed] [Google Scholar]
- Guzmán P., Ecker J.R. (1990). Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S.K., Hunter T. (1995). Protein kinases 6. The eukaryotic protein kinase superfamily: Kinase (catalytic) domain structure and classification. FASEB J. 9: 576–596 [PubMed] [Google Scholar]
- Hanks S.K., Quinn A.M. (1991). Protein kinase catalytic domain sequence database: Identification of conserved features of primary structure and classification of family members. Methods Enzymol. 200: 38–62 [DOI] [PubMed] [Google Scholar]
- He K., Gou X., Yuan T., Lin H., Asami T., Yoshida S., Russell S.D., Li J. (2007). BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr. Biol. 17: 1109–1115 [DOI] [PubMed] [Google Scholar]
- Johnson L.N., Noble M.E., Owen D.J. (1996). Active and inactive protein kinases: Structural basis for regulation. Cell 85: 149–158 [DOI] [PubMed] [Google Scholar]
- Jones J.D., Dangl J.L. (2006). The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Kosugi S., Ohashi Y. (2000). Cloning and DNA-binding properties of a tobacco Ethylene-Insensitive3 (EIN3) homolog. Nucleic Acids Res. 28: 960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Reyes A., Du Y., Koornneef A., Proietti S., Körbes A.P., Memelink J., Pieterse C.M., Ritsema T. (2010). Ethylene signaling renders the jasmonate response of Arabidopsis insensitive to future suppression by salicylic acid. Mol. Plant Microbe Interact. 23: 187–197 [DOI] [PubMed] [Google Scholar]
- Liu Y., Koornneef M., Soppe W.J. (2007). The absence of histone H2B monoubiquitination in the Arabidopsis hub1 (rdo4) mutant reveals a role for chromatin remodeling in seed dormancy. Plant Cell 19: 433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O., Piqueras R., Sánchez-Serrano J.J., Solano R. (2003). ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 15: 165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Wu S., Gao X., Zhang Y., Shan L., He P. (2010). A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc. Natl. Acad. Sci. USA 107: 496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya A., Albert P., Shinya T., Desaki Y., Ichimura K., Shirasu K., Narusaka Y., Kawakami N., Kaku H., Shibuya N. (2007). CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 104: 19613–19618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pré M., Atallah M., Champion A., De Vos M., Pieterse C.M., Memelink J. (2008). The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol. 147: 1347–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J.L., et al. (2008). Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J. 27: 2214–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen J.P., Chang J.H., Staskawicz B.J., Michelmore R.W. (1999). Constitutively active Pto induces a Prf-dependent hypersensitive response in the absence of avrPto. EMBO J. 18: 3232–3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A., Alvarez-Venegas R., Avramova Z. (2008). An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat. Protoc. 3: 1018–1025 [DOI] [PubMed] [Google Scholar]
- Schulze B., Mentzel T., Jehle A.K., Mueller K., Beeler S., Boller T., Felix G., Chinchilla D. (2010). Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J. Biol. Chem. 285: 9444–9451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa G., D’Ascenzo M., Martin G.B. (2000). Thr38 and Ser198 are Pto autophosphorylation sites required for the AvrPto-Pto-mediated hypersensitive response. EMBO J. 19: 2257–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao F., Golstein C., Ade J., Stoutemyer M., Dixon J.E., Innes R.W. (2003). Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 301: 1230–1233 [DOI] [PubMed] [Google Scholar]
- Shiu S.H., Bleecker A.B. (2001). Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. USA 98: 10763–10768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R., Stepanova A., Chao Q., Ecker J.R. (1998). Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel S.H., Johnson J.S., Dong X. (2007). Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc. Natl. Acad. Sci. USA 104: 18842–18847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Frederick R.D., Zhou J., Halterman D.A., Jia Y., Martin G.B. (1996). Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science 274: 2060–2063 [DOI] [PubMed] [Google Scholar]
- Thomma B.P., Eggermont K., Penninckx I.A., Mauch-Mani B., Vogelsang R., Cammue B.P.A., Broekaert W.F. (1998). Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 95: 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma B.P., Eggermont K., Tierens K.F., Broekaert W.F. (1999). Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 121: 1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma B.P., Nürnberger T., Joosten M.H. (2011). Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell 23: 4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K., Katagiri F. (2010). Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol. 13: 459–465 [DOI] [PubMed] [Google Scholar]
- Tsuda K., Sato M., Glazebrook J., Cohen J.D., Katagiri F. (2008). Interplay between MAMP-triggered and SA-mediated defense responses. Plant J. 53: 763–775 [DOI] [PubMed] [Google Scholar]
- Veronese P., Chen X., Bluhm B., Salmeron J., Dietrich R., Mengiste T. (2004). The BOS loci of Arabidopsis are required for resistance to Botrytis cinerea infection. Plant J. 40: 558–574 [DOI] [PubMed] [Google Scholar]
- Veronese P., Nakagami H., Bluhm B., Abuqamar S., Chen X., Salmeron J., Dietrich R.A., Hirt H., Mengiste T. (2006). The membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens. Plant Cell 18: 257–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Goshe M.B., Soderblom E.J., Phinney B.S., Kuchar J.A., Li J., Asami T., Yoshida S., Huber S.C., Clouse S.D. (2005). Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 receptor kinase. Plant Cell 17: 1685–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D.X., Feys B.F., James S., Nieto-Rostro M., Turner J.G. (1998). COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091–1094 [DOI] [PubMed] [Google Scholar]
- Yanagisawa S., Yoo S.D., Sheen J. (2003). Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425: 521–525 [DOI] [PubMed] [Google Scholar]
- Zarei A., Körbes A.P., Younessi P., Montiel G., Champion A., Memelink J. (2011). Two GCC boxes and AP2/ERF-domain transcription factor ORA59 in jasmonate/ethylene-mediated activation of the PDF1.2 promoter in Arabidopsis. Plant Mol. Biol. 75: 321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., et al. (2010). Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 7: 290–301 [DOI] [PubMed] [Google Scholar]
- Zheng Z.Y., Qamar S.A., Chen Z.X., Mengiste T. (2006). Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 48: 592–605 [DOI] [PubMed] [Google Scholar]
- Zhou L., Jang J.C., Jones T.L., Sheen J. (1998). Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc. Natl. Acad. Sci. USA 95: 10294–10299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C., Kunze G., Chinchilla D., Caniard A., Jones J.D., Boller T., Felix G. (2006). Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.