Abstract
Obesity is an independent risk factor for breast cancer, and obese breast cancer patients exhibit a higher risk for larger tumor burden and increased metastasis. Obesity, as associated with metabolic syndrome, results in an increase in circulating insulin-like growth factor (IGF), which acts as a mitogen. In addition, higher plasma level of adipocytokine leptin is associated with obesity. In the present study, we show that cotreatment with leptin and IGF-I significantly increases proliferation as well as invasion and migration of breast cancer cells. We found a novel bidirectional crosstalk between leptin and IGF-I signaling; IGF-I induced phosphorylation of leptin receptor (Ob-Rb) and leptin induced phosphorylation of IGF-I receptor (IGF-IR), whereas cotreatment induced synergistic phosphorylation and association of Ob-Rb and IGF-IR along with activation of downstream effectors, Akt and extracellular signal–regulated kinase. Leptin increased phosphorylation of IGF signaling molecules insulin-receptor substrate (IRS)-1 and IRS-2. Interestingly, we found that leptin and IGF-I cotreatment synergistically transactivated epidermal growth factor receptor (EGFR), depending on the proteolytic release of EGFR ligands, as the broad-spectrum matrix metalloproteinase inhibitor GM6001 could inhibit this effect. Using clinically relevant EGFR inhibitors, erlotinib and lapatinib, we found that inhibition of EGFR activation effectively inhibited leptin- and IGF-I–induced invasion and migration of breast cancer cells. Taken together, these data suggest a novel bidirectional crosstalk between leptin and IGF-I signaling that transactivates EGFR and promotes the metastatic properties as well as invasion and migration of breast cancer cells. Our findings indicate the possibility of using EGFR inhibitors erlotinib and lapatinib to counter the procancerous effects of leptin and IGF-I in breast cancers.
Introduction
Obesity is considered a serious health problem because it is associated with a variety of disorders, including carcinogenesis (1, 2). Epidemiologic studies have shown that obese women are more likely to have metastatic breast cancer when they are first diagnosed and have a poor final outcome (3, 4). Several hypotheses have been proposed to explain this association. Whereas particular emphasis has been placed on the increased production of estrogen from peripheral aromatization of androgens in adipose tissue (5), obesity has also been associated with metabolic syndrome and increased levels of insulin-like growth factor (IGF-I; ref. 6). Recent studies have put forth obesity as an endocrine tumor and placed high levels of adipocytokine leptin and its autocrine, paracrine, and endocrine functions at center stage (7, 8) to explain the molecular effects of obesity.
Leptin, a product of the obese (ob) gene, is a multifunctional adipocytokine (9) with wide-ranging biological activities including appetite regulation, bone formation, reproductive function, and angiogenesis (9). In recent years, many labs including ours have shown that leptin increased proliferation of breast and many other cancer cells via multiple signaling pathways (refs. 10–12; reviewed in ref. 13). Leptin receptors were not detectable in normal mammary epithelial cells by immunohistochemistry, whereas carcinoma cells showed positive staining for leptin receptor in 83% of cases (14). Importantly, overexpression of leptin was observed in 92% of breast tumors examined whereas none of the normal breast epithelium examined showed leptin overexpression (14). Another noteworthy study showing a positive relationship between blood leptin levels and breast cancer risk also found that the degree of leptin mRNA expression in the peritumoral adipose tissue was significantly higher in the breast cancer patients than in the control women (15). One recent study also showed that leptin and leptin receptor are overexpressed in primary and metastatic invasive ductal breast carcinoma compared with noncancer mammary tissue (16). Collectively, these studies suggest the importance of leptin signaling in breast carcinogenesis.
Several studies of different populations, genders, and age groups have found a nonlinear relationship between circulating concentrations of IGF-I and body mass index (BMI), with the highest concentrations of IGF-I at a BMI between 24 and 27 kg/m2 (reviewed in ref. 17). High IGF-I levels positively correlate with increased breast cancer risk (18, 19). Overexpression of IGF-I leads to excessive proliferation and survival signals for the development of breast tumor (20). IGF-I receptor (IGF-IR) is overexpressed in ~50% of primary breast tumors compared with normal tissue, indicating that these carcinomas have enhanced responses to the mitogenic and antiapoptotic effects of IGF-I (21). Inactivation of IGF-IR results in reduced breast tumor growth and metastasis in vivo (22). Recent advances in signal transduction biology have put forth crosstalk between different membrane receptor as a well-established concept. Given the importance of obesity-related increased levels of IGF-I and leptin in breast carcinogenesis and overexpression of both IGF-IR and Ob-Rb in breast tumors (14, 21), we hypothesized that leptin and IGF-I signaling might interact and synergize to produce enhanced procancerous effects. Intriguingly, we found a novel bidirectional crosstalk between leptin and IGF-I signaling that leads to synergistic transactivation of epidermal growth factor (EGF) receptor (EGFR). We further investigated the effects of leptin and IGF-I signaling on the malignant properties of breast cancer cells, including invasion and migration, and the importance of EGFR transactivation.
Materials and Methods
Antibodies
Antibodies for phospho-Akt, Akt, phospho-extracellular signal–regulated kinase (ERK), ERK, IGF-IR, p-Tyr, EGFR, insulin-receptor substrate (IRS)-1, and IRS-2 were purchased from Cell Signaling Technology. Antibodies for short and long forms of leptin receptors Ob-R (C-20), Ob-R (B-3), and Ob-R (H-300) were purchased from Santa Cruz Biotechnology.
Cell cultures, reagents, and treatments
The human breast cancer cell lines MCF-7, MDA-MB-231, and MDA-MB-468 were maintained in DMEM supplemented with 10% fetal bovine serum (FBS; Gemini Bio-Products) and 2 µmol/L l-glutamine (Invitrogen). HCC-1806 cells were maintained in RPMI supplemented with 10% FBS and 2 µmol/L l-glutamine. MDA-MB-231, MDA-MB-468, and HCC-1806 are triple-negative breast cancer cells as they lack expression of estrogen receptor and progesterone receptor and also lack overexpression of HER2 (23). For treatment, cells were seeded at a density of 1× 106 per 100-mm tissue culture dish. After 24 h of serum starvation, the complete culture media were changed to serum-free media containing treatments as indicated. Cultures were treated with human recombinant leptin (Sigma-Aldrich) at 100 ng/mL (12) and/or IGF-I (Sigma-Aldrich) at 100 ng/mL (24). An EGFR tyrosine kinase inhibitor, AG1478 (Sigma-Aldrich), was used at 250 nmol/L for indicated durations (25). The broad-spectrum matrix metalloproteinase (MMP) inhibitor GM6001 (25) was purchased from Calbiochem. EGFR inhibitors lapatinib (GlaxoSmithKline) and erlotinib (OSI Pharmaceuticals, Inc.) were used at 2.5 µmol/L for indicated durations (26, 27). For electric cell-substrate impedance sensing (ECIS) migration assay, ECIS cell cultureware was purchased from Applied BioPhysics.
Immunoprecipitation of Ob-Rb, IGF-IR, and EGFR
For immunoprecipitation (11, 12), whole-cell lysate from breast cancer cells was incubated with specific antibodies for Ob-R (C-20; specific for long form of leptin receptor), IGF-IR, or EGFR and the mixture was rotated slowly at 4°C for 16 h. A total of 20 µL of packed protein A/G agarose beads were added and mixture was incubated at 4°C for 1 h with rotation. The beads were collected by gentle centrifugation and washed twice with 1.5 mL ice-cold buffer [50 mmol/L Tris-Cl (pH 7.4), 150 mmol/L NaCl, 1 mmol/L EDTA, 1% NP40, 0.25% Na-deoxycholate, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 10 µg/mL aprotinin, 10 µg/mL leupeptin]. After the final wash, the precipitated protein-bead complexes were resuspended in SDS sample loading buffer, fractionated by SDS-PAGE, and transferred onto nitrocellulose membrane. Immunodetection was done by blocking the membranes for 1 h in TBS buffer [20 mmol/L Tris-Cl (pH 7.5), 137 mmol/L NaCl, 0.05% Tween 20] containing 5% powdered nonfat milk followed by addition of the primary antibody, as indicated, in TBS for 2 h at room temperature. Specifically bound primary antibodies were detected with peroxidase-coupled secondary antibodies and developed by enhanced chemiluminescence (ECL system, Amersham Pharmacia Biotech, Inc.) according to the manufacturer’s instructions.
