Abstract
Background and Aims
Caloric Restriction (CR) is the most robust and reproducible intervention for slowing aging, and maintaining health and vitality in animals. Previous studies found that CR is associated with changes in specific biomarkers in monkeys that were also associated with reduced risk of mortality in healthy men. In this study we examine the association between other potential biomarkers related to CR and extended lifespan in healthy humans.
Methods
Based on the Baltimore Longitudinal Study of Aging, “Long-lived” participants who survived to at least 90 years of age (n=41, cases) were compared with “Short-lived” participants who died between 72–76 years of age (n=31, controls) in the nested case control study. Circulating levels of ghrelin, insulin, leptin, interleukin 6, adiponectin and testosterone were measured from samples collected between the ages 58 to 70 years. Baseline differences between groups were examined with t-test or Wilcoxon test, and mixed effects general linear model was used for a logistic model to differentiate the two groups with multiple measurements on some subjects.
Results
At the time of biomarkers evaluation (58–70 years), none of the single biomarker levels were significantly different between the two groups. However, after combining information from multiple biomarkers by adding the z-transformed values, the global score differentiated the long-and short-lived participants (p=0.05).
Conclusions
In their sixties, long lived and short lived individuals do not differ in biomarkers that have been associated with caloric restriction in animals. However, difference between the groups was only obtained when multiple biomarker dysregulation was considered.
Keywords: caloric restriction, aging, longevity, biomarkers
INTRODUCTION
Caloric Restriction (CR) is the most robust and reproducible intervention for slowing aging, increasing lifespan, and maintaining health and vitality in animal models (1, 2). Although most studies of CR have been conducted in rodents and lower animals, there is some evidence that CR can significantly increase lifespan also in animals that live longer than rodents, such as dogs and cows (3, 4). In addition, some data from rhesus monkeys suggest that CR may also be relevant for primates, including humans (5–8).
The few studies conducted rigorously in humans have produced conflicting results, but even assuming that CR could be beneficial in humans, few individuals would be willing and/or able to maintain the 25–40% reduction in food intake over the bulk of the adult lifespan necessary for meaningful benefits. For this reason, the concept of CR mimetics was introduced in 1998 by Lane and co-workers (9) who hypothesized that the same beneficial effects of CR could be obtained by targeting metabolic and stress response pathways usually affected by CR without the need to restrict caloric intake. This research area has attracted increased interest (10, 11) and a number of candidate CR mimetics have been investigated in animals and humans. The identification of candidate CR mimetics as well as other aging interventions has increased the demand for candidate biomarkers that could be used to assess effects of any intervention. A major desired feature of a candidate biomarker would be the ability to predict longevity.
Low plasma insulin and body temperature, as well as maintenance of higher plasma dehydroepiansterone-sulfate(DHEA-S) levels have been shown to correlate with increased survival in male participants of the Baltimore Longitudinal Study of Aging (BLSA) (12). Interestingly, changes of these biomarkers in the same direction were also detected after CR in rhesus monkeys (12).
To further expand our understanding of these findings, we compared a group of participants in the BLSA who survived to greater than 90 years of age with a group of participants who died between ages 72–76 years. All BLSA participants considered had been evaluated in their 60s, were healthy, and at that time had donated a blood sample. Based on previous literature, we selected potential biomarkers that are known to be affected by CR and are involved in energy homeostasis and lipid metabolism, including the following (with ↑ indicating CR-related increase and ↓ indicating CR-related decrease) insulin ↓ (12), ghrelin ↑ (13), leptin ↓ (14–16) and adiponectin ↑ (17). In addition, we also examined interleukin (IL)-6 ↓, which is a biomarker of inflammation as well as testosterone ↑, which is a biomarker of anabolic hormone, because they are associated with aging, age-related conditions and mortality (16, 18–21). Consequently, the aim of this study was to compare the level of above mentioned individual biomarkers between long- and short-lived healthy men and women.
METHODS
Study population
The Baltimore Longitudinal Study of Aging (BLSA) is an ongoing longitudinal study carried out with community-dwelling volunteers with above average education, income and access to medical care as well as general health consciousness. A general description of the sample, the recruitment criteria and implementation of the BLSA, have been previously reported (22, 23).
