Abstract
Objectives
This study sought to elucidate the mechanisms responsible for the benefits of small muscle mass exercise training in patients with chronic heart failure (CHF).
Background
How central cardiorespiratory and/or peripheral skeletal muscle factors are altered with small muscle mass training in CHF is unknown.
Methods
We studied muscle structure and oxygen (O2) transport and metabolism at maximal cycle (whole body) and knee-extensor exercise (KE) (small muscle mass) in 6 healthy controls and 6 patients with CHF who then performed 8 weeks of KE training (both legs, separately) and repeated these assessments.
Results
Pre-training cycling and KE peak leg O2 uptake (VO2peak) were ~17% and ~15% lower, respectively, in the patients compared to controls. Structurally, KE training increased quadriceps muscle capillarity and mitochondrial density by ~21 and ~25%, respectively. Functionally, despite not altering maximal cardiac output, KE training increased maximal O2 delivery (~54%), arterial-venous O2 (a–v O2) difference (~10%), and muscle O2 diffusive conductance (DMO2) (~39%) (assessed during KE), thereby increasing single leg VO2peak by ~53%, to a level exceeding that of the untrained controls. Post-training, during maximal cycling, O2 delivery (~40%), a–v O2 difference (~15%), and DMO2 (~52%) all increased, yielding an increase in VO2peak of ~40%, matching the controls.
Conclusions
In the face of continued central limitations, clear improvements in muscle structure, peripheral convective and diffusive O2 transport, and subsequently O2 utilization support the efficacy of local skeletal muscle training as a powerful approach to combat exercise intolerance in CHF.
Keywords: oxygen supply, oxygen utilization, cardiac output, blood flow, skeletal muscle, hyperoxia
Introduction
Diminished maximal exercise capacity is a defining symptom of chronic heart failure (CHF), limiting physical activity and impairing quality of life (1). Regular exercise in patients with CHF improves quality of life and reduces symptoms, hospitalization, disability, and even perhaps mortality (2–4). Traditionally, this attenuated exercise capacity has been attributed predominantly to the “central” hemodynamic limitations associated with the failing cardiac pump, but it is now evident that “peripheral” factors also contribute to this exercise limitation, as first highlighted by the work of Poole and Musch in a rat model of CHF (5,6) and later confirmed in humans (7). However, due to the multi-factorial determinants of maximal exercise and its associated O2 consumption, VO2peak, the mechanisms responsible for the beneficial effects of exercise training, and therefore exercise prescription, in patients with CHF are not yet completely understood.
Traditional cardiac rehabilitation has employed whole body exercise which challenges a large muscle mass and therefore taxes the central circulation. This approach has consistently yielded significant improvements in exercise capacity in patients with CHF (3,8). However, as whole body exercise induces a complex interaction between central hemodynamic and peripheral responses, such an observation leaves doubt as to the role of central and peripheral adaptations in response to exercise training. In an attempt to address this uncertainty, several studies have employed small muscle mass training, unlikely to stimulate central hemodynamic adaptations, and then challenged the patients with whole body exercise (9–11). Although this innovative approach has revealed that muscle specific training can indeed improve whole body exercise capacity in patients with CHF (9–11), the indirect physiological assessments used in these studies mean that the mechanisms responsible for this positive outcome have yet to be determined.
Consequently, utilizing direct intramuscular and intravascular measurements, this study sought to determine the physiologic mechanisms responsible for the anticipated improvement in whole body exercise capacity following 8 weeks of isolated KE training in patients with CHF. Specifically, it was hypothesized that small muscle mass training will 1) stimulate intramuscular structural changes that are conducive to O2 transport and oxidative metabolism (e.g. increased capillarity and mitochondrial density), 2) significantly improve muscle O2 diffusional conductance, 3) significantly enhance skeletal muscle O2 delivery, and subsequently 4) improve VO2peak, assessed both locally (across the muscle) and centrally (across the lungs), despite only a minimal central hemodynamic challenge and therefore nominal central adaptation. We further hypothesized that improvement in exercise capacity would be evident not only during KE but also in whole body (cycling) exercise. If these hypotheses are supported by experimental data, they will provide direct evidence of the important role that skeletal muscle convective and diffusive O2 transport play in limiting exercise capacity in patients with CHF and provide direction for future pharmacologic and rehabilitative interventions in this population.
Methods
Subjects
Six male clinically stable patients with CHF (NYHA class II–III, Weber VO2max Class C/B (12)) and 6 healthy male controls volunteered and gave written informed consent to participate in this study, which had been approved by the University of California, San Diego Human Subjects Protection Program. Mean left ventricular ejection fraction in the CHF patients was 25 ± 3%. Other than β-blockers, that were withheld for 48 hours prior to the studies, patient medications were not altered throughout the study. Particular care was taken to match patients with CHF and controls in terms of age, sex, and, especially, physical activity by questionnaire and interview (table 1).
Table 1.
Characteristics of patients with CHF and controls.
| CHF pre | CHF post | Controls | |
|---|---|---|---|
| Age (yrs) | 54 ± 14 | 54 ± 14 | 51 ± 8 |
| Height (cm) | 182 ± 6 | 182 ± 6 | 179 ± 7 |
| Body Mass (kg) | 100 ± 4# | 101 ± 4# | 90 ± 11 |
| Knee extensor (one leg) Muscle Mass (kg) |
2.6 ± 0.3 | 3.0 ± 0.4*# | 2.4 ± 0.4 |
| NYHA class | II–III | II–III | - |
| Medications | % using | % using | % using |
| Digoxin | 100% | 100% | 0% |
| Diuretics | 100% | 100% | 0% |
| Long-acting nitrates | 80% | 80% | 0% |
| Statins | 60% | 60% | 0% |
| Aspirin | 80% | 80% | 0% |
| β-blockers | 100% | 100% | 0% |
| Warfarin | 40% | 40% | 0% |
| ACE inhibitors | 80% | 80% | 0% |
| Ca++ Channel blockers | 40% | 40% | 0% |
NYHA, New York Heart Association; Results are expressed as mean ±SD.
P<0.05 (post vs pre);
P<0.05 (CHF vs controls).
Catheter placement, experimental and training protocol
Upon arrival at the laboratory, following a series of familiarization sessions, radial arterial and common femoral venous catheters were placed. In addition, a thermocouple sensor was placed in the common femoral vein, as previously described (13). Following these procedures, all subjects undertook four exercise tests in a balanced design, each separated by at least 1.5 hours for recovery (Cycle and KE in both normoxia and hyperoxia (100% O2)). In each trial, exercise intensity was incremented progressively every 2 minutes to exhaustion. On a separate day, a percutaneous biopsy of vastus lateralis muscle was obtained (Bergstrom needle) from the CHF patients and controls, as previously described (14). These catheter-based and biopsy studies were then repeated in the CHF patients following 8 weeks of supervised KE training (3 times/week, varied intensity - with overall intensity progressively increased based upon biweekly assessments, 50 min/session/leg), as previously described (15–17). Exercise training compliance was evaluated as a % of training sessions attended. Control subjects did not undergo training and thus were studied only once.
Exercise modalities
Cycle exercise was performed on an electromagnetically-braked cycle ergometer (Lode Excalibur Sport, Quinton Instruments Inc. Groningen, The Netherlands). During KE, the subject was seated on an adjustable chair with the ankle of one leg attached by a rigid bar to a cycle ergometer (Monark), as previously described ((18);Figure 1).
