Abstract
Objective
To evaluate the relationship between missteps and falls and to identify factors associated with missteps to potentially generate a broader picture of fall risk.
Design
Prospective, observational cohort.
Setting
General community.
Subjects
A sample of healthy, community-living older adults (N=266; ages: 70–90y) who were cognitively intact and walked independently.
Interventions
Not applicable.
Main Outcome Measures
Baseline testing of gait, motor function, cognitive function, affect, and balance confidence was followed by a 12-month period in which subjects completed a daily log documenting the number of falls and missteps (defined as a trip, slip, or other loss of balance in which recovery occurred to prevent a fall).
Results
Mean ± SD participant age was 76.4±4.3 years. Among the participants, 20.7% reported at least 1 misstep and 25.6% of the participants reported at least 1 fall during the 12 months. Among subjects who had multiple falls, missteps were more common than falls by a ratio of 3:1 (P<.001). Subjects who reported multiple missteps were more likely to fall prospectively (relative risk=3.89). Missteps were associated with higher scores on the Geriatric Depression Scale (P=.009) and increased anxiety (P=0.014), but were not associated with other known risk factors of falls, including gait and cognitive function.
Conclusions
The self-report of missteps may be a valuable tool in the research of falls and fall risk and may provide a way to identify patients at risk for falls before they fall.
Keywords: falls, missteps, aging, cognitive function, gait
Falls are a significant cause of morbidity and a common debilitating problem among older adults.1–5 To a large extent, research and the clinical evaluation of fall risk relies on self-report of falls as a marker of fall risk.1,4–8 While this approach has been widely and successfully used, it suffers from several limitations: (1) the difference between a nonfaller and someone who experiences a single, random fall is rather crude and does not allow for quantification of subtle alterations of imbalance; and (2) one would prefer to identify persons at risk for falls before a fall is experienced.9 While many fall risk factors have already been described (eg, muscle strength, gait impairment, cognitive dysfunction),1–3 fall risk stratification remains a challenge.
Greater sensitivity may be obtained by asking subjects about missteps.10,11 One study in patients with Parkinson's disease found that falls were more common than near falls or missteps,10 while another study observed that missteps were more frequent.11 Patients with myotonic dystrophy type 1 reported missteps much more frequently (9.6 per subject) than controls (2.2 per subject).12 Yet among healthy older adults, the relationship between falls and missteps is still largely unknown. If missteps (here used to include near falls, trips, stumbles, and other balance losses) are associated with increased fall risk, measuring these events might help to provide a broader picture of fall risk, in addition to fall history.
The purpose of the present study was to investigate the properties of self-report of missteps in a relatively healthy cohort of older adults and to gain insight into the subject-specific characteristics of older adults who experience missteps. Specifically, we examined the relationship between missteps and falls to assess discriminate validity and to study the association between missteps and other factors commonly associated with fall risk.
METHODS
Subjects
Subjects were 266 older adult men and women who were participating in a prospective study designed to examine the relationship between gait and cognitive function. Subjects were recruited from local senior centers, via flyers, advertising, and word of mouth. An initial phone interview identified community-dwelling, independent ambulators who were between the ages of 70 and 90 years and were free from disease likely to directly impact gait (eg, vestibular, orthopedic, neurologic disease). After the phone interview, eligible subjects underwent a thorough evaluation that lasted about 3 hours and included a neurologic examination, a cognitive assessment, and clinical testing by a certified physical therapist. Demographic information and fall history during the 12-month period prior to participating in the study were also obtained. Subjects completed forms that detailed their medical history and all prescription medications and these were reviewed to complete the Charlson Comorbidity Index, which quantified disease burden (higher scores indicate greater comorbidity).13 Subjects were excluded if they had acute illness (eg, if they were being treated for any illness other than a chronic condition), brain surgery, major depression (as defined by Diagnostic and Statistics Manual criteria), history of stroke, or if they scored less then 25 on the MMSE.14 The study was approved by the local human studies committee and informed written consent was obtained prior to enrollment.
