Abstract
Please cite this paper as: Morens and Taubenberger. (2010) Historical thoughts on influenza viral ecosystems, or behold a pale horse, dead dogs, failing fowl, and sick swine. Influenza and Other Respiratory Viruses 4(6), 327–337.
Objectives To understand human influenza in a historical context of viral circulation in avian species, mammals, and in the environment.
Design Historical review.
Setting Global events in a variety of circumstances over more than 3,000 years time.
Sample Comprehensive review of the historical literature including all major publications on pandemic and panzootic influenza.
Main Outcome Measures Influenza pandemics, panzootics, major epidemics and epizootics, and instances of interspecies transmission of influenza A.
Results Extensive documentation of human and animal influenza over many centuries suggests that influenza A viruses have adapted to a variety of species and environmental milieu and are capable of switching between many different hosts under widely varying circumstances.
Conclusions The genetic elements of influenza A viruses circulate globally in an extensive ecosystem comprised of many avian and mammalian species and a spectrum of environments. Unstable gene constellations found in avian species become stable viruses only upon switching to secondary hosts, but may then adapt and circulate independently. It may be desirable to think of influenza A viruses as existing and evolving in a large ecosystem involving multiple hosts and environments. Implications for understanding human influenza are discusssed.
Keywords: Equine, history, influenza
The importance of understanding how pandemic influenza A viruses (IAV) emerge, and the nature of their relationship with IAV of birds and mammals, has been emphasized by the 2009–2010 H1N1 swine‐origin influenza pandemic, and by 13 years of enzootic/epizootic/human “spill‐over” circulation of highly pathogenic H5N1 avian IAV (HPAI) that have not yet, despite grave concerns, become pandemic. The threat of animal influenza is, however, not new [Figure 1, references (1, 2)]. Thousands of possible outbreaks, spanning centuries, and even millennia have been reported but never examined systematically. Lacking such organized historical information, in this brief review we nevertheless draw attention to events, themes, and trends in the occurrence of (apparent) non‐human influenza as reported in scientific and non‐scientific sources up to the beginning of the “microbial era”. 3 In doing so, we attempt to broaden the picture of influenza and to suggest that it is useful to view influenza viruses as existing and circulating within a large complex ecosystem which includes not only wild waterfowl, but also various other avian and mammalian hosts and environmental milieux.
Figure 1.

The Race Track (Death on a Pale Horse), by Albert Pinkham Ryder (1847–1917), ca. 1896, oil on canvas, Cleveland Museum of Art. Katherine Anne Porter’s (1890–1980) classic novella Pale Horse, Pale Rider, 2 which describes both the 1918 death of her lover and her own near death from influenza has fixed in the imagination of many readers an association between the chilling biblical imagery of the “pale horse” (Revelation 6:8) and the enormous death toll of the 1918–1919 pandemic.
Current understanding of influenza as an avian enzoonosis
All pandemic influenza viruses, like those of 1918, 1957, 1968, and 2009, ultimately acquired some or all of their gene segments from the avian IAV gene pool. 4 Additionally, zoonotic infections with both low pathogenicity (LPAI) and HPAI viruses have sporadically occurred. 5 Diverse IAV are widely distributed in their natural reservoir, numerous wild avian species – especially the aquatic Anseriformes and Charadriiformes waterfowl – around the world, 6 , 7 , 8 , 9 , 10 where they infect predominantly the lower intestinal tract 9 , 11 and are transmitted via the fecal‐oral route. Although not reservoir hosts of avian IAV, domesticated Galliformes (e.g., chickens, turkeys, quails) are susceptible, after adaptation, to infection with wild bird‐derived IAV. 12 , 13 Avian IAV are antigenically and genetically diverse; all 16 identified hemagglutinin (HA) and nine neuraminidase (NA) subtypes have been identified in a great variety of combinations. 8 , 9 , 14 The existence of a large number of different HA–NA subtype combinations, without evidence of genetic linkage among the specific segments, indicates that mixed IAV infection and reassortment in wild birds is extremely common 15 , 16 , and it further suggests that in wild birds IAV exist as transient genome constellations. 15 It is also likely that IAV can be maintained in the environment, e.g., in pond water and mud; 17 , 18 , 19 the significance of this in relation to the complex ecobiology of IAV is still poorly understood 14 but remains an important subject for further research.
As will be discussed, IAV maintained in wild birds have frequently switched to such new hosts as horses, swine, humans, and most frequently domestic poultry. But the mechanisms by which avian IAV cross species barriers, whether causing “dead‐end” infections or onward transmission in the new hosts, are largely unknown. Stable host switching likely involves acquisition of numerous mutations, depending on the virus and the host species 12 , 13 , 20 and is likely achieved by a variety of different evolutionary pathways, leading to different sets of genetic changes in each such event. Because adaptation to a new host seems to limit return to the wild bird IAV gene pool, 13 , 21 emergent viruses that begin as transient gene constellations in their natural avian hosts must go on to evolve as distinct and stable eight‐segment genome configurations within the new host. 5 , 15 Although the historical record is silent on the question, similar mechanisms may well have been causing influenza viral emergences for many centuries.
Influenza infections in animals: historical background
A major problem with review of the historical literature for evidence of animal influenza is that the further one goes back in time, the more difficult it becomes provisionally to identify influenza. Before the 16th century, epidemics of all kinds were usually recorded by laymen without medical vocabularies, and without appreciation of etiology, pathogenesis, natural history, distinctive/pathognomonic signs, or any of the modern ways in which we now diagnose, identify, and classify specific diseases. Even such distinct diseases as measles and smallpox were long confused with each other.
Human influenza can nevertheless be provisionally recognized historically when there is evidence of such unmistakable clinical and epidemiologic features as explosive epidemic febrile coughing that kills the young, the old, the infirm, and pregnant women. Looking for historical evidence of animal influenza is more complicated. Until the late 1700s, there were no veterinarians or veterinary schools, and until the late 19th century no organized poultry industry. Reports of animal diseases generally came as anecdotes or “asides” from physicians, farmers, or interested citizens; diseases we now recognize to be of different etiologies were frequently confused with each other, and until the 20th century many believed that what we now know to be different diseases based on their different etiologies were instead single diseases whose manifestations differed under the influence of various ill‐defined environmental, or telluric/atmospheric/climatic influences. The quality of this patchwork animal disease record remains poor until the late 19th century. Reference works on the historical occurrences of human and animal influenza are widely available, 1 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 including an important multivolume series begun by Corradi 40 but often depend for primary source material on subjective and unscientific information such as speculative epidemic/epizootiologic treatises, eye witness accounts, diaries, and hearsay.
Claims for animal influenza in the ancient literature
The classical literatures of Greece, Rome, and other ancient civilizations, as well as records from the Dark and Early Middles Ages, are packed with reports of human and animal disease outbreaks. In most cases, the diseases in question are so vaguely described that identification with modern diseases is difficult or impossible. For example, Homer’s The Iliad describes an epidemic of 9‐ day duration at the beginning of the Trojan War (ca. 1194 BC) that first affected horses and dogs, then men. Some 19th‐ and 20th‐century observers considered this disease to be influenza, 31 , 41 , 42 even though The Iliad does not mention cough or typical epidemiologic features. On similarly shaky ground is the description by veterinary physician Absyrtus of an alleged equine influenza epizootic in 330 AD. 43
A major epidemic of influenza‐like disease followed Carolman’s (Charlemagne’s) army across Europe in 876–877, having first arrived in Italy to spread northward, a directional pattern that was documented repeatedly for certain influenza pandemics occurring between 1510 and 1761, and also killing dogs and birds. 44 , 46 Another epidemic “cough that spread like the plague” 44 in 927 AD [but long confused by scholars with an epidemic 100 years earlier, in 826–827 AD 45 ] again first struck Italy and spread northward to affect the whole European continent, and again caused illness and death in humans, dogs, and birds. 29 , 32 Scholars have speculated that this was the first recorded pandemic of influenza, a possibility consistent with its appearance in southern Europe and its rapid spread northward.