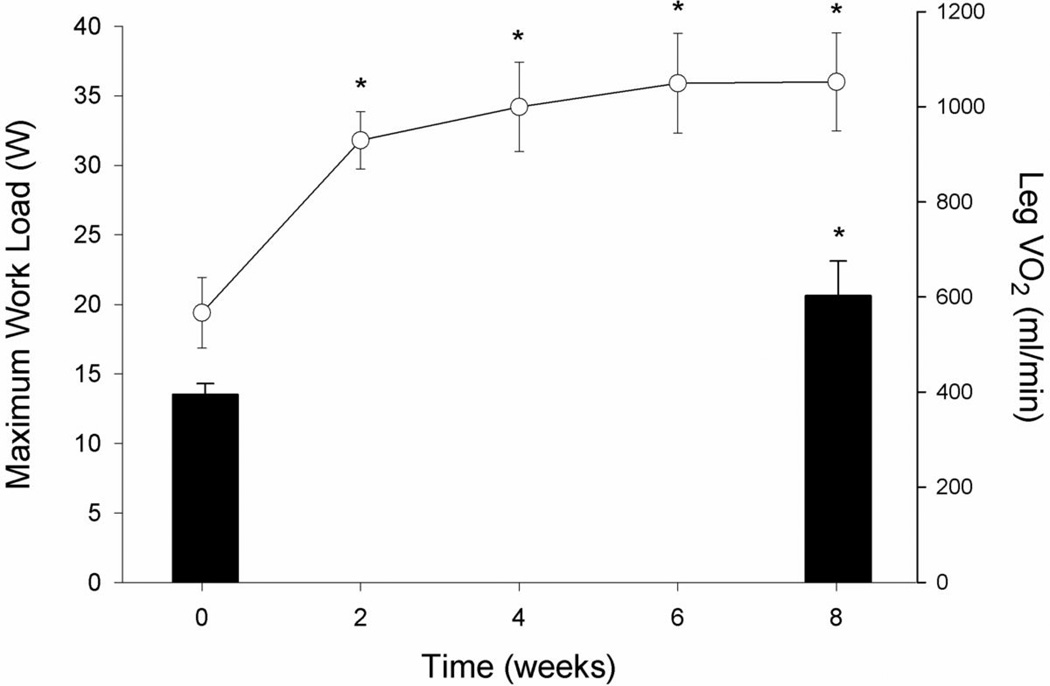
Figure 1. The time course for maximal knee-extensor (KE) work rate improvement over 8 weeks of KE training and pre and post training muscle VO2peak in patients with CHF.
Line plot represents maximum work load assessed every 2 weeks (left y axis) and bars represent leg VO2 assessed before and after exercise training (right y axis). *P<0.05 vs pre training condition.
Measurements and Calculations
Mixed expired O2 and CO2, expiratory air flow and ECG were continuously recorded and digitized (Parvo Medics, Salt Lake City, UT). Radial arterial and common femoral venous blood samples were collected at rest and during the third minute of each incremental work rate. At each level of work, the following variables were measured from these samples: PO2, PCO2, pH (IL model 1302, pH/blood gas analyzer, Instrumentation Laboratories, Milan, Italy), oxyhemoglobin saturation, hemoglobin concentration ([Hb]) (IL 482 co-oximeter), and lysed whole-blood lactate concentrations [La] (YSI 23L blood lactate analyzer; Yellow Springs Instruments, Yellow Springs, OH). Blood gas values were corrected to the temperature measured in the common femoral vein. In addition, common femoral venous blood flow (thermodilution) and arterial and venous pressures were measured (18). Muscle O2 diffusional conductance (DMO2) and mean capillary PO2 (PcapO2) were calculated as described previously (19,20). Briefly, a numerical integration procedure is used to determine that value of DMO2, assumed constant along the capillary, that produces the measured femoral venous PO2, given the measured arterial PO2. Additional explicit assumptions of this calculation are: 1) mitochondrial PO2 is negligibly small at VO2max and 2) the only explanation of O2 remaining in the femoral venous blood is diffusion limitation of O2 efflux from the muscle microcirculation. PcapO2 is the numerical average of all PO2 values computed, equally spaced in time, along the capillary from the arterial to the venous end. Further technical aspects of these measurements and subsequent calculations have been previously provided in detail (19,20).
Cardiac output
Cardiac output was measured in duplicate at rest and during exercise using an open-circuit acetylene (C2H2) uptake technique, as described previously (21).
Catecholamines
Epinephrine and norepinephrine (Ne) were extracted from plasma using a cis-diol-specific affinity gel, acylated, derivatized enzymatically and then assayed by competitive ELISA using the microtiter plate format, as previously described (22). The rate of Ne spillover was determined as described previously (23) using the following equation:
where Cv and Ca are plasma Ne concentrations in the common femoral vein and radial artery, respectively. Ee is the fractional extraction of epinephrine, and LPF is the leg plasma flow, determined from leg blood flow and hematocrit.
Muscle mass
With thigh length, circumferences, and skin-fold measurements, thigh volume was calculated to allow an estimate of quadriceps muscle mass, as utilized previously (18,24). During cycle exercise, the amount of working muscle mass was estimated based on the reported ratio of quadriceps muscle mass to other leg muscles, in a similar fashion as we have previously (25).
Muscle biopsy handling
The pericutaneous biopsy samples from the vastus lateralis (~ 80–100 mg) were equally divided for histochemistry and microscopy, with the former being oriented for future sectioning and then frozen while the latter were glutaraldehyde-fixed.
Histochemistry
Eight mm-thick transverse sections were cut at −24°C on a cryostat (Jung-Reichert Cryocut 1800) and kept at −20°C until histochemical processing, which was performed within a week of sectioning. After 5 min fixation in a Guth and Samaha fixative at room temperature, sections were incubated at 37°C for 1 hr in lead (Pb)-ATPase staining medium to simultaneously stain for fiber types I and II and capillaries (26).
Tissue preparation for microscopy
The glutaraldehyde-fixed samples were completely cut into thin longitudinal strips and processed for electron microscopy as described previously (27). Electron micrographs for morphometry were taken on 70 mm films with a Zeiss 10 electron microscope.
Morphometry
The relative cross-sectional area and number of type I and type II fibers was estimated under a light microscope (250×) on histochemical sections by point-counting using an eyepiece square grid test A100 (28). Capillary density (i.e. capillary number per fiber cross-sectional area), capillary-to-fiber ratio (i.e. capillary number per fiber number), capillary number around a fiber and fiber cross-sectional area were measured by point-counting on 1µm-thick sections examined at a magnification of 400× with a light microscope. The volume density of mitochondria per volume of muscle fiber was estimated by point-counting using electron microscopy at a final magnification of 49,000× on ultrathin transverse sections.
Statistical Analysis
Data were analysed using parametric statistics, following mathematical confirmation of normal distribution using Shapiro-Wilk tests. Within-group subject assessments (CHF) were achieved using paired sample t-tests or ANOVA for repeated measurements, while CHF patient and control data were compared using unpaired t-tests or ANOVA, where appropriate. Following a significant main effect and interaction with an ANOVA, t-tests were employed to make post-hoc comparisons. Due to the limited sample size and large number of measurements, post-hoc comparisons were not corrected for multiple comparisons and this is a recognized limitation of the statistical analyses employed. The relationship between selected variables was identified using a Pearson Product Moment Correlation. Statistical significance was set at P<0.05. Data are expressed as means ± standard error (SE).