Western blot
Whole-cell lysate was prepared by scraping cells in 250 µL of ice-cold modified radioimmunoprecipitation assay buffer [50 mmol/L Tris-Cl (pH 7.4), 150 mmol/L NaCl, 1 mmol/L EDTA, 1% NP40, 0.25% Nadeoxycholate, 1 mmol/L PMSF, 10 µg/mL aprotinin, 10 µg/mL leupeptin, 1 mmol/L Na3VO4, and 1 mmol/L NaF; refs. 10, 11]. The lysate was rotated 360 degrees for 1 h at 4°C followed by centrifugation at 12,000 × g for 10 min at 4°C to clear the cellular debris. Total protein was quantified with the Bradford protein assay kit (Bio-Rad). Equal amount of protein was resolved on SDS-polyacrylamide gel and transferred onto nitrocellulose membrane; Western blot analysis was done with the previously described antibodies. Immunodetection was done using enhanced chemiluminescence (ECL system, Amersham Pharmacia Biotech) according to the manufacturer’s instructions.
Cell viability assay
Cell viability assay was done (12) by estimating reduction of 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxyanilide (XTT) using a commercially available kit (Roche Applied Science) following the manufacturer’s instructions. Breast cancer cells were plated in 96-well plates at an initial density of 4 × 103 per well for 24 h. After 16 h of serum starvation, the culture media were changed to serum-free media containing treatments as indicated. XTT labeling reagent was added to each culture well to attain a final concentration of 0.3 mg/mL. After a 4-h exposure at 37°C, absorbance was measured at 450 and 690 nm using a 96-well plate reader (SPECTRAmax PLUS, Molecular Devices). Pilot experiments verified that the cell densities used in the experiments were within the linear range of the XTT assay. A standard curve was prepared using cell densities from 1 × 103 to 1 × 106, and the result was calculated with respect to the number of cells.
Mitogen-activated protein kinase and Akt activity assay
Mitogen-activated protein kinase (MAPK) and Akt were immunoprecipitated with the specific antibodies following the immunoprecipitation protocol as described above. MAPK and Akt activities were measured using MAPK activity assay kit (Chemicon International) and Akt activity assay kit (Calbiochem) following the manufacturers’ instructions.
Tumor cell invasion assay
For an in vitro model system for metastasis, we performed a Matrigel invasion assay by using a Matrigel invasion chamber from BD Biocoat Cellware (10, 11). MDA-MB-231 and MDA-MB-468 cells were seeded at a density of 1 × 105 per insert and cultured overnight. After 16 h of serum starvation, the culture media were changed to serum-free media containing treatments as indicated. Triplicate wells were used for each treatment. Cells were treated with human recombinant leptin and IGF-I at 100 ng/mL, alone and in combination. In other sets of experiments, cells were treated with either 2.5 µmol/L lapatinib or 2.5 µmol/L erlotinib alone and in combination with leptin and IGF-I. After 24-h incubation, cells remaining above the insert membrane were removed by gentle scraping with a sterile cotton swab. Cells that invaded through the Matrigel to the bottom of the insert were fixed in methanol for 10 min. After washing in PBS, the cells were stained with H&E. The insert was subsequently washed in PBS, briefly air-dried, and mounted. The slides were coded to prevent counting bias, and the number of invaded cells on representative sections of each membrane was counted under a light microscope. The number of invaded cells for each experimental sample represents the average of triplicate wells. All experiments were done at least thrice.
Migration assay
To perform migration assays (11), cells were plated into 24-well cell culture plates precoated with human fibronectin (5 µg/cm2; Sigma). Cells were allowed to grow in 10% FBS–containing DMEM to confluence, washed with serum-free medium, and serum starved for 16 h. A 1-mm-wide scratch was made across the cell layer with a sterile pipette tip. After washing with serum-free medium twice, DMEM containing 10 µg/mL human fibronectin was added to replace matrix depleted with the scratch. Plates were photographed immediately after scratching. Cells were treated with human recombinant leptin and/or IGF-I at 100 ng/mL. In other sets of experiments, cells were treated with lapatinib or erlotinib at 2.5 µmol/L alone and in combination with leptin and IGF-I. Plates were photographed after 24 h at the identical location of initial image. All experiments were done at least thrice.
ECIS wound-healing assays
Wound-healing assays were done using the ECIS (Applied BioPhysics) technology (11). For wound-healing assays, cells were grown to confluence on ECIS plates and serum starved for 16 h. The ECIS plates were submitted to an elevated voltage pulse of 40-kHz frequency, 3.5-V amplitude, and 30-s duration, which led to the death and detachment of cells present on the small active electrode, resulting in a wound normally healed by cells surrounding the small active electrode that have not been submitted to the elevated voltage pulse. Cells were immediately treated with leptin and/or IGF-I at 100 ng/mL. In other sets of experiments, cells were treated with lapatinib or erlotinib at 2.5 µmol/L alone and in combination with leptin or IGF-I. Wound healing was then assessed by continuous resistance measurements for 24 h.
Statistical analysis
All experiments were independently done thrice in triplicates. Statistical analysis was done using Microsoft Excel software. Significant differences were analyzed using Student’s t test and two-tailed distribution. Data were considered to be statistically significant if P < 0.05. Data are expressed as mean ± SE between triplicate experiments.
Results
Combined treatment with leptin and IGF-I increases proliferation as well as migration and invasion of breast cancer cells
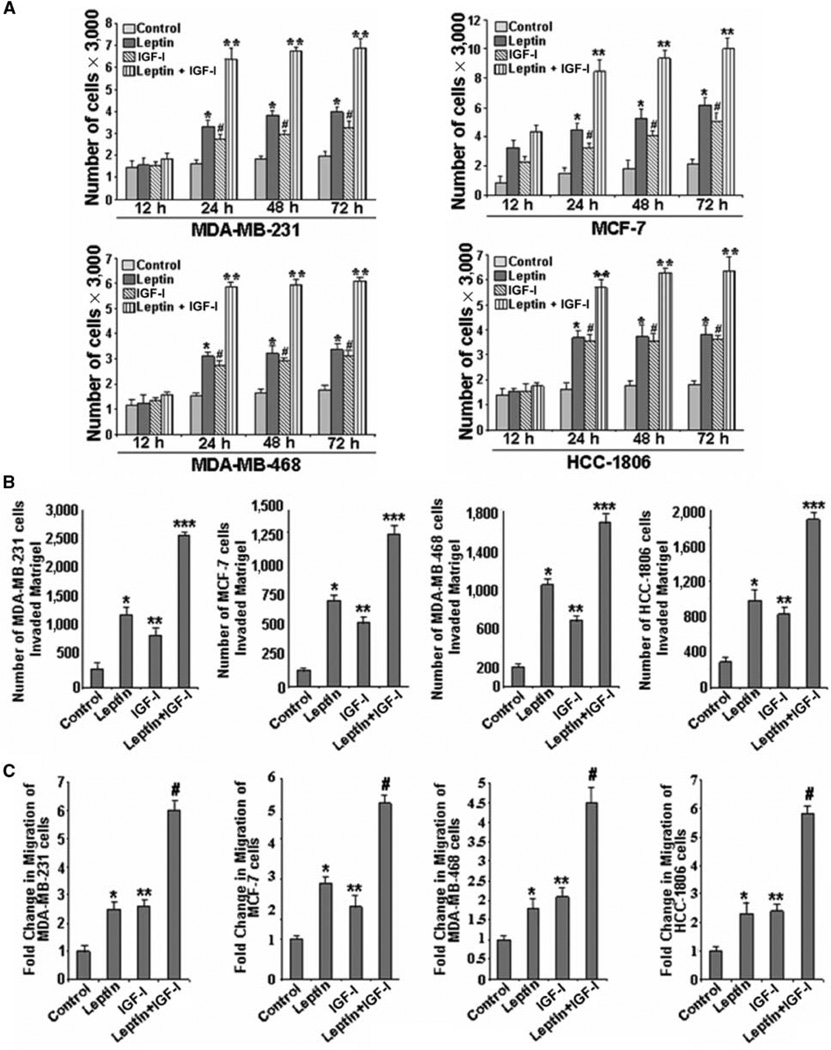
Recently, we and others showed that leptin increases proliferation and growth of breast cancer cells (refs. 10–12; reviewed in ref. 13). IGF-I is a known mitogen whose overexpression promotes tumor growth (18–21). It is suggested that IGF-I can act in an endocrine, paracrine, or autocrine fashion to regulate cell growth, survival, and differentiation and can synergize with other growth factors to produce enhanced mitogenic effects (19). We examined the effect of cotreatment of leptin and IGF-I on breast cancer cell proliferation using an anchorage-dependent cell proliferation assay. We found that cotreatment with leptin and IGF-I significantly increases proliferation of breast cancer cells in comparison with either treatment alone (Fig. 1A). Generally, IGF-I does not stimulate growth in estrogen receptor–negative cell lines; however, many estrogen receptor–negative cell lines depend on IGF-IR for tumorigenesis and metastasis (17, 28). We observed that IGF-I stimulated growth in estrogen receptor–negative breast cancer cells, albeit the level of stimulation was lesser than that in estrogen receptor–positive breast cancer cells. Cancer progression is a multistep process that involves invasion of basement membrane by tumor cells and migration to points far from a given primary tumor mass leading to metastasis. We also examined the effect of combined treatment of leptin and IGF-I on invasion and migration properties of breast cancer cells. As shown in Fig. 1B and C, breast cancer cells treated with both leptin and IGF-I exhibited significantly increased invasion and migration potential as compared with cells treated with either agent alone.
