Abstract
The problem of immunotolerance to GM3, an important tumor-associated trisaccharide antigen, seriously hinders its usage in cancer vaccine development. To solve this problem, the keyhole limpet hemocyanin (KLH) conjugates of a series of GM3 derivatives were synthesized and screened as therapeutic cancer vaccines. First, the β-linked anomeric azides of differently N-acylated GM3 analogs were prepared by a highly convergent procedure. Next, a pentenoyl group was linked to the reducing end of the carbohydrate antigens following selective reduction of the azido group. The linker was thereafter ozonolyzed to give an aldehyde functionality permitting the conjugation of the antigens to KLH via reductive amination. Finally, the immunological properties of the resultant glycoconjugates were studied in C57BL/6 mice by assessing the titers of specific antibodies induced by the GM3 analogs. While KLH-GM3 elicited low levels of immune response, the KLH conjugates of N-propionyl, N-butanoyl, N-iso-butanoyl and N-phenylacetyl GM3’s induced robust immune reactions with antibodies of multiple isotypes, indicating significantly improved and T-cell dependent immune responses that lead to isotype switching, affinity maturation and the induction of immunological ‘memory’. It was suggested that GM3PhAc-KLH is a promising vaccine candidate for glycoengineered immunotherapy of cancer with GM3 as the primary target.
Keywords: carbohydrate, sialic acid, GM3, glycoconjugate, cancer vaccine
Introduction
It is well established that oncological transformations are accompanied by the change of cell surface glycosylation patterns.1,2 The abnormal glycans expressed on tumor cells are known as tumor-associated carbohydrate antigens (TACAs). Since the early 1970’s, many TACAs have been identified2–6 and become useful molecular templates and targets in the design and development of therapeutic cancer vaccines.4–10 Traditionally, cancer vaccines are created by coupling TACAs to a carrier protein, such as keyhole limpet hemocyanin (KLH). Resultant glycoconjugates can be employed to immunize cancer patients to fight tumors that bear the same antigens. This method has witnessed success with some TACAs, and a few cancer vaccines thus developed are now in clinical trials.11 However, a majority of TACAs are not functional due to the problem of immunotolerance, namely, the indifference of patients’ immune system to TACAs and their protein conjugates.
To overcome this problem, we have recently explored a new strategy12 that is based upon metabolic engineering of cell surface N-acetylneuraminic acid (Neu5Ac) which was pioneered by Bertozzi13–17 and Reutter.18,19 Neu5Ac is a member of natural sialic acids and is significantly overexpressed by many tumors.20 The underlining concept of our strategy is to first immunize cancer patients with a synthetic vaccine consisted of an artificial derivative or analog of a natural TACA that has its sialic acid residues chemically modified. After an immune response specific to the artificial antigen is established, the patients are treated with correspondingly modified mannosamine, which serves as a biosynthetic precursor of modified sialic acid, to initiate the expression of the artificial antigen in place of the natural TACA on tumors. The activated immune system will thereby eliminate the marked tumor cells. The concept is further supported by recent results from Jennings’ group.21
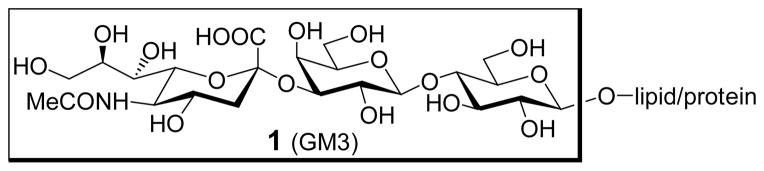
In principle, this strategy should be applicable to various TACAs, provided that two conditions are fulfilled. First, there must be an effective synthetic vaccine that can induce a specific immune response in cancer patients. Second, there must be a proper protocol that can be used to engineer cancer cells to express the same artificial TACA. This work is focused on the first issue, namely the identification of an artificial antigen for vaccine design, using GM3 (Figure 1), a sialylated TACA, as the target molecule.1,2
Figure 1.
