Abstract
Abdominal pain is a common symptom of inflammatory bowel disease (IBD: Crohn’s disease, ulcerative colitis). Pain may arise from different mechanisms, which can include partial blockage and gut distention as well as severe intestinal inflammation. A majority of patients suffering from acute flares of IBD will experience pain, which will typically improve as disease activity decreases. However, a significant percentage of IBD patients continue experiencing symptoms of pain despite resolving inflammation and achieving what appears to be clinical remission. Current evidence suggests that sensory pathways sensitize during inflammation, leading to persistent changes in afferent neurons and central nervous system pain processing. Such persistent pain is not only a simple result of sensory input. Pain processing and even the activation of sensory pathways is modulated by arousal, emotion, and cognitive factors. Considering the high prevalence of iatrogenic as well as essential neuropsychiatric comorbidities including anxiety and depression in IBD patients, these central modulating factors may significantly contribute to the clinical manifestation of chronic pain. The improved understanding of peripheral and central pain mechanisms is leading to new treatment strategies that view pain as a biopsychosocial problem. Thus, improving the underlying inflammation, decreasing the excitability of sensitized afferent pathways, and altering emotional and/or cognitive functions may be required to more effectively address the difficult and disabling disease manifestations.
Keywords: sensitization, hyperalgesia, inflammatory bowel disease
PREVALENCE AND IMPACT OF PAIN ON IBD
Pain is an important manifestation of inflammation, as inflammatory cytokines and mediators sensitize primary afferent neurons. It should thus not be surprising that pain is 1 of the presenting symptoms in about 50%–70% of patients experiencing the initial onset or exacerbations of inflammatory bowel disease (IBD).1,2 However, ongoing and/or severe inflammation does not suffice to explain pain in IBD patients, as about 20% of patients in clinical and even endoscopic remission continue experiencing significant symptoms. Up to one-sixth of IBD patients are chronically treated with opioids. 3–5 Physicians and patients typically think of pain as an alarm symptom that is triggered by high intensity and potentially noxious stimuli. This physiologic role of pain as an indicator of impending injury or harm is certainly relevant for IBD patients, as shown by the increase in pain prevalence and severity during disease flares. Pain may be the only symptom of disease activity in some patients. Thus, newly developing discomfort and/or a change in symptoms should always trigger appropriate diagnostic tests to determine whether it is due to an exacerbation of the underlying disease (Fig. 1). Not only inflammation, but also obstructions can manifest as pain and may necessitate specific, typically surgical interventions (Fig. 2). Inflammation or stricture formation requiring more intense medical or even surgical therapy likely explain why significant pain requiring analgesic therapy is a negative prognostic indicator.5 When confounding factors, such as immunomodulator or steroid use, are taken into account, this increased mortality risk was no longer significant, supporting the relevance of pain as an alarm symptom.
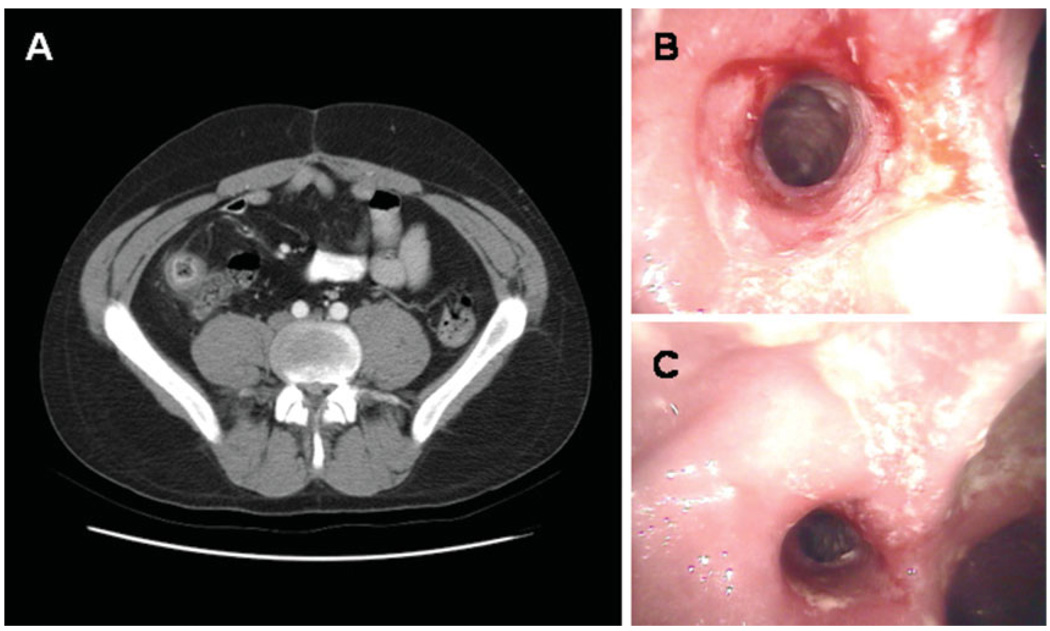
FIGURE 1.
Computerized tomography (A) and colonoscopic findings (B,C) in a patient with worsening abdominal pain, but no other symptoms of disease exacerbation. The CT scan shows wall thickening and enhancement in the neoterminal ileum, which was inflamed and strictured on colonoscopy.
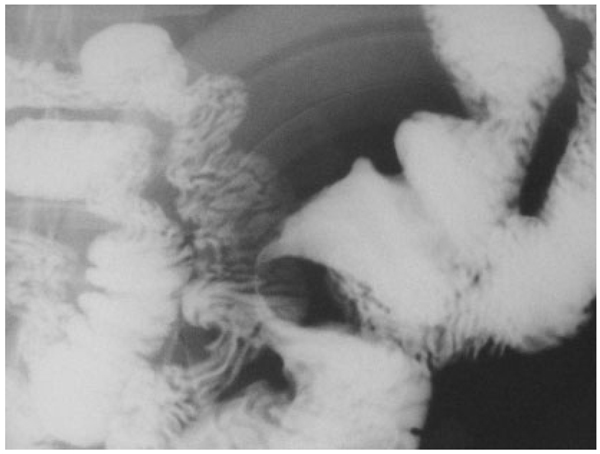
FIGURE 2.
Filiform stricture in a patient with isolated small bowel Crohn’s disease.
However, a subgroup of IBD patients without evidence of active inflammation, obstruction, or other biologically defined abnormalities continue to have pain. More than other sensory input, pain is associated with a significant emotional component. Detailed psychophysical studies suggest that this affective dimension is especially prominent in visceral pain.6 Thus, pain or fear of pain constitute a significant burden for IBD patients and can impair their quality of life.3,7 Despite its prevalence and impact on affected individuals, few studies have addressed pain management in IBD patients. Traditional nonsteroidal antiinflammatory drugs or the more selective COX-2-specific agents have been linked to a higher likelihood of disease exacerbation.8,9 Chronic use of narcotics in benign disorders is controversial, as it is linked to the potential for abuse as well as significant adverse reactions involving gastrointestinal function and dysmotility.3,4,10 Perhaps 1 of the most concerning findings regarding narcotic treatment of pain in Crohn’s disease (CD) emerged from an analysis of the TREAT registry, which prospectively followed over 6000 patients, half of whom received biologic therapy with the agent infliximab.5 Use of chronic narcotic analgesics by CD patients was associated with significantly increased morbidity and mortality on multivariate logistic regression, suggesting that these agents may unfortunately mask underlying organic and potentially serious health problems. Thus, while novel therapies have significantly improved our ability to treat the underlying inflammatory processes and their complications, pain management options continue to remain limited for patients suffering from IBD. In the following sections we will discuss the current understanding of mechanisms that may contribute to the pathogenesis of chronic pain in IBD with the goal to identify potential targets for possible innovative therapies.