RESULTS
The Shapiro-Wilk tests revealed P values >0.05; thus, the null hypothesis that the data were normally distributed was not rejected and parametric statistics were employed.
Subject Characteristics
Prior to exercise training, the only statistically significant different anthropometric characteristic between the patients and controls was body mass, which was higher in patients. Post-training, body mass was unchanged while quadriceps muscle mass was 15% greater (Table 1). As a consequence of the KE training-induced increase in cycle VO2max (Table 2), the patients who were, pre-training, predominantly categorized as Weber VO2max Class C (12) were now predominantly Class A and B.
Table 2.
Cardiorespiratory and metabolic responses to maximal normoxic cycle and kneeextensor exercise (KE) in patients with chronic heart failure and controls.
| CYCLE | KE | |||||
|---|---|---|---|---|---|---|
| CHF pre | CHF post | Controls | CHF pre | CHF post | Controls | |
| Work load (watts) | 115 ± 13# | 141 ± 16* | 148 ± 8 | 19 ± 2# | 37 ± 5* | 35 ± 4 |
| Pulm VO2 (L·min−1) | 1.63 ± 0.09# | 2.02 ± 0.15* | 2.14 ± 0.10 | 0.76 ± 0.08# | 1.08 ± 0.05* | 1.12 ± 0.09 |
| Pulm VO2 (ml·Kg−1·min−1) | 15.3 ± 1.2# | 18.6 ± 1.6*# | 24.1 ± 1.1 | 7.0 ± 0.4# | 9.9 ± 0.7* | 12.7±1.2 |
| RER | 1.21 ± 0.02# | 1.08 ±0.06 | 1.05 ± 0.02 | 1.11 ± 0.12# | 1.06 ± 0.06# | 0.86 ± 0.03 |
| Cardiac Output (L·min−1) | 13.6 ± 1.2# | 14.3 ± 1.4# | 16.8 ± 0.8 | 9.0 ± 1.2 | 9.4 ± 1.1 | 10.9 ± 1.2 |
| HR (b·min−1) | 155 ± 5 | 149 ± 9 | 156 ± 9 | 101 ± 6# | 107 ± 7 | 116 ± 6 |
| Leg blood flow (L·min−1) | 4.62 ± 0.46 | 5.49 ± 0.52* | 5.19 ± 0.37 | 3.09 ± 0.24 | 4.27 ± 0.46*# | 3.6 ± 0.24 |
| Leg O2 delivery (L·min−1) | 0.86 ± 0.10# | 1.05 ± 0.09* | 1.06 ± 0.07 | 0.53 ± 0.03# | 0.79 ± 0.09* | 0.7 ± 0.04 |
| Leg VO2 (L·min−1) | 0.62 ± 0.04# | 0.87 ± 0.07* | 0.77 ± 0.06 | 0.39 ± 0.02# | 0.60 ± 0.07*# | 0.49 ± 0.03 |
| DMO2 (ml·min−1·mmHg−1) | 14.0 ± 0.9# | 21.3 ± 3.4* | 18.7 ± 1.3 | 10.1 ± 0.8# | 14.0 ± 2.3* | 12.1 ± 0.9 |
| Fem arterial pressure (mmHg) | 123 ± 15 | 118 ± 8 | 132 ± 14 | 113 ± 3# | 123 ± 7# | 149 ± 9 |
| Fem venous pressure (mmHg) | 11 ± 1# | 16 ± 2* | 19 ± 3 | 16 ± 2 | 18 ± 2 | 16 ± 4 |
| Leg Vasc Res (mmHg ml sec) | 1.50 ± 0.22 | 1.14 ± 0.11* | 1.48 ± 0.25 | 1.89 ± 0.20# | 1.58 ± 0.23*# | 2.31 ± 0.24 |
| CaO2 (ml·100ml−1) | 18.8 ± 1.0# | 19.2 ± 0.5# | 20.4 ± 0.2 | 17.4 ± 1.0# | 18.6 ± 0.9 | 19.6 ± 0.3 |
| CaO2–CvO2(ml·100ml−1) | 13.7 ± 0.8# | 15.8 ± 0.2* | 15.3 ± 0.8 | 13.0 ± 0.8 | 14.3 ± 1.1* | 13.8 ± 0.5 |
| [La]a (mM) | 6.3 ± 0.9 | 6.8 ± 1.4 | 6.4 ± 0.8 | 2.7 ± 0.3 | 3.4 ± 0.5 | 3.2 ± 0.3 |
| PcapO2 (mmHg) | 42.4 ± 1.1 | 41.2 ± 2.8 | 41.3 ± 2.0 | 38.5 ± 0.8# | 43.6 ± 2.4* | 41.3 ± 0.8 |
Pulm VO2, pulmonary VO2; RER, respiratory exchange ratio; HR, heart rate; leg VO2, one-leg O2 uptake; DMO2, muscle O2 diffusional conductance; Leg Vasc Res, one-leg vascular resistance; CaO2, arterial O2 content; CaO2–CvO2, arterial - venous O2 content difference; [La]a, arterial lactate concentration; PcapO2, calculated partial pressure of O2 in the capillaries of the exercising muscle. Results are expressed as mean ±SE.
P<0.05 (post vs pre);
P<0.05 (CHF vs controls).
Exercise Training Compliance
The individualized approach to training with supervision in the laboratory resulted in a 98% compliance to the prescribed exercise regimen. A single patient did not complete the post training tests due to sickness and so was excluded from all analyses.
Maximal KE exercise pre- and post-KE training
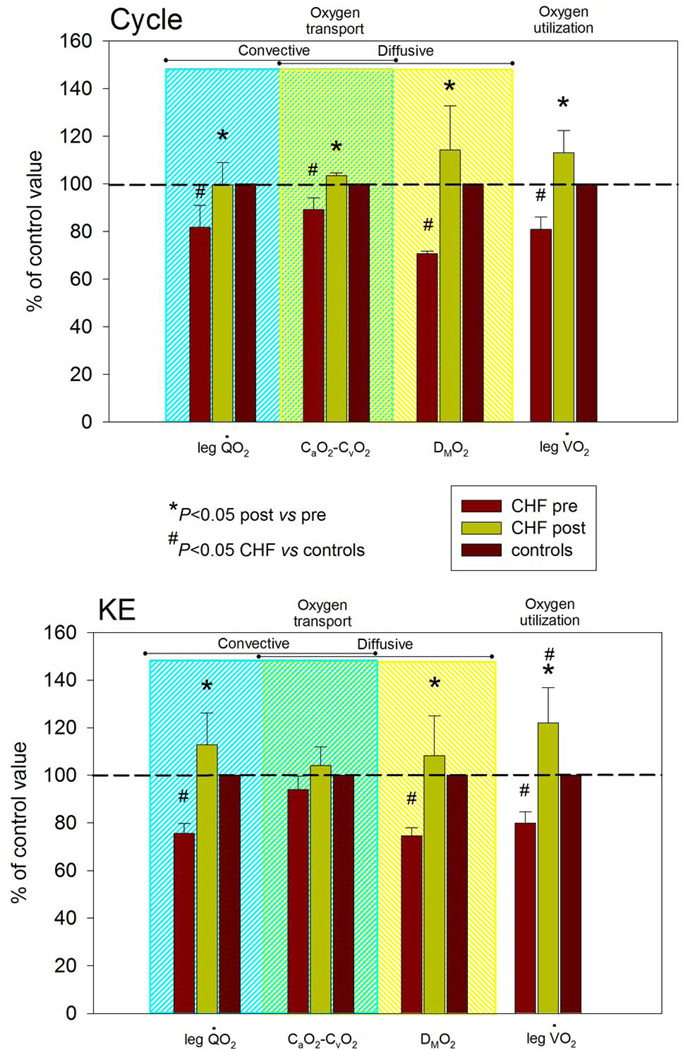
Maximum work rate during single leg KE increased substantially in the first two weeks of training and continued to rise over the next four weeks, with little measurable increase in the final two weeks of training (Figure 1), on average almost doubling from 19 to 37 watts to equal that of the (untrained) control subjects. Pre-training, cardiac output attained during maximal KE in the patients with CHF was not significantly different than in controls and was not altered by KE training (Table 2). Pre-training, maximum leg O2 delivery was significantly lower than in controls, but post-training patient maximum leg O2 delivery increased to equal that of the controls, in both cases driven by differences in blood flow and not O2 content (Table 2). Leg vascular resistance at maximal exercise was significantly attenuated after exercise training, however, the assessment of Ne spillover across the muscle bed did not implicate a reduction in muscle sympathetic nerve activity (Table 3). Pre-training, DMO2 at maximal KE was significantly lower in CHF patients than controls. Post-training, DMO2, although tending to be higher, was not significantly different from controls (P = 0.1) (Table 2, Figures 2 and 3). Pre-training, KE leg VO2peak was significantly lower in patients compared to controls. However, post-training, patient leg VO2peak increased and was significantly greater than in controls (Table 2, Figures 1 and 2).