The present study was conducted as a case control study with cases defined as “Long-lived” subjects who survived to at least 90 years of age (n=41) and controls defined as “Short-lived” subjects who died between the ages of 72–76 years (n=31). All enrolled subjects had at least one BLSA visit when they were between 58 and 70 years of age and at that time had donated a blood sample. Subjects were excluded if they had diabetes mellitus, a diagnosis of coronary artery disease or cancer at the time of the blood sample. The BLSA protocol is approved by the Medstar Research IRB which complies with the principles stated in the Declaration of Helsinki. All subjects signed an IRB-approved informed consent.
Mortality
Mortality data were collected by intermittent telephone follow-up of inactive participants and correspondence with participants and their relatives. Every year, regular searches of the National Death Index were conducted to ascertain the vital status of the participants. For deceased BLSA subjects, the cause of death was determined by the consensus of three physicians reviewing all available information, including death certificates, letters from physicians and families, medical records, and autopsy reports. The cause of death was classified as “cardiovascular”, “cancer”, “other” or “unknown”. In addition, in the long-lived group there were three subjects who were still alive at the last assessment of death.
Laboratory measures
Blood samples were obtained from participants between 07:00 and 09:30 a.m. after a night of fasting. All blood samples were aliquoted at the same day and then stored at −80 C degrees. All the hormones and biomedical assays were performed at the Laboratory of Clinical Investigation and its Diabetes Section, National Institute on Aging, Baltimore, MD. Plasma ghrelin was measured by a radioimmunoassay (RIA) (Phoenix Pharmaceuticals, Belmont, CA) with intra-assay and inter-assay variations of 6.7% and 7.8%, respectively. Plasma insulin was measured by enzyme-linked immunosorbent assay (ELISA) (Alpco Diagnostics, Salem, NH) with intra-assay variations of 4.8–9.0% and inter-assay variations of 2.6–3.6%. Plasma leptin was measured using ELISA (LINCO Research, St. Charles, MO) with intra-assay variations of 1.09–4.98% and inter-assay variations of 3.89–5.33%. Plasma IL-6 was measured with ELISA (R & D systems, Minneapolis, MN) with intra-assay variations of 6.9–7.8% and inter-assay variations of 6.5–9.6%. Plasma adiponectin was measured by RIA (LINCO) with intra-assay and inter-assay variation of 1.78–6.21% and 6.9–9.25%, respectively. Plasma testosterone was measured by RIA (Diagnostic Systems Laboratories, Inc., Webster, TX) with intra-assay variation 7.8–9.6%, and inter-assay variation 8.4–9.1%. Fasting plasma glucose was measured by the glucose oxidase method (Beckman Instruments Inc., Fullerton, CA).
Other measures
Body height and weight were objectively measured, and body mass index (BMI) was calculated by dividing body weight in kilograms by the square of height in meters (kg/m2). Waist circumference was measured in centimeters with an inelastic tape at the narrowest part of the torso at the end of expiration. Blood pressure measurement was performed in the morning, after a light breakfast, with participants in the seated position during a medical history and physical examination, according to a standard protocol. Heart rate as beats per minute was recorded after blood pressure measurement from the radial pulse.
Physical activity was assessed using a questionnaire that asked about the amount of time spent doing specific leisure time activities. Amount of physical activity was expressed in metabolic equivalent (MET)*minutes as previously reported (24). Muscle mass was estimated as fat free mass (1-body fat mass) from anthropometric measures using a previously published formula for fat mass (25) derived by comparing DEXA estimates of fat mass with anthropometric measurements from 469 BLSA visits.
Statistical analysis
Study population characteristics at age 58–70 years are reported as mean values for continuous variables and proportions for categorical variables. Differences between groups were examined with t-test for normally distributed continuous variables and Wilcoxon test for skewed variables. To examine the combined effect of multiple biomarkers on longevity, each log transformed measure was z-scored with positive values coded according to the direction that, based on the literature, was hypothesized to be associated with CR. Then, the measurements were summed to represent a combined effect.
To assess whether the combined z-score would differentiate the two groups, and because some subjects had data from more than one sample available, a mixed effects logistic general linear model was used with a random subject term to account for the repeated measurements. Because of the distributional differences in testosterone between men and women, a nonparametric statistic, the permutation method, was used which only considers the data at hand. The permutation test estimates what the sampling distribution of the statistic is if the null hypothesis were true (26). Conceptually it considers all possible combinations of group assignment for subjects within the study, by creating a large number of datasets by randomly assigning a subject to either the short- or long-lived group (based on the number of subjects who were actually in each group). The idea of permutation is to calculate a reference distribution (for this study, the regression coefficient) that represents the null distribution of the parameter. We have used a 1000 randomly drawn datasets. The actual statistic (regression coefficient) obtained from the data is then compared to the permuted null distribution. Because significant differences between these two data sets were seen in this study, the permuted data and likelihood ratio was used to compare z-scores for short- and long-lived group.