PERIPHERAL MECHANISMS OF VISCERAL PAIN
Changing luminal contents and ongoing motor activity continuously activate intrinsic and extrinsic afferent neurons that project to the enteric and central nervous system, respectively. Detailed physiologic studies have shown that most of the sensory neurons respond to low-intensity stimuli, such as distension, flow, or chemical signals within the gut lumen.11 Despite the ongoing afferent barrage, healthy individuals rarely sense (ie, consciously perceive) signals emanating from their intestinal tract. Intense stimuli are required to trigger sensations that, more often than not, are felt as uncomfortable or even painful. This relationship between stimulus intensity and perception is changed in disease states: low-intensity stimuli that would normally not be perceived are felt as painful and noxious (Fig. 3A). Thus, a change in the stimulus–response function sensitizes afferent pathways. Experimental and clinical data have provided important information about underlying mechanisms and identified some potential treatment targets.
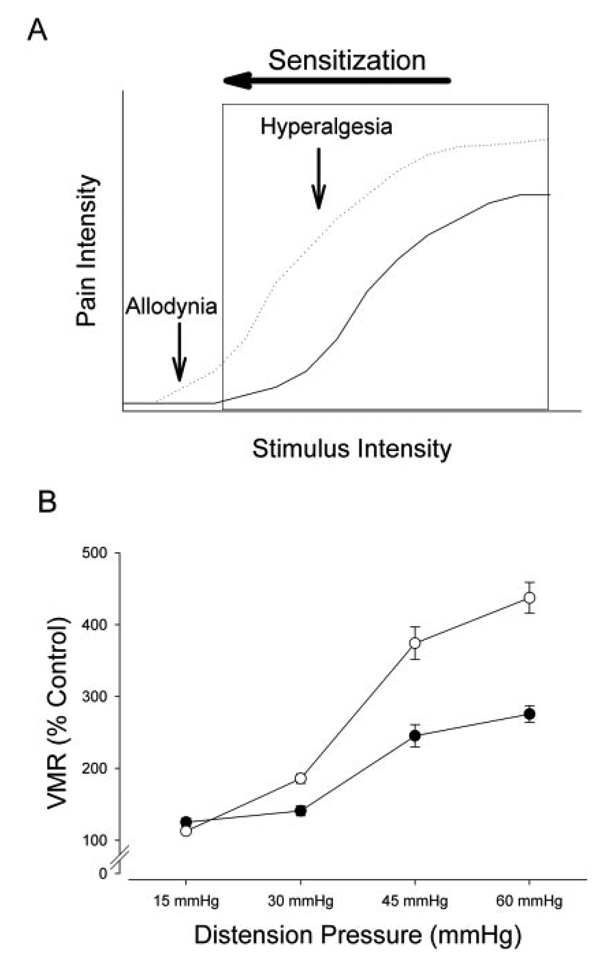
FIGURE 3.
Colitis causes visceral hypersensitivity. Potentially noxious stimuli (eg, colorectal distension) trigger responses that encode stimulus intensity, as shown in panel A. Once a threshold is passed, pain is perceived as indicated by the box. In many disorders associated with pain the stimulus response relationship is shifted to the left (sensitization), as indicated by the dotted line. Previously innocuous stimuli cause pain (allodynia) and the perceived pain intensity of noxious stimuli is increase (hyperalgesia). (B) An example of sensitization due to experimental colitis in mice. Animals were tested at baseline and 3 days after induction of colitis. Responses to colorectal distension were assessed using electromyographic recordings of the abdominal wall muscles (VMR: visceromotor response). Filled circles indicate baseline conditions, open circles represent findings on day 3 after induction of colitis.
Experimental Data
Several rodent models of intestinal inflammation have been established to identify possible mechanisms that contribute to the development of chronic IBD. As pain cannot be directly assessed in these animal models, investigators typically rely on surrogate measures, such as reflexes or behavioral responses that are triggered by high-intensity stimuli. Under control conditions, distension pressures need to exceed 20 mmHg before one can observe consistent increases in such nocifensive responses. However, colonic inflammation shifts the normal stimulus–response relationship, leading to such reflex or behavioral responses during low-intensity stimulation as well as raising the magnitude of responses over the entire range of stimulus intensities (Fig. 3B).12–14
Complex behavioral or reflex responses cannot identify the role of peripheral sensitization because the experimental readout is a combination of changes in both the central nervous system (CNS) and peripheral sensory neurons. Two different approaches have been used to more clearly define changes in primary sensory neurons: 1) single-fiber recordings from the isolated and dissected visceral nerves, and 2) direct recordings from the cell body of dorsal root ganglion (DRG) neurons projecting to the gastrointestinal tract. Both approaches independently show an increase in excitability with spontaneous activity at baseline and higher response frequency during stimulation that has been attributed to changes in the properties and expression of ion channels (see below).15–17 Inflammation also indirectly affects primary sensory neurons. Enteroendocrine cells and other epithelial cells within the mucosa can release mediators, such as serotonin or ATP, which increase during inflammation and also activate sensory neurons through serotonin and purinergic receptors, respectively.18–20 This combination of increased neuronal excitability and enhanced release of mucosal signals provides the basis of peripheral sensitization observed during visceral inflammation.
Considering concerns about chronic opioid therapy, animal models of colitis have been studied to develop alternative treatment options for patients with visceral pain. In addition to exhibiting responses to inflammation that are similar to those seen in humans, rodent models have allowed the identification of unique characteristics of populations of sensory neurons that innervate the gut. These features are being used to develop new drugs that can target those neurons responsible for chronic visceral pain in a manner that will not affect normal function. As a first step in identifying unique receptors/channels on visceral afferents, various laboratories have injected neuronal markers to label both afferents that project to various gastrointestinal organs.21–24 Interestingly, there are common features of sensory neurons innervating different organs including colon, stomach, and pancreas. All of these structures are densely innervated by sensory fibers that express calcitonin gene-related peptide (CGRP). This peptide, a potent vasodilator, is released by peripheral sensory nerve terminals in response to noxious and nonnoxious stimulation and can have effects on vasculature and other structures. This property highlights 1 of the underappreciated aspects of visceral (and most somatic) sensory neurons; ie, in addition to detecting internal (visceral) or external (environmental) stimuli, sensory neurons release a number of compounds including CGRP and substance P (SP) that can have peripheral motor and/or autocrine/paracrine effects on other cells or sensory neurons. Under normal conditions these compounds contribute to homeostatic regulation, but in disease states, including those with an inflammatory component, they may exacerbate symptoms (“neurogenic inflammation”). Dramatic demonstration of the importance of the sensory neurons in contributing to the onset of disease comes from mouse models of type I diabetes. In diabetes-prone NOD (nonobese diabetic) mice, elimination of a subset of sensory neurons blocks the development of inflammation and destruction of islet cells.25 The sensory neurons that were ablated in these studies express the vanilloid receptor, TRPV1. TRPV1 is a nonselective cation channel that has been shown to be required for inflammatory hyperalgesia in a number of organs including pancreas, skin, and colon.26–29 Most TRPV1-expressing neurons release SP and/or CGRP and it is thought that the release of these compounds may drive inflammatory responses. TRPV1 may play a central role in setting the overall sensitivity of colon afferents. In mice lacking TRPV1 gene expression, there is a significant reduction of colonic sensitivity to distension.26 In addition, the hypersensitivity that develops in response to inflammatory mediators in wild-type mice is absent in TRPV1-deficient mice. Moreover, pharmacologic inhibition of the TRPV1 blocks the development of hyperalgesia during colitis.26,30 Whereas TRPV1 has received considerable attention recently, no single channel is likely to be responsible for colonic hypersensitivity. Deletion of a member of acid sensitive ion channels (ASIC3) or another member of the TRP (transient receptor potential) family (TRPV4) blunt or completely block the development of hyperalgesia in response to colonic inflammation.26,31
We have identified some of the molecular mechanisms involved in peripheral sensitization. Yet the results described in the preceding sections also raise questions about signaling pathways leading to the functional changes in primary afferents neurons. Serotonin, prostaglandin, bradykinin, ATP, or other signaling molecules can be released during colonic inflammation or in response to injury and rapidly alter neuron properties. However, the changes are transient in nature and cannot sufficiently explain the lasting changes seen experimentally. Neurotrophic factors have emerged as potential mediators for these prolonged effects. Most of these molecules produce sensitization of sensory neurons that can convert nonnoxious stimuli into pain-producing stimuli, as discussed above. Among the most potent of these molecules are the growth factors in the glial cell-line-derived neurotrophic factor (GDNF) family and nerve growth factor (NGF). These growth factors act on sensory neurons by upregulating the expression of ion channels such as TRPV1 and/or altering the properties of these channels. For example, a single injection of NGF combined with artemin (a member of the GDNF family) can produce cutaneous hypersensitivity lasting for up to 6 days.32
NGF and members of the GDNF family have attracted significant attention because their high affinity receptors are found on sensory neurons that also express TRPV1, ASIC3, TRPA1 (a molecule related to TRPV1 and also found to be required for inflammatory hyperalgesia), and TRPV4, as well other molecules that are preferentially found in nociceptive neurons.33 Consistent with a role in the development of hypersensitivity, NGF levels increase during colitis and neutralizing NGF antibodies block the enhanced responses to colorectal distension during colitis.12,34,35
Taken together, experimental evidence certainly supports a role of inflammation in the pathogenesis of visceral pain. Drug companies are working on therapies for persistent pain that involve antibodies targeting neurotrophic factors, including NGF. Based on the success of treatments using humanized TNFα-antibodies in IBD patients, investigators are optimistic that a similar approach might work to block growth factors that contribute to hyperalgesia associated with IBD.