Table 3.
Arterial and venous epinephrine, norepinephrine (Ne), and calculated Ne spillover from the muscle during maximal cycle and knee-extensor exercise (KE) in controls and patients with chronic heart failure.
| CYCLE | KE | |||||
|---|---|---|---|---|---|---|
| CHF pre | CHF post | Controls | CHF pre | CHF post | Controls | |
| [e]a (nM) | 2.2 ± 0.5 | 3.6 ± 1.5 | 1.0 ± 0.12 | 0.9 ± 0.3 | 1.3 ± 0.5 | 0.61± 0.1 |
| [e]v (nM) | 1.8 ± 0.48 | 3.4 ± 1.5 | 0.75 ± 0.12 | 0.8 ± 0.3 | 1.1 ± 0.42 | 0.49 ± 0.1 |
| [Ne]a (nM) | 27.2 ± 6.1# | 30.1 ± 5.5# | 18.3 ± 3.5 | 6.0 ± 0.9 | 8.0 ± 0.8 | 8.6 ± 2.2 |
| [Ne]v (nM) | 24.0 ± 4.3# | 32.1 ± 4.4# | 17.6 ± 5.2 | 6.9 ± 1.1 | 8.5 ± 1.2 | 9.3 ± 2.1 |
| Ne Spillover (nM·min−1) | 10.7 ± 3.1 | 10.2 ± 3.7 | 7.9 ± 2.3 | 4.3 ± 2.4 | 5.0 ± 2.7 | 4.9 ± 1.7 |
[e]a, arterial epinephrine concentration; [e]v, femoral venous epinephrine concentration; [Ne]a, arterial norepinephrine concentration; [Ne]v, femoral venous norepinephrine concentration; Ne spillover, norepinephrine spillover in one leg. Results are expressed as mean ±SE.
= P < 0.05 (CHF vs. controls).
Figure 2. A comparison of oxygen transport and utilization parameters assessed at maximal cycle (upper panel) and knee-extensor exercise (KE) (lower panel) both before and after KE training in patients with chronic heart failure (CHF) (n=5) normalized to values from healthy controls (n=8).
leg V̇ O2, one-leg O2 uptake;Q̇ O2, one-leg O2 delivery; CaO2–CvO2, arterial-venous O2 content difference.
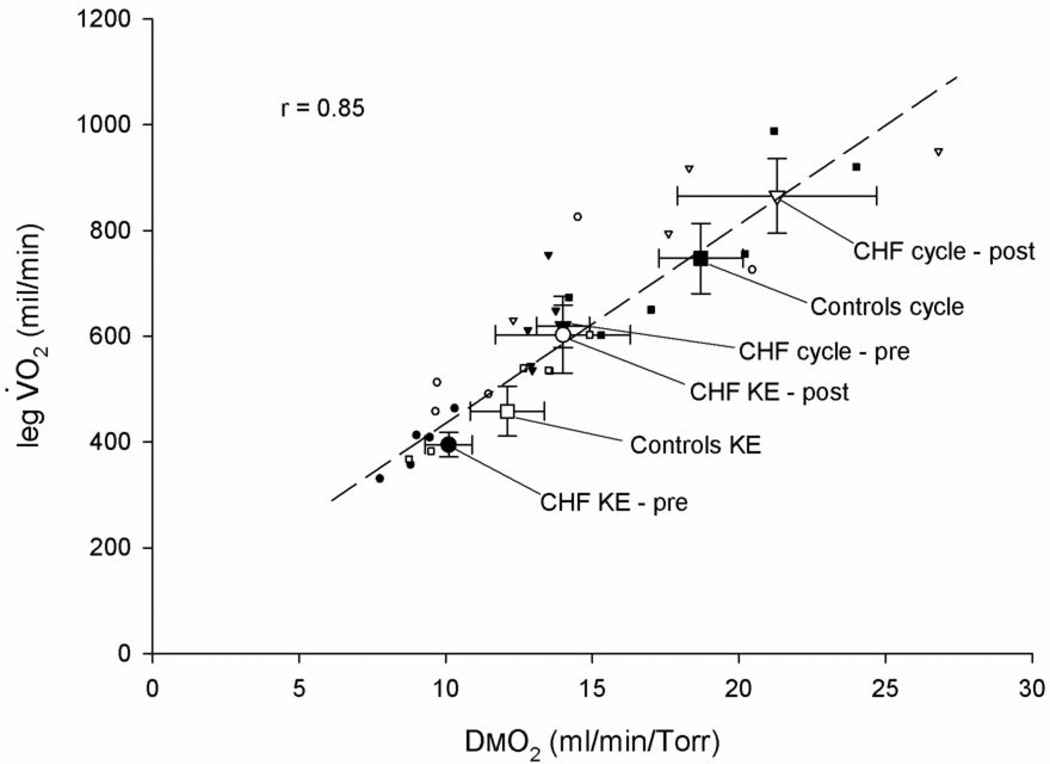
Figure 3. The improvement in skeletal muscle diffusional conductance (DMO2) in relation to leg VO2peak during maximal bike and knee-extensor exercise (KE) afforded by 8 weeks of KE training in patients with CHF, compared with healthy controls.
Note the correlation coefficient only represents the relationship between the individual data.
Maximal cycle exercise pre- and post-KE extensor training
Pre-training, cardiac output attained during maximal cycle exercise in the patients was significantly lower than in the controls and was unaffected by training (Table 2). Pre-training, maximum leg cycle O2 delivery in the patients was also significantly lower than in controls, but post-training, maximum leg O2 delivery increased to equal that of the controls, in both cases driven by differences in blood flow and not O2 content (Table 2). Leg vascular resistance at maximal exercise was significantly attenuated after exercise training, however, as with KE exercise, Ne spillover across the muscle bed did not suggest a reduction in muscle sympathetic nerve activity (Table 3). Pre-training, DMO2 at maximal cycle exercise in the patients with CHF was significantly lower than in controls. Post-training, DMO2, although tending to be higher, was not statistically different from controls (P = 0.1) (Table 2, Figures 2 and 3). Both pulmonary and leg VO2peak were significantly lower in the patients compared to controls during maximal cycle exercise pre-training, however, after training, both cycling pulmonary VO2peak and leg VO2peak increased to equal that of controls (Table 2 and Figures 1 and 2).