The Barthel Activities of Daily Living Index,15 the Frenchay Activities Index,16 the PASE,17 and the relative components of the SF-3618 characterized disability, lifestyle and functional independence, physical activity levels and self-report of general health, respectively. Higher scores on these 4 tests reflect better health and functional abilities. The motor portion (part III) of the UPDRS quantified extra-pyramidal signs, such as resting tremor and rigidity19–22; lower scores reflect better motor function.23 Quadriceps muscle strength (average of left and right) was measured using a digital hand-held dynamometera.
Prospective Assessment of Missteps and Falls
After the baseline assessment, self-report of missteps and falls was collected from subjects using a calendar that subjects filled out daily and returned every month by mail using prepaid and preaddressed envelopes. Subjects were instructed to keep the calendar in a convenient place and to record the number of falls and missteps that occurred after every event or at the end of each day.7,24,25 A fall was defined as unintentionally coming to rest on a lower surface.26 A misstep was defined as a trip, slip, or other loss of balance in which recovery occurred to prevent a fall. About 80% of the diaries were returned on time. If participants failed to return the diary, a telephone call was placed to obtain the missing information, typically within 1 month.
Cognitive Assessment
At baseline, the Mindstreamsb computerized neuropsychologic test battery quantified executive function, attention, memory, and visual spatial orientation.27,28 Age and education adjusted composite indices of these different cognitive domains were computed as previously established.27,29–33 Higher scores indicate better cognitive function.
Performance-based Measures of Balance and Mobility
The Berg Balance Scale Test and the Dynamic Gait Index provided performance-based measures of balance and mobility34–36 (higher scores indicate better function). The Timed Up & Go test37 assessed functional mobility and fall risk.1 The Pull test (item 29 of the motor portion of the UPDRS) measured ability to recover from a postural perturbation38; higher scores reflect decreased postural control (eg, 1-indicates retropulsion, but with unaided recovery; 2-the subject would fall if not caught). We also measured average swing time and swing time variability using force-sensitive insoles.30,32
Assessment of Affect and Balance Confidence
The Activities-specific Balance Confidence Scale was administered to assess fear of falling (higher scores indicate less fear).39,40 The Geriatric Depression Scale (30-question version) measured depressive symptoms and emotional well-being.41 The State-Trait Anxiety Inventory quantified anxiety.42
Statistical Analysis
The characteristics of the study participants were described using appropriate descriptive statistics including frequencies, proportions, and means. Spearman correlation coefficients were used to quantify the bivariate associations between the number of missteps reported and independent, potential explanatory measures. The Student t test and paired t tests were used to compare subgroups and relative risk was determined to evaluate fall risk as a function of missteps. Mean values are reported as mean ± SD. P values reported are based on 2-tailed comparisons. Statistical analyses were performed using SPSS 14.0 for Windowsc.
RESULTS
Subject Characteristics
As summarized in table 1, the participants were generally healthy (eg, low Charlson Co-Morbidity Indices, mean MMSE near 30). Scores on the SF-36, PASE, and Frenchay Activities Index were consistent with activity levels typical of functionally independent, healthy elderly individuals. Functional balance tests (ie, Dynamic Gait Index and Berg Balance score) revealed near-perfect scores and Timed Up & Go times were low, also consistent with good mobility.
Table 1.