Clinical Data
Consistent with a potentially sensitizing effect of inflammation on afferent nerve function, small studies showed rectal hypersensitivity, with the severity of inflammation inversely correlating with pain thresholds.36,37 In view of the potential importance of NGF in the development of pain, investigators studied NGF levels in human diseases and showed increases several neoplastic and inflammatory disorders associated with pain, including IBD.38–40 While there is no direct and simple link between NGF increases and changes in function and/or structure of afferent neurons, chronic intestinal inflammation is also associated with an increase nerve fiber expressing the capsaicin receptor TRPV1 and the purinergic P2X3 receptor measured in mucosal biopsies.41–43 Thus, the data fit into the general framework defined by the more detailed experiments with animal models of human disease.
Several small studies and case series have demonstrated autonomic or sensory neuropathies in IBD patients.44–47 Iatrogenic causes, such as metronidazole or thalidomide, or micronutrient deficiencies are certainly important in the pathogenesis of such neuropathies. However, causal factors cannot be identified in some patients, suggesting that demyelinating or small fiber neuropathy may be a rare extraintestinal manifestation of IBD. Considering the small and often retrospective studies, the association between neuropathy and pain has not been clearly established.
Clinical Implications
While IBD is a biologically defined illness, pain in IBD patients is mediated by more than the biologically defined abnormalities. Thus, pain is an alarm symptom that should trigger appropriate evaluations if newly developed or significantly changing in intensity or character. Yet pain may become a continuing problem even if the disease is controlled. There is no question that peripheral sensitization plays a major role in pain and discomfort during acute flares. Under such circumstances, treatment should target the underlying inflammation rather than sensory neurons that convey alarm signals to the CNS. However, sensory changes may persist beyond the acute event. In patients with irritable bowel syndrome (IBS), an acute and self-limited infection can be the initial trigger, leading to the manifestation of the functional disorder.48 This relationship between inflammation and chronic pain development may also explain persistent symptoms of IBD patients in clinical remission. So, can we decrease the excitability of primary afferent neurons? Despite the potential contribution of peripheral sensitization, only 2 treatment strategies have emerged that target visceral afferent pathways. Rectal instillation of lidocaine enemas transiently alleviated pain in small studies with IBS patients.49,50 While not specific to visceral afferents and certainly not convenient, this strategy may be beneficial and acceptable in a small subgroup of patients with significant and temporarily limited symptoms, such as rectal pain, tenesmus, or urgency. The second strategy relies on peripherally acting μ-opioid agonists. Kappa opioid receptors are expressed on primary visceral afferent neurons and exert an analgesic effect in animal models of visceral pain without causing constipation or other adverse effects on the gastrointestinal tract that are typically mediated by activation of μ-opioid receptors.51,52 A recent multicenter trial in IBS patients suggests a potential benefit of asimadoline.53 However, the benefit was only seen in a post-hoc subgroup analysis of patients with diarrhea and moderate pain. As the findings also contradict results from an earlier trial,54 additional investigations are needed to determine the true potential of peripherally acting μ-opioid agonists as analgesic agents for patients with acute or chronic gastrointestinal pain.
CENTRAL MECHANISMS OF VISCERAL PAIN
In the preceding section we discussed the effects of acute or chronic inflammation, such as colitis, on visceral sensory neurons projecting to the CNS. However, these peripheral mechanisms alone are not sufficient to explain the differential manifestation of pain in IBD patients. Subjective symptoms poorly correlate with biological markers of disease activity, suggesting that factors other than inflammation and sensitization of afferent pathways contribute to the clinical manifestation of the disorder.55 As peripheral sensitization increases the excitability of primary afferents, we can certainly expect secondary changes in higher-order sensory neurons in the spinal cord or brain. The brain as the ultimate source of perception not only receives sensory input, it can also modulate input and alter visceral function, thus creating a complex interrelationship between gastrointestinal function, sensory input, perception, cognitive, and emotional processing.
Experimental Data
A mild and transient form of acute colitis can trigger robust visceral hypersensitivity, even after the initially increased excitability of primary colorectal afferents has normalized. 56 Thus, visceral sensory processing must have changed with these changes, outlasting the altered properties of primary afferent neurons. Indeed, the temporal and spatial summation of increased input may activate N-methyl-D-as-partate (NMDA) receptors on second- or higher-order sensory neurons within the CNS. Activation of NMDA receptors allows calcium influx, which in turn functions as a second messenger within cells and can lead to lasting increases in excitability through phosphorylation of ion channels or changes in gene transcription.57
Pain referral to distant cutaneous areas is 1 of the hallmarks of visceral pain. Convergent input of cutaneous and visceral input onto a single second-order neuron provides the anatomical basis for this pain referral. Inflammation, such as colitis, will sensitize primary colonic afferents. The resulting increase in visceral input will enhance the excitability of secondary sensory neurons within the spinal cord. As these second-order neurons receive convergent input from other sites, such as skin, input from those sites may also be processed differently, resulting in secondary hypersensitivity within the referral area and/or wider areas of pain referral (Fig. 4).13,58 Such cross-sensitization can be blocked by NMDA antagonists, supporting the importance of NMDA receptors in central sensitization.59,60
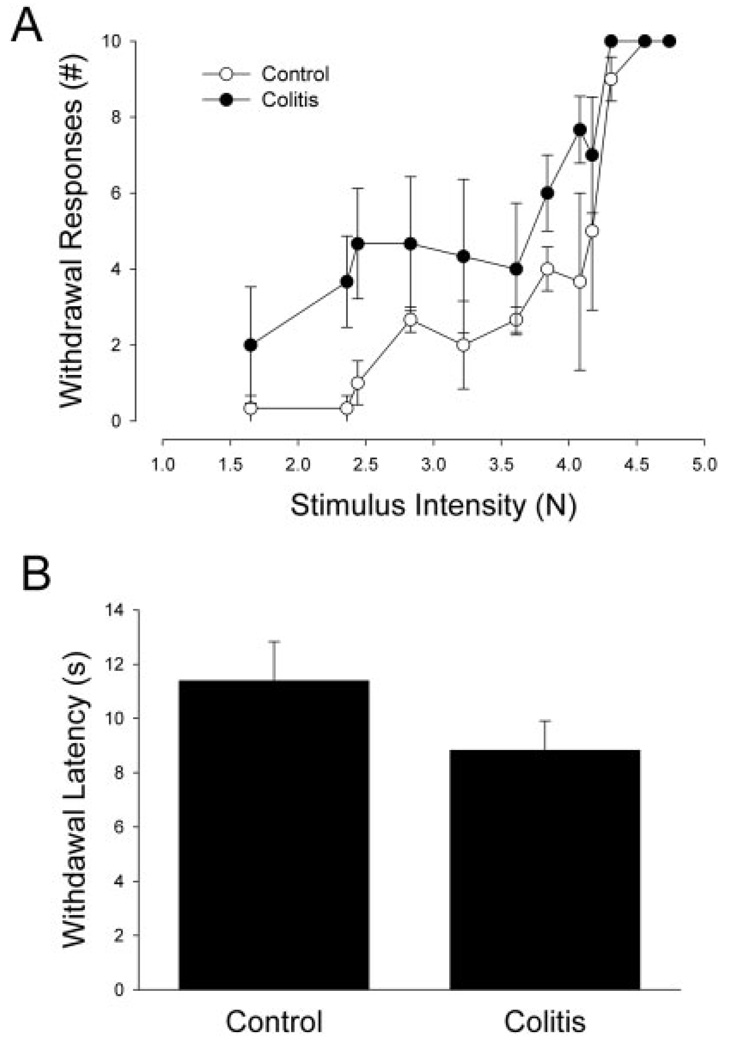
FIGURE 4.