An a priori power calculation, based on 50 subjects in the older and 43 subjects in the younger group, found an effect size of 0.5 at an alpha of 0.05 and power of 0.8 based on ghrelin. All analyses and graphs were completed using R version 2.10.0 (R Project for Statistical Computing, http://www.r-project.org).
RESULTS
At the time of their initial evaluation (58–70 years of age) long- and short-lived participants were similar for body weight, height, waist circumference, glucose and diastolic blood pressure (Table 1). In addition, the two groups were similar for physical activity and estimated fat free mass. The only significant difference between groups was higher systolic blood pressure in short-lived participants (p=0.05).
Table 1.
Subject Characteristics at Age 58–70 Years Among Short-lived and Long-lived Participants.
| Short-lived (Died at Age 72–76) | Long-lived (Survived to Age 90) | P | |
|---|---|---|---|
| Subjects, n (W/M) | 31 (4/27) | 41 (2/39) | |
| Mean (SD) | Mean (SD) | ||
| Age at Evaluation, years | 63.9(3.2) | 64.9 (3.5) | 0.29 |
| Age Death/Censor, years | 73.5 (1.2) | 92.4 (2.6) | <.001 |
| Weight, kg | 77.3 (9.3) | 75.7 (8.3) | 0.43 |
| Height, cm | 174.1 (6.5) | 173.7 (5.9) | 0.78 |
| BMI, kg/m2 | 25.5 (2.7) | 25.1 (2.4) | 0.48 |
| Waist circumference, cm | 90.3 (10.0) | 87.1 (6.4) | 0.25 |
| Systolic BP, mmHg | 139.5 (19.7) | 130.1 (18.7) | 0.05 |
| Diastolic BP, mmHg | 85.1 (12.9) | 80.1 (11.9) | 0.10 |
| Heart Rate, beats/minute | 76.2 (11.4) | 72.7 (8.5) | 0.17 |
| Fasting Plasma Glucose, mg/dL | 101.7 (11.1) | 100.9 (7.7) | 0.75 |
| Physical activity, MET*min | 371.6 (225.0) | 368.4 (353) | 0.97 |
| Fat free mass, kg | 55.2 (7.5) | 55.4 (5.4) | 0.94 |
| Biomarkers | |||
| Ghrelin, ρg/mL | 99.8 (68.5) | 102.3 (72.6) | 0.91 |
| Insulin, μU/mL | 5.4 (3.8) | 5.1 (4.2) | 0.75 |
| Leptin, ng/mL | 26.6 (38.4) | 15.0 (12.0) | 0.11 |