The emphasis in these early accounts on horses, birds, and dogs is curious but inconclusive in implicating influenza. Domestic animals like horses and dogs were ubiquitous and probably suffered from many endemic and some epidemic illnesses that were considered indistinguishable from each other at the time. It would not be surprising if, during epidemic prevalences, coincidental observations of clinically similar illnesses in domestic animals would be noted by observers who lacked a scientific understanding of infection. References to avian deaths might also reflect formulaic Greek literary traditions attributing bird deaths to epidemics of any sort, or arguments favoring miasmatic spread. While tantalizing, many references to deaths of birds and domestic animals associated with possible human influenza outbreaks in the ancient literature are ultimately inconclusive.
Influenza in the middle ages
Although some historians consider the first influenza pandemic to have occurred earlier [frequently mentioned candidates include the European epidemics of 927, 1173 and 1386–1387 5 , 45 ], there is little doubt that the three pandemics of the 16th century, occurring in 1510, 1557, and 1580, ushered in a major influenza pandemic era (Figure 2). Although several writers commented non‐specifically about ill animals, 43 , 47 to our knowledge none of them reported specific influenza‐like illnesses in large domestic animals. A curious footnote was however recorded by Daniel Sennert (1572–1637), the physician who first scientifically described scarlet fever, in noting that: “…names applied to [the 1580 pandemic disease] included febrile catarrh, suffocating fever, epidemic catarrh, coughing epidemic, [and] contagious headache. The Germans called [it] ‘the chirp’, the sheep cough, the sheep disease, [and] the chicken malady, because sick persons were suffering [as with] the coryza of chickens” [(48); Figure 3]. The identity of the disease causing “chicken coryza” noted in 1580 is not known, although the same term had been used at the time of the 1557 pandemic, possibly in referring to croup‐like respiratory signs in children. 49 Neither HPAI nor Newcastle disease was recognized until more than 300 years later. We are unaware of other reports of similar avian illnesses in the voluminous literature on the 1580 pandemic which, however, has rarely been examined systematically, and never to our knowledge for evidence of animal disease.
Figure 2.
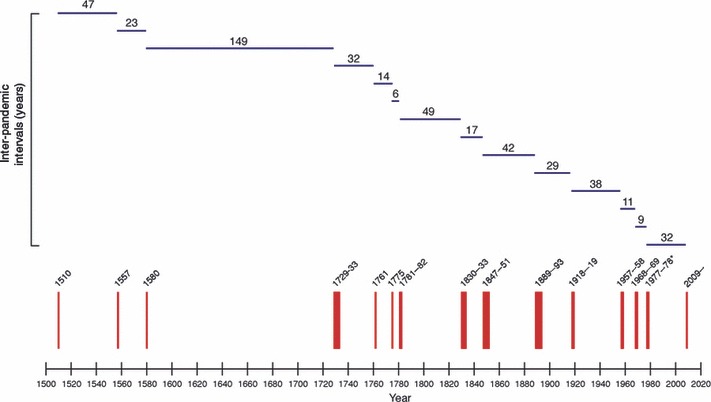
Influenza pandemics since 1510 [modified from (5)].
Figure 3.

The first page of Chapter 17, “On the catarrh and coughing epidemic”, in Daniel Sennert’s account of the 1580 influenza pandemic. 48 Sennert refers obliquely to a “coryza of chickens”, but it is not known whether avian influenza occurred during the pandemic.
An association between human and equine influenzas, 1648–1916
An H7N7 equine IAV was first isolated during a 1956 epizootic 50 but is now likely extinct; 51 an H3N8 equine IAV, detected in the early 1960s 52 still circulates enzootically to cause significant disease and economic burden. 53 Equine H3N8 viruses can infect humans experimentally, 54 , and it has been noted by several groups that human birth cohorts from the late 19th century, particularly those born before about 1893, demonstrated serologic reactivity with equine H3N8 viruses many decades later. 55
Before the virologic era, equine epizootics highly suggestive of influenza were documented repeatedly, beginning in 1299 with an equine epizootic 43 coincident with a widespread European influenza epidemic. In this epizootic: “the horse carried his head drooping, would eat nothing, and ran from the eyes”. 22 Twenty‐nine years later, in 1328, an equine epizootic in Yemen, later attributed to influenza, 1 , 56 coincided with a major European epidemic of apparent influenza. In 1404, yet another equine epizootic was associated with a major European influenza epidemic. 34 , 38 , 45
Between 1581 and 1728, a 147‐ year period in which pandemic influenza seemingly disappeared, 5 countless local and regional influenza outbreaks were documented in Europe, the New World, and elsewhere. During this time, a close temporal–geographic association between equine and human influenza‐like disease activity began to be documented in hundreds of medical, epidemiologic, and other publications. The first well‐documented occurrence in the Renaissance followed a modest 1647–1648 human outbreak at the end of the Thirty Years’ War in Spain, in French soldiers in Germany, and in their cavalry horses. 1 , 23 , 43 A decade later, in 1657–1658, one of the era’s most significant European influenza epidemics caused high human mortality [Oliver Cromwell (1599–1658) apparently died of it] accompanied by cases of influenza‐like illnesses in horses. 35
From 1658 until the early 20th century, outbreaks consistent with equine influenza were associated with major human outbreaks, epidemics, and pandemics more often than not. Considering several hundred published reports of major influenza occurrences between 1688 and 1888 that we have examined (obviously subject to errors related to, among other things, the possibility of not recognizing outbreaks that occurred), and considering only those with reasonably complete historical information, by our count there were 112 (of 200) years in which either significant epidemics/pandemics or equine epizootics of apparent influenza were documented in Europe. Of these 112 years, combined equine epizootics/epidemics were documented in 67; equine epizootics only in 25; and epidemics only in 20. The pattern in the western hemisphere during the same interval was somewhat different: of 56 years in which human or equine influenza was documented, both horses and humans were involved in 21 years; humans only in 25 years, and horses only in 10 years.
Regardless of geographic locale, equine influenza typically appeared about 3 weeks before human influenza, 42 , 57 a pattern first noted in 1688 in England and Ireland. 43 But in a few instances, this pattern was reversed, e.g., in 1776 and 1780 human outbreaks preceded equine outbreaks. 31 , 42 , 43 , 47 , 57 Commenting on a 1688 influenza epidemic, one chronicler wrote: “…an epidemic catarrh followed all over Europe, beginning among horses and ending with men as is frequently the case”, 57 then observed the same pattern five and again 10 years later [1693 and 1698 1 , 57 ]. Well before the year 1700, a strong association between human and equine influenza had been widely observed and widely accepted as a typical pattern of influenza occurrence.