The structure of GM3 antigen
GM3 is one of the most abundant TACAs on several types of tumors, such as malignant melanoma and neuroectodermal tumors.22 Consequently, an effective vaccine based on GM3 can be widely useful. Unfortunately, GM3 is poorly immunogenic, and attempts to break its immunotolerance by traditional techniques, such as linkage to KLH covalently, only achieved limited success.22,23 Even though the incorporation of GM3 into “very small size proteoliposomes” made of lipoproteic extracts from bacteria could improve the immunogenicity of GM3,24 the system is complex and difficult to control.
We anticipate that our strategy might circumvent the immunotolerance problem of GM3 and result in effective cancer immunotherapy based on GM3. Therefore, we have synthesized the natural GM3 and its artificial N-acyl derivatives, linked them to KLH, and studied the immunological properties of the resultant glycoconjugates.
Results and Discussion
Design of modified GM3 antigens and glycoconjugate vaccines
The major objectives of this work are to understand how chemical modifications of GM3 would affect its immunogenicity and to establish effective vaccines for cancer immunotherapy using GM3. In this context, we are especially interested in modifying the sialic acid residue of GM3, because previous studies suggest that chemically modified sialic acids and sialo carbohydrates are significantly more immunogenic than their natural counterparts.25–28 More importantly, Bertozzi,13–17 Reutter18,19 and recently Varki29 have demonstrated that a variety of cells, including tumor cells,12,21 can uptake unnatural N-acyl mannosamines to biosynthesize artificial sialic acids and sialoglycoconjugates and present them onto cell surfaces. Thus, metabolic engineering of GM3 on tumor cells may be conveniently achieved by incorporating unnatural sialic acids, which will meet the second requirement of our new immunotherapy. A caveat is that in the design of modified sialo antigens we need to bear in mind that the correspondingly N-modified mannosamines have to be available to the enzymes involved in the biosynthesis of sialic acid.
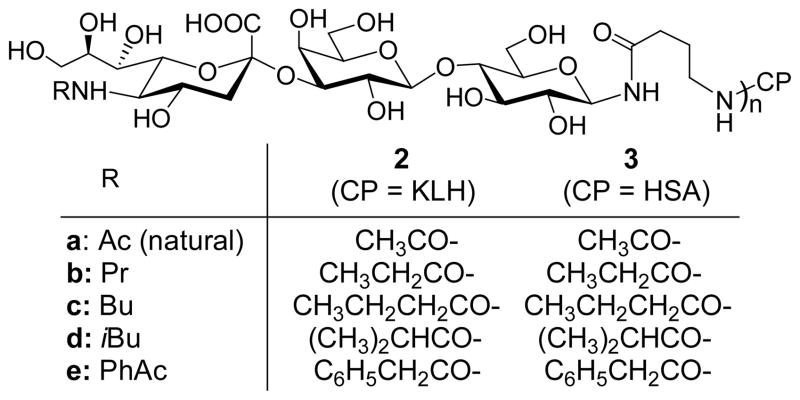
We have designed and studied four unnatural N-acyl derivatives of GM3 (Figure 2), including N-propionyl GM3 (GM3NPr, b), N-butanoyl GM3 (GM3NBu, c), N-iso-butanoyl GM3 (GM3NiBu, d) and N-phenylacetyl GM3 (GM3NPhAc, e), which comprise a range of structural variations. GM3NPr and natural GM3 have a small structural difference, as the propionyl group is only one carbon longer than an acetyl group. Nevertheless, a similar structural variation of polysialic acid could substantially improve the immunogenicity.25,26 Therefore, it is interesting to examine how a propionyl group would affect the immunological properties of GM3. Butanoyl, iso-butanoyl and phenylacetyl groups are structurally more different from an acetyl group, and we expect that their derivatives would be even more immunogenic. Additionally, all acyl groups in GM3NPr, GM3NBu, GM3NiBu and GM3NPhAc are more hydrophobic than the acetyl group, which we hope would further improve the immunogenicity. Our studies on sialic acid itself showed that hydrophobic substitutes could often promote immunogenicity.30
Figure 2.