Figure 1.
Cotreatment with leptin and IGF-I increases proliferation as well as migration and invasion of breast cancer cells. A, leptin and IGF-I increase proliferation of cells in an anchorage-dependent proliferation assay. MDA-MB-231, MCF-7, MDA-MB-468, and HCC-1806 breast cancer cells were serum starved for 24 h, followed by treatment with 100 ng/mL leptin, 100 ng/mL IGF-I alone, and the combination for 12, 24, 48, and 72 h. XTT assays were then done as described in Materials and Methods. Both leptin and IGF-I increased proliferation of breast cancer cells in a time-dependent manner and combined treatment resulted in further increase in proliferation. Columns, mean of three independent experiments done in triplicates; bars, SE. *, P < 0.001, compared with untreated control cells; #, P < 0.005, compared with untreated control cells; **, P < 0.001, compared with cells treated with leptin alone. B, leptin and IGF-I increase invasion of breast cancer cells. MDA-MB-231, MCF-7, MDA-MB-468, and HCC-1806 breast cancer cells were cultured in Matrigel invasion chambers and serum starved for 24 h, followed by treatment with 100 ng/mL leptin, 100 ng/mL IGF-I alone, and the combination for 24 h. The number of cells that invaded through the Matrigel was counted in five different regions. The slides were blinded to remove counting bias. Columns, mean of three independent experiments done in triplicates; bars, SE. *, P < 0.005, compared with untreated control cells; **, P < 0.005, compared with untreated control cells; ***, P < 0.001, compared with cells treated with leptin alone. C, leptin and IGF-I up-regulate migration of breast cancer cells. MDA-MB-231, MCF-7, MDA-MB-468, and HCC-1806 breast cancer cells were grown to confluence on fibronectin-coated surface, serum starved for 24 h, and scratched with a pipette tip. The plates were photographed immediately following scratching. Culture media were replaced with media containing 100 ng/mL leptin, 100 ng/mL IGF-I alone, and the combination for 24 h. The plates were photographed at the identical location of the initial image at 24 h posttreatment. The fold change in migration was calculated. Columns, mean of three independent experiments done in triplicates; bars, SE. *, P < 0.005, compared with untreated control cells; **, P < 0.005, compared with untreated control cells; #, P < 0.001, compared with cells treated with leptin alone.
Crosstalk between leptin and IGF-I signaling involves association between leptin receptor and IGF-IR in breast cancer cells
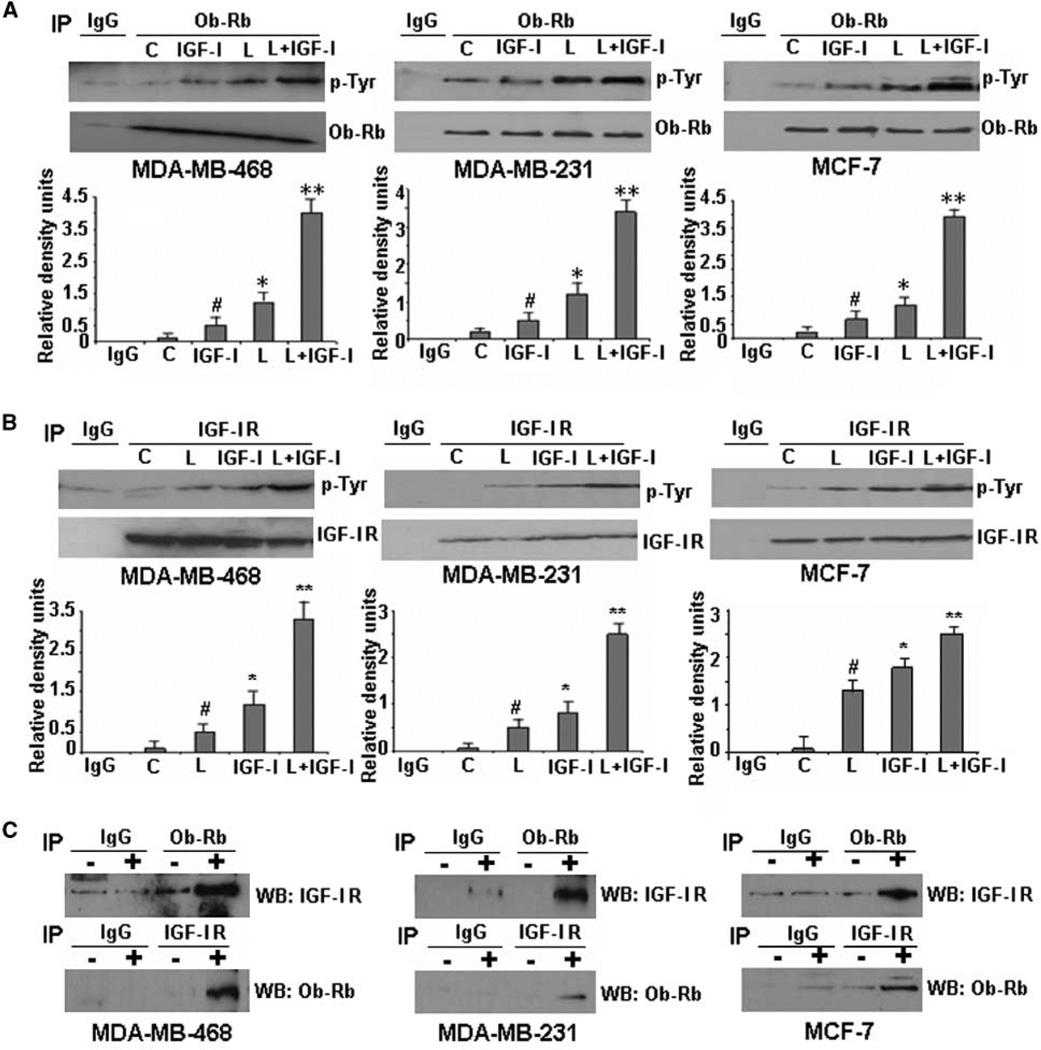
Binding of leptin to leptin receptor (Ob-Rb) phosphorylates conserved tyrosine residues at Y985, Y1077, and Y1138 (29). These phosphorylation events are important for subsequent signaling events including Janus-activated kinase (JAK) and signal transducer and activator of transcription-3 activation (29). We first examined the effect of leptin and IGF-I on phosphorylation of leptin receptor (Ob-Rb) in breast cancer cells and found that leptin treatment increased the phosphorylation of Ob-Rb in MDA-MB-468, MDA-MB-231, and MCF-7 cells as shown by immunoprecipitation of Ob-Rb followed by immunoblotting with an anti-phosphotyrosine antibody. Interestingly, IGF-I treatment also resulted in Ob-Rb phosphorylation albeit a little less robust than leptin treatment. However, the combined treatment resulted in synergistic increase in Ob-Rb phosphorylation (Fig. 2A). Binding of IGF-I leads to activation of IGF-IR initiating autophosphorylation of the receptor at tyrosine residues 1131, 1135, and 1136 (30). We found that IGF-I treatment initiated tyrosine phosphorylation at IGF-IR in MDA-MB-468, MDA-MB-231, and MCF-7 cells as expected. Intriguingly, displaying a bidirectional crosstalk with IGF-I signaling, leptin treatment increased phosphorylation of IGF-IR and combined treatment led to a synergistic increase in phosphorylation of IGF-IR (Fig. 2B). Immunoblots were reprobed with antibodies against Ob-Rb and IGF-IR to show that the increase in tyrosine phosphorylation at receptors was not due to any change in the level of immunoprecipitated protein. Phosphorylated tyrosine bands shown in all cases correspond to the expected size band (Ob-Rb and IGF-IR). Next, we examined if IGF-IR and Ob-Rb interact in the presence of leptin and IGF-I. Coimmunoprecipitation analysis showed that IGF-IR coimmuno-precipitated with Ob-Rb in MDA-MB-468, MDA-MB-231, and MCF-7 cells treated with leptin and IGF-I. Conversely, Ob-Rb was detected in IGF-IR immunoprecipitates (Fig. 2C). Interestingly, no association of Ob-Rb and IGF-IR was observed in the absence of ligands.
Figure 2.