Catecholamines
Resting arterial norepinephrine, an index of CHF severity, was significantly higher in the patients both before (4.7 ± 2.9 nM) and after training (4.2 ± 2.6 nM) compared to controls (3.2 ± 0.5 nM) and was not significantly reduced by the exercise training. During cycling, the assessment of arterial and venous catecholamines at maximal exercise revealed a tendency for greater arterial and venous epinephrine and Ne and calculated Ne spillover across the quadriceps in the patients with CHF. However, likely due to large individual variations, this effect only achieved significance for arterial and venous Ne on the cycle ergometer, both before and after training. During KE, the catecholamine data were more similar between patients and controls and there was no apparent impact of training (Table 3).
Muscle Structure
Prior to training, the muscle characteristics for both patients with CHF and controls were very similar, with no significant difference in fiber cross sectional area, % area of type I and II fibers, capillary density, capillary-to-fiber ratio or number of capillaries around a fiber between patients and controls. However, mitochondrial volume density was significantly lower in the patients with CHF. As a consequence of training, the patients exhibited a significant increase in fiber cross-sectional area, capillary-to-fiber ratio, number of capillaries around a fiber, and mitochondrial volume density. This increase in mitochondrial volume density was such that, post training, there was no longer a difference between patients and controls, as was the case with all other structural variables measured (Table 4).
Table 4.
Vastus lateralis muscle characteristics
| CHF pre | CHF post | Controls | |
|---|---|---|---|
| Fiber cross-sectional area (µm2) | 3458 ±204 | 4074 ±215* | 3853 ±606 |
| % area of type I fibers | 34 ±4 | 41 ±3* | 41 ±4 |
| % area of type II fibers | 66 ±4 | 59 ±3* | 59 ±4 |
| Capillary density (capillaries·mm−2) | 498 ±23 | 484 ±23 | 415 ±46 |
| Capillary-to-fiber ratio | 1.6 ±0.1 | 1.8 ±0.1* | 1.5 ±0.10 |
| Number of capillaries around a fiber | 3.6 ±0.2 | 4.3 ±0* | 3.8 ±0.1 |
| Mitochondrial volume density (%) | 3.8 ±0.3# | 4.9 ±0.4* | 4.4 ±0.4 |
P<0.05 (post vs pre);
P<0.05 (CHF vs controls)
100% O2 breathing
At both peak cycle exercise and KE prior to and following training, hyperoxic breathing in the patients with CHF increased CaO2 by 8 –10 % (P < 0.05), with no significant effect on leg blood flow. Therefore, leg O2 delivery also increased by 5 - 0% (P < 0.05), but leg VO2peak was unaffected by this increased O2 availability in each scenario.
Correlative analyses
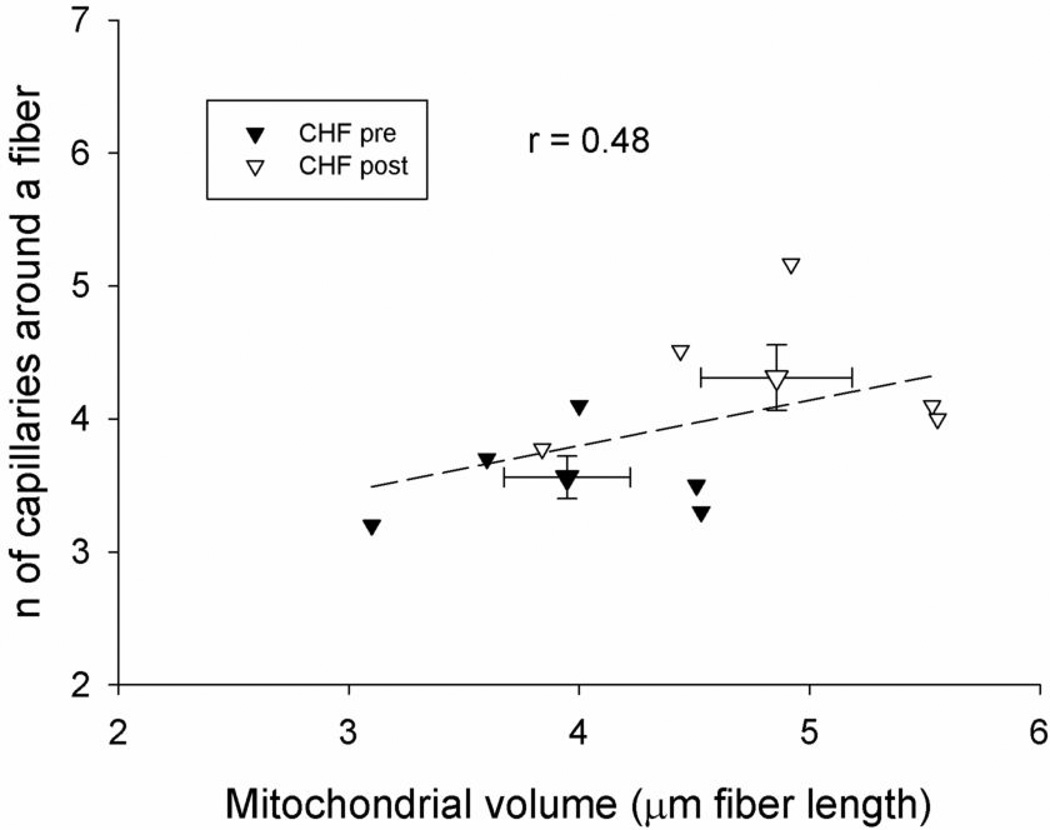
With the inclusion of all normoxic scenarios studied (i.e. CHF patients and controls on the cycle and KE and patients again on these two modalities after training) there was a wide range of values of DMO2 and leg VO2peak data. A Pearson’s Correlation analysis revealed that there was a strong and significant relationship (r=0.85) between DMO2 and leg VO2peak across all these paradigms (Figure 3). An additional correlation, of note, was the relationship between mitochondrial volume and number of capillaries around a fiber. Specifically, the combination of both pre and post KE training data for the CHF patients revealed a significant correlation between these two physiologically important variables (Figure 4).
Figure 4. Correlation between the number of capillaries around a fiber and mitochondrial volume in patients with CHF before and after 8 weeks of knee-extensor training.
Note the correlation coefficient only represents the relationship between the individual data.
DISCUSSION
Here, with the intent to elucidate the mechanisms responsible for improvements in whole body exercise capacity when exercise training of a small muscle mass is employed in patients with CHF, we studied the determinants of VO2peak during both KE and cycle exercise before and after 8 weeks of KE training. As anticipated, this intervention had no effect upon maximal cardiac output, as this exercise training paradigm only minimally stresses the heart and was therefore unlikely to stimulate central hemodynamic adaptations. In contrast there were a multitude of significant peripheral structural and functional adaptations that contributed to improved patient exercise capacity, both during maximal small muscle mass and whole body exercise. Specifically, in addition to significant training-induced muscle morphometric changes, both convective and diffusive O2 transport were increased at maximal KE and cycle exercise, yielding a significant increase in VO2peak in each scenario. These peripheral structural and O2 transport improvements, without a change in cardiac output, provides evidence of significant peripheral vascular and metabolic plasticity in this population that can be developed in isolation with small muscle mass training and then harnessed to the benefit of whole body exercise capacity. Additionally, these findings highlight the importance of skeletal muscle specific adaptations in patients with CHF allowing the contributions of these peripheral factors to be partitioned, perhaps guiding clinical interventions in the future.