Subject Characteristics and Correlations Between Missteps and These Characteristics (N=266)
| Variables | Subject Characteristics | Correlations | |
|---|---|---|---|
| Background measures |
Mean ± SD (or %) | R (P) | |
| Age (y) | 76.4±4.3 | 0.09 (0.17) | |
| Sex (% women) | 58.5% | 0.01 (0.87) | |
| Body mass index (kg/m2) | 26.6±3.6 | −0.12 (0.066) | |
| MMSE | 28.7±1.2 | −.019 (0.756) | |
| Education (y) | 13.6±3.7 | 0.028 (0.655) | |
| Fell in past year (%) | 26.4% | 0.073 (0.258) | |
| Motor part of the UPDRS | 4.1±2.8 | 0.34 (0.57) | |
| SF-36 Health | 69.1±17 | 0.02 (0.78) | |
| PASE* | 113±66.4 | −0.06 (0.31) | |
| Charlson Comorbidity Index | 0.8±1.0 | −0.02 (0.73) | |
| Prescription medications (no.) | 3.7±2.4 | 0.11 (0.07) | |
| Quadriceps strength (kg) | 20.7±7.6 | −0.14 (0.051) | |
| Cognitive function |
Executive function index | 99.1±10.8 | −0.01 (0.84) |
| Attention index | 98.7±12.9 | −0.05 (0.46) | |
| Memory index | 99.2±12.2 | 0.07 (0.27) | |
| Visual-spatial function index | 97.0±15.9 | −0.02 (0.74) | |
| Balance confidence & affect |
ABC Scale | 91.8±10.5 | −0.11 (0.07) |
| Geriatric Depression Scale | 5.1±4.5 | 0.16 (0.02) | |
| Trait Anxiety Inventory | 33.7±8.6 | 0.15 (0.02) | |
| State Anxiety Inventory | 31.8±9.9 | 0.17 (0.01) | |
| Performance- based measures of mobility |
Dynamic Gait Index | 22.8±1.5 | −0.31 (0.62) |
| Berg Balance Score | 54.1±2.4 | −0.02 (0.75) | |
| Timed Up & Go (s) | 9.5±1.7 | −0.02 (0.74) | |
| Pull test | 0.4±0.6 | 0.08 (0.20) | |
| Usual-walking measures of gait |
Gait speed (m/s) | 1.3±0.2 | 0.02 (0.77) |
| Average swing time (%) | 37.6±2.3 | 0.05 (0.47) | |
| Swing time variability (%) | 2.2±1.0 | −0.05 (0.41) |
Missteps and Falls
Fifty-five (20.7%) participants reported at least 1 misstep. The number of missteps reported per subject over the 12-month study period ranged from 0 to 15 (table 2), averaging 0.6±1.7 missteps per subject. Of all the participants, 25.6% reported at least 1 fall during the prospective follow-up and 9% reported multiple falls, on average, 0.4±0.8 falls (range, 0–4) per subject. Missteps were attributed to slips, trips, and stumbles over obstacles, but there was a wide variety of contributing causes cited by participants.
Table 2.
Frequency of Self-Report of Missteps Over the 1-year Study Period
| Missteps | Number of subjects | % of subjects |
|---|---|---|
| 0 missteps | 211 | 79.3 |
| 1 misstep | 25 | 9.4 |
| 2 missteps | 13 | 4.9 |
| 3 or missteps | 17 | 6.4 |
The number of missteps reported was positively correlated with the number of prospective falls reported (r=.38; P<.001). To better understand the association of missteps with other factors, we focused our analysis on subjects who reported multiple missteps. These subjects were more likely to fall prospectively (relative risk=3.89) and subjects without multiple missteps were less likely to have any falls (P<.001). Eighty-two percent of subjects who had less than 2 missteps did not report any falls. In subjects who had both multiple missteps and multiple falls, multiple missteps were more common than falls by a ratio of 3.0:1.0 (P<.001); in other words, among subjects with multiple missteps, 47% did not have multiple falls. Among participants who had no history of falls in the 12 month prior to the beginning of the study, the number of missteps reported (0.6±1.9) tended to be higher than the number of prospective falls (0.4±0.8) during the 12-month study (P=.061).