Evidence of crosstalk between leptin receptor (Ob-Rb) and IGF-IR. Leptin and IGF-I act synergistically to increase phosphorylation of Ob-Rb (A) and IGF-IR (B) in MDA-MB-468, MDA-MB-231, and MCF-7 breast cancer cells. Cells were serum starved for 24 h, followed by treatment with 100 ng/mL leptin (L), 100 ng/mL IGF-I alone (IGF-I), and the combination (L + IGF-I) for 30 min. C, untreated controls. Total protein was isolated and equal amounts of proteins were subjected to immunoprecipitation with specific antibodies for leptin receptor (A) or IGF-IR (B). Immunoprecipitates were resolved by SDS-PAGE and subjected to immunoblot analysis with a mouse monoclonal antibody against phospho-tyrosine. Immunoprecipitation with IgG was included as control (IgG). Leptin and IGF-I treatment increased phosphorylation of Ob-Rb whereas combined treatment induced further increase in Ob-Rb phosphorylation (A). Increased phosphorylation of IGF-IR was observed in cells treated with leptin and IGF-I alone, whereas combined treatment induced a synergistic increase in IGF-IR phosphorylation (B). Phosphorylated tyrosine bands shown in all cases correspond to the expected size band (Ob-Rb and IGF-IR). The membranes were reblotted with Ob-Rb and IGF-IR antibodies as control. Representative blots of multiple independent experiments. The histogram is the densitometric analysis of the Western blot signals normalized to total Ob-Rb or IGF-IR. #, P < 0.05, compared with untreated controls; *, P < 0.05, compared with untreated controls; **, P < 0.01, compared with leptin-treated cells. C, association of Ob-Rb and IGF-IR occurs in breast cancer cells. MDA-MB-468, MDA-MB-231, and MCF-7 cells were serum starved for 24 h, followed by no treatment (−) or combined treatment with 100 ng/mL leptin (L) and 100 ng/mL IGF-I for 30 min (+). Total protein was isolated and equal amounts of proteins were subjected to immunoprecipitation with specific antibodies for Ob-Rb. The immunoprecipitates were probed with anti–IGF-IR antibody. In a reverse experiment, equal amounts of proteins were immunoprecipitated with anti–IGF-IR antibody and immunoprecipitates were probed with anti–Ob-Rb antibody. Immunoprecipitation with IgG was included as control (IgG). Both Ob-Rb and IGF-IR were coimmunoprecipitated in the presence of IGF-I and leptin, indicating the association of Ob-Rb and IGF-IR.
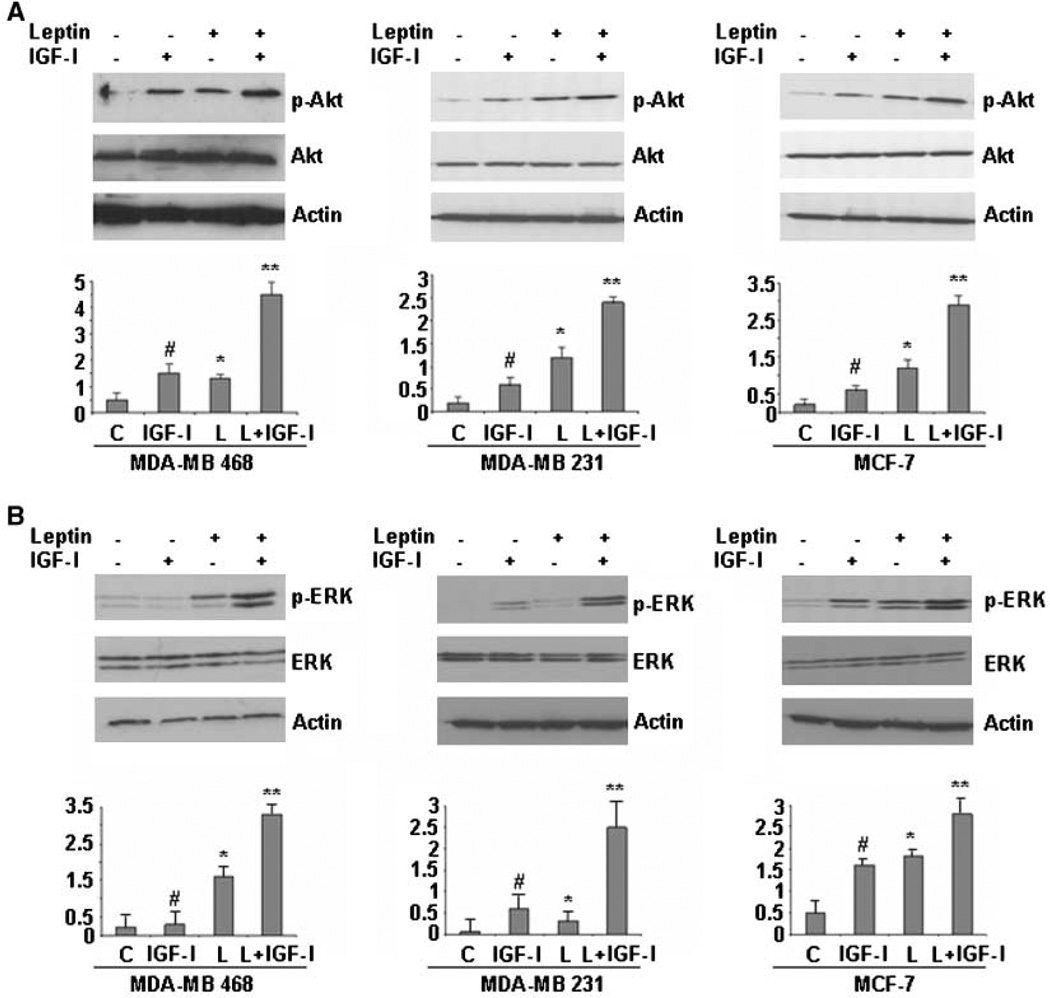
Downstream signaling of leptin and IGF-I involves activation of phosphatidylinositol 3-kinase/Akt and MAPK signaling (29, 30). We next examined if leptin and IGF-I can coactivate these downstream signaling molecules. Leptin treatment led to increased phosphorylation of Ser473 on Akt (Fig. 3A) and Thr202 and Tyr204 on p42 ERK and p44 ERK (Fig. 3B) in MDA-MB-468, MDA-MB-231, and MCF-7 cells. IGF-I treatment also increased phosphorylation of Akt and ERK when compared with untreated control cells. Interestingly, combined treatment of leptin and IGF-I induced a robust increase in phosphorylation of Akt and ERK. Leptin and IGF-I treatment had no effect on total ERK and Akt protein expression levels (Fig. 3A and B). Together, these data suggest that a bidirectional crosstalk occurs between leptin and IGF-I signaling involving association of Ob-Rb and IGF-IR and activation of downstream signaling molecules.
Figure 3.
Combined treatment with leptin and IGF-I leads to a synergistic increase in phosphorylation of both Akt and ERK in breast cancer cells. MDA-MB-468, MDA-MB-231, and MCF-7 cells were serum starved for 24 h, followed by treatment with 100 ng/mL leptin, 100 ng/mL IGF-I alone, and the combination for 30 min. Total protein was isolated from MDA-MB-468, MDA-MB-231, and MCF-7 cells and equal amounts of proteins were subjected to immunoblot analysis with specific antibodies against phospho-Akt (p-Akt) and Akt (A) or phospho-ERK (p-ERK) and ERK (B). Increased phosphorylation of Akt was observed in MDA-MB-468, MDA-MB-231, and MCF-7 cells treated with leptin or IGF-I, whereas combined treatment induced further increase in Akt phosphorylation. Leptin and IGF-I treatment increased phosphorylation of ERK, whereas combined treatment induced a synergistic increase in ERK phosphorylation. The membranes were reblotted with ERK, Akt, and anti-actin antibodies as control. Representative blots of multiple independent experiments. The representative histogram is the densitometric analysis of the Western blot signals showing fold increase in levels of phospho-Akt and phospho-ERK with respect to total Akt and ERK. #, P < 0.01, compared with untreated controls; *, P < 0.05, compared with untreated controls; **, P < 0.01, compared with leptin-treated cells.