Small muscle mass exercise training-induced changes in O2 transport
Previously (7), building upon the initial work of Poole and Musch in a rat model of CHF (5,6), we have documented the contributions of both the convective (bulk delivery of O2) and diffusive (movement of O2 from hemoglobin to mitochondria) elements in determining VO2peak in CHF patients and contrasted these findings with age and activity matched healthy controls. In the current study we have the opportunity to elucidate the effect of small muscle mass training, which did not alter maximal cardiac output, in patients with CHF, on the convective and diffusive components of O2 transport that ultimately determine VO2peak, again with age and activity matched controls as a reference (tables 1 and 2). Here, the main findings are that deficits in O2 delivery and DMO2 that result in an attenuated VO2peak are evident equally at maximal exercise, whether performed on a cycle or KE ergometer, and can be restored to, or even exceed (i.e. KE VO2peak), levels of normal but untrained controls by 8 weeks of KE training (figures 2, 3, and 5).
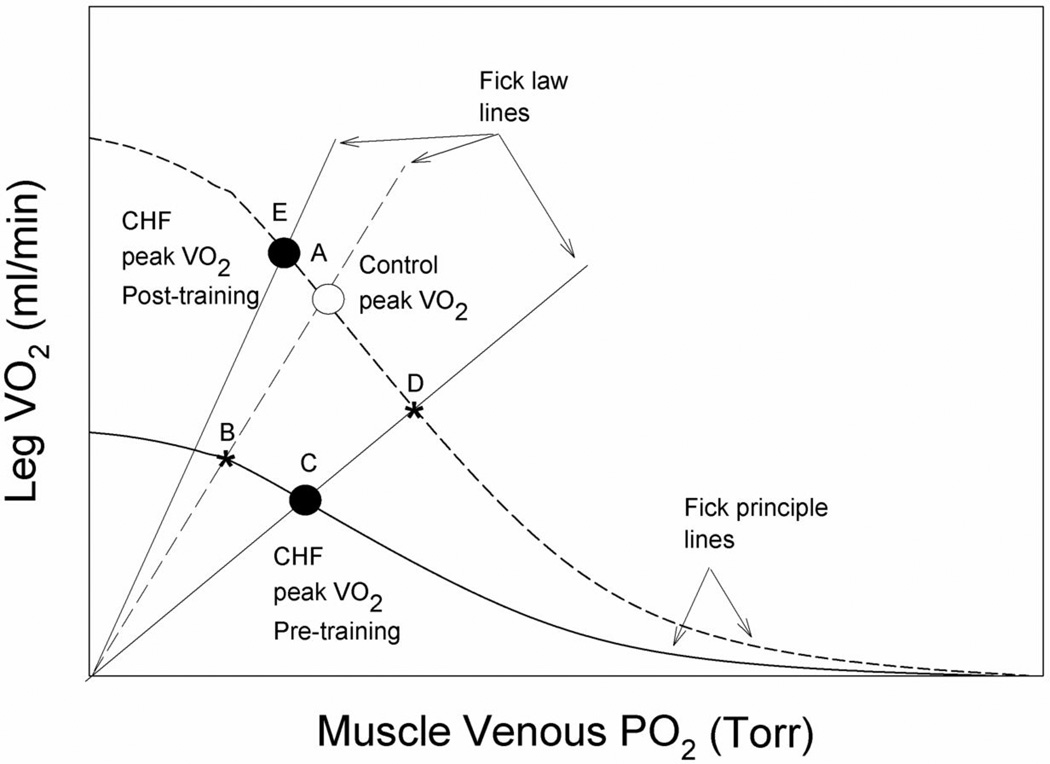
Figure 5. A schematic illustration of the convective and diffusive components that interact to determine peak oxygen uptake (VO2peak) in both chronic heart failure and control subjects during cycle (large muscle mass) and knee-extensor exercise (KE, small muscle mass) and the subsequent changes as a consequence KE training.
Dotted lines represent Fick law and Principle lines for the controls (intersecting at A; control VO2peak), while the solid lines represent the patients with CHF. Following training both groups share the dotted Fick Principle line. In both exercise modalities, prior to training, the patients with CHF exhibited attenuated convective and diffusive oxygen transport as evidenced by their VO2peak being defined by the intercept of lower Fick law and principle lines (C). With B indicating the less severe reduction in VO2peak had the patients only revealed a reduction in convective O2 transport. KE training corrected both of these deficits, without increasing cardiac output, restoring both skeletal muscle convective and diffusive O2 transport and therefore allowing VO2peak to equal or exceed (KE) that of the healthy controls (E). Letter D represents the consequence of exercise training if the increase in VO2peak had only been driven by an increase in convective O2 transport (C to D).
One method to better understand these interactions is by the representation of both the diffusive component of O2 transport, that is defined by Fick’s law of diffusion (VO2 = DMO2 * K * PvO2, where K is a constant that takes into account the proportionality between mean capillary PO2 and PvO2 and the assumption that intracellular PO2 is very close to zero (13), and the convective component, that is defined by the Fick Principle (VO2 = blood flow * (CaO2 – CvO2)), in a model that links VO2peak and effluent muscle PO2 (PvO2) to explain limitations to maximal exercise capacity (Figure 4)(29). At this point, it is important to recognize that based upon our previous work (7) and the current findings, the same schematic can be used to illustrate the results of both KE and cycle exercise studies. This is because, despite the fact that maximal cycle exercise undoubtedly completely taxed cardiac output capacity and KE had a cardiac reserve (7), prior to exercise training both the convective and diffusive components of O2 transport of the patients were attenuated, regardless of exercise modality (Figures 2 and 5) and then were restored in both modalities in a remarkably similar fashion as a consequence of KE training.
With this model of the determinants of VO2peak, it can be seen (Figure 5) that if before KE training the only difference between the controls and the CHF patients were the reduced convective component of O2 transport (Table 2), then VO2peak would have fallen from A to B as a result of this pathology (6). However, in addition to the lower convective component of O2 transport, the patients with CHF also revealed a significantly attenuated DMO2 (diffusive component) (Figures 2, 3, and 5) which actually resulted in an even greater fall in peak VO2peak from A to C (Figure 5). Likewise, this graphical illustration of the current data reveals that the consequence of exercise training was the result of not only a blood flow driven increase in convective O2 transport (C to D) or the restoration of DMO2 to that of controls (C to B) or higher, but a combination of these phenomenon (C to E) that resulted in the large increase in VO2peak (Figures 1, 2, 3, and 5). These findings have several important implications. First, that patients with CHF exhibit significant plasticity in terms of their ability to respond to exercise training both in terms of skeletal muscle blood flow changes and diffusional O2 transport. Second, both of these KE training-induced adaptations (augmented peripheral convective and diffusive O2 transport) translate into improvements in whole body exercise capacity (cycle) without a requisite improvement in cardiac function.