Acute experimental colitis causes hypersensitivity of the left foot tested with small filaments (mechanical hypersensitivity; A) or a radiant heat source (thermal hypersensitivity; B).
As already indicated, the CNS not only receives and processes peripheral input, it can also modulate afferent information through descending pathways. Neurons within brainstem nuclei (rostral ventromedial medulla and periaqueductal gray) project through the bulbospinal tract to spinal afferents and can facilitate or inhibit reactions to noxious visceral stimuli, such as colorectal distension.61 These brainstem regions are also connected to the locus coeruleus, linking them to stress and arousal.62 Evolutionarily, such a link makes sense, as it provides a structural basis for a decrease in pain perception during acute stress, such as “fight or flight” reactions. Because of its potential importance as a filter for sensory, especially noxious input, this system has been labeled descending noxious inhibitory control (DNIC). Interestingly, current data suggest a differential activation of these modulating filters during acute and chronic (“unphysiologic”) stress, with acute stress blunting but chronic stress enhancing responses to noxious stimuli.62 Experimental evidence certainly supports a role of stressors in the development of visceral hyperalgesia.63–65 Stress typically activates the hypothalamic–pituitary–adrenal axis, triggering increases in corticosteroids and activation of the sympathetic nervous system. Stereotactic injection of corticosteroids into the amygdala increased anxiety and results in visceral hypersensitivity in rats, which was associated with decreased activation of descending inhibitory pathways.66,67 The role of stress in inflammatory disorders is more complex, as chronic stress can also modulate intestinal inflammation, which in turn may sensitize primary afferent neurons and further alter sensory input.68–70
Clinical Data
Considering the complexity of cognitive and emotional pain processing, animal experiments can certainly only provide limited insight into central responses to visceral pain in humans. Despite this conceptual shortcoming, investigations performed in healthy human volunteers and IBD patients showed findings consistent with data obtained in animal studies. As described above, temporal and/or spatial summation can alter spinal processing of visceral input. To investigate the effect of temporal summation, Ness et al71 performed repetitive colorectal distensions in human volunteers, which caused a progressive increase in the area of referred pain and in the subjective pain ratings. A small study of patients with CD without active colonic disease showed an increased viscerosomatic referral during rectal distension compared to controls comparable to results seen in IBS patients.72 However, pain thresholds were higher than those of IBS patients. As viscerosomatic pain referral is due to convergent input onto spinal neurons, these provide evidence of central sensitization at the level of the spinal cord.
Descending inhibitory control mechanisms have been studied in patients with different chronic pain syndromes. Most investigations demonstrated impaired activation of inhibitory circuits in patients compared to healthy controls, which may contribute to the pathogenesis of chronic pain and also explain the frequent coexistence of different pain syndromes affecting more than a single area of the body in a single patient.72–78 IBD may cause extraintestinal manifestations associated with pain, complicating systematic studies to define the coexistence of multiple pain syndromes in these patients as a potential marker of impaired modulation of nociceptive input. The current evidence remains inconclusive, with only some studies suggesting an increased incidence of fibromyalgia or noninflammatory joint pain.79–81 Extensive psychophysical characterizations of colorectal sensation that even included functional brain imaging suggest a more effective activation of descending inhibitory control mechanisms in IBD patients with quiescent disease, as patients showed higher discomfort thresholds in response to rectal distension compared to patients with IBS, a finding that correlated with stronger activation of medullary structures. 37,82 However, studies were performed in small groups, with selection bias potentially skewing results. The reason for this note of caution lies in several studies suggesting a high prevalence of psychological problems in IBD patients, with emotion potentially linking pain and IBD. Current evidence indicates that emotional problems contribute to the development of chronic pain syndromes.
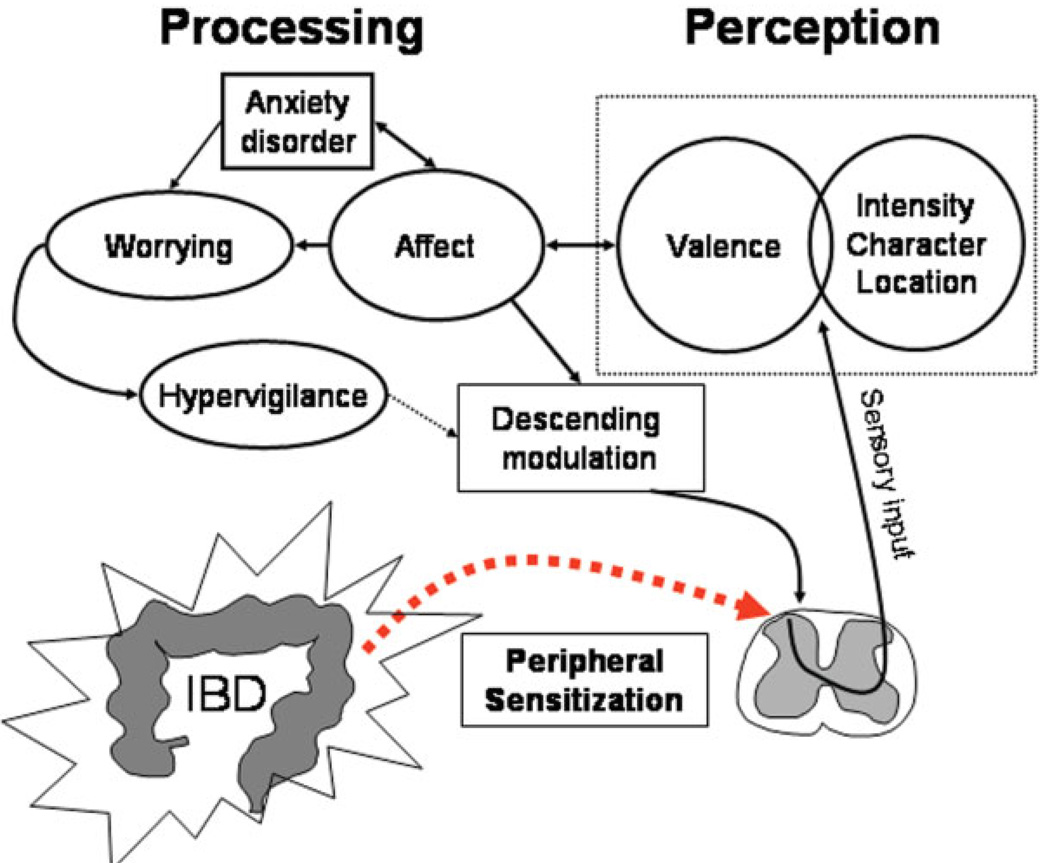
Two distinct abnormalities underlie increased pain in response to visceral stimuli: hypersensitivity with lowered thresholds during experimental stimulation and hypervigilance with increased focus on and more negative rating of visceral sensory input.83 Affect and hypervigilance relate to each other in a complex fashion, as pain triggers emotional responses and cognitive processing of pain potentially increases anxiety, leading to worries and further enhancement of vigilance.84 Consistent with the importance of emotional processing, anxiety is a predictor for the development of IBS in individuals suffering from an acute gastrointestinal infection. 85 These findings may also be relevant in IBD patients, many of whom suffer from psychiatric problems.86–91 Similar to the role of anxiety in the development of IBS, mood disorders are associated with persistent symptoms in patients who are in clinical remission of their underlying IBD.88,92–94 In a large epidemiologic study, the presence of pain was significantly associated with depression.91 Thus, pain and other symptoms in IBD patients are initiated by sensitization of afferent pathways due to the underlying inflammation. The associated affective response and coexisting emotional problems, most notably anxiety, influence arousal and cognitive processing, which, in turn, modulate descending inhibitory pathways, thereby potentially further enhancing sensory input (Fig. 5).