Although these were non‐pandemic years, the equine association appears to have been about as strong during major (e.g., European‐wide) epidemics as during more local outbreaks. When the next pandemic appeared (in 1729), equine cases were again seen. 58 It was also noted that older horses and horses that had had influenza in the past were often immune. 59 With better epizootic documentation, and occurring with or without coincident epidemics, it was eventually noted, between 1815 and 1873, that major equine epizootics occurred in almost exact 4‐ year cycles, 60 presumably reflecting equine birth cohort susceptibility. Moreover, it came to be understood that although human non‐pandemic influenza in this era was often a seasonal (winter) event, when epidemics were associated with equine epizootics they tended to occur in the spring or fall, the usual seasons in which equine influenza prevailed. Conceivably, during the warmer months horses and humans alike were more likely to be traveling and moving about in agricultural work, and thereby more likely to acquire and transmit influenza.
In 1727, a year of low‐level endemic and epidemic influenza in Europe, an apparently new phenomenon was documented: an extremely explosive equine epizootic that far overshadowed the associated epidemic. Large scale European equine epizootics or panzootics recurred in 1750 and 1760. 1 , 41 , 60 , 61 In 1750: “About the middle or latter end of December, the most epidemic and universally spreading disease among horses that anyone living remembered [and] analogous to [human epidemic] influenza…now particularly attacked the horses…[and] spread through all England in almost an instant, and…raged in Denmark at the same time…there was scarce an instance of a horse in town or country but had it…It vanished about the middle of January [1751]”. 61
The 1872 western hemispheric equine panzootic
The most explosive equine panzootic ever documented was first recognized in pastured horses in three farm communities north of Toronto in late September, 1872, at a time when human influenza was not prevalent. 43 , 60 From Toronto, it spread quickly along rail lines, with separate paths going eastward into Upstate New York and westward into Michigan, radiating outward from these new foci in every direction, eventually spreading via shipping routes to the Caribbean and Central America. By some accounts, the panzootic stopped at Panama, where there were no horses. 62 Ill horses exported to Europe were quarantined in port; although enzootic and epizootic equine influenza prevailed in Europe that year, principally in England and Germany, there is no good evidence of importation from America.
Horses, mules, and circus/menagerie zebras were all affected, and there were numerous outbreaks and single cases of mild influenza in humans as well, often linked to equine exposures. 63 National epidemics of influenza in 1873 and 1874 that seemed to spare old people were linked by some physicians to the equine epizootic, 64 but as mild seasonal influenza had already been prevailing in some locales in late 1872, a causal association is speculative. The human form of the epizootic disease became popularly known throughout the United States as the “epizooty” or “zooty”, 63 a term that ended up as a plot device in a famous novel published exactly a century later. 65
Onset of the disease in horses was acute with fever and a “short, dry, husky cough” 43 , 66 followed within about 2 days by loosening cough, nasal discharge, swollen eyelids, labored breathing, loss of appetite, increased thirst, painful swallowing, weakness, dullness, and prostration. Complications included pneumonia and dropsy: “oedematous swellings of the limbs, beneath the chest and belly, and in the lower part of the head”. 43 Necropises of horses that died acutely revealed evidence consistent with primary viral pneumonia in some instances and secondary bacterial pneumonia in others. 43 Almost all urban epizootics were explosive with urban attack rates approaching 100%. The death rate was in the range of 2%, including not only acute deaths from pneumonia but also dropsical complications, the latter occurring in about 1–15% of surviving horses and associated with a fatality rate of 20–50%. Dropsy appeared as a “trailing” epizootic about 3 weeks after the peak of epizootic influenza; it predominated in some cities, but not in others, and was widely observed to occur in older horses that had been over worked or poorly cared for when ill with influenza (The Chicago Daily Tribune, 14 and 15 November 1872).
The 1872 panzootic literally shut down the United States for several weeks, preventing travel, transportation, mail delivery, and delivery of goods and provisions. Truck deliveries ground to a halt as winter approached, causing coal prices to skyrocket across the nation (The Chicago Daily Tribune, 17 November 1872; The New York Times, 27 November 1872). Physicians were unable to reach their patients (The New York Times, 16 November 1872). Fire stations around the country either brought in oxen from the countryside or trained teams of young men to pull fire wagons. Nevertheless, a fire in Boston’s financial district got out of control, and much of the city burned down on 9 November [Figure 4; (67)], allegedly in part because slower moving fire wagons drawn by teams of young men could not respond quickly enough (Boston, 11 November 1872). Equine epizootics were common across the United States for the next 30 years, but with the exception of major epizootics in 1880–1881 and 1900–1901, they tended to be local, to affect mostly young horses, and to feature much lower attack rates. 68 , 69 One of the last widespread equine influenza epizootics in the United States occurred in the winter of 1915–1916, concurrent with a major epidemic season. 70
Figure 4.

The Boston fire of 9 November 1872 burned down much of the financial district of Boston. 67 Among other factors, the devastation of the fire has been attributed to the ongoing influenza panzootic (Boston, 11 November 1872). Because most of the equine work force was incapacitated, fire stations throughout the United States recruited teams of men to pull fire wagons. The slow response times of the Boston teams are believed by many to have led to the fire getting out of control. Above, onlookers on Devonshire Street assemble around Steamer Number 10, from the Fire House on Mount Vernon Street. At far right a fireman with a hose sprays the ruins.
Were the countless reports of influenza‐like diseases in horses, recorded over centuries, really influenza? We are unaware of pathological material available before modern times that might answer this question definitively. Circumstantial but arguably strong evidence for equine influenza includes the consistency of clinical signs, the temporal–geographic association with human influenza, high explosivity and rapid horse‐to‐horse spread – with increased explosivity in crowded stables – and a consistent incubation period for both equine and human secondary cases. Eighteenth and nineteenth century authorities believed the two diseases were the same disease and believed them to be transmitted in both directions. The infrequency in the differential diagnosis of other clinically consistent respiratory diseases of high explosivity is also of note.
At a time when horses were ubiquitous in virtually all rural and urban settings, it is thus conceivable that they played a role in influenza virus maintenance and evolution analogous to that of pigs in the modern era. Although the cause of the 1889 influenza pandemic is unknown, various archeserologic data suggest the possibility of an H3 HA, and possibly also an N8 NA. 54 , 55 , 71 , 72 , 73 Equine influenza‐like disease was common in the 1889 pandemic, but the virus clearly spread globally by means of person‐to‐person spread. 74 The possibility that the 1889 pandemic was caused by an H3N8 equine influenza virus that had become adapted to humans, and which then spread pandemically, is highly speculative but cannot yet be ruled out. Alternatively, if the 1889 pandemic virus was truly of H3N8 subtype, it is conceivable that, in a manner analogous to the persistent “classical” swine H1N1 virus derived from the 1918 pandemic virus, it was at some point transmitted from humans to horses and the virus or some of its gene segments may persist in horses today.