Missteps and Potential Contributing Factors
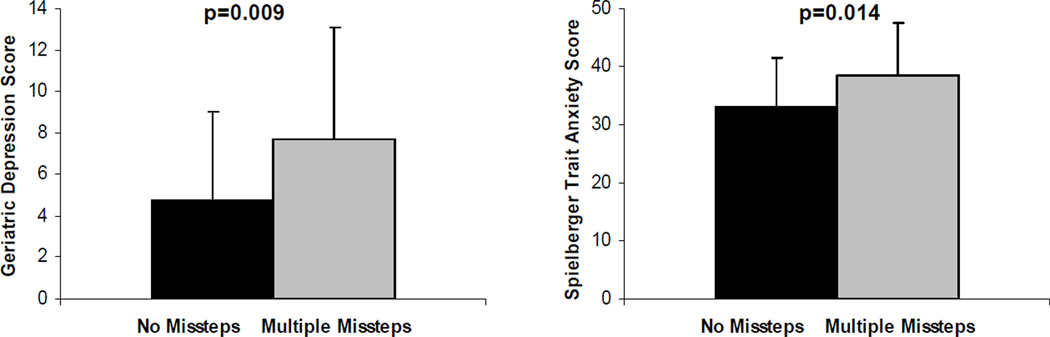
Among the outcome measures examined, only the Geriatric Depression Scale (r=.16, P=.016) and anxiety trait and state scores (r=.15, P=.019; r=.16, P=.010, respectively) were significantly correlated with missteps, and even these measures were weakly correlated (fig 1). Similar patterns were observed when comparing subjects who experienced multiple missteps to those who experienced none (table 3). For example, Geriatric Depression Scale scores were different in subjects who had no missteps compared to multiple missteppers (P=.009), while gait speed was similar in these 2 groups.
Fig 1.
Factors associated with missteps. (A) Scores on the Geriatrics Depression Scale were higher, reflecting increased depressive symptoms, among the subgroup of subjects who reported multiple missteps (n=30), compared to subjects who reported none (n=211). (B) Scores on the Spielberger Test, were also higher, reflecting increased anxiety, in the subgroup of subjects who reported multiple missteps.
Table 3.
Differences Between Subjects Who Reported No Missteps Compared to Those Who Reported 2 or More Missteps (N=266)
| Variable | No Missteps (n=211) |
Multiple Missteps (n=30) |
P | |
|---|---|---|---|---|
| Background measures |
Age (y) | 76.1±4.1 | 77.3±4.7 | 0.17 |
| Sex (% women) | 58.5% | 57.9% | 0.88 | |
| Body mass index (kg/m2) | 26.8±3.8 | 25.4±2.8 | 0.07 | |
| MMSE | 28.7±1.2 | 28.7±1.2 | 0.76 | |
| Education (y) | 13.5±3.6 | 14.1±4.0 | 0.39 | |
| Fell in year prior to study (%) | 26.4% | 30% | 0.528 | |
| Motor Part of UPDRS | 4.1±2.8 | 4.4±2.7 | 0.57 | |
| SF-36 Health | 68.9±17 | 66.9±18 | 0.56 | |
| PASE | 115±69 | 100±60 | 0.26 | |
| Charlson Comorbidity Index | 0.8±1 | 0.8±1 | 0.96 | |
| Prescription medications (no.) | 3.6±2.4 | 4.3±2.3 | 0.13 | |
| Quadriceps strength (kg) | 21.2±7.7 | 19.6±7.4 | 0.33 | |
| Cognitive function |
Executive function index | 99.1±10.9 | 100±11.5 | 0.66 |
| Attention index | 98.8±13.0 | 98.4±11.9 | 0.88 | |
| Memory index | 98.7±12.3 | 100±10.9 | 0.46 | |
| Visual-spatial function index | 97.1±15.4 | 95.1±17.2 | 0.54 | |
| Balance confidence and affect |
ABC Scale | 92.2±10.5 | 88.0±10.3 | 0.04 |
| Geriatric Depression Scale | 4.8±4.3 | 7.7±5.4 | 0.001 | |
| Trait Anxiety Inventory | 33.0±8.4 | 38.3±9.0 | 0.002 | |
| State Anxiety Inventory | 31.0±9.3 | 37.2±11.9 | 0.001 | |
| Performance- based measures of mobility |
Dynamic Gait Index | 22.8±1.5 | 22.4±1.9 | 0.12 |
| Berg Balance Score | 54.1±2.3 | 53.9±2.7 | 0.55 | |
| Timed Up & Go (s) | 9.6±1.7 | 9.7±1.7 | 0.63 | |
| Pull test | 0.4±0.6 | 0.6±0.7 | 0.26 | |
| Usual-walking measures of gait |
Gait speed (m/s) | 1.3±0.21 | 1.3±0.23 | 0.73 |
| Average swing time (%) | 37.6±2.4 | 38.1±1.8 | 0.31 | |
| Swing time variability (%) | 2.2±1.0 | 2.2±0.88 | 0.89 |
Whereas reported missteps were significantly correlated with reported falls, the occurrence of prospective falls did not correlate with scores on the Geriatric Depression Scale (P>.48) or anxiety measures (P>.20). Participants with multiple falling episodes had clinical characteristics known to be associated with fall risk including relatively increased age, higher Timed Up & Go times, increased scores on part III of the UPDRS and the Pull test, lower quadriceps strength, and poorer attention, and executive function. These differences were not observed when comparing subjects with multiple missteps to those with none (see table 3 and fig 2).
Fig 2.
Factors not associated with missteps that were associated with falls. (A) Age, (B) Timed Up & Go times, (C) executive function index.
DISCUSSION
The key findings of this study are as follows: (1) self-report of missteps is significantly associated with self-report of falls. (2) Factors associated with missteps include depressive symptoms and anxiety. (3) Risk factors associated with falls are not strongly associated with missteps. This suggests that missteps are a relevant marker of fall risk, but not simply a redundant copy of self-report of falls.