Structures of N-modified GM3 and conjugate vaccines
Our plan was to couple the N-acyl derivatives of GM3 to a carrier protein to form glycoconjugate vaccines, which may provide T cell “help” for B cell responses.31 B cells that express membrane-bound antibodies specific for derivatized GM3 should exhibit antibody-enhanced uptake of the glycoconjugate, allowing enhanced B cell presentation of the carrier protein to T cells. This mechanism drives T cell responses that promote carbohydrate-specific B cells to proliferate, undergo isotype class switching and affinity maturation and differentiate into long-lived memory B cells. Thus, conjugation of carbohydrate antigens to carrier protein enhances not only the magnitude but also the quality of antibody responses.31
KLH, a well-established carrier protein for experimental cancer vaccines,6 was employed to form glycoconjugate vaccines 2a–2e (Figure 2), as KLH conjugates are often highly immunogenic and induce IgG antibody responses that are desirable for cancer immunotherapy. Alternatively, human serum album (HSA) conjugates 3a–3e were prepared as capture reagents for ELISA assays in immunological studies, which would avoid detection of antibodies specific for KLH.
A pentenoyl group was utilized as linker to conjugate GM3 antigens to proteins. We have recently demonstrated that as a linker pentenoyl group has some advantages.32 For instance, the linker and the antigens are coupled by a relatively stable amide bond through a selective reaction between pentenoyl anhydride and glycosyl amines. Moreover, since the coupling reaction can be achieved at the final stage of synthesis, protection tactics required for oligosaccharide preparation can be significantly simplified. Ozonolysis of the double bond of the pentenoyl group eventually gives an aldehyde functionality that facilitates the conjugation of GM3 antigens to proteins via reductive amination.
Consequently, glycoconjugates 2a–2e and 3a–3e were synthesized for studying the immunological properties of N-modified GM3 antigens.
Synthesis of N-acyl GM3 antigens and their protein conjugates
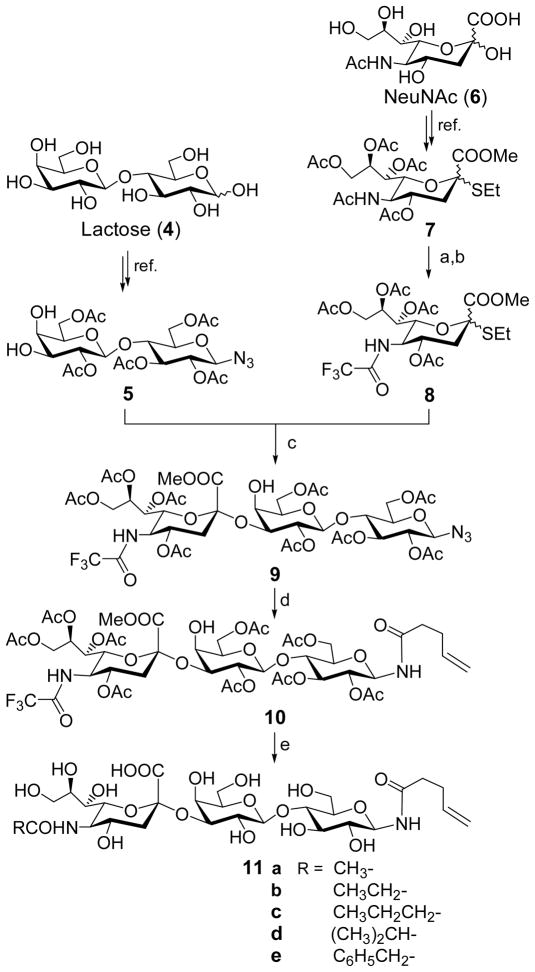
Despite the significant progress in carbohydrate chemistry in recent years, the chemical synthesis of complex oligosaccharides remains a significant undertaking. One of the major problems in the synthesis of GM3 and its derivatives is that the sialylation reactions employed to introduce the sialic acid residue usually give very poor yields and stereoselectivity.33–39 To address this problem we planned to examine a new strategy developed by Boons and coworkers,40,41 in which an N-trifluoroacetylneuraminic acid derivative was utilized as the glycosyl donor to afford significantly improved results. The use of trifluoroacetyl group as a protecting group of the amino group also has other advantages in our synthesis. For instance, a trifluoroacetamido group can be easily deprotected under mild conditions, which can simplify the design of protection tactics and the final stage modification of GM3.