Influenza in dogs, cats, and other mammals
Influenza‐like illnesses in dogs and cats, apparently acquired from either horses or, less commonly, humans, have been reported for centuries during epidemics and equine epizootics, 1 , 62 probably representing in most instances occasional “dead‐end” host transmission. Apparent canine influenza was reported, for example, during the 1675–1676, the 1760, and the 1767 epidemics/equine epizootics, 1 , 43 , 57 , 59 , 61 and substantial outbreaks of possible influenza in dogs were seen during the 1782 pandemic, anecdotally associated with transmission to parrots, 75 and the Australian epidemic of 1851–1852. 76 Similar canine and feline illnesses and deaths were reported frequently in the 1872 equine panzootic as well and have more recently been reported in the 2009 H1N1 pandemic. Influenza cases and deaths in racing greyhounds have been recognized for over a century (The San Francisco Call, 11 November 1901; Figure 5). Equine H3N8 viruses have recently become stably adapted to dogs 77 and are now evolving in dogs by antigenic drift, 78 establishing another extra‐avian IAV reservoir. Different lineages of this virus are now spreading internationally, 79 a situation without recognized precedent in the historical literature. Other species such as large cats, ferrets, minks, seals, and other mammals have also occasionally been infected.
Figure 5.

During a 1900–1901 equine influenza epizootic, the US champion racing greyhound For Freedom died of “an attack of the epizooty, which is prevalent here among horses” (The San Francisco Call, 11 November 1901).
Influenza in pigs
Despite the strong historical association between human and equine influenza, and the extensive documentation of many swine diseases over several centuries, 1 , 33 including swine outbreaks in association with poultry outbreaks, we have been unable to identify any epizootics that appear highly consistent with swine influenza until the fall wave of the 1918 influenza pandemic. 80 At that time, the pandemic virus seems to have been transmitted from humans to pigs, thereby splitting off into two lineages, one human, the other porcine, from which viral descendants were isolated in the 1930s. 81 , 82 These viral lineages persist today, having both contributed genes to the 2009 H1N1 reasssortant pandemic virus. 83 Conceivably, in the modern era, pigs have replaced the once ubiquitous horse as a secondary extra‐human mammalian influenza reservoir.
Numerous influenza subtypes isolated from pigs cause either enzootic infections or self‐limited outbreaks globally. Since 1998, several lineages of triple reassortant viruses containing genes from “classical” swine H1N1, human H3N2, and avian influenza viruses have emerged to cause enzootic disease in pigs in the United States and elsewhere. 84 , 85 Other fully avian or human IAV‐derived viruses, or reassortant viruses containing genes of avian, swine, and/or human IAV origin, have been associated with less widespread disease in swine, e.g., H1N1, H1N2, H1N7, H2N3, H3N1, H3N2, H3N3, H4N6, and H9N2 subtypes. 86 Equine H3N8 viruses were recently isolated from pigs in China without evidence of stable host switching. 87
In the late 1970s, yet another H1N1 lineage emerged to cause enzootic disease in Eurasia by genomic adaptation of an avian IAV. 88 , 89 , 90 Still in circulation, this virus played a reassortment role in the emergence of the 2009 pandemic H1N1 virus, 91 which caused swine outbreaks in many places around the world 92 after apparent back transmission from humans to pigs. The history, genetic makeup, and behavior of this virus 4 suggest significant risk of future swine virus epidemics/pandemics. Other swine‐adapted IAV have caused zoonotic infections of at least 50 humans, with seven fatalities, between 1958 and 2005, 93 including a 1976 outbreak at Fort Dix, New Jersey, associated with human‐to‐human transmission, in which classical swine H1N1 infected 230 soldiers and caused one death. 94
Because pigs are susceptible to infection with both avian and human IAV strains, 10 , 88 they have been considered a possible intermediate host (“mixing vessel”) for the generation of IAV of pandemic potential. 10 , 95 This dual susceptibility was previously attributed to the presence of both sialic acid (SA)‐α2,3‐galactose (“avian‐like”) and SA‐α2,6‐galactose (“human‐like”) linkages on the glycocalyx of epithelial cells lining the swine upper airway and trachea. 96 However, recent lectin histochemistry studies have shown little SA‐α2,3‐galactose in the upper airway, 97 thus swine likely have an SA receptor pattern similar to that of humans. 98 , 99
Influenza in poultry
In the past decade, many documented human infections with avian IAV have occurred, prominently in association with H5N1 HPAI epizootics in Eurasia and Africa. 100 H5N1 HPAI viruses initially caused a 1996 poultry epizootic in southern China, followed within a year by a Hong Kong epizootic that caused 18 human cases and six deaths. 101 , 102 H5N1 strains continued to circulate thereafter in China, reappeared in epizootic form in 2003, and then spread widely, 103 resulting in the appearance and spread of genetically and antigenically diverse strains, including reassortants with other avian IAV. 104 The H5N1 panzootic is unique in causing the deaths from infection and culling of millions of poultry in 64 countries on three continents, 105 and in producing, since 2003, 499 human spill‐over cases with 295 deaths in 15 countries (WHO, as of 8 June 2010), and rare instances of self‐limited human‐to‐human transmission. 106 The H5N1 panzootic is also unique in causing infections and deaths in numerous wild bird species 12 and occasional infections of wild and domestic mammals. 107 , 108
In 2003, an H7N7 HPAI virus caused a poultry epizootic in the Netherlands and spread regionally. Before the epizootic was contained, at least 86 poultry workers and three of their contacts had become infected and developed conjunctivitis with or without influenza‐like illnesses; one of them died. 109 Similarly, two persons developed influenza conjunctivitis during an outbreak of H7N3 HPAI in Canada in 2004. 110 During the past decade, at least eight other major poultry epizootics have occurred, caused either by novel emerging H5 or H7 subtype HPAI viruses, or in one instance by an H9N2 LPAI virus, 12 leading to occasional human infections. 109
Did human infection with avian IAV occur before the virologic era? As noted earlier, the vague references to bird deaths and disappearances during historical influenza epidemics, and the curious passage of Sennert noting “coryza of chickens” in the 1580 pandemic, 48 are of uncertain meaning. An oft‐cited fatal 1776 poultry epizootic associated with an influenza pandemic was probably not of influenza origin 43 and was not fatal to humans, nor were other temporally associated poultry outbreaks that were consistent with influenza such as one that spread across Northern Italy in 1789. 111 In any case, the historical record indicates that poultry influenza outbreaks have never been shown to cause more than a limited number of human spill‐over cases.
HPAI (then called “fowl plague”) was recognized as a disease entity in 1878 112 and between 1901 and 1903 was shown to be caused by a filterable agent. 113 , 114 , 115 , 116 Epizootiologic links were soon made to transmission from pet birds to humans, and to epizootics of pneumo‐enteritis in pigs, 115 although it is not certain that these diseases were influenza. Fowl plague virus was identified as influenza A in 1955. 117 Isolation of IAV from wild ducks in 1974 7 led to the realization that wild aquatic birds are the natural reservoir for IAV. 10
With this historical picture in mind, it is curious to note that between 15 November and 15 December 1872, 6 years before the recognition of “fowl plague”, an explosive, fatal, and still mysterious epizootic in poultry, prairie chickens, turkeys, ducks, and geese occurred over much of the populated United States in temporal–geographic association with the panzootic of equine influenza that had just begun. 43 , 60 First recognized in Poughkeepsie, New York, on 15 November (The New York Times, 16 November 1872), whole flocks were usually struck simultaneously, with fatality at or near 100%. Prominent features included upper respiratory signs of “a cold or influenza”, slimy discharge emitted from the beak, dark streaks in the neck, “dizziness” or “staggering fits”, crawling away from the flock into holes or corners, and in most cases death within 12–18 hours (The [New York] World, 17 November 1872; The New York Herald, 18 November 1872; The Iowa State Reporter, 20 November 1872; The Janesville [Wisconsin] Gazette, 22 November 1872).