FIGURE 5.
Conceptual model of pain in IBD patients. The inflammatory process sensitizes visceral afferent neurons, leading to increased central input and perception. The affective dimension of pain (valence) triggers emotional responses, which can result in enhanced worrying and hypervigilance, impairing descending inhibitory control mechanisms.
Stress may be an important link between inflammation, emotion, and pain. Several studies reported an increased stress exposure or a perception of increased stress in IBD patients, leading to the question of whether stress may contribute to the manifestation or exacerbations of the disease. 86,87,95 Such a causal role is theoretically supported by stress-induced changes in inflammatory activity.96 While the impact of stress on disease activity is controversial, many studies support a role of stress on symptoms. Subjective distress tolerance and perceived stress predict the development of IBS symptoms after acute, but self-limited gastrointestinal inflammation.97,98 Once manifest, increased stress carries a negative prognostic value for patients with functional gastrointestinal disorders.99 Experimental stressors acutely lower rectal pain thresholds in IBS patients but not controls, which is likely due to impaired activation of descending pain-modulating pathways.100,101 Such a relationship has not been examined in IBD patients. The high prevalence of stress suggests that similar mechanisms may be responsible for the development of chronic pain or other persistent symptoms in IBD patients. However, the interaction between stress and disease-related symptoms is reciprocal rather than causal, as the experience of a chronic disease and its associated symptoms itself constitute stress.
Clinical Implications
CNS processing highlights the importance of psychosocial contributions in the development of chronic pain, even if the underlying disease is biologically defined. Considering the important interaction between affect and pain, antidepressants have been tried extensively in many disorders associated with chronic pain. Current evidence supports the use of tricyclic antidepressants and agents affecting serotonin and norepinephrine reuptake, while the more specific serotonin reuptake inhibitors seem to be less effective.102 Antidepressant drugs are commonly prescribed in patients with functional bowel disorders. Despite their frequent use, published results are inconclusive, with many underpowered studies, lack of appropriate control groups, and different outcome measures.103–106 A post-hoc “per protocol” analysis in 1 of the largest trials took into account actual use rather than treatment assignment to desipramine, showing a potential benefit of the tricyclic.106,107 While up to 20% of IBD patients are treated for depression, no systematic study has addressed the impact of such treatments on gastrointestinal symptoms. 108,109
Several studies have clearly demonstrated that psychological treatments are very effective in treating individuals with functional gastrointestinal disorders.110–115 Based on the high prevalence of emotional disorders in IBD patients, such treatments have also been examined in patients with CD and ulcerative colitis (UC). Supportive individual or group psychotherapy is not effective.116–118 Initial investigations have shown promising results with cognitive behavioral therapy in treating depression and abdominal pain in children and adolescents suffering from CD and UC, making it a potentially attractive alternative approach for pain management in adult IBD patients.119–121 Similarly, hypnosis, which is effective in decreasing anxiety and pain across a variety of illnesses, has shown some benefit for IBD patients.122–124
CONCLUSION
Medical treatment of CD and UC has greatly improved over the past several years and will be effective in the majority of patients. However, for a subgroup of patients, symptoms of abdominal pain often persist after improvement or even resolution of the mucosal inflammation and continue to impair the quality of life. Pain and discomfort are among the most significant problems for the often young patients who suffer from these chronic illnesses. While more mechanistic insight and systematic and appropriately powered studies are needed, current evidence supports a complex interaction between the sensitizing effects of inflammation on afferent pathways and the emotional and cognitive processing of the increased visceral input. This model requires a more comprehensive approach to the “palliative”—meaning symptom-oriented—treatment of IBD patients. Such a comprehensive strategy may include gut-specific analgesics that are currently under development, having shown initial benefit in early trials. Perhaps more important, the psychological burden of the disease needs to be recognized and addressed more effectively, as emotional factors significantly contribute to the pathogenesis of chronic pain and other symptoms. Considering the importance of central processing, specifically designed psychological interventions have also shown promise. While time- and labor-intensive, recent studies focusing on ways to decrease the associated costs may facilitate the more widespread use of such therapies.125
REFERENCES
- 1.Aghazadeh R, Zali MR, Bahari A, et al. Inflammatory bowel disease in Iran: a review of 457 cases. J Gastroenterol Hepatol. 2005;20:1691–1695. doi: 10.1111/j.1440-1746.2005.03905.x. [DOI] [PubMed] [Google Scholar]
- 2.Wagtmans MJ, Verspaget HW, Lamers CB, et al. Crohn’s disease in the elderly: a comparison with young adults. J Clin Gastroenterol. 1998;27:129–133. doi: 10.1097/00004836-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Cross RK, Wilson KT, Binion DG. Narcotic use in patients with Crohn’s disease. Am J Gastroenterol. 2005;100:2225–2229. doi: 10.1111/j.1572-0241.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 4.Edwards JT, Radford-Smith GL, Florin TH. Chronic narcotic use in inflammatory bowel disease patients: prevalence and clinical characteristics. J Gastroenterol Hepatol. 2001;16:1235–1238. doi: 10.1046/j.1440-1746.2001.02468.x. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–630. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Strigo IA, Bushnell MC, Boivin M, et al. Psychophysical analysis of visceral and cutaneous pain in human subjects. Pain. 2002;97:235–246. doi: 10.1016/S0304-3959(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 7.Lix LM, Graff LA, Walker JR, et al. Longitudinal study of quality of life and psychological functioning for active, fluctuating, and inactive disease patterns in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1575–1584. doi: 10.1002/ibd.20511. [DOI] [PubMed] [Google Scholar]
- 8.Matuk R, Crawford J, Abreu MT, et al. The spectrum of gastrointestinal toxicity and effect on disease activity of selective cyclooxygenase-2 inhibitors in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:352–356. doi: 10.1097/00054725-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi K, Smale S, Premchand P, et al. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:196–202. doi: 10.1016/s1542-3565(05)00980-8. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan MA, Korelitz BI. Narcotic dependence in inflammatory bowel disease. J Clin Gastroenterol. 1988;10:275–278. doi: 10.1097/00004836-198806000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Bielefeldt K, Christianson JA, Davis BM. Basic and clinical aspects of visceral sensation: transmission to the CNS. Neurogastroenterol Motil. 2005;17:488–499. doi: 10.1111/j.1365-2982.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- 12.Delafoy L, Gelot A, Ardid D, et al. Interactive involvement of brain derived neurotrophic factor, nerve growth factor, and calcitonin gene related peptide in colonic hypersensitivity in the rat. Gut. 2006;55:940–945. doi: 10.1136/gut.2005.064063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamb K, Zhong F, Gebhart GF, et al. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G451–G457. doi: 10.1152/ajpgi.00353.2005. [DOI] [PubMed] [Google Scholar]