A priori, it was not clear whether missteps would be positively or negatively associated with fall risk. In order for a fall to take place, loss of balance must be followed by inadequate recovery. Missteps or near falls might occur more often than falls because these events require only the initial sudden loss of balance. Because missteps result from a successful recovery response, they may occur more frequently in people with less frequent falls, due to a healthy recovery response. Alternatively, missteps might reflect poor balance and therefore could be a symptom of underlying risk factors that lead to loss of balance. The results of the present study support this second possibility and suggest that among relatively healthy older adults, missteps are not a sign of health, but are related to falls.
Subjects who reported both missteps and falls also reported more missteps than fall events. This raises the possibility that many falls may begin as missteps and as postural control declines over time, a loss of balance that once resulted in a misstep may be more likely to become a fall. Laboratory investigations of the relationship between trips and falls identified several parameters that contribute to balance recovery after postural disturbance43,44; in one study, the incidence of trip-related falls was most closely related to the trip frequency.45 This association is consistent with the data from the present study, that is, subjects with multiple self-reported missteps had a higher risk of falling prospectively.
It is somewhat surprising that many of the risk factors traditionally associated with falls (eg, gait, balance, muscle strength, age, and sex) were not associated with missteps. Executive function was associated with falls in the present study, consistent with previous findings,32,46–49 while executive function and attention did not correlate with missteps (P=.848, P=.464, respectively). There was also a tendency toward higher (worse) scores on the Pull test in subjects with multiple falls, but not in those with multiple missteps. This suggests that postural recovery and cognitive function play a role in determining whether a subject will have a misstep or a fall once balance has been challenged, but play less of a role in the generation of the misstep itself. This is consistent with previous work which also showed a weak trend between measures of postural stability and the ability of healthy older adults to recover from postural disturbances under laboratory conditions.43
This study relies on the self-reporting of missteps and is heavily dependent on subjects’ recollection of events. While there was significant correlation between missteps and falls, most subjects reported neither event during the 12-month study and the total number of missteps reported was surprisingly small. It is possible that objective assessment by a monitoring device or observation by a third party would capture missteps more accurately. Though the daily calendar method is more reliable than simple recall,7 self-report still relies on a subject’s memory and the personal drive to follow the instructions. Because a fall is typically a more memorable event than a misstep, subjects may have under-reported missteps more than they under-reported falls. Also, subjects who have a history of falls may be more vigilant in their gait, and therefore may remember missteps more than people who do not fall. Because of the difficulties inherent in self-report and heterogeneity in the responses, we did not quantitatively characterize the specific circumstances of each misstep. Despite these limitations, the strengths of the present investigation include the large number of subjects and the fact that this study examines the association between the self-report of missteps and falls in a population of healthy older adults. Future work to further elucidate this association could include prospective studies to explore the relationship between missteps and falls in patient populations with a higher fall risk. Such studies could also investigate the circumstances surrounding missteps and falls to determine if there are conditions that make a subject more likely to recover from a misstep.