Catecholamines and O2 delivery
Neurohumoral activation, including increased sympathetic nervous system activity (SNA), is a hallmark of advanced CHF and those patients with the greatest SNA have the poorest chance of survival (30). So strong is this link, virtually every pharmacologic therapy proven to increase survival in CHF interrupts this increase in SNA (31). Exercise training, on the other hand, has clear beneficial effects, but data regarding the effect of exercise training on SNA in CHF have been equivocal. For example direct SNA measurements with mircroneurography revealed a clear reduction in resting SNA in patients with CHF following exercise training (32), while a study, more similar to the current work, evaluated patients with CHF before and after 8 weeks of two-legged KE training. This study yielded many apparent benefits, but training did not alter resting Ne levels (33). The current study supports the latter findings, with resting arterial norepinephrine levels being significantly higher than the healthy controls before KE training and this difference was maintained following the exercise intervention, although it should be noted that there was a tendency for levels to decrease. Additionally, during exercise there was evidence of relatively elevated Ne levels in the patients with CHF and a tendency for greater vascular resistance, attenuated blood flows, and consequently significantly attenuated O2 delivery prior to exercise training, which was reversed by KE training despite unremarkable changes in Ne levels (Tables 2 and 4). Thus, although somewhat indirect, the current data fail to support an obligatory association between the benefits of exercise training and a reduction in SNA in patients with CHF. It is, of course, plausible that other exercise training-induced vascular adaptations, such as improved vascular endothelial function, were responsible for the reduction in vascular resistance in the patients with CHF, but this is beyond the scope of the current study. Also in terms of all the catecholamine data, it is interesting to note the much more similar findings in the patients and controls during KE exercise compared to cycle exercise, which is likely a consequence of the far less globally taxing nature of KE exercise.
Skeletal muscle structural plasticity
A significant strength of the current study was the potential to not only perform the functional assessment VO2peak and dissect changes in the determining factors of this important variable, but also to directly examine concomitant changes in muscle structure (Table 4). Other studies of skeletal muscle structure and function within and between species have revealed design features that are uniform throughout muscles of widely varying metabolic demand. Such studies have revealed that the size of the capillary-to-fiber interface is matched to mitochondrial volume/fiber length in response to stimuli such as chronic hypoxia (34), electrical stimulation (35), and physical activity (36). These observations suggest another regulated design feature in skeletal muscle is the matching of structural capacity for O2 flux to fiber metabolic demand, as we have previously reported (36). The current data (Figure 4), in which mitochondrial volume and capillarity and the changes in these variables due to KE training appear to be well related, again reveals a positive and physiologically expected relationship in patients with CHF. Thus, not only does this study document a a degree of normalcy in these patients in terms of the components of O2 supply and demand, but it also emphasizes the fact that they retain the plasticity to respond to an exercise training stimulus (Figure 4).
Skeletal muscle metabolic reserve and exercise training
Previously, we have documented that by switching from a large (cycle) to a small muscle mass (KE) exercise modality a cardiac reserve was available to both patients with CHF and controls (7) and this was again the case in these subjects (Table 2). This central reserve, with the appropriate normalization to muscle mass involved in the exercise (25), translated into a clear improvement in muscle blood flow and metabolic capacity in patients with CHF and controls that was accessible during KE (Table 2). This observation is of specific interest in light of the many previous studies that have suggested CHF-induced skeletal muscle exhibits a shift in fiber type distribution, reduced oxidative capacity, reduced mitochondrial based enzymes, decreased mitochondrial volume density, all as a consequence of either muscle atrophy or myopathy, or both (1,37–39). Therefore, in this population, it is a significant observation that there still appears to be a metabolic reserve at maximal exercise, apparent when skeletal muscle is freed from the restraints imposed by the failing heart and that KE training can augment this reserve.
By increasing O2 availability, breathing 100% O2, we have previously also documented that patients with CHF can exhibit a skeletal muscle metabolic reserve (7). That is, when breathing hyperoxia which resulted in an elevated arterial O2 content, these patients were able to achieve a 5% greater cycle work rate and a 7% increase in VO2peak and interestingly this hyperoxic benefit was not achieved during KE. The current patients contrast with this previous report, responding more in line with the healthy controls, who failed to increase VO2peak above that attained in normoxia when breathing hyperoxia in both modalities (7). This failure to improve exercise capacity when provided with greater O2 availability (40,41), suggests that in these patients ambient O2 was either perfectly matched or in excess of basal muscle metabolic capacity. This sets the stage for a reassessment following an exercise-induced increase in metabolic capacity. Somewhat disappointingly, 8 weeks of KE training and subsequent testing in hyperoxia did not significantly elevate VO2peak above the level achieved in normoxia either during KE or cycle exercise, despite clear increases in mitochondrial volume (Tables 2 and 4). A potential explanation for this conundrum may be related to the actual delivery of this increased O2 in the microcirculation and the subsequent matching of O2 supply and metabolic demand in this population, but this hypothesis is not addressed in the current study and will therefore require further investigation.
Experimental consideration
Although β-blockers were withheld from the patients for 48 hours prior to testing, to allow an unrestrained heart response to maximal exercise, other patient medications were not altered throughout the study. As documented in Table 1, the medication regimen of these patients was considerable, many with direct vascular consequences. Therefore, although unavoidable for ethical reasons, the potential impact of these medications and possible interaction with the KE training cannot be disregarded. This is a significant limitation of both the current study and the majority of work performed in this population. It should also be noted that, as with the majority of studies in patients with CHF, this work focused upon patients with a NYHA Classification of II and III and not the most impacted patients (Class IV). This was not due to concern regarding exercise testing, but rather a consequence of subject availability due to the more common use of left ventricular assist devices and early heart transplantation in the most severely affected patients. This may have skewed the data in terms of the potential impact of CHF on exercise capacity and exercise training-induced plasticity, however, due to the aforementioned interventions NYHA Class II and III CHF patients are now the predominant category in this population.
CONCLUSIONS
In conclusion, despite continued central hemodynamic limitations, clear improvements in muscle structure, peripheral convective and diffusive O2 transport, and subsequently O2 utilization, both reveal the mechanisms of, and provide support for, the efficacy of local skeletal muscle training as a powerful approach to decrease exercise intolerance in patients with CHF. These mechanistic findings may have important practical consequences in terms of guiding future pharmacologic and rehabilitative interventions in this population.
ACKNOWLEDGEMENTS
The Authors wish to thank all the patients and the subjects of this study, for their committed participation and sacrifice to be a part of this involved study.
Funding: This research was funded in part by grants from NIH National Heart, Lung, and Blood Institute (HL 09183), and the Tobacco-Related Disease Research Program (15RT-0100).