- 14.Eijkelkamp N, Kavelaars A, Elsenbruch S, et al. Increased visceral sensitivity to capsaicin after DSS-induced colitis in mice: spinal cord c-fos expression and behavior. Am J Physiol Gastrointest Liver Physiol. 2007;293:G749–G757. doi: 10.1152/ajpgi.00114.2007. [DOI] [PubMed] [Google Scholar]
- 15.Beyak MJ, Ramji N, Krol KM, et al. Two TTX-resistant NA+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol. 2004;287:G845–G855. doi: 10.1152/ajpgi.00154.2004. [DOI] [PubMed] [Google Scholar]
- 16.Stewart TM, Beyak MJ, Vanner SJ. Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia neurons. J Physiol (Lond) 2003;552.3:797–807. doi: 10.1113/jphysiol.2003.046409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coldwell JR, Phillis BD, Sutherland K, et al. Increased responsiveness of rat colonic splanchnic afferents to 5-HT after inflammation and recovery. J Physiol (Lond) 2007;579:203–213. doi: 10.1113/jphysiol.2006.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wynn G, Ma B, Ruan HZ, et al. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G647–G657. doi: 10.1152/ajpgi.00020.2004. [DOI] [PubMed] [Google Scholar]
- 19.Linden DR, Chen J-X, Gershon MD, et al. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- 20.O’Hara JR, Ho W, Linden DR, et al. Enteroendocrine cells and 5-HT availability are altered in mucosa of guinea pigs with TNBS ileitis. Am J Physiol. 2004;287:G998–G1007. doi: 10.1152/ajpgi.00090.2004. [DOI] [PubMed] [Google Scholar]
- 21.Zhong F, Christianson JA, Davis BM, et al. Dichotomizing axons in spinal and vagal afferents of the mouse stomach. Dig Dis Sci. 2008;53:194–203. doi: 10.1007/s10620-007-9843-z. [DOI] [PubMed] [Google Scholar]
- 22.Robinson DR, Mcnaughton PA, Evans ML, et al. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 23.Christianson JA, Traub RJ, Davis BM. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. J Comp Neurol. 2006;494:246–259. doi: 10.1002/cne.20816. [DOI] [PubMed] [Google Scholar]
- 24.Fasanella KE, Christianson JA, Chanthaphavong RS, et al. Distribution and neurochemical identification of pancreatic afferents in the mouse. J Comp Neurol. 2008;509:42–52. doi: 10.1002/cne.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razavi R, Chan Y, Afifiyan FN, et al. Trpv1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 26.Jones RCW, III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karai L, Brown DC, Mannes AJ, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–1352. doi: 10.1172/JCI20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petho G, Izydorczyk I, Reeh PW. Effects of TRPV1 receptor antagonists on stimulated ICGRP release from isolated skin of rats and TRPV1 mutant mice. Pain. 2004;109:284–290. doi: 10.1016/j.pain.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 29.Wick EC, Hoge SG, Grahn SW, et al. Transient receptor potential vanilloid 1, calcitonin gene-related peptide, and substance P mediate nociception in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G959–G969. doi: 10.1152/ajpgi.00154.2005. [DOI] [PubMed] [Google Scholar]
- 30.Miranda A, Nordstrom E, Mannem A, et al. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience. 2007;148:1021–1032. doi: 10.1016/j.neuroscience.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sipe WEB, Brierley SM, Martin CM, et al. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1288–G1298. doi: 10.1152/ajpgi.00002.2008. [DOI] [PubMed] [Google Scholar]
- 32.Malin SA, Molliver DC, Koerber HR, et al. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26:8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 34.Barada KA, Mourad FH, Sawah SI, et al. Up-regulation of nerve growth factor and interleukin-10 in inflamed and non-inflamed intestinal segments in rats with experimental colitis. Cytokine. 2007;37:236–245. doi: 10.1016/j.cyto.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Delafoy L, Raymond F, Doherty AM, et al. Role of nerve growth factor in the trinitrobenzene sulfonic acid-induced colonic hypersensitivity. Pain. 2003;105:489–497. doi: 10.1016/S0304-3959(03)00266-5. [DOI] [PubMed] [Google Scholar]
- 36.Drewes AM, Frokjaer JB, Larsen E, et al. Pain and mechanical properties of the rectum in patients with active ulcerative colitis. Inflamm Bowel Dis. 2006;12:294–303. doi: 10.1097/01.MIB.0000209365.09189.04. [DOI] [PubMed] [Google Scholar]
- 37.Chang L, Munakata J, Mayer EA, et al. Perceptual responses in patients with inflammatory and functional bowel disease. Gut. 2000;47:497–505. doi: 10.1136/gut.47.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Mola FF, Friess H, Zhu ZW, et al. Nerve growth factor and TRK high affinity receptor (TRKA) gene expression in inflammatory bowel disease. Gut. 2000;46:670–679. doi: 10.1136/gut.46.5.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friess H, Zhu ZW, Di Mola FF, et al. Nerve growth factor and its high-affinity receptor in chronic pancreatitis. Ann Surg. 1999;230:615–624. doi: 10.1097/00000658-199911000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider MB, Standop J, Ulrich A, et al. Expression of nerve growth factors in pancreatic neural tissue and pancreatic cancer. J Histochem Cytochem. 2001;49:1205–1210. doi: 10.1177/002215540104901002. [DOI] [PubMed] [Google Scholar]
- 41.Yiangou Y, Facer P, Baecker PA, et al. Atp-gated ion channel p2×3 is increased in human inflammatory bowel disease. Neurogastroenterol Motil. 2001;13:365–369. doi: 10.1046/j.1365-2982.2001.00276.x. [DOI] [PubMed] [Google Scholar]
- 42.Yiangou Y, Facer P, Dyer NHC, et al. Vanilloid receptor 1 immuno-reactivity in inflamed human bowel. Lancet. 2001;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- 43.Yiangou Y, Facer P, Smith JAM, et al. Increased acid-sensing ion channel asic-3 in inflamed human intestine. Eur J Gastroenterol Hepatol. 2001;13:891–896. doi: 10.1097/00042737-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Gondim FA, Brannagan TH, Sander HW, et al. Peripheral neuropathy in patients with inflammatory bowel disease. Brain. 2005;128:867–879. doi: 10.1093/brain/awh429. [DOI] [PubMed] [Google Scholar]
- 45.Nemni R, Fazio R, Corbo M, et al. Peripheral neuropathy associated with Crohn’s disease. Neurology. 1987;37:1414–1417. doi: 10.1212/wnl.37.8.1414. [DOI] [PubMed] [Google Scholar]
- 46.Ohlsson B, Sundkvist G, Lindgren S. Subclinical sympathetic neuropathy appears early in the course of Crohn’s disease. BMC Gastroenterol. 2007;7:33. doi: 10.1186/1471-230X-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Straub RH, Antoniou E, Zeuner M, et al. Association of autonomic nervous hyperreflexia and systemic inflammation in patients with Crohn’s disease and ulcerative colitis. J Neuroimmunol. 1997;80:149–157. doi: 10.1016/s0165-5728(97)00150-1. [DOI] [PubMed] [Google Scholar]
- 48.Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662–1671. doi: 10.1016/s0016-5085(03)00324-x. [DOI] [PubMed] [Google Scholar]
- 49.Verne GN, Robinson ME, Vase L, et al. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105:223–230. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 50.Verne GN, Sen A, Price DD. Intrarectal lidocaine is an effective treatment for abdominal pain associated with diarrhea-predominant irritable bowel syndrome. J Pain. 2005;6:493–496. doi: 10.1016/j.jpain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Joshi SK, Su X, Porreca F, et al. Kappa-opioid receptor agonists modulate visceral nociception at a novel, peripheral site of action. J Neurosci. 2000;20:5874–5879. doi: 10.1523/JNEUROSCI.20-15-05874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sengupta JN, Su X, Gebhart GF. Kappa, but not mu or delta, opioids attenuate responses to distention of afferent fibers innervating the rat colon. Gastroenterology. 1996;111:968–980. doi: 10.1016/s0016-5085(96)70064-1. [DOI] [PubMed] [Google Scholar]
- 53.Mangel AW, Bornstein JD, Hamm LR, et al. Clinical trial: Asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:239–249. doi: 10.1111/j.1365-2036.2008.03730.x. [DOI] [PubMed] [Google Scholar]