| IL-6, pg/mL | 2.2 (1.4) | 2.2 (2.2) | 0.95 |
| Adiponectin, μg/mL | 14.7 (8.6) | 12.2 (6.6) | 0.21 |
| Testosterone, ng/mL | 5.5 (5.6) | 5.9 (5.5) | 0.83 |
| Cause of Death, % | 0.006 | ||
| Cardiovascular | 39 | 24 | |
| Cancer | 23 | 5 | |
| Other | 39 | 41 | |
| Unknown | 0 | 22 | |
| Alive | 0 | 7 |
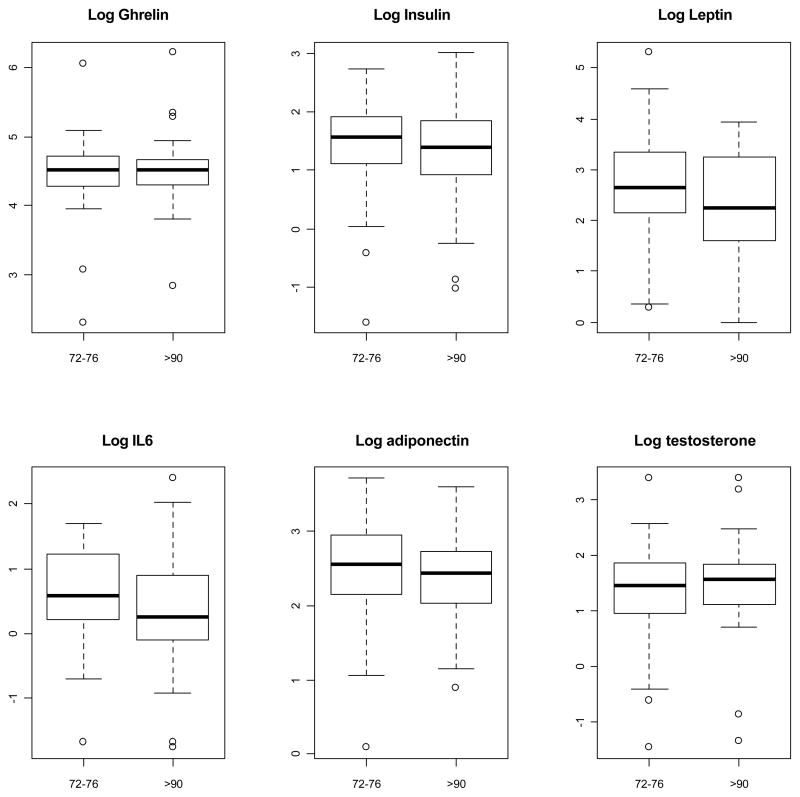
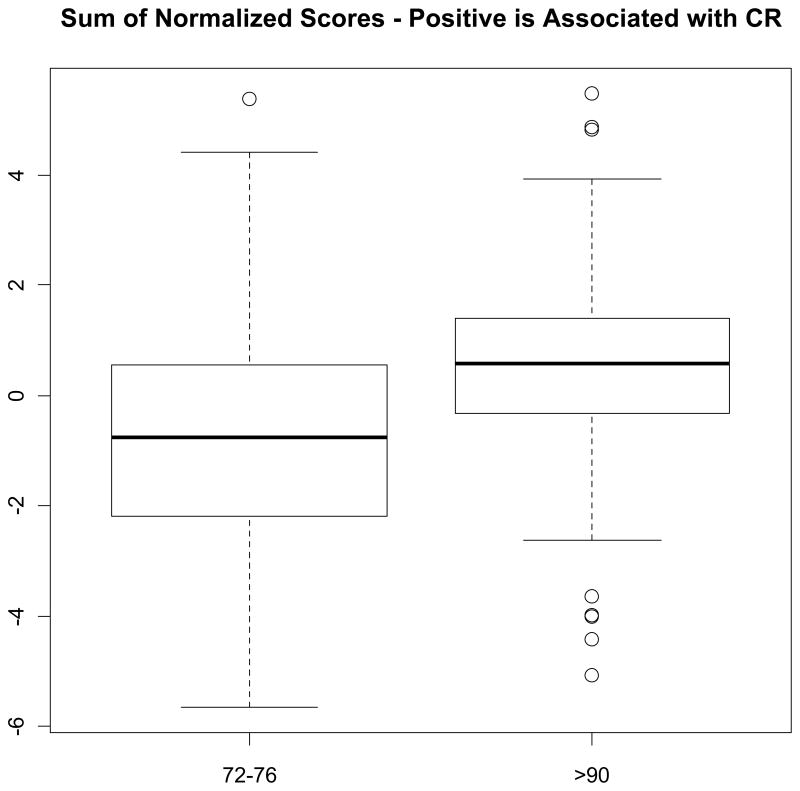
Taken separately, none of the six biomarkers were different between the two groups (Figure 1). However, long-lived participants tended to have non-significantly lower insulin, leptin and IL6, and higher testosterone values. The global score that combined information across the five biomarkers, ghrelin, insulin, leptin, IL6, and adiponectin, was borderline significantly higher in the long-lived compared to the short-lived BLSA participants (p=0.09 by likelihood ratio and p=0.05 by permutation) and when testosterone was included (p=0.22 by likelihood ratio and p=0.05 by permutation). In addition to take into account all available observations, by using the logistic regression models the global score significantly differentiated the groups both excluding (p=0.06 by loglikelihood and p=0.03 by permutation) and including testosterone in the index (p=0.05 by loglikelihood and p=0.02 by permutation) (Figure 2).
Figure 1.
Boxplot Comparison of Biomarkers Between Short-lived and Long-lived Participants.
Figure 2.
Boxplot Comparison of Sum of Normalized Scores Between Short-Lived and Long-Lived Participants *.
* Sum of normalized scores is based on Z-scores of ghrelin, insulin, leptin, IL6, adiponectin and testosterone. Each log transformed measure was z-scored with positive values coded according to the direction that was hypothesized based on the literature to be associated with CR (p=0.05 by loglikelihood and p=0.02 by permutation test).