The present findings suggest that self-report of missteps may have clinical utility. A subject with frequent missteps could be identified as high-risk before a first fall and may benefit from interventions to prevent recurrent falls. Indeed, we found that among participants who experienced no falls in the past, missteps tended to be more common than falls and that subjects who reported multiple missteps were also more likely to fall in the future. These findings are similar to results in patients with neurologic disease,11,12 which reported missteps occurring more frequently than falls.
CONCLUSIONS
The present findings suggest that self-report of missteps may enhance our ability to discriminate among older adults with different levels of fall risk and allow for the identification of high-risk individuals before they have a first fall. Thus, this measure may have advantages over the more traditional reliance on the self-report of falls alone.
Acknowledgements
The authors are indebted to the participants and staff of the "Holchim Rachok" project for their invaluable contribution to this study.
Supported by the National Institutes of Health (grant no. AG-14100), by the Israel Ministry of Absorption and by the European Union Sixth Framework Program (grant no. FET 018474-2, Dynamic Analysis of Physiological Networks, DAPHNet, and STREP 045622 SENSing and ACTION to support mobility in Ambient Assisted Living, SENSACTION-AAL).
List of Abbreviations
- MMSE
Mini-Mental State Examination
- PASE
Physical Activity Scale for the Elderly
- SF-36
Medical Outcomes Study 36-Item Short-Form Health Survey
- UPDRS
Unified Parkinson's Disease Rating Scale
- ABC
Activities-specific Balance Confidence
Footnotes
Presented to the American Geriatrics Society, Washington DC, May 2008.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
Suppliers
MSE 100; Chatillon & Sons Inc, P.O. Box. 35668, Greensboro, NC
NeuroTrax Corp, 211 Warren St, Ste 213, Newark, NJ 07103.
SPSS Inc, 233 S Wacker Dr, 11th Fl, Chicago, IL 60606.
References
- 1.AGS Guidelines. Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49:664–672. [PubMed] [Google Scholar]
- 2.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;(35 Suppl 2):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 3.Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ. Will my patient fall? JAMA. 2007;297:77–86. doi: 10.1001/jama.297.1.77. [DOI] [PubMed] [Google Scholar]
- 4.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 5.Pickering RM, Grimbergen YA, Rigney U, et al. A meta-analysis of six prospective studies of falling in Parkinson's disease. Mov Disord. 2007;22:1892–1900. doi: 10.1002/mds.21598. [DOI] [PubMed] [Google Scholar]
- 6.Anstey KJ, von SC, Luszcz MA. An 8-year prospective study of the relationship between cognitive performance and falling in very old adults. J Am Geriatr Soc. 2006;54:1169–1176. doi: 10.1111/j.1532-5415.2006.00813.x. [DOI] [PubMed] [Google Scholar]
- 7.Mackenzie L, Byles J, D'Este C. Validation of self-reported fall events in intervention studies. Clin Rehabil. 2006;20:331–339. doi: 10.1191/0269215506cr947oa. [DOI] [PubMed] [Google Scholar]
- 8.Thurman DJ, Stevens JA, Rao JK. Practice parameter: assessing patients in a neurology practice for risk of falls (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2008;70:473–479. doi: 10.1212/01.wnl.0000299085.18976.20. [DOI] [PubMed] [Google Scholar]
- 9.Voermans NC, Snijders AH, Schoon Y, Bloem BR. Why old people fall (and how to stop them) Pract Neurol. 2007;7:158–171. doi: 10.1136/jnnp.2007.120980. [DOI] [PubMed] [Google Scholar]