- 54.Szarka LA, Camilleri M, Burton D, et al. Efficacy of on-demand asimadoline, a peripheral kappa-opioid agonist, in females with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:1268–1275. doi: 10.1016/j.cgh.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henriksen M, Jahnsen J, Lygren I, et al. Ulcerative colitis and clinical course: Results of a 5-year population-based follow-up study (the Ibsen study) Inflamm Bowel Dis. 2006;12:543–550. doi: 10.1097/01.MIB.0000225339.91484.fc. [DOI] [PubMed] [Google Scholar]
- 56.Jones RCW, Otsuka E, Wagstrom E, et al. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distension in the mouse. Gastroenterology. 2007;133:184–194. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 57.Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol Pain. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 58.Ness TJ, Gebhart GF. Characterization of neuronal responses to noxious visceral and somatic stimuli in the medial lumbosacral spinal cord of the rat. J Neurophysiol. 1987;57:1867–1892. doi: 10.1152/jn.1987.57.6.1867. [DOI] [PubMed] [Google Scholar]
- 59.Miranda A, Peles S, Rudolph C, et al. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1189. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Peles S, Miranda A, Shaker R, et al. Acute nociceptive somatic stimulus sensitizes neurones in the spinal cord to colonic distension in the rat. J Physiol (Lond) 2004;560:291–302. doi: 10.1113/jphysiol.2004.069070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhuo M, Gebhart G. Facilitation and attenuation of a visceral nociceptive reflex from the rostroventral medulla in the rat. Gastroenterology. 2002;122:1007–1019. doi: 10.1053/gast.2002.32389. [DOI] [PubMed] [Google Scholar]
- 62.Imbe H, Murakami S, Okamoto K, et al. The effects of acute and chronic restraint stress on activation of ERK in the rostral ventromedial medulla and locus coeruleus. Pain. 2004;112:361–371. doi: 10.1016/j.pain.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 63.Coutinho SV, Plotsky PM, Sablad M, et al. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 64.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 65.Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–G53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 66.Greenwood-Van Meerveld B, Gibson M, Gunter W, et al. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res. 2001;893:135–142. doi: 10.1016/s0006-8993(00)03305-9. [DOI] [PubMed] [Google Scholar]
- 67.Qin C, Greenwood-Van Meerveld B, Myers DA, et al. Corticosterone acts directly at the amygdala to alter spinal neuronal activity in response to colorectal distension. J Neurophysiol. 2003;89:1343–1352. doi: 10.1152/jn.00834.2002. [DOI] [PubMed] [Google Scholar]
- 68.Veenema AH, Reber SO, Selch S, et al. Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology. 2008;149:2727–2736. doi: 10.1210/en.2007-1469. [DOI] [PubMed] [Google Scholar]
- 69.Collins S, Mchugh K, Jacobson K, et al. Previous inflammation alters the response of the rat colon to stress. Gastroenterology. 1996;111:1509–1515. doi: 10.1016/s0016-5085(96)70012-4. [DOI] [PubMed] [Google Scholar]
- 70.Collins SM. Stress and the gastrointestinal tract: IV. Modulation of intestinal inflammation by stress: basic mechanisms and clinical relevance. Am J Physiol Gastrointest Liver Physiol. 2001;280:G315–G318. doi: 10.1152/ajpgi.2001.280.3.G315. [DOI] [PubMed] [Google Scholar]
- 71.Ness TJ, Metcalf AM, Gebhart GF. A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain. 1990;43:377–386. doi: 10.1016/0304-3959(90)90035-C. [DOI] [PubMed] [Google Scholar]
- 72.Bernstein CN, Niazi N, Robert M, et al. Rectal afferent function in patients with inflammatory and functional intestinal disorders. Pain. 1996;66:151–161. doi: 10.1016/0304-3959(96)03062-x. [DOI] [PubMed] [Google Scholar]
- 73.Sandrini G, Serrao M, Rossi P, et al. The lower limb flexion reflex in humans. Prog Neurobiol. 2005;77:353–395. doi: 10.1016/j.pneurobio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 74.Dunckley P, Wise RG, Fairhurst M, et al. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005;25:7333–7341. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song GH, Venkatraman V, Ho KY, et al. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain. 2006;126:79–90. doi: 10.1016/j.pain.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 76.Wilder-Smith CH, Schindler D, Lovblad K, et al. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conboy LA, Wasserman RH, Jacobson EE, et al. Investigating placebo effects in irritable bowel syndrome: a novel research design. Contemp Clin Trials. 2006;27:123–134. doi: 10.1016/j.cct.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 78.Lembo T, Naliboff BD, Matin K, et al. Irritable bowel syndrome patients show altered sensitivity to exogenous opioids. Pain. 2000;87:137–147. doi: 10.1016/S0304-3959(00)00282-7. [DOI] [PubMed] [Google Scholar]
- 79.Buskila D, Odes LR, Neumann L, et al. Fibromyalgia in inflammatory bowel disease. J Rheumatol. 1999;26:1167–1171. [PubMed] [Google Scholar]
- 80.Palm O, Moum B, Jahnsen J, et al. Fibromyalgia and chronic widespread pain in patients with inflammatory bowel disease: a cross sectional population survey. J Rheumatol. 2001;28:590–594. [PubMed] [Google Scholar]
- 81.Palm O, Bernklev T, Moum B, et al. Non-inflammatory joint pain in patients with inflammatory bowel disease is prevalent and has a significant impact on health related quality of life. J Rheumatol. 2005;32:1755–1759. [PubMed] [Google Scholar]
- 82.Mayer EA, Berman S, Suyenobu B, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 83.Naliboff BD, Munakata J, Fullerton S, et al. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lackner JM, Quigley BM. Pain catastrophizing mediates the relationship between worry and pain suffering in patients with irritable bowel syndrome. Behav Res Ther. 2005;43:943–957. doi: 10.1016/j.brat.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 85.Moss-Morris R, Spence M. To “lump” or to “split” the functional somatic syndromes: can infectious and emotional risk factors differentiate between the onset of chronic fatigue syndrome and irritable bowel syndrome? Psychosom Med. 2006;68:463–469. doi: 10.1097/01.psy.0000221384.07521.05. [DOI] [PubMed] [Google Scholar]
- 86.Jones MP, Wessinger S, Crowell MD. Coping strategies and interpersonal support in patients with irritable bowel syndrome and inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:474–481. doi: 10.1016/j.cgh.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 87.Graff LA, Walker JR, Lix L, et al. The relationship of inflammatory bowel disease type and activity to psychological functioning and quality of life. Clin Gastroenterol Hepatol. 2006;4:1491–1501. doi: 10.1016/j.cgh.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 88.Farrokhyar F, Marshall JK, Easterbrook B, et al. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: prevalence and impact on health. Inflamm Bowel Dis. 2006;12:38–46. doi: 10.1097/01.mib.0000195391.49762.89. [DOI] [PubMed] [Google Scholar]
- 89.Guthrie E, Jackson J, Shaffer J, et al. Psychological disorder and severity of inflammatory bowel disease predict health-related quality of life in ulcerative colitis and Crohn’s disease. Am J Gastroenterol. 2002;97:1994–1999. doi: 10.1111/j.1572-0241.2002.05842.x. [DOI] [PubMed] [Google Scholar]
- 90.Kurina LM, Goldacre MJ, Yeates D, et al. Depression and anxiety in people with inflammatory bowel disease. J Epidemiol Community Health. 2001;55:716–720. doi: 10.1136/jech.55.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fuller-Thomson E, Sulman J. Depression and inflammatory bowel disease: Findings from two nationally representative Canadian surveys. Inflamm Bowel Dis. 2006;12:697–707. doi: 10.1097/00054725-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 92.Simren M, Axelsson J, Gillberg R, et al. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol. 2002;97:389–396. doi: 10.1111/j.1572-0241.2002.05475.x. [DOI] [PubMed] [Google Scholar]