Concentrated mostly in the Northeast and Midwest, the epizootic spread outward with tremendous speed from the upper Hudson River area of New York State. According to the newspapers, it “follow[ed] in the tracks of the dread [equine] Epizootic” (The Richwood [Ohio] Gazette, 28 November 1872), allegedly spreading “all over the country” by 19 November (Indiana Progress, 21 November 1872) and without exception occurring in locales that were or had been experiencing equine influenza. Outbreaks in poultry often occurred immediately after they had been allowed to roost or peck in stables that held ill or recently ill horses (The New York Herald, 19 November 1872; The [Baltimore] Sun, 21 November 1872; The Janesville [Wisconsin] Gazette, 27 November 1872; The [Davenport, Iowa] Daily Gazette, 28 November 1872).
The epizootic poultry disease – amply recorded in the national press, but apparently not studied scientifically – could have been caused by other agents. “Chicken cholera” (linked to Pasteurella multocida in 1878) was well known in 1872 but was considered to be a different disease than the 1872 epizootic disease (The [New York] World, 19 November 1872). Newcastle disease was not recognized until 1926, does not seriously affect horses, pigs, or humans, and in any case seems somewhat less consistent with epizootic features described in 1872. The consistent clinical features and epidemiologic evidence, coupled with a remarkable temporal–geographic association between equine influenza and poultry outbreaks of an explosive and fatal illness, appear consistent with influenza transmission. Although “back‐transmission” of a mammalian‐adapted influenza virus to an avian host would seem unlikely based on current (but limited) understanding of IAV host switching, it is noteworthy that both classical swine H1N1 and the 2009 H1N1 pandemic virus have caused symptomatic epizootics in turkeys; 118 , 119 138 years after it occurred, the poultry epizootic associated with the 1872 equine influenza epizootic remains a mysterious footnote in the ever mysterious history of influenza.
Conclusions
Although IAV are rightly considered to be avian viruses resident in a large reservoir comprised of many species of wild waterfowl and shorebirds, they are uniquely promiscuous in undergoing an extraordinary degree of genetic change within their primary hosts, and within the host’s aquatic environment – comprised of water, mud, and organic material – and they frequently switch hosts to infect many other avian and mammalian species. The genetic elements of IAV circulate globally in an extensive ecosystem comprised of many avian and mammalian species and a spectrum of environments around the world. In their natural reservoirs, IAV can be thought of not as stable viral entities but as transient genome constellations. 15 Genomic stability seems to be an unnatural state and may be only attainable when an eight‐gene segment constellation finds its way into an “unnatural” secondary host. What IAV can do when they become stabilized by adaptation is not fully explored, but the breadth of the viral repertoire is inferred by the historical literature suggesting viral infection of, adaptation to, and onward transmission by a variety of hosts including humans, horses, pigs, dogs, and domestic poultry. It may be desirable to more broadly conceive of IAV as being adapted not just to a few avian reservoir hosts but to a complex environment populated by many different species in a variety of stabilizing environmental milieux. The historical literature on influenza, though frustrating in its general lack of corroboration by modern scientific methods, suggests that IAV may be more evolutionarily flexible than we have realized. It would not be surprising if additional secondary reservoir hosts were involved in viral maintenance, evolution, and transmission. Influenza history suggests that there is still much to learn, and many surprises probably still await us.
Acknowledgements
We thank Betty Murgolo and the staff of the Collection, Management & Delivery Branch, Document Delivery Section, NIH Library, and the staff of the History of Medicine Division, National Library of Medicine, for research support; and John Weddle for assistance with image reproduction.
References
- 1. Fleming G. Animal Plagues: Their History, Nature, and Prevention. London: Chapman and Hall, 1871. [Google Scholar]
- 2. Porter K. Pale Horse, Pale Rider; Three Short Novels. New York: Harcourt, Brace and Company, 1939. [Google Scholar]
- 3. Koch R. Die Aetiologie der Milzbrand‐Krankheit, begründet auf die Entwicklungsgeschichte der Bacillus Anthracis. Beiträge zur Biologie der Pflanzen (Breslau [Wrocław]) 1876; 2:277–310. [Google Scholar]
- 4. Morens DM, Taubenberger JK, Fauci AS. The persistent legacy of the 1918 influenza virus. N Engl J Med 2009; 361(3):225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taubenberger JK, Morens DM. Pandemic influenza – including a risk assessment of H5N1. Rev Sci Tech 2009; 28(1):187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Easterday BC, Trainer DO, Tumova B, Pereira HG. Evidence of infection with influenza viruses in migratory waterfowl. Nature 1968; 219(5153):523–524. [DOI] [PubMed] [Google Scholar]
- 7. Slemons RD, Johnson DC, Osborn JS, Hayes F. Type‐A influenza viruses isolated from wild free‐flying ducks in California. Avian Dis 1974; 18(1):119–124. [PubMed] [Google Scholar]
- 8. Krauss S, Walker D, Pryor SP et al. Influenza A viruses of migrating wild aquatic birds in North America. Vector Borne Zoonotic Dis 2004; 4(3):177–189. [DOI] [PubMed] [Google Scholar]
- 9. Munster VJ, Fouchier RA. Avian influenza virus: of virus and bird ecology. Vaccine 2009; 27(45):6340–6344. [DOI] [PubMed] [Google Scholar]
- 10. Webster R, Bean W, Gorman O, Chambers T, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev 1992; 56(1):152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stallknecht DE, Kearney MT, Shane SM, Zwank PJ. Effects of pH, temperature, and salinity on persistence of avian influenza viruses in water. Avian Dis 1990; 34(2):412–418. [PubMed] [Google Scholar]
- 12. Alexander DJ. An overview of the epidemiology of avian influenza. Vaccine 2007; 25(30):5637–5644. [DOI] [PubMed] [Google Scholar]
- 13. Swayne DE. Understanding the complex pathobiology of high pathogenicity avian influenza viruses in birds. Avian Dis 2007; 51(1 Suppl.):242–249. [DOI] [PubMed] [Google Scholar]
- 14. Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus AD, Fouchier RA. Global patterns of influenza A virus in wild birds. Science 2006; 312(5772):384–388. [DOI] [PubMed] [Google Scholar]