Abbreviations
- CHF
chronic heart failure
- VO2
oxygen uptake
- KE
knee-extensor exercise
- CaO2
arterial O2 concentration
- DMO2
O2 conductance
- Ne
norepinephrine
- QO2
oxygen delivery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No relationships with industries to declare
REFERENCES
- 1.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 3.Piepoli MF, Davos C, Francis DP, Coats AJ, ExTra MC. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) Br Med J. 2004;328:189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail. 2006;8:841–850. doi: 10.1016/j.ejheart.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Richardson TE, Kindig CA, Musch TI, Poole DC. Effects of chronic heart failure on skeletal muscle capillary hemodynamics at rest and during contractions. J Appl Physiol. 2003;95:1055–1062. doi: 10.1152/japplphysiol.00308.2003. [DOI] [PubMed] [Google Scholar]
- 6.Poole DC, Musch TI. Pulmonary and peripheral gas exchange during exericse. In: Roca J, Rodriguez-Roisin R, Wagner PD, editors. Pulmonary and peripheral gas exchange in health and disease: Marcel Dekker Inc. 2000. pp. 469–523. [Google Scholar]
- 7.Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: Partitioning the contributors. J Am Coll Cardiol. 2009;55:1945–1954. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hambrecht R, Fiehn E, Weigl C, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–2715. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 9.Magnusson G, Gordon A, Kaijser L, et al. High intensity knee extensor training, in patients with chronic heart failure. Major skeletal muscle improvement. Eur Heart J. 1996;17:1048–1055. doi: 10.1093/oxfordjournals.eurheartj.a015001. [DOI] [PubMed] [Google Scholar]
- 10.Tyni-Lenne R, Dencker K, Gordon A, Jansson E, Sylven C. Comprehensive local muscle training increases aerobic working capacity and quality of life and decreases neurohormonal activation in patients with chronic heart failure. Eur J Heart Fail. 2001;3:47–52. doi: 10.1016/s1388-9842(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 11.Tyni-Lenne R, Gordon A, Jensen-Urstad M, Dencker K, Jansson E, Sylven C. Aerobic training involving a minor muscle mass shows greater efficiency than training involving a major muscle mass in chronic heart failure patients. J Card Fail. 1999;5:300–307. doi: 10.1016/s1071-9164(99)91334-9. [DOI] [PubMed] [Google Scholar]
- 12.Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation. 1982;65:1213–1223. doi: 10.1161/01.cir.65.6.1213. [DOI] [PubMed] [Google Scholar]
- 13.Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest. 1995;96:1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- 15.Richardson RS, Wagner H, Mudaliar SR, Saucedo E, Henry R, Wagner PD. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;279:H772–H778. doi: 10.1152/ajpheart.2000.279.2.H772. [DOI] [PubMed] [Google Scholar]
- 16.Lawrenson L, Hoff J, Richardson RS. Aging attenuates vascular and metabolic plasticity but does not limit improvement in muscle VO2 max. Am J Physiol Heart Circ Physiol. 2004;286:H1565–H1572. doi: 10.1152/ajpheart.01070.2003. [DOI] [PubMed] [Google Scholar]
- 17.Lawrenson L, Poole JG, Kim J, Brown CF, Patel PM, Richardson RS. Vascular and Metabolic Response to Isolated Small Muscle Mass Exercise: The Effect of Age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–H1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- 18.Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- 19.Agusti AGN, Roca J, Barbera JA, Casademont J, Rodriguezroisin R, Wagner PD. Effect of Sampling Site On Femoral Venous Blood Gas Values. J Appl Physiol. 1994;77:2018–2022. doi: 10.1152/jappl.1994.77.4.2018. [DOI] [PubMed] [Google Scholar]
- 20.Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE, Wagner PD. Hyperoxia increases leg maximal oxygen uptake. J Appl Physiol. 1993;75:2586–2594. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- 21.Barker RC, Hopkins SR, Kellogg N, et al. Measurement of cardiac output during exercise by open-circuit acetylene uptake. J Appl Physiol. 1999;87:1506–1512. doi: 10.1152/jappl.1999.87.4.1506. [DOI] [PubMed] [Google Scholar]
- 22.Wadley GD, Lee-Young RS, Canny BJ, et al. Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. Am J Physiol Endocrinol Metab. 2006;290:E694–E702. doi: 10.1152/ajpendo.00464.2005. [DOI] [PubMed] [Google Scholar]
- 23.Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ, Saltin B. Norepinephrine spillover from skeletal muscle during exercise: role of muscle mass. Am J Physiol. 1989;257:H1812–H1818. doi: 10.1152/ajpheart.1989.257.6.H1812. [DOI] [PubMed] [Google Scholar]
- 24.Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol. 1969;204:63P–66P. [PubMed] [Google Scholar]
- 25.Richardson RS, Grassi B, Gavin TP, et al. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. J Appl Physiol. 1999;86:1048–1053. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- 26.Rosenblatt JD, Kuzon WM, Jr, Plyley MJ, Pynn BR, McKee NH. A histochemical method for the simultaneous demonstration of capillaries and fiber type in skeletal muscle. Stain Technol. 1987;62:85–92. doi: 10.3109/10520298709107973. [DOI] [PubMed] [Google Scholar]
- 27.Mathieu-Costello O. Capillary tortuosity and degree of contraction or extension of skeletal muscles. Microvasc Res. 1987;33:98–117. doi: 10.1016/0026-2862(87)90010-0. [DOI] [PubMed] [Google Scholar]
- 28.Weibel E. Practical methods for biological morphometry. London-New York-Torronto: Academic Press; 1979. [Google Scholar]
- 29.Wagner PD. New ideas on limitations to VO2max. Exerc Sport Sci Rev. 2000;28:10–14. [PubMed] [Google Scholar]
- 30.Cohn JN, Levine TB, Olivari MT, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 31.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 32.Roveda F, Middlekauff HR, Rondon MU, et al. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol. 2003;42:854–860. doi: 10.1016/s0735-1097(03)00831-3. [DOI] [PubMed] [Google Scholar]
- 33.Gordon A, Tyni-Lenne R, Jansson E, Kaijser L, Theodorsson-Norheim E, Sylven C. Improved ventilation and decreased sympathetic stress in chronic heart failure patients following local endurance training with leg muscles. J Cardiac Fail. 1997;3:3–12. doi: 10.1016/s1071-9164(97)90002-6. [DOI] [PubMed] [Google Scholar]
- 34.Mathieu-Costello O, Agey PJ, Wu L, Szewczak JM, MacMillen RE. Increased fiber capillarization in flight muscle of finch at altitude. Respir Physiol. 1998;111:189–199. doi: 10.1016/s0034-5687(97)00119-9. [DOI] [PubMed] [Google Scholar]
- 35.Mathieu-Costello O, Agey PJ, Wu L, Hang J, Adair TH. Capillary-to-fiber surface ratio in rat fast-twitch hindlimb muscles after chronic electrical stimulation. J Appl Physiol. 1996;80:904–909. doi: 10.1152/jappl.1996.80.3.904. [DOI] [PubMed] [Google Scholar]
- 36.Poole DC, Mathieu-Costello O. Relationship between fiber capillarization and mitochondrial volume density in control and trained rat soleus and plantaris muscles. Microcirc. 1996;3:175–186. doi: 10.3109/10739689609148286. [DOI] [PubMed] [Google Scholar]
- 37.Harrington D, Anker SD, Chua TP, et al. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30:1758–1764. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 38.Massie BM, Simonini A, Sahgal P, Wells L, Dudley GA. Relation of systemic and local muscle exercise capacity to skeletal muscle characteristics in men with congestive heart failure. J Am Coll Cardiol. 1996;27:140–145. doi: 10.1016/0735-1097(95)00416-5. [DOI] [PubMed] [Google Scholar]
- 39.Mancini DM, Walter G, Reichek N, et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 40.Haseler LJ, Lin AP, Richardson RS. Skeletal muscle oxidative metabolism in sedentary humans: 31P-MRS assessment of O2 supply and demand limitations. J Appl Physiol. 2004;97:1077–1081. doi: 10.1152/japplphysiol.01321.2003. [DOI] [PubMed] [Google Scholar]
- 41.Cardus J, Marrades R, Roca J, et al. Effects of FiO2 on leg VO2 during cycle ergometry in sedentary subjects. Med Sci Sports Exerc. 1998;30:697–703. doi: 10.1097/00005768-199805000-00009. [DOI] [PubMed] [Google Scholar]