Synergistic effect of leptin and IGF-I requires transactivation of EGFR in breast cancer cells
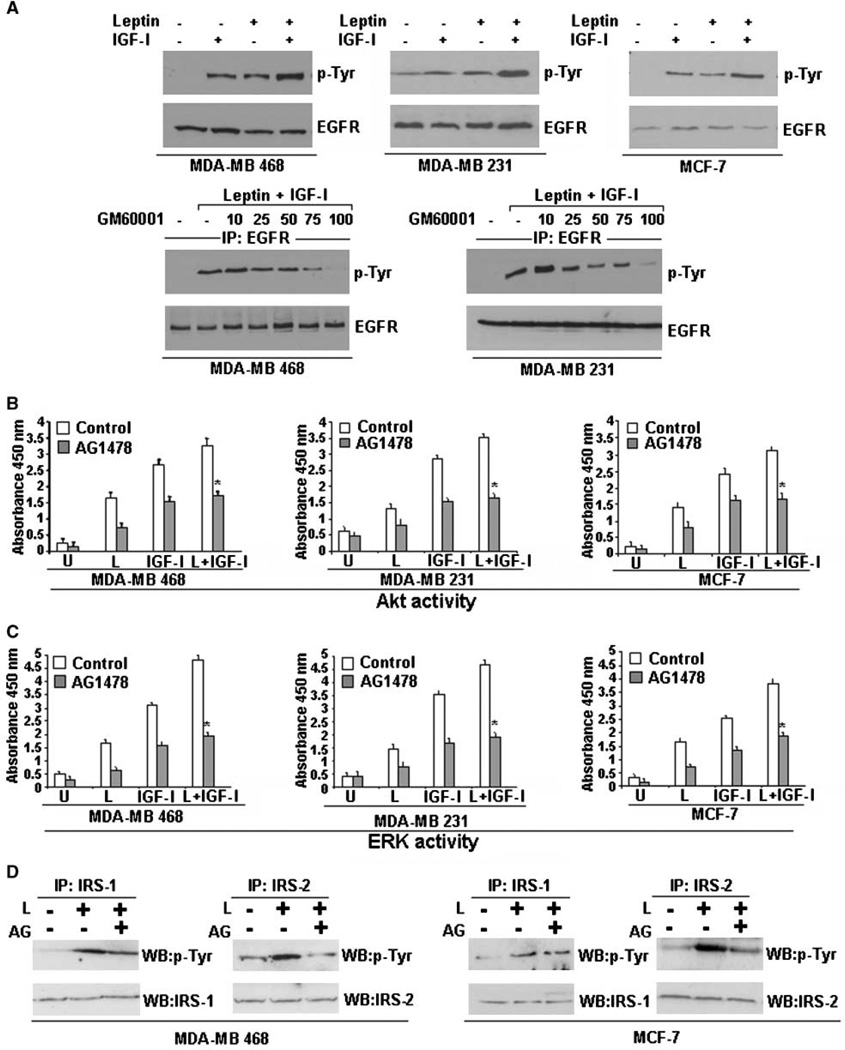
Transactivation of EGFR in response to activation of G-protein–coupled receptors, IGF-I, E-cadherin, and integrins can have important physiologic consequences (31–33). Increased phosphorylation of EGFR in response to leptin was recently reported in human gastric cancer cells (25). Treatment of breast cancer cells with either leptin or IGF-I resulted in increased phosphorylation of EGFR, as shown by immunoprecipitation of EGFR followed by immunoblotting with an anti-phosphotyrosine antibody. Phosphorylated tyrosine bands shown in all cases correspond to the expected size band (EGFR). Interestingly, combined treatment with leptin and IGF-I induced a synergistic increase in phosphorylation of EGFR in MDA-MB-468, MDA-MB-231, and MCF-7 cells (Fig. 4A). We next examined the mechanism of transactivation of EGFR in response to combined treatment of leptin and IGF-I. G-protein–coupled receptor ligand–induced EGFR transactivation is known to require MMP activation resulting in cleavage of the membrane-anchored growth factor precursor pro-HB-EGF in some cells (34). We found that preincubation of MDA-MB-468 and MDA-MB-231 cells with MMP inhibitor, GM6001, inhibited leptin- and IGF-I–induced tyrosine phosphorylation of EGFR in a dose-dependent manner (Fig. 4A).
Figure 4.
Leptin and IGF-I require EGFR activation for their biological effects. A, leptin and IGF-I increase EGFR phosphorylation. MDA-MB-468, MDA-MB-231, and MCF-7 cells were serum starved for 24 h, followed by treatment with 100 ng/mL leptin, 100 ng/mL IGF-I alone, and the combination for 30 min. Total protein was isolated and equal amounts of proteins were subjected to immunoprecipitation with a specific antibody for EGFR. Immunoprecipitates were resolved by SDS-PAGE and subjected to immunoblot analysis with a mouse monoclonal antibody against phospho-tyrosine. Phosphorylated tyrosine bands shown in all cases correspond to the expected size band (EGFR). Leptin and IGF-I treatment induced a little increase in phosphorylation of EGFR, whereas combined treatment with leptin and IGF-I induced a synergistic increase in EGFR phosphorylation. The membranes were reblotted with anti-EGFR antibody as control. Leptin and IGF-I require MMP activation for EGFR transactivation. MDA-MB-468 and MDA-MB-231 cells were serum starved for 24 h, pretreated with 10 to 100 µmol/L of GM6001 or an equal volume of vehicle (DMSO) for 30 min, and stimulated with combined treatment of 100 ng/mL leptin and IGF-I for 30 min. Total cell lysates were isolated and immunoprecipitated with EGFR; immunoprecipitates were immunoblotted with anti-phosphotyrosine antibody. The membranes were reblotted with anti-EGFR antibody as control. Pretreatment with GM6001 inhibited leptin- and IGF-I–induced EGFR phosphorylation, indicating the requirement of MMP activation. B to D, EGFR inhibition inhibits leptin- and IGF-I–induced Akt and ERK activation and inhibits leptin-induced IRS-1 and IRS-2 activation. MDA-MB-468, MDA-MB-231, and MCF-7 cells were serum starved for 24 h, followed by treatment with 100 ng/mL leptin, 100 ng/mL IGF-I alone, and the combination. Cells were pretreated with 250 nmol/L AG1478 (EGFR inhibitor) for 45 min, followed by leptin and/or IGF-I treatment as indicated. Cell lysates were prepared; Akt and ERK were immunoprecipitated with anti-Akt and anti-ERK antibodies and subjected to Akt and ERK activity assay as detailed in Materials and Methods. Pretreatment with AG1478 inhibits Akt and ERK stimulation in response to combined treatment with leptin and IGF-I. Columns, mean of three independent experiments done in triplicates; bars, SE. *, P < 0.001, AG1478-pretreated and leptin + IGF-I–treated cells compared with leptin + IGF-I–treated cells. IRS-1 and IRS-2 were immunoprecipitated with anti–IRS-1 or anti–IRS-2 antibodies and subjected to immunoblot analysis with p-Tyr antibody. Phosphorylated tyrosine bands shown in all cases correspond to the expected size band (IRS-1 and IRS-2). EGFR inhibition blocks leptin-induced activation of IRS-1 and IRS-2 in MDA-MB-468 and MCF-7 cells.
We next sought to determine the biological importance of the transactivation of EGFR in the context of synergistic effect of leptin and IGF-I on breast cancer cell proliferation and activation of downstream signaling molecules. The biological effect of blocking EGFR with an EGFR inhibitor, AG1478, was studied by cell proliferation assay. MDA-MB-468, MDA-MB-231, and MCF-7 cells were pretreated with AG1478 followed by stimulation with leptin and IGF-I together. Interestingly, pretreatment with AG1478 significantly inhibited the stimulatory effect of leptin and IGF-I on breast cancer cell proliferation (Supplementary Fig. S1). In addition, inhibition of EGFR activation using AG1478 inhibited the synergistic effect of combined treatment of leptin and IGF-I on Akt and ERK activation (Fig. 4B and C). On activation, IGF-IR further directly phosphorylates various intracellular substrates such as IRS-1 and IRS-2 (30). Leptin induced phosphorylation of IRS-1 and IRS-2 in MDA-MB-468 and MCF-7 cells, showing further evidence of crosstalk between leptin and IGF-I signaling. Phosphorylated tyrosine bands shown in all cases correspond to the expected size band (IRS-1 and IRS-2). Leptin-induced phosphorylation of IRS-1 and IRS-2 could be inhibited by inhibition of EGFR activation. Interestingly, inhibition of EGFR activation inhibited leptin-induced phosphorylation of IRS-2 more efficiently compared with IRS-1 (Fig. 4D). IGF-I treatment induced IRS-1 and IRS-2 phosphorylation. Combined treatment of leptin and IGF-I synergistically increased phosphorylation of IRS-1 and IRS-2. Inhibition of EGFR activation inhibited the stimulatory effect of IGF-I and leptin on IRS-2 more effectively than IRS-1 (Supplementary Fig. S2). These results show that transactivation of EGFR is essential for the synergistic effect of leptin and IGF-I on breast cancer cell proliferation and activation of Akt, ERK, IRS-1, and IRS-2. In addition, these data suggest that transactivation of EGFR is upstream of the activation of the ERK and Akt pathways, revealing the hierarchy of these events, and disruption of EGFR activation may be a valid therapeutic approach to counter the effects of leptin and IGF-I on breast cancer cells.