DISCUSSION
Using data from the BLSA we tested the hypothesis that in the sixth decade of life, individuals who survive to the age of 90 and those who died before the age of 76 already show subtle differences in biomarkers that in animal models are affected by CR. Interestingly, we did not find any significant differences between the two groups for any of the six biomarkers, ghrelin, insulin, leptin, IL6, adiponectin and testosterone, examined in this study. However, levels of insulin, leptin and IL6 were directionally lower and testosterone higher in the long-lived group than in the short-lived group which is in agreement with the knowledge about these markers and CR and aging (12, 14–16, 27, 28). Nevertheless, when all the measures were considered together, the combined score differentiated the long- and short-lived groups in a manner that was consistent with the expectations observed in CR studies (11, 12).
Our finding about the directional association between combination of biomarkers related to CR and longevity may be important and concurs with the current thinking about aging as a dysregulation in multiple systems (29). Recently several epidemiological studies have utilized this approach and shown that the number of dysregulated systems is more predictive of negative health outcomes, such as muscle strength decline, frailty and mortality, than dysregulation in an individual system (30–34).
A significant difference in cause of death was observed between short and long-lived group. Although the short-lived group died more often for cardiovascular diseases, the causes of death are consistent with known population differences between 70 and 90 year-olds. The biomarkers compared in this study were measured at the time when the subjects were in their 60s and they were considered “healthy” and had no evidence for cardiovascular disease, diabetes or cancer. It is intrinsic for our study hypothesis that higher values in z-scores, at age when the subjects had no clinical evidence of disease, would lead to less serious disease and a later death from a different cause.
It is possible that due to a small sample size we did not have statistical power to detect clear differences between long- and short-lived individuals for the individual markers. An a priori power calculation was based on the parameter that would show the greatest change, and thus we are underpowered for the remaining factors. Due to the absence of adequate blood samples, our sample size was smaller than the projected sample of 50 subjects in the long-lived group. Further studies in larger population with multiple measures of potential CR mimetic markers are warranted to confirm our findings.
CONCLUSIONS
A case-control study including generally healthy men and women showed that in their sixties, long- and short lived individuals do not differ in biomarkers that have been associated with CR. However, directional difference between groups was obtained when multiple biomarker dysregulation was considered. Examining biomarkers related to CR may also help in understanding the processes that make CR work. Additional studies with representative populations are needed to confirm these findings and examine other potential markers of CR that are predictive of survival.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging. Data for these analyses were obtained from the Baltimore Longitudinal Study of Aging, a study performed by the National Institute on Aging.
References
- 1.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 2.Mehta LH, Roth GS. Caloric restriction and longevity: the science and the ascetic experience. Ann N Y Acad Sci. 2009;1172:28–33. doi: 10.1111/j.1749-6632.2009.04409.x. [DOI] [PubMed] [Google Scholar]
- 3.Kealy R, Lawler D, Ballam J, et al. Effects of diet restriction on lifespan and age-related changes in dogs. J Am Vet Med Assoc. 2002;220:1315–1320. doi: 10.2460/javma.2002.220.1315. [DOI] [PubMed] [Google Scholar]
- 4.Pinney D, Stephens D, Pope L. Lifetime effects of winter supplemental feed level and age at first parturition on range beef cows. J Anim Sci. 1972;34:1067–1074. doi: 10.2527/jas1972.3461067x. [DOI] [PubMed] [Google Scholar]
- 5.Lane MA, Mattison J, Ingram DK, Roth GS. Caloric restriction and aging in primates: Relevance to humans and possible CR mimetics. Microsc Res Tech. 2002;59(4):335–338. doi: 10.1002/jemt.10214. [DOI] [PubMed] [Google Scholar]
- 6.Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38(1–2):35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 8.Ingram DK, Roth GS, Lane MA, et al. The potential for dietary restriction to increase longevity in humans: extrapolation from monkey studies. Biogerontology. 2006;7(3):143–148. doi: 10.1007/s10522-006-9013-2. [DOI] [PubMed] [Google Scholar]