- 15. Dugan VG, Chen R, Spiro DJ et al. The evolutionary genetics and emergence of avian influenza viruses in wild birds. PLoS Pathog 2008; 4(5):e1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang R, Soll L, Dugan V et al. Examining the hemagglutinin subtype diversity among wild duck‐origin influenza A viruses using ethanol‐fixed cloacal swabs and a novel RT‐PCR method. Virology 2008; 375(1):182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ito T, Okazaki K, Kawaoka Y, Takada A, Webster RG, Kida H. Perpetuation of influenza A viruses in Alaskan waterfowl reservoirs. Arch Virol 1995; 140(7):1163–1172. [DOI] [PubMed] [Google Scholar]
- 18. Lang AS, Kelly A, Runstadler JA. Prevalence and diversity of avian influenza viruses in environmental reservoirs. J Gen Virol 2008; 89(Pt 2):509–519. [DOI] [PubMed] [Google Scholar]
- 19. Brown JD, Goekjian G, Poulson R, Valeika S, Stallknecht DE. Avian influenza virus in water: infectivity is dependent on pH, salinity and temperature. Vet Microbiol 2009; 136(1–2):20–26. [DOI] [PubMed] [Google Scholar]
- 20. Salomon R, Webster RG. The influenza virus enigma. Cell 2009; 136(3):402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campitelli L, Mogavero E, De Marco MA et al. Interspecies transmission of an H7N3 influenza virus from wild birds to intensively reared domestic poultry in Italy. Virology 2004; 323(1):24–36. [DOI] [PubMed] [Google Scholar]
- 22. Rusii L. Hippiatria Sive Marescalia. Basiliensi [Basel]: Christianum Wechelum, 1532. [Google Scholar]
- 23. De Solleysel J. Le Parfait Mareschal, Qui Enseigne A Connoistre La Beauté, La Bonté, Et Les Defauts Des Chevaux, Les Signes & les Causes des maladies: les moyens de les prévenir, leur guerison, & le bon ou mauvais usage de la Purgation & de la Saignée. La maniere de les conserver dans les Voyages, de les nourit & de les penser selon l’ordre. La Ferrure sur les desseins des Fers, qui rétabliront les méchans pieds, & conserveront les bons. Ensemble Un Traité du Haras, pour élever de beaux & de bons poulains; & les Préceptes pour bien Emboucher le Chevaux: Avec les Figures necessaires. Neuvième Edition. Bruxelles: Lambert Marchant, 1691. [Google Scholar]
- 24. Gibson W. A New Treatise on the Diseases of Horses. London: A. Millar, 1754. [Google Scholar]
- 25. Osmer W. A Dissertation on Horses. London: T. Waller, 1756. [Google Scholar]
- 26. Saillant [C‐J]. Tableau historique et raisonné des épidémies catarrhales, vulgairement dites la grippe; depuis 1510 jusques & y compris celle de 1780, avec l’indication des traitemens curatifs & des moyens propres à s’en préserver. Paris: Didot jeune, 1780. [Google Scholar]
- 27. De Villalba J. Epidemiologia española; ó, Historia cronológica de las pestes, contagios, epidemias y epizootias que han acaecido en España desde la venida de los cartagineses, hasta el año 1801, con noticia de algunas otras enfermedades de esta especie que han sufrido los españoles en otros reynos, y de los autores nacionales que han escrito sobre esta materia, así en la península como fuera de ella. Madrid: Mateo Repullés, 1802. [Google Scholar]
- 28. Schnurrer F. Geographische Nosologie; oder die Lehre von den Veränderungen der Krankheiten in den verschiedenen Gegenden der Erde, in Verbindung mit physischer Geographie und Natur‐Geschichte des Menschen. Stuttgart: Steinkopf, 1813. [Google Scholar]
- 29. Schnurrer F. Chronik der Seuchen, in Verbindung mit den gleichzeitigen Vorgängen in der physischen Welt und in der Geschichte der Menschen. Tübingen: Osiander, 1823, 1825. [Google Scholar]
- 30. Gluge G. Die Grippe oder Influenza. Eine historisch‐pathologische Abhandlung; in Hecker JFC. (ed.): Neue wissenschaftliche Annalen der gesammten Heilkunde, Dritter Band. Berlin: Theod[or] Christ[ian] Friedr[ich] Enslin, 1836; 129–171. [Google Scholar]
- 31. Thompson T. Annals of Influenza or Epidemic Catarrhal Fever in Great Britain From 1510 to 1837. London: Sydenham Society, 1852. [Google Scholar]
- 32. Parkes E. Influenza; in Reynolds J. (ed.): A System of Medicine. London: MacMillan and Company, 1866; 27–50. [Google Scholar]
- 33. Fleming G. Animal Plagues: Their History, Nature, and Prevention. Volume II. (From A. D. 1800–1844). London: Baillière, Tindall, and Cox, 1882. [Google Scholar]
- 34. Zeviani GV. Sul catarrho epidemico opuscolo. Memorie di Matematica e di Fisica (Societa Italiana delle scienze; Modena) 1804; 11:476–530. [Google Scholar]
- 35. Creighton C. Influenza; in Creighton C. (ed.): A History of Epidemics in Great Britain from AD 664 to the Extinction of Plague. Cambridge: University Press, 1891; 397–413. [Google Scholar]
- 36. Paulet J‐J. Recherches historiques & physiques sur les maladies epizootiques, avec les moyens d’y rémedier, dans tous les cas. Paris: Rualt, 1775. [Google Scholar]
- 37. Leichtenstern O. Influenza; in Nothnagel H. (ed.): Cholera asiatica und Cholera nostras, von C Liebermeister. Influenza und Dengue, von O Leichtenstern. Der Keuchhusten Der Bostock’sche Sommerkatarrh, das sogenannte Heufieber, von Georg Sticker. Influenza and dengue. Wien: Alfred Hölder, 1896; 1–198. [Google Scholar]
- 38. Finkler D. Influenza; in Stedman T. (ed.): Twentieth Century Practice: An International Encyclopedia of Modern Medical Science By Leading Authorities of Europe and America. Volume XV. Infectious Diseases. New York: William Wood & Company, 1898; 3–249. [Google Scholar]
- 39. Creighton C. Influenzas and epidemic agues A History of Epidemics in Great Britain. Volume II. From the Extinction of Plague to the Present Time. Cambridge: University Press, 1894; 300–433. [Google Scholar]
- 40. Corradi A. Annali delle epidemie occorse in Italia dalle prime memorie fino al 1850. Parte Prima. Dalle Prime Memorie Fino al 1500. Bologna: Gamberini e Parmeggiani, 1865. [Google Scholar]
- 41. Hamer WH. The Milroy lectures on epidemic diseases in England – the evidence of variability and persistence of type. Lecture II. Lancet 1906; 1:655–662. [Google Scholar]
- 42. Clarkson F. The history of influenza. Canad M Month 1920; 4:59–63. [Google Scholar]
- 43. Law J. Influenza in horses; in Agriculture Commission (ed.): Report of the Commissioner of Agriculture for the Year 1872. Washington, DC: Government Printing Office, 1874; 203–248. [Google Scholar]
- 44. Wilson J, Da Costa J. Influenza; In Wilson J, Da Costa J. (eds): A Treatise on the Continued Fevers. New York: William Wood & Company, 1881; 10–45. [Google Scholar]
- 45. Clemow F. Epidemic influenza. Proceedings of the Society of Medical Officers of Public Health. Public Health 18891890; 2:358–367. [Google Scholar]
- 46. Anonymous . Annals of Fulda [Annales Fuldenses]. Manchester: Manchester University Press, 1992. [Google Scholar]
- 47. Short T. A general chronological history of the air, weather, seasons, meteors, &c Sundry Places and Different Times…: With Some of Their Most Remarkable Effects on Animal (Especially Human) Bodies, and Vegetables; in Two Volumes. London: T. Longman, A. Millar, 1749. [Google Scholar]
- 48. Sennerto D. De Catarrho & Tussi epidemia; in Sennert D. (ed.) De Febribus Libri IV. Wittbergæ: Zachariam Schurerum, 1619; 1055–1064. [Google Scholar]