Inhibition of EGFR disrupts leptin- and IGF-I–induced invasion and migration of breast cancer cells
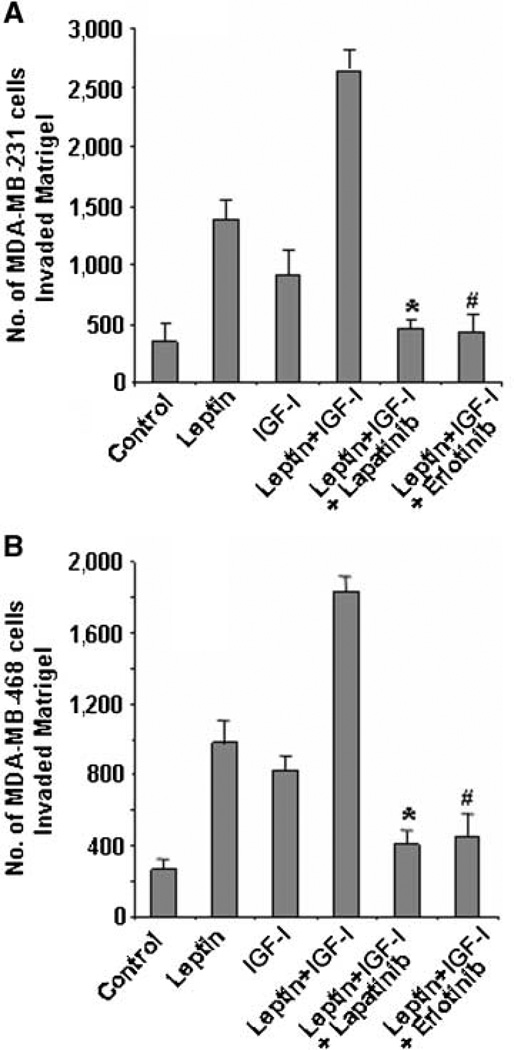
Invasion and metastasis are the key biological features of carcinoma cell behavior. In addition to examining the effect of EGFR inhibitor AG1478 on leptin- and IGF-I–induced breast cancer cell proliferation, we also examined EGFR blockade [using clinically relevant EGFR inhibitors, lapatinib (26) and erlotinib (27)] as a potential strategy for inhibiting the synergistic effect of leptin and IGF-I on invasion and migration. Lapatinib is a small-molecule inhibitor of the EGFR and HER2 tyrosine kinase domains that inhibits baseline and ligand-stimulated activity of EGFR and HER2 and blocks downstream signaling (26, 35). EGFR inhibitor erlotinib is currently approved for non–small cell lung cancer, colon cancer, pancreatic cancer, and head and neck cancer (35). We showed that combined treatment of leptin and IGF-I significantly increased the invasion potential of breast cancer cells (Fig. 1B). Pretreatment with lapatinib or erlotinib significantly inhibited the combined stimulatory effect of leptin and IGF-I on MDA-MB-468 and MDA-MB-231 breast cancer cell invasion potential (Fig. 5).
Figure 5.
Lapatinib and erlotinib inhibit leptin- and IGF-I–induced increased invasion of breast cancer cells. MDA-MB-231 (A) and MDA-MB-468 (B) cells were cultured in Matrigel invasion chambers and serum starved for 24 h, followed by treatment with 100 ng/mL leptin, 100 ng/mL IGF-I alone, leptin + IGF-I, leptin + IGF-I + 2.5 µmol/L lapatinib, and leptin + IGF-I + 2.5 µmol/L erlotinib for 24 h. The number of cells that invaded through the Matrigel was counted in five different regions. The slides were blinded to remove counting bias. Columns, mean of three independent experiments done in triplicates; bars, SE. *, P < 0.001, compared with leptin + IGF-I–treated cells. #, P < 0.001, compared with leptin + IGF-I–treated cells.
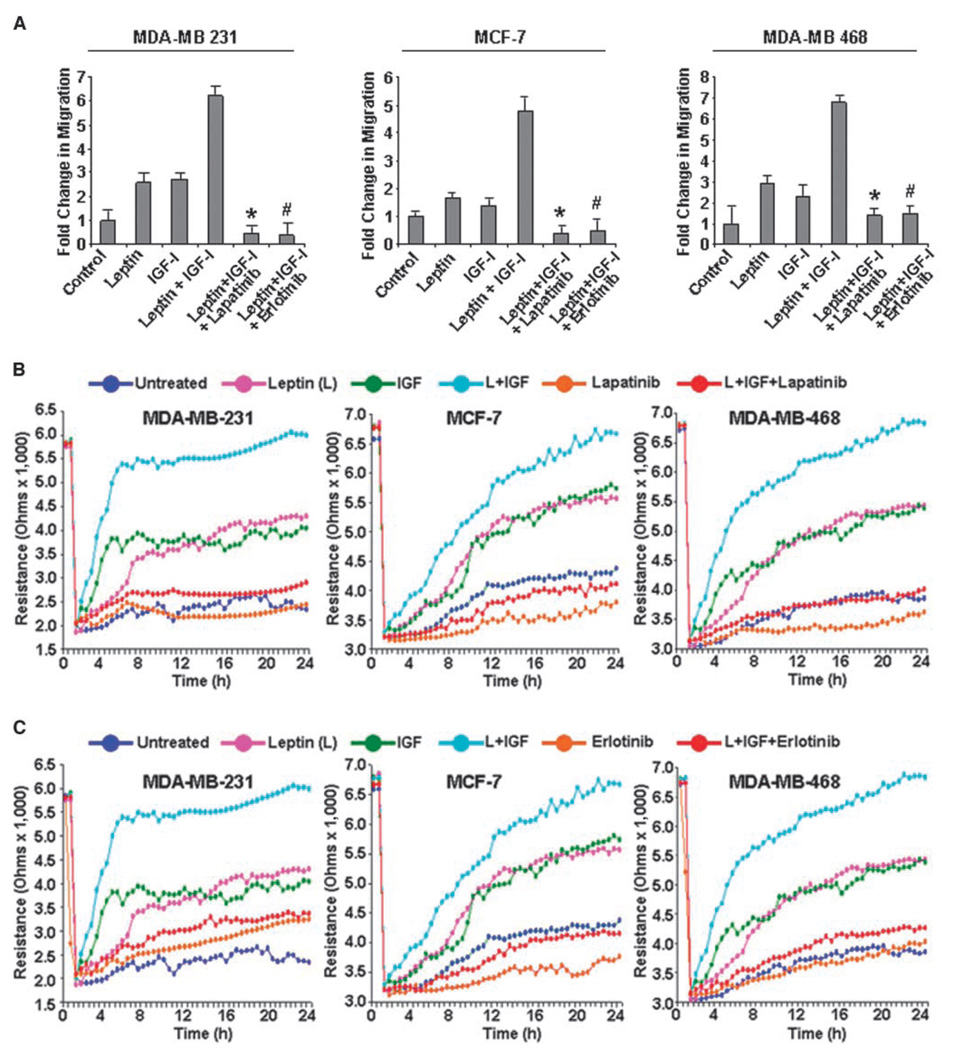
We next examined the effect of lapatinib and erlotinib treatment on the combined stimulatory effect of leptin and IGF-I on migration of breast cancer cells. As shown in Fig. 6A, combined treatment with leptin and IGF-I increased the migration of MDA-MB-231, MCF-7, and MDA-MB-468 cells, whereas erlotinib and lapatinib treatment resulted in significant inhibition. Next, we performed a quantitative real-time impedance assay using an ECIS-based technique (11) to follow the migration of MDA-MB-231, MCF-7, and MDA-MB-468 cells (Fig. 6B and C). MDA-MB-231, MCF-7, and MDA-MB-468 cells treated with leptin and IGF-I displayed an increase in resistance, showing increased migration in comparison with untreated cells, whereas cells treated with both leptin and IGF-I together rapidly increased to reach the resistance values of the nonwounded cells at the start of the experiment. This increase in migration in response to combined treatment of leptin and IGF-I was inhibited by lapatinib (Fig. 6B) or erlotinib treatment (Fig. 6C). We found that leptin and IGF-I induced phosphorylation of IRS-1 and IRS-2, whereas inhibition of EGFR activation inhibited phosphorylation of IRS-2 more efficiently compared with IRS-1 (Fig. 4D). IRS-2 activation plays a critical role in growth factor–induced migration of breast cancer cells (30); therefore, EGFR inhibition dramatically inhibited leptin- and IGF-I–induced migration. These results collectively show that transactivation of EGFR by IGF-I and leptin is indeed a crucial component of the signaling machinery used by the leptin receptor and IGF-IR in promoting invasion and migration of breast cancer cells.