- 10.Gray P, Hildebrand K. Fall risk factors in Parkinson's disease. J Neurosci Nurs. 2000;32:222–228. doi: 10.1097/01376517-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Stack E, Ashburn A. Fall events described by people with Parkinson's disease: implications for clinical interviewing and the research agenda. Physiother Res Int. 1999;4:190–200. doi: 10.1002/pri.165. [DOI] [PubMed] [Google Scholar]
- 12.Wiles CM, Busse ME, Sampson CM, et al. Falls and stumbles in myotonic dystrophy. J Neurol Neurosurg Psychiatry. 2006;77:393–396. doi: 10.1136/jnnp.2005.066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability? Int Disabil Stud. 1988;10:64–67. doi: 10.3109/09638288809164105. [DOI] [PubMed] [Google Scholar]
- 16.Turnbull JC, Kersten P, Habib M, et al. Validation of the Frenchay Activities Index in a general population aged 16 years and older. Arch Phys Med Rehabil. 2000;81:1034–1038. doi: 10.1053/apmr.2000.7162. [DOI] [PubMed] [Google Scholar]
- 17.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 18.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Louis ED, Tang MX, Schupf N, Mayeux R. Functional correlates and prevalence of mild parkinsonian signs in a community population of older people. Arch Neurol. 2005;62:297–302. doi: 10.1001/archneur.62.2.297. [DOI] [PubMed] [Google Scholar]
- 20.Bennett DA, Shannon KM, Beckett LA, Wilson RS. Dimensionality of parkinsonian signs in aging and Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 1999;54:M191–M196. doi: 10.1093/gerona/54.4.m191. [DOI] [PubMed] [Google Scholar]
- 21.Bennett DA, Beckett LA, Murray AM, et al. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334:71–76. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RS, Schneider JA, Beckett LA, Evans DA, Bennett DA. Progression of gait disorder and rigidity and risk of death in older persons. Neurology. 2002;58:1815–1819. doi: 10.1212/wnl.58.12.1815. [DOI] [PubMed] [Google Scholar]
- 23.Fahn S, Elton R. Members of the UPDRS development committee. Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent developments in Parkinson's disease. Florham Park: Macmillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- 24.Close J, Ellis M, Hooper R, et al. Prevention of falls in the elderly trial (PROFET): a randomised controlled trial. Lancet. 1999;353:93–97. doi: 10.1016/S0140-6736(98)06119-4. [DOI] [PubMed] [Google Scholar]
- 25.Cumming RG, Thomas M, Szonyi G, et al. Home visits by an occupational therapist for assessment and modification of environmental hazards: a randomized trial of falls prevention. J Am Geriatr Soc. 1999;47:1397–1402. doi: 10.1111/j.1532-5415.1999.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 26.Buchner DM, Hornbrook MC, Kutner NG, et al. Development of the common data base for the FICSIT trials. J Am Geriatr Soc. 1993;41:297–308. doi: 10.1111/j.1532-5415.1993.tb06708.x. [DOI] [PubMed] [Google Scholar]
- 27.Dwolatzky T, Whitehead V, Doniger GM, et al. Validity of a novel computerized cognitive battery for mild cognitive impairment. BMC Geriatr. 2003;3:4. doi: 10.1186/1471-2318-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schweiger A, Doniger GM, Dwolatzky T, Jaffe D, Simon ES. Reliability of a novel computerized neuropsychological battery for mild cognitive impairment. Acta Neuropsychologica. 2003;1:407–413. [Google Scholar]
- 29.Schweiger A, Abramovitch A, Doniger GM, Simon ES. A clinical construct validity study of a novel computerized battery for the diagnosis of ADHD in young adults. J Clin Exp Neuropsychol. 2007;29:100–111. doi: 10.1080/13803390500519738. [DOI] [PubMed] [Google Scholar]