Another important issue in the preparation of the protein conjugates of GM3 and its derivatives is that a pentenoyl group as the appropriate linker has to be attached to the GM3 antigens. For this purpose, we planned to have the reducing end of derivatized GM3’s capped by an azido group that can serve as a latent amino group. Thus, after the azido group is selectively reduced under mild conditions to give a primary amine, the pentenoyl group can be introduced to the GM3 reducing end even in the presence of free hydroxyl groups. The azido group can also serve as a protecting group, because an azido group is stable to most reactions involved in the oligosaccharide synthesis.
Meanwhile, since a trifluoroacetyl group can be selectively removed in the presence of a pentenoyl group, it will facilitate a procedure of introducing the linker at an early stage before the deprotection and acylation of the amino group on C-5. Deprotecting and modifying the sialic acid residue at the latest stage can lead to a highly convergent synthetic design as shown in Scheme 1.
Scheme 1.
Reagents and conditions: (a) MsOH (cat.), MeOH, reflux, 24 h; then CF3COOMe, Et3N, MeOH; (b) Ac2O, pyridine, 84% (two steps); (c) NIS, TfOH, MS 4Å, MeCN, ca. 63%; (d) Lindlar catalyst, H2, MeOH-EtOAc (1:1), 4-pentenoic anhydride, 6 h, 90%. (e) 0.5 N NaOH, 10 h; then various anhydride, rt, 83–94%.
The glycosyl acceptor 5 and donor 8 were prepared from lactose (4) and Neu5Ac (6) according to reported procedures.32,42 The azido group was attached to the glycosyl acceptor segment at an early stage. The thioglycoside of sialic acid (7) was smoothly converted into a trifluoroacetyl derivative 8 in 2 steps. The sialylation of 5 by 8 under the conventional conditions40,41 afforded trisaccharide 9 in a good yield (ca. 63%) and excellent stereoselectivity, but the purification process was very tedious due to the partial overlapping of 9 with the excessive substrate 5 on the column. Because we found that after introduction of the linker the product was more easily purified, we directly applied the partially purified 9 to the next step. The selective reduction of the azido group of 9 and acylation of the resultant amine were performed in one pot to afford the N-trifluoroacetyl GM3 conjugate 10 in an excellent yield (90%). The 1H NMR data of 10 (H-3e: δ 2.64) showed that the sialic acid residue was α-linked33–38,40,41 and the glycosylamino bond had a β-configuration (H-1: δ 4.90, J = 9.0 Hz). The α-configuration of sialic acid was further confirmed in the deprotected product 11 that showed a downfield shift of H-3e NMR signal (δ 2.63) compared to that of the β-isomer (δ < 2.40). It is worthy pointing out that this is the first synthesis of a glycosylazido derivative of GM3, although a number of procedures for GM3 and other derivatives have been described previously.33–39
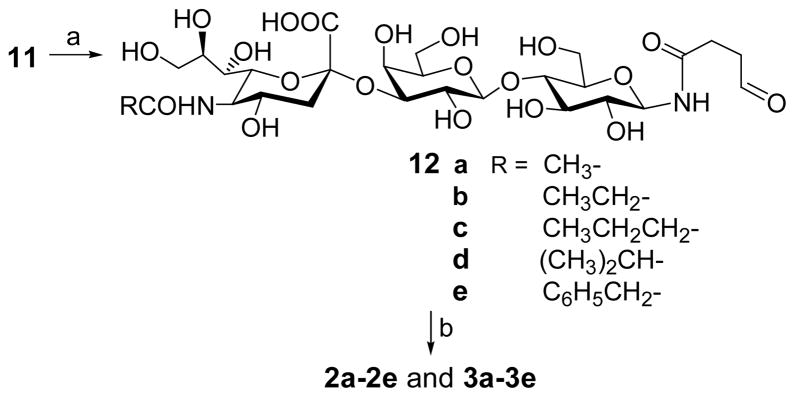
After 10 was obtained, its trifluoroacetyl group was removed with 0.5 N NaOH solution at room temperature. Under this condition, the hydroxyl and carboxyl groups were also deprotected, but the linker was unaffected. The resultant free amino group was then selectively acylated in methanol by acetic, propionic, butyric, iso-butyric and phenylacetic anhydrides respectively to afford the N-acyl derivatives of GM3 11a–11e. The products were purified on a Biogel P2 column, and their identities and purities were characterized with MS and high resolution NMR.