Figure 6.
Inhibition of EGFR with lapatinib and erlotinib inhibits leptin- and IGF-I–induced increased migration of breast cancer cells. A, MDA-MB-231, MCF-7, and MDA-MB-468 breast cancer cells were grown to confluence on fibronectin-coated surface, serum starved for 24 h, and scratched with a pipette tip. The plates were photographed immediately following scratching. Culture media were replaced with media containing 100 ng/mL leptin, 100 ng/mL IGF-I alone, leptin + IGF-I, leptin + IGF-I + 2.5 µmol/L lapatinib, and leptin + IGF-I + 2.5 µmol/L erlotinib for 24 h. The plates were photographed at the identical location of the initial image at 24 h posttreatment. The fold change in migration was calculated. Columns, mean of three independent experiments done in triplicates; bars, SE. *, P < 0.005, compared with leptin + IGF-I–treated cells; #, P < 0.001, compared with leptin + IGF-I–treated cells. B and C, MDA-MB-231, MCF-7, and MDA-MB-468 cells were grown to confluence in ECIS plates, serum starved for 16 h, and subjected to an elevated voltage pulse of 40-kHz frequency at 3.5-V amplitude for 30 s to incite a wound. The cells were immediately treated with 100 ng/mL leptin, 100 ng/mL IGF-I alone, leptin + IGF-I, leptin + IGF-I + 2.5 µmol/L lapatinib, and leptin + IGF-I + 2.5 µmol/L erlotinib. The wound was then allowed to be healed by cells surrounding the small active electrode that did not undergo the elevated voltage pulse. Resistance was measured before and after the elevated voltage pulse application as described in Materials and Methods. The measurements were stopped 24 h after the creation of wound. All the experiments were done thrice in triplicates.
Discussion
The following novel findings are described in this study: (a) Combined treatment with leptin and IGF-I increases proliferation of breast cancer cells. (b) Leptin stimulates phosphorylation of IGF-IR whereas IGF-I increases phosphorylation of Ob-Rb. (c) Ob-Rb and IGF-IR associate in the presence of leptin and IGF-I. (d) Leptin and IGF-I treatment synergistically induces transactivation of EGFR via MMP activation. (e) The synergistic effect of leptin and IGF-I on invasion and migration of breast cancer cells requires transactivation of EGFR. These results suggest that a bidirectional crosstalk exists between Ob-Rb and IGF-IR, which involves transactivation of EGFR, and targeting EGFR may be a suitable therapeutic strategy for breast cancer progressing in the presence of leptin and IGF-I.
Interaction between growth factor signaling pathways have previously been described, such as crosstalk between IGF-I and EGF (24, 36). Our studies show for the first time that crosstalk occurs between leptin and IGF-I signaling, and importantly, this is bidirectional. High levels of leptin can influence the IGF-I signaling cascade, inducing phosphorylation of IGF-IR; similarly, IGF-I can influence leptin signaling molecules by phosphorylating Ob-Rb. Physical interaction between membrane receptors such as IGF-IR and HER2 has previously been reported in MCF-7 breast cancer cells (37) and in trastuzumab-resistant breast cancer cells (24). Interestingly, this interaction was hierarchical in which IGF-IR directs HER2 phosphorylation and physical association (24, 37). The association between Ob-Rb and IGF-IR in breast cancer cells in response to IGF-I and leptin treatment is the first report of association between Ob-Rb and IGF-IR. Our studies support the concept that high levels of leptin and IGF-I associated with obesity can act synergistically to influence breast cancer cells and increase the negative influence of obesity on breast carcinogenesis.
Activation of EGFR provides a potent survival signal in many cell types, and this activation has been seen in response to a variety of stimulations including IGF-I (24) and leptin (25). It is suggested that a significant component of IGF-IR-mediated survival signaling in epithelial cells occurs through transactivation of EGFR (37). Recently, transactivation of EGFR was found to be involved in leptin-mediated activation of JAK2 and ERK1/2 in human gastric cancer cells (25). We found that leptin and IGF-I synergistically increased the activation of EGFR in breast cancer cells. Importantly, we found that inhibition of EGFR activation using EGFR inhibitor AG1478 inhibits leptin- and IGF-I–induced activation of downstream signaling molecules as well as the biological actions of leptin and IGF-I. These results show that EGFR transactivation is an important step in the leptin and IGF-I crosstalk in breast cancer cells, which can be potentially used for clinical intervention.
Targeted therapies against EGFR have been quite disappointing (38–40) in contrast to targeted therapies against the HER2/neu receptor, which have been very effective against breast tumors exhibiting HER2 gene amplification (41, 42). It is important to note that, thus far, EGFR-targeted therapies have been used indiscriminately against all subtypes of breast carcinoma, perhaps missing a subtype that might benefit more from this treatment. The molecular classification of breast cancer has been recently redefined by gene microarray analysis, which identified distinct subtypes of breast cancer. These subtypes display distinct gene expression signatures and clinical outcomes (43–45). A high percentage of triple-negative (lacking expression of estrogen receptor and progesterone receptor and lacking overexpression of HER2) breast tumors were found to express EGFR, raising the possibility that this subgroup can benefit from EGFR-targeted therapy (46, 47). The dual tyrosine kinase inhibitor lapatinib has been shown to inhibit HER2 and EGFR signaling and to block the signal transduction effects of HER-family ligands transforming growth factor α, heregulin, and EGF (26, 35). Erlotinib was shown to exhibit greater growth inhibition in high-HER2-expressing than in low-HER2-expressing breast cancer cells (27). We show here for the first time that lapatinib and erlotinib successfully inhibit invasion and migration of triple-negative cells induced by combined treatment of leptin and IGF-I.
Our results become more important in light of the recent epidemiologic studies showing (a) the high prevalence of triple-negative breast cancer in African American women (48), and (b) that African American breast cancer patients are more likely to have advanced disease at diagnosis, higher risk of recurrence, more aggressive tumor, and poorer overall prognosis in comparison with White American women (49). Epidemiologic studies indicate that these differences can be partly explained by the fact that African American women more frequently exhibit endocrine and metabolic changes associated with upper body obesity. These changes include high levels of leptin and IGF-I and perturbations of other components associated with metabolic syndrome (reviewed in ref. 49). Taken together, these studies indicate that obese women with triple-negative breast cancers may have the worst prognosis of any subtype of breast cancer. We found that the novel bidirectional crosstalk between leptin and IGF-I signaling augments triple-negative breast cancer cell migration and invasion potential. It is important to note that triple-negative breast cancers are not only highly aggressive and highly proliferative cancers but they also they have the worst clinical prognosis, partly due to the lack of targeted therapy. Our study show that triple-negative and hormone-positive breast cancer can be effectively targeted with EGFR inhibitors, and these inhibitors can neutralize the procancerous effects of leptin and IGF-I.
Supplementary Material
Acknowledgments
Grant support: National Institutes of Diabetes, Digestive and Kidney Diseases, NIH grants K01DK076742 (N.K. Saxena), R01DK061941 and R01DK071594 (D. Merlin), and DK064399, R01DK062092, and R01DK075397 (F.A. Anania); the Wilbur and Hilda Glenn Foundation (R.M. O’Regan and D. Sharma); Georgia Cancer Coalition (R.M. O’Regan); National Cancer Institute Center for Cancer Nanotechnology Excellence (R.M. O’Regan); Congressionally Directed Medical Research Programs, Breast Cancer Research Program grant BC 030963 (D. Sharma); Susan G. Komen for the Cure grant BCTR 0503526 (D. Sharma); Emory University Research Council (D. Sharma); and Mary K. Ash Foundation (D. Sharma).