- 30.Yogev G, Giladi N, Peretz C, et al. Dual tasking, gait rhythmicity, and Parkinson's disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22:1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 31.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164:541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- 32.Springer S, Giladi N, Peretz C, et al. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord. 2006;21:950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- 33.Leitner Y, Doniger GM, Barak R, Simon ES, Hausdorff JM. A novel multi-domain computerized cognitive assessment for attention deficit hyperactivity disorder: evidence for widespread and circumscribed cognitive deficits. J Child Neurol. 2007;22:264–276. doi: 10.1177/0883073807299859. [DOI] [PubMed] [Google Scholar]
- 34.Berg K, Wood-Dauphinee S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995;27:27–36. [PubMed] [Google Scholar]
- 35.Chiu YP, Fritz SL, Light KE, Velozo CA. Use of item response analysis to investigate measurement properties and clinical validity of data for the dynamic gait index. Phys Ther. 2006;86:778–787. [PubMed] [Google Scholar]
- 36.Whitney SL, Hudak MT, Marchetti GF. The dynamic gait index relates to self-reported fall history in individuals with vestibular dysfunction. J Vestib Res. 2000;10:99–105. [PubMed] [Google Scholar]
- 37.Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 38.Hunt AL, Sethi KD. The pull test: a history. Mov Disord. 2006;21:894–899. doi: 10.1002/mds.20925. [DOI] [PubMed] [Google Scholar]
- 39.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–M34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 40.Peretz C, Herman T, Hausdorff JM, Giladi N. Assessing fear of falling: can a short version of the Activities-specific Balance Confidence scale be useful? Mov Disord. 2006;21:2101–2105. doi: 10.1002/mds.21113. [DOI] [PubMed] [Google Scholar]
- 41.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 42.Spielberger C. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Pr; 1983. State-Trait Anxiety Inventory. Self-evaluation questionnaire (form Y) [Google Scholar]
- 43.Pavol MJ, Owings TM, Foley KT, Grabiner MD. Mechanisms leading to a fall from an induced trip in healthy older adults. J Gerontol A Biol Sci Med Sci. 2001;56:M428–M437. doi: 10.1093/gerona/56.7.m428. [DOI] [PubMed] [Google Scholar]
- 44.Lockhart TE. An integrated approach towards identifying age-related mechanisms of slip initiated falls. J Electromyogr Kinesiol. 2008;8:205–217. doi: 10.1016/j.jelekin.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavol MJ, Owings TM, Foley KT, Grabiner MD. Gait characteristics as risk factors for falling from trips induced in older adults. J Gerontol A Biol Sci Med Sci. 1999;54:M583–M590. doi: 10.1093/gerona/54.11.m583. [DOI] [PubMed] [Google Scholar]
- 46.Van lersel B, Kessels R, Bloem B, Verbeek A, Olde Rikkert M. Executive function influences gait and balance in community-living elderly people. J Gerontol A Biol Sci Med Sci. 2008;63:1344–1349. doi: 10.1093/gerona/63.12.1344. [DOI] [PubMed] [Google Scholar]
- 47.Yogev G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hausdorff JM, Doniger GM, Springer S, et al. A common cognitive profile in elderly fallers and in patients with Parkinson's disease: the prominence of impaired executive function and attention. Exp Aging Res. 2006;32:411–429. doi: 10.1080/03610730600875817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alexander NB, Hausdorff JM. Linking thinking, walking and falling. J Gerontol A Biol Sci Med Sci. 2008;63:1325–1328. doi: 10.1093/gerona/63.12.1325. [DOI] [PubMed] [Google Scholar]