- 93.Minderhoud IM, Oldenburg B, Wismeijer J, et al. IBS-like symptoms in patients with inflammatory bowel disease in remission; relationships with quality of life and coping behavior. Dig Dis Sci. 2004;49:469–474. doi: 10.1023/b:ddas.0000020506.84248.f9. [DOI] [PubMed] [Google Scholar]
- 94.Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. Gen Hosp Psychiatry. 1996;18:220–229. doi: 10.1016/0163-8343(96)00036-9. [DOI] [PubMed] [Google Scholar]
- 95.Maunder RG, Levenstein S. The role of stress in the development and clinical course of inflammatory bowel disease: epidemiological evidence. Curr Mol Med. 2008;8:247–252. doi: 10.2174/156652408784533832. [DOI] [PubMed] [Google Scholar]
- 96.Mawdsley JE, Rampton DS. Psychological stress in ibd: New insights into pathogenic and therapeutic implications. Gut. 2005;54:1481–1491. doi: 10.1136/gut.2005.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruigomez A, Garcia Rodriguez L, Panes J. Risk of irritable bowel syndrome after an episode of bacterial gastroenteritis in general practice: influence of comorbidities. Clin Gastroenterol Hepatol. 2007;5:465–469. doi: 10.1016/j.cgh.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 98.Nicholl BI, Halder SL, Macfarlane GJ, et al. Psychosocial risk markers for new onset irritable bowel syndrome—results of a large prospective population-based study. Pain. 2008;137:147–155. doi: 10.1016/j.pain.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bennett EJ, Tennant CC, Piesse C, et al. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut. 1998;43:256–261. doi: 10.1136/gut.43.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murray CDR, Flynn J, Ratcliffe L, et al. Effect of acute physical and psychological stress on gut autonomic innervation in irritable bowel syndrome. Gastroenterology. 2004;127:1695–1703. doi: 10.1053/j.gastro.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 101.Tracey I, Dunckley P. Importance of anti- and pro-nociceptive mechanisms in human disease. Gut. 2004;53:1553–1555. doi: 10.1136/gut.2004.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jann MW, Slade JH. Antidepressant agents for the treatment of chronic pain and depression. Pharmacotherapy. 2007;27:1571–1587. doi: 10.1592/phco.27.11.1571. [DOI] [PubMed] [Google Scholar]
- 103.Tabas G, Beaves M, Wang J, et al. Paroxetine to treat irritable bowel syndrome not responding to high-fiber diet: a double-blind, placebo-controlled trial. Am J Gastroenterol. 2004;99:914–920. doi: 10.1111/j.1572-0241.2004.04127.x. [DOI] [PubMed] [Google Scholar]
- 104.Kuiken SD, Tytgat GN, Boeckxstaens GE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: a double blind, randomized, placebo-controlled trial. Clin Gastroenterol Hepatol. 2003;1:219–228. doi: 10.1053/cgh.2003.50032. [DOI] [PubMed] [Google Scholar]
- 105.Tack J, Broekaert D, Fischler B, et al. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut. 2006;55:1095–1103. doi: 10.1136/gut.2005.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 107.Halpert A, Dalton CB, Diamant NE, et al. Clinical response to tricyclic antidepressants in functional bowel disorders is not related to dosage. Am J Gastroenterol. 2005;100:664–671. doi: 10.1111/j.1572-0241.2005.30375.x. [DOI] [PubMed] [Google Scholar]
- 108.Mikocka-Walus AA, Turnbull DA, Moulding NT, et al. “It doesn’t do any harm, but patients feel better”: a qualitative exploratory study on gastroenterologists’ perspectives on the role of antidepressants in inflammatory bowel disease. BMC Gastroenterol. 2007;7:38. doi: 10.1186/1471-230X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mikocka-Walus AA, Turnbull DA, Moulding NT, et al. Controversies surrounding the comorbidity of depression and anxiety in inflammatory bowel disease patients: a literature review. Inflamm Bowel Dis. 2007;13:225–234. doi: 10.1002/ibd.20062. [DOI] [PubMed] [Google Scholar]
- 110.Blanchard EB, Lackner JM, Sanders K, et al. A controlled evaluation of group cognitive therapy in the treatment of irritable bowel syndrome. Behav Res Ther. 2007;45:633–648. doi: 10.1016/j.brat.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 111.Lackner JM, Lou Coad M, Mertz HR, et al. Cognitive therapy for irritable bowel syndrome is associated with reduced limbic activity, GI symptoms, and anxiety. Behav Res Ther. 2006;44:621–638. doi: 10.1016/j.brat.2005.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lackner JM, Mesmer C, Morley S, et al. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. J Consult Clin Psychol. 2004;72:100–113. doi: 10.1037/0022-006X.72.6.1100. [DOI] [PubMed] [Google Scholar]
- 113.Gonsalkorale WM, Miller V, Afzal A, et al. Long term benefits of hypnotherapy for irritable bowel syndrome. Gut. 2003;52:1623–1629. doi: 10.1136/gut.52.11.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lea R, Houghton LA, Calvert EL, et al. Gut-focused hypnotherapy normalizes disordered rectal sensitivity in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:635–642. doi: 10.1046/j.1365-2036.2003.01486.x. [DOI] [PubMed] [Google Scholar]
- 115.Soo S, Forman D, Delaney BC, et al. A systematic review of psychological therapies for nonulcer dyspepsia. Am J Gastroenterol. 2004;99:1817–1822. doi: 10.1111/j.1572-0241.2004.30086.x. [DOI] [PubMed] [Google Scholar]
- 116.Maunder RG, Esplen MJ. Supportive-expressive group psychotherapy for persons with inflammatory bowel disease. Can J Psychiatry. 2001;46:622–626. doi: 10.1177/070674370104600706. [DOI] [PubMed] [Google Scholar]
- 117.Von Wietersheim J, Kessler H. Psychotherapy with chronic inflammatory bowel disease patients: a review. Inflamm Bowel Dis. 2006;12:1175–1184. doi: 10.1097/01.mib.0000236925.87502.e0. [DOI] [PubMed] [Google Scholar]
- 118.Jantschek G, Zeitz M, Pritsch M, et al. Effect of psychotherapy on the course of Crohn’s disease. Results of the German prospective multicenter psychotherapy treatment study on Crohn’s disease. German study group on psychosocial intervention in Crohn’s disease. Scand J Gastroenterol. 1998;33:1289–1296. doi: 10.1080/00365529850172386. [DOI] [PubMed] [Google Scholar]
- 119.Szigethy E, Carpenter J, Baum E, et al. Case study: longitudinal treatment of adolescents with depression and inflammatory bowel disease. J Am Acad Child Adolesc Psychiatry. 2006;45:396–400. doi: 10.1097/01.chi.0000198591.45949.a4. [DOI] [PubMed] [Google Scholar]
- 120.Szigethy E, Kenney E, Carpenter J, et al. Cognitive-behavioral therapy for adolescents with inflammatory bowel disease and subsyndromal depression. J Am Acad Child Adolesc Psychiatry. 2007;46:1290–1298. doi: 10.1097/chi.0b013e3180f6341f. [DOI] [PubMed] [Google Scholar]
- 121.Szigethy E, Whitton SW, Levy-Warren A, et al. Cognitive-behavioral therapy for depression in adolescents with inflammatory bowel disease: a pilot study. J Am Acad Child Adolesc Psychiatry. 2004;43:1469–1477. doi: 10.1097/01.chi.0000142284.10574.1f. [DOI] [PubMed] [Google Scholar]
- 122.Mawdsley JE, Jenkins DG, Macey MG, et al. The effect of hypnosis on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Am J Gastroenterol. 2008;103:1460–1469. doi: 10.1111/j.1572-0241.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 123.Keefer L, Keshavarzian A. Feasibility and acceptability of gut-directed hypnosis on inflammatory bowel disease: a brief communication. Int J Clin Exp Hypn. 2007;55:457–466. doi: 10.1080/00207140701506565. [DOI] [PubMed] [Google Scholar]
- 124.Miller V, Whorwell PJ. Treatment of inflammatory bowel disease: a role for hypnotherapy? Int J Clin Exp Hypn. 2008;56:306–317. doi: 10.1080/00207140802041884. [DOI] [PubMed] [Google Scholar]
- 125.Lackner JM, Jaccard J, Krasner SS, et al. Self-administered cognitive behavior therapy for moderate to severe irritable bowel syndrome: clinical efficacy, tolerability, feasibility. Clin Gastroenterol Hepatol. 2008;6:899–906. doi: 10.1016/j.cgh.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]