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Newell B, Proust K, Dyball R, McManus P. Seeing obesity as a systems problem. N SW Public Health Bull. 2007;18:214–218. doi: 10.1071/nb07028. [DOI] [PubMed] [Google Scholar]
- 2.Irigaray P, Newby JA, Lacomme S, Belpomme D. Overweight/obesity and cancer genesis: more than a biological link. Biomed Pharmacother. 2007;61:665–678. doi: 10.1016/j.biopha.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Berclaz G, Li S, Price KN, et al. Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol. 2004;15:875–884. doi: 10.1093/annonc/mdh222. [DOI] [PubMed] [Google Scholar]
- 5.Grodin JM, Siiteri PK, MacDonald PC. Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab. 1973;36:207–214. doi: 10.1210/jcem-36-2-207. [DOI] [PubMed] [Google Scholar]
- 6.Pasquali R, Vicennati V, Gambineri A, Pagotto U. Hormones and pathophysiology of obesity. Eat Weight Disord. 2001;6:9–20. [PubMed] [Google Scholar]
- 7.Dizdar O, Alyamac E. Obesity: an endocrine tumor? Med Hypotheses. 2004;63:790–792. doi: 10.1016/j.mehy.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 8.Housa D, Housova J, Vernerova Z, Haluzik M. Adipocytokines and cancer. Physiol Res. 2005;55:233–244. doi: 10.33549/physiolres.930848. [DOI] [PubMed] [Google Scholar]
- 9.Huang L, Li C. Leptin: a multifunctional hormone. Cell Res. 2000;10:81–92. doi: 10.1038/sj.cr.7290038. [DOI] [PubMed] [Google Scholar]
- 10.Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin mediates a proliferative response in human endometrial cancer cells by activating multiple signal transduction pathways. Endocr Relat Cancer. 2006;13:629–640. doi: 10.1677/erc.1.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saxena NK, Sharma D, Ding X, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxena NK, Vertino PM, Anania FA, Sharma D. Leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to cyclin D1 promoter via activation of stat3. J Biol Chem. 2007;282:13316–13325. doi: 10.1074/jbc.M609798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schäffler A, Schölmerich J, Buechler C. Mechanisms of disease: adipokines and breast cancer—endocrine and paracrine mechanisms that connect adiposity and breast cancer. Nat Clin Pract Endocrinol Metab. 2007;3:345–354. doi: 10.1038/ncpendmet0456. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OBR) in human breast cancer. Clin Cancer Res. 2004;10:4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- 15.Tessitore L, Vizio B, Jenkins O, et al. Leptin expression in colorectal and breast cancer patients. Int J Mol Med. 2000;5:421–426. doi: 10.3892/ijmm.5.4.421. [DOI] [PubMed] [Google Scholar]
- 16.Garofalo C, Koda M, Cascio S, et al. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12:1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 17.Renehan AG, Harvie M, Howell A. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and breast cancer risk: eight years on. Endocr Relat Cancer. 2006;13:273–278. doi: 10.1677/erc.1.01219. [DOI] [PubMed] [Google Scholar]
- 18.Muti P, Quattrin T, Grant BJ, et al. Fasting glucose is a risk factor for breast cancer: a prospective study. Cancer Epidemiol Biomarkers Prev. 2002;11:1361–1368. [PubMed] [Google Scholar]
- 19.Zhang X, Yee D. Tyrosine kinase signalling in breast cancer: insulin-like growth factors and their receptors in breast cancer. Breast Cancer Res. 2000;2:170–175. doi: 10.1186/bcr50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pacher M, Seewald MJ, Mikula M, et al. Impact of constitutive IGF1/IGF2 stimulation on the transcriptional program of human breast cancer cells. Carcinogenesis. 2007;28:49–59. doi: 10.1093/carcin/bgl091. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu C, Hasegawa T, Tani Y, et al. Expression of insulin-like growth factor 1 receptor in primary breast cancer: immunohistochemical analysis. Hum Pathol. 2004;35:1537–1542. doi: 10.1016/j.humpath.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Sachdev D, Hartell JS, Lee AV, Zhang X, Yee D. A dominant negative type I insulin-like growth factor receptor inhibits metastasis of human cancer cells. J Biol Chem. 2004;279:5017–5024. doi: 10.1074/jbc.M305403200. [DOI] [PubMed] [Google Scholar]
- 23.Neve RM, Chin K, Fridlyand J, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJ. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 25.Shida D, Kitayama J, Mori K, Watanabe T, Nagawa H. Transactivation of epidermal growth factor receptor is involved in leptin-induced activation of janus-activated kinase 2 and extracellular signal-regulated kinase 1/2 in human gastric cancer cells. Cancer Res. 2005;65:9159–9163. doi: 10.1158/0008-5472.CAN-05-0598. [DOI] [PubMed] [Google Scholar]
- 26.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 27.Emlet DR, Brown KA, Kociban DL, et al. Response to trastuzumab, erlotinib, and bevacizumab, alone and in combination, is correlated with the level of human epidermal growth factor receptor-2 expression in human breast cancer cell lines. Mol Cancer Ther. 2007;6:2664–2674. doi: 10.1158/1535-7163.MCT-07-0079. [DOI] [PubMed] [Google Scholar]
- 28.Bartucci M, Morelli C, Mauro L, Ando S, Surmacz E. Differential insulin-like growth factor I receptor (ER)-positive MCF-7 and ER-negative MDA-MB-231 breast cancer cells. Cancer Res. 2001;61:6747–6754. [PubMed] [Google Scholar]
- 29.Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004;81:223–241. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Grothey A, Voigt W, Schöber C, Müller T, Dempke W, Schmoll HJ. The role of insulin-like growth factor I and its receptor in cell growth, transformation, apoptosis, and chemoresistance in solid tumors. J Cancer Res Clin Oncol. 1999;125:166–173. doi: 10.1007/s004320050259. [DOI] [PubMed] [Google Scholar]
- 31.Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. Transactivation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in COS-7 cells. J Biol Chem. 2000;275:22583–22589. doi: 10.1074/jbc.M002915200. [DOI] [PubMed] [Google Scholar]
- 32.Maudsley S, Pierce KL, Zamah AM, et al. The βl2-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem. 2000;275:9572–9580. doi: 10.1074/jbc.275.13.9572. [DOI] [PubMed] [Google Scholar]
- 33.Pece S, Gutkind JS. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem. 2000;275:41227–41233. doi: 10.1074/jbc.M006578200. [DOI] [PubMed] [Google Scholar]
- 34.Prenzel N, Zwick E, Daub H, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 35.Reid A, Vidal L, Shaw H, de Bono J. Dual inhibition of ErB1 (EGFR/HER1) and ErB2 (HER2/neu) Eur J Cancer. 2007;43:481–489. doi: 10.1016/j.ejca.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Gilmore AP, Valentijn AJ, Wang P, et al. Activation of BAD by therapeutic inhibition of epidermal growth factor receptor and transactivation by insulin-like growth factor receptor. J Biol Chem. 2002;277:27643–27650. doi: 10.1074/jbc.M108863200. [DOI] [PubMed] [Google Scholar]
- 37.Balañá ME, Labriola L, Salatino M, et al. Activation of ErbB-2 via a hierarchical interaction between ErbB-2 and type I insulin-like growth factor receptor in mammary tumor cells. Oncogene. 2001;20:34–47. doi: 10.1038/sj.onc.1204050. [DOI] [PubMed] [Google Scholar]
- 38.Averbuch S, Kcenler M, Morris C, Wakeling A. Therapeutic potential of tyrosine kinase inhibitors in breast cancer. Cancer Invest. 2003;21:782–791. doi: 10.1081/cnv-120023776. [DOI] [PubMed] [Google Scholar]
- 39.Green MR. Targeting targeted therapy. N Engl J Med. 2004;350:2191–2193. doi: 10.1056/NEJMe048101. [DOI] [PubMed] [Google Scholar]
- 40.Mass RD. The HER receptor family: a rich target for therapeutic development. Int J Radiat Oncol Biol Phys. 2004;58:932–940. doi: 10.1016/j.ijrobp.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 41.Nanda R. Targeting the human epidermal growth factor receptor 2 (HER2) in the treatment of breast cancer: recent advances and future directions. Rev Recent Clin Trials. 2007;2:111–116. doi: 10.2174/157488707780599375. [DOI] [PubMed] [Google Scholar]
- 42.Petrelli F, Cabiddu M, Cazzaniga ME, Cremonesi M, Barni S. Targeted therapies for the treatment of breast cancer in the post-trastuzumab era. Oncologist. 2008;13:373–381. doi: 10.1634/theoncologist.2007-0173. [DOI] [PubMed] [Google Scholar]
- 43.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 44.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 46.Siziopikou KP, Ariga R, Proussaloglou KE, Gattuso P, Cobleigh M. The challenging estrogen receptor-negative/progesterone receptor-negative/HER-2-negative patient: a promising candidate for epidermal growth factor receptor-targeted therapy? Breast J. 2006;12:360–362. doi: 10.1111/j.1075-122X.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- 47.Siziopikou KP, Cobleigh M. The basal subtype of breast carcinomas may represent the group of breast tumors that could benefit from EGFR-targeted therapies. Breast. 2007;16:104–107. doi: 10.1016/j.breast.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Ihemelandu CU, Naab TJ, Mezghebe HM, et al. Basal cell-like (triple-negative) breast cancer, a predictor of distant metastasis in African American women. Am J Surg. 2008;195:153–158. doi: 10.1016/j.amjsurg.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 49.Rose DP, Haffner SM, Baillargeon J. Adiposity, the metabolic syndrome, and breast cancer in African-American and white American women. Endocr Rev. 2007;28:763–777. doi: 10.1210/er.2006-0019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.