- 49. Sprengel K. Versuch einer pragmatischen Geschichte der Arzneikunde. Dritter Theil. Halle: Johann Jacob Gebauer, 1801. [Google Scholar]
- 50. Sovinová O, Tůmová B, Poutska F, Němec J. Isolation of a virus causing respiratory disease in horses. Acta Virol 1958; 2(1):52–61. [PubMed] [Google Scholar]
- 51. Madić J, Matrtinović S, Naglić T, Hajsig D, Cvetnić S. Serological evidence for the presence of A/equine‐1 influenza virus in unvaccinated horses in Croatia. Vet Rec 1996; 138:68. [DOI] [PubMed] [Google Scholar]
- 52. Waddell GH, Teigland MB, Sigel MM. A new influenza virus associated with equine respiratory disease. J Am Vet Med Assoc 1963; 143:587–590. [PubMed] [Google Scholar]
- 53. Paillot R, Hannant D, Kydd JH, Daly JM. Vaccination against equine influenza: quid novi? Vaccine 2006; 24(19):4047–4061. [DOI] [PubMed] [Google Scholar]
- 54. Alford R, Kasel J, Lehrich J, Knight V. Human responses to experimental infection with influenza A/equi 2 virus. Am J Epidemiol 1967; 86(1):185–192. [DOI] [PubMed] [Google Scholar]
- 55. Minuse E, McQueen J, Davenport F. Studies of antibodies to 1956 and 1963 equine influenza viruses in horses and man. J Immunol 1965; 94(4):563–566. [PubMed] [Google Scholar]
- 56. Fleming G. Influenza in horses. J Comp Med & Vet Arch 1891; 12:129–137. [PMC free article] [PubMed] [Google Scholar]
- 57. Forster T. Illustrations of the Atmospherical Origin of Epidemic Diseases, and of Its Relation to Their Predisponent Constitutional Causes, Exemplified by Historical Notices and Cases, and on the Twofold Means of Prevention, Mitigation, and Cure, and of the Powerful Influence of Change of Air, as a Principal Remedy. To which are Appended, Popular Rules for the Maintenance of Health, 2nd edn Chelmsford: Meggy & Chalk, 1829. [Google Scholar]
- 58. Moore V. Influenza; in Moore V. (ed.) The Pathology and Differential Diagnosis of Infectious Diseases of Animals. Ithaca, New York: Taylor & Carpenter, 1906; 440–445. [Google Scholar]
- 59. Bissett C. An Essay on the Medical Constitution of Great Britain. To Which are Added Observations on the Weather, and the Diseases Which Appear in the Period Included Betwixt the First of January 1758, and the Summer Solstice in 1760. Together With A Narrative of the Throat Distemper, and the Miliary Fever, Which Were Epidemical in the Duchy of Cleveland, in 1760. Likewise, Observations on the Effects of Some Antihelminthics, Particularly the Great Bastard, Black Hellibore, or Bear’s‐Foot. London: A. Millar, D. Wilson, 1762. [Google Scholar]
- 60. Judson A. Report on the origin and progress of the epizootic among horses in 1872, with a table of mortality in New York. Illustrated with maps Board of Health of the City of New York (eds). Third Annual Report of the Board of Health of the Health Department of the City of New York, April 11, 1872, to April 30, 1873. New York: D. Appleton and Company, 1873; 250–291. [Google Scholar]
- 61. Rutty J. A Chronological History of the Weather & Seasons, and of the Prevailing Diseases in Dublin, With Their various Periods, Successions, and Revolutions, during the Space of Forty Years. With A comparative View of the Difference of the Irish Climate and Diseases, and those of England and other Countries. London: Robinson and Roberts, 1770. [Google Scholar]
- 62. Anonymous . Veterinary science; in Ripley G, Dana CA. (eds): The American Cyclopædia: A Popular Dictionary of General Knowledge. New York: D. Appleton and Company, 1876: 331–336. [Google Scholar]
- 63. Hutton T, Galen Treichler G, Seip G et al. On the epidemic influenza. Med & Surg Reporter, Phila 1873; 28(14 [no. 840]):275–281. [Google Scholar]
- 64. Tyrrell G. The epidemic influenza. Pac Med Surg J. 1873; 6:213–225. [Google Scholar]
- 65. Willingham C. Rambling Rose. New York: Delacorte Press; 1972. [Google Scholar]
- 66. Fricke A, Leidy J. The horse epidemic of October and November 1872. With a statement of microscopical appearances of specimens. Phil Med Times 1873; 3:211–213. [Google Scholar]
- 67. Sammarco A. Images of America: The Great Boston Fire of 1872. Charleston, South Carolina: Arcadia Publishing, 1997. [Google Scholar]
- 68. Tibbals N. Appendix. Report on diseases of horses In: Board of Health of the City of New Haven (ed). The Fifth Annual Report of the Board of Health of the City of New Haven 1877. New Haven: Tuttle, Morehouse & Taylor, 1878; 65–67. [Google Scholar]
- 69. Soper G. Influenza in horses and in man. NY Med J 1919; 109:720–724. [Google Scholar]
- 70. Mathers G. The streptococcus in acute epidemic respiratory infection of horses, so‐called equine influenza, stable fever, shipping fever, equine typhoid fever, contagious pleuropneumonia, etc. J Infect Dis 1918; 22:74–79. [Google Scholar]
- 71. Kendal AP, Minuse E, Maassab HF, Hennessy AV, Davenport FM. Influenza neuraminidase antibody patterns of man. Am J Epidemiol 1973; 98(2):96–103. [DOI] [PubMed] [Google Scholar]
- 72. Dowdle WR. Influenza A virus recycling revisited. Bull World Health Organ 1999; 77(10):820–828. [PMC free article] [PubMed] [Google Scholar]
- 73. Dowdle WR. Influenza pandemic periodicity, virus recycling, and the art of risk assessment. Emerg Infect Dis 2006; 12(1):34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Clemow F. The recent pandemic of influenza: its place of origin and mode of spread. Lancet 1894; 1:139–143. [Google Scholar]
- 75. Barrier C. De la maladie des chiens Instructions et observations sur les maladies des animaux domestiques. In: Chabert P, Flandrin P.], Huzard J‐B. Instructions et observations sur les maladies des animaux domestiques. Tome V. Deuxième ed. Paris: Huzard, An XII [1804]; 123–148. [Google Scholar]
- 76. Parkes E. Influenza; In Reynolds J, Hartshorne H. (eds): System of Medicine. Philadelphia: Henry C. Lea’s Son, 1880; 33–47. [Google Scholar]
- 77. Crawford P, Dubovi E, Castleman W et al. Transmission of equine influenza to dogs. Science 2005; 310:482–485. [DOI] [PubMed] [Google Scholar]
- 78. Hoelzer K, Murcia PR, Baillie GJ et al. Intra‐host evolutionary dynamics of canine influenza virus in naive and partially immune dogs. J Virol 2010; 84:5329–5335. Epub 2010 March 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dubovi E, Njaa B. Canine influenza. Vet Clin Small Anim 2008; 38:827–835. [DOI] [PubMed] [Google Scholar]
- 80. Koen JS. A practical method for field diagnosis of swine diseases. Am J Vet Med 1919; 14:468–470. [Google Scholar]
- 81. Shope RE. The etiology of swine influenza. Science 1931; 73(1886):214–215. [DOI] [PubMed] [Google Scholar]
- 82. Taubenberger JK, Reid AH, Janczewski TA, Fanning TG. Integrating historical, clinical and molecular genetic data in order to explain the origin and virulence of the 1918 Spanish influenza virus. Philos Trans R Soc Lond B Biol Sci 2001; 356(1416):1829–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Morens DM, Taubenberger JK. Understanding influenza backward. JAMA 2009; 302(6):679–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Webby RJ, Swenson SL, Krauss SL, Gerrish PJ, Goyal SM, Webster RG. Evolution of swine H3N2 influenza viruses in the United States. J Virol 2000; 74(18):8243–8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Olsen CW. The emergence of novel swine influenza viruses in North America. Virus Res 2002; 85(2):199–210. [DOI] [PubMed] [Google Scholar]