- 9.Lane M, Ingram D, Roth G. 2-Deoxy-D-glucose feeding in rats mimics physiological effects of calorie restriction. J Anti Aging Med. 1998;1:327–337. [Google Scholar]
- 10.Lane MA, Ingram DK, Roth GS. The serious search for an anti-aging pill. Sci Am. 2002;287(2):36–41. doi: 10.1038/scientificamerican0802-36. [DOI] [PubMed] [Google Scholar]
- 11.Lane MA, Roth GS, Ingram DK. Caloric restriction mimetics: a novel approach for biogerontology. Methods Mol Biol. 2007;371:143–149. doi: 10.1007/978-1-59745-361-5_11. [DOI] [PubMed] [Google Scholar]
- 12.Roth GS, Lane MA, Ingram DK, et al. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297(5582):811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Youm YH, Nakata C, Dixit VD. Chronic caloric restriction induces forestomach hypertrophy with enhanced ghrelin levels during aging. Peptides. 2007;28(10):1931–1936. doi: 10.1016/j.peptides.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komatsu T, Chiba T, Yamaza H, et al. Effect of leptin on hypothalamic gene expression in calorie-restricted rats. J Gerontol A Biol Sci Med Sci. 2006;61(9):890–898. doi: 10.1093/gerona/61.9.890. [DOI] [PubMed] [Google Scholar]
- 15.Shimokawa I, Higami Y. Leptin signaling and aging: insight from caloric restriction. Mech Ageing Dev. 2001;122(14):1511–1519. doi: 10.1016/s0047-6374(01)00284-6. [DOI] [PubMed] [Google Scholar]
- 16.Chiba T, Yamaza H, Higami Y, Shimokawa I. Anti-aging effects of caloric restriction: Involvement of neuroendocrine adaptation by peripheral signaling. Microsc Res Tech. 2002;59(4):317–324. doi: 10.1002/jemt.10211. [DOI] [PubMed] [Google Scholar]
- 17.Zhu M, Miura J, Lu LX, et al. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol. 2004;39(7):1049–1059. doi: 10.1016/j.exger.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41(2):176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 19.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 20.Orwoll E, Lambert LC, Marshall LM, et al. Endogenous testosterone levels, physical performance, and fall risk in older men. Arch Intern Med. 2006;166(19):2124–2131. doi: 10.1001/archinte.166.19.2124. [DOI] [PubMed] [Google Scholar]
- 21.Lehtonen A, Huupponen R, Tuomilehto J, et al. Serum testosterone but not leptin predicts mortality in elderly men. Age Ageing. 2008;37(4):461–464. doi: 10.1093/ageing/afn048. [DOI] [PubMed] [Google Scholar]
- 22.Shock NW, Greulich R, Andres R, et al. U.S. Govt. Printing Office. Normal Human Aging: The Baltimore Longitudinal Study of Aging. 1984. NIH Publication 84–2450. [Google Scholar]
- 23.Lissner L, Andres R, Muller DC, Shimokata H. Body weight variability in men: metabolic rate, health and longevity. Int J Obes. 1990;14(4):373–383. [PubMed] [Google Scholar]
- 24.Talbot LA, Metter EJ, Fleg JL. Leisure-time physical activities and their relationship to cardiorespiratory fitness in healthy men and women 18–95 years old. Med Sci Sports Exerc. 2000;32(2):417–425. doi: 10.1097/00005768-200002000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 26.Ludbrook J, Dudley H. Why Permutation Tests Are Superior to t and F Tests in Biomedical Research. The American Statistician. 1998;52(2):127–132. [Google Scholar]
- 27.Maggio M, Basaria S, Ble A, et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91(1):345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- 28.Araujo AB, Travison TG, Bhasin S, et al. Association Between Testosterone and Estradiol and Age-Related Decline in Physical Function in a Diverse Sample of Men. J Am Geriatr Soc. 2008;56(11):2000–2008. doi: 10.1111/j.1532-5415.2008.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrucci L, Giallauria F, Schlessinger D. Mapping the road to resilience: novel math for the study of frailty. Mech Ageing Dev. 2008;129(11):677–679. doi: 10.1016/j.mad.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maggio M, Lauretani F, Ceda GP, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Arch Intern Med. 2007;167(20):2249–2254. doi: 10.1001/archinte.167.20.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cappola AR, Xue QL, Fried LP. Multiple hormonal deficiencies in anabolic hormones are found in frail older women: the Women’s Health and Aging studies. J Gerontol A Biol Sci Med Sci. 2009;64(2):243–248. doi: 10.1093/gerona/gln026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gruenewald TL, Seeman TE, Karlamangla AS, Sarkisian CA. Allostatic Load and Frailty in Older Adults. J Am Geriatr Soc. 2009 doi: 10.1111/j.1532–5415.2009.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64(10):1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stenholm S, Maggio M, Lauretani F, et al. Anabolic and Catabolic Biomarkers As Predictors of Muscle Strength Decline: The InCHIANTI Study. Rejuvenation Res. 2010;13(1):3–11. doi: 10.1089/rej.2009.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]