- 86. Reperant LA, Rimmelzwaan GF, Kuiken T. Avian influenza viruses in mammals. Rev Sci Tech 2009; 28(1):137–159. [DOI] [PubMed] [Google Scholar]
- 87. Tu J, Zhou H, Jiang T et al. Isolation and molecular characterization of equine H3N8 influenza viruses from pigs in China. Arch Virol 2009; 154(5):887–890. [DOI] [PubMed] [Google Scholar]
- 88. Brown IH. The epidemiology and evolution of influenza viruses in pigs. Vet Microbiol 2000; 74(1–2):29–46. [DOI] [PubMed] [Google Scholar]
- 89. Pensaert M, Ottis K, Vandeputte J, Kaplan MM, Bachmann PA. Evidence for the natural transmission of influenza A virus from wild ducks to swine and its potential importance for man. Bull World Health Organ 1981; 59(1):75–78. [PMC free article] [PubMed] [Google Scholar]
- 90. Dunham EJ, Dugan VG, Kaser EK et al. Different evolutionary trajectories of European avian‐like and classical swine H1N1 influenza A viruses. J Virol 2009; 83(11):5485–5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Garten RJ, Davis CT, Russell CA et al. Antigenic and genetic characteristics of swine‐origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325(5937):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pasma T, Joseph T. Pandemic (H1N1) 2009 infection in swine herds, Manitoba, Canada. Emerg Infect Dis 2010; 16(4):706–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis 2007; 44(8):1084–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gaydos JC, Top FH Jr, Hodder RA, Russell PK. Swine influenza A outbreak, Fort Dix, New Jersey, 1976. Emerg Infect Dis 2006; 12(1):23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Scholtissek C, Burger H, Bachmann PA, Hannoun C. Genetic relatedness of hemagglutinins of the H1 subtype of influenza A viruses isolated from swine and birds. Virology 1983; 129(2):521–523. [DOI] [PubMed] [Google Scholar]
- 96. Ito T, Couceiro JN, Kelm S et al. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol 1998; 72(9):7367–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Van Poucke SG, Nicholls JM, Nauwynck HJ, Van Reeth K. Replication of avian, human and swine influenza viruses in porcine respiratory explants and association with sialic acid distribution. Virol J 2010; 7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nicholls JM, Bourne AJ, Chen H, Guan Y, Peiris JS. Sialic acid receptor detection in the human respiratory tract: evidence for widespread distribution of potential binding sites for human and avian influenza viruses. Respir Res 2007; 8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature 2006; 440(7083):435–436. [DOI] [PubMed] [Google Scholar]
- 100. Peiris JS, De Jong MD, Guan Y. Avian influenza virus (H5N1): a threat to human health. Clin Microbiol Rev 2007; 20(2):243–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Claas EC, Osterhaus AD, Van Beek R et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 1998; 351(9101):472–477. [DOI] [PubMed] [Google Scholar]
- 102. Subbarao K, Klimov A, Katz J et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 1998; 279(5349):393–396. [DOI] [PubMed] [Google Scholar]
- 103. Guan Y, Smith GJ, Webby R, Webster RG. Molecular epidemiology of H5N1 avian influenza. Rev Sci Tech 2009; 28(1):39–47. [DOI] [PubMed] [Google Scholar]
- 104. Guan Y, Poon LL, Cheung CY et al. H5N1 influenza: a protean pandemic threat. Proc Natl Acad Sci USA 2004; 101(21):8156–8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Webster RG, Hulse‐Post DJ, Sturm‐Ramirez KM et al. Changing epidemiology and ecology of highly pathogenic avian H5N1 influenza viruses. Avian Dis 2007; 51(1 Suppl.):269–272. [DOI] [PubMed] [Google Scholar]
- 106. Ungchusak K, Auewarakul P, Dowell SF et al. Probable person‐to‐person transmission of avian influenza A (H5N1). N Engl J Med 2005; 352(4):333–340. [DOI] [PubMed] [Google Scholar]
- 107. Keawcharoen J, Oraveerakul K, Kuiken T et al. Avian influenza H5N1 in tigers and leopards. Emerg Infect Dis 2004; 10(12):2189–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kuiken T, Rimmelzwaan G, Van Riel D et al. Avian H5N1 influenza in cats. Science 2004; 306(5694):241. [DOI] [PubMed] [Google Scholar]
- 109. Fouchier RA, Schneeberger PM, Rozendaal FW et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci USA 2004; 101(5):1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tweed SA, Skowronski DM, David ST et al. Human illness from avian influenza H7N3, British Columbia. Emerg Infect Dis 2004; 10(12):2196–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Baronio G. Essai sur la maladie épizootique qui a regné sur les volailles, dans la Lombardie, pendant l’été de 1789; in Chabert P, Flandrin [P], Huzard [J‐B] (eds): Instructions et observations sur les maladies des animaux domestiques Tome IV. Deuxième ed. Paris: Huzard, An X [1802]; 207–225. [Google Scholar]
- 112. Perroncito E. Epizoozia tifoide nei gallinacei. Ann Accad Agric Torino 1878; 21:87–126. [Google Scholar]
- 113. Lode A, Gruber F. Bakteriologische Studien über die Aetiologie einer epidemischen Erkrankung der Hühner in Tirol (1901). Centralblatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten. 1 Abteilung: Medizinische-hygienische Bakteriologie und tierische Parasitenkunde 1901; 30:593–604. [Google Scholar]
- 114. Centanni E. Die Vogelpest. Beitrag zu dem durch Kerzen filtrierbaren Virus. Centralblatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten. 1 Abteilung: Medizinische-hygienische Bakteriologie und tierische Parasitenkunde 1902; 31:145–152. [Google Scholar]
- 115. Maggiora A, Valenti G. Ueber eine Seuche von exsudtivem Typhys bei Hühnern. I. Mittheilung. Zeitschrift für Hygiene und Infektionskrankheiten; medizinische Mikrobiologie, Immunologie und Virologie 1903; 42:185–243. [Google Scholar]
- 116. Taubenberger JK, Hultin JV, Morens DM. Discovery and characterization of the 1918 pandemic influenza virus in historical context. Antivir Ther 2007; 12(4 Pt B):581–591. [PMC free article] [PubMed] [Google Scholar]
- 117. Schäfer W. Vergleichende sero‐immunologische Untersuchungen über die Viren der Influenza und klassichen Geflügelpest [Comparative sero‐immunological investigations on the viruses of influenza and classical fowl plague]. Z Naturforsch 1955; 10b:81–91. [Google Scholar]
- 118. Mathieu C, Moreno V, Retamal P et al. Pandemic (H1N1) 2009 in breeding turkeys, Valparaiso, Chile. Emerg Infect Dis 2010; 16(4):709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hinshaw VS, Webster RG, Naeve CW, Murphy BR. Altered tissue tropism of human‐avian reassortant influenza viruses. Virology 1983; 128(1):260–263. [DOI] [PubMed] [Google Scholar]