GM3 derivatives were conjugated to the carrier proteins through a two-step procedure (Scheme 2). First, each GM3 derivative was treated with ozone in methanol to selectively break the carbon-carbon double bond of the linker and give an aldehyde, as shown in 12a–12e. The products were purified on a Biogel P2 column, and their NMR spectra clearly showed a signal of the hydrated aldehyde at about 5.0 ppm and the disappearance of the double bond signals. The coupling of 12 to carrier proteins, including KLH and HSA, was accomplished by reductive amination carried out in a 0.1 M NaHCO3 buffer in the presence of NaBH3CN. The GM3 derivatives were employed in excess, and the glycoconjugates were readily separated from the unreacted GM3 derivatives on a Biogel A0.5 column. The product that gave positive results in both assays for sugar and protein respectively and was naturally the first component eluted out of the column was collected, dialyzed against distilled water, and finally freeze dried to afford the glycoconjugates as white fluffy solids.
Scheme 2.
Reagents and conditions: (a) O3, MeOH, −70 °C, 0.5 h; then Me2S, to rt, 2 h, 85–90%; (b) KLH or HSA, NaBH3CN, 0.1 N NaHCO3, 37 °C, 3d.
The sialic acid contents of the resultant glycoconjugates were analyzed by Svennerholm43 method and then converted into the levels of carbohydrate loading according the equation presented in the experimental section. In general, the coupling reactions were very efficient and gave glycoconjugates containing 11–18% (w/w) of carbohydrates (Table 1). These levels of antigen loading are at the ideal range for glycoconjugates being used as vaccines in immunological studies26 and as capture reagents in ELISA assays.
Table 1.
Carbohydrate loading of glycoconjugates
| sample | KLH conjugates
|
HSA conjugates
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2a (Ac) | 2b (Pr) | 2c (Bu) | 2d (iBu) | 2e (PhAc) | 3a (Ac) | 3b (Pr) | 3c (Bu) | 3d (iBu) | 3e (PhAc) | |
| loading (%) | 12.4 | 13.5 | 12.8 | 13.5 | 10.8 | 15.0 | 15.4 | 17.5 | 13.2 | 11.9 |
Immunological studies of protein conjugates of N-acyl GM3 antigens
The immunogenicity of the KLH conjugates of N-acyl GM3 derivatives was investigated in C57BL/6 mice, a well characterized inbred strain that is appropriate for further studies. As mentioned earlier, conjugating GM3 derivatives to a carrier protein, namely KLH herein, allows B cells to present GM3 derivative-specific immunogenic peptides, generated by proteolytic cleavage of the KLH molecule, to T cells, which should lead to a significantly improved immune response.31
The immunizations made use of Ribi adjuvant, an oil emulsion system that is approved for use in humans.44 At day 27 and day 35 following intraperitoneal immunizations (see Procedures), animal sera were withdrawn and pooled with respect to the individual group and date. We assessed the total antigen-specific antibody titers of the pooled sera by means of ELISA. Then, the titers of specific antibody isotypes, including IgM, IgG1, IgG2a and IgG3, were assessed. The isotypes of antibody have different capacities for inducing the effector function important to anti-tumor response, e.g. antibody dependant cell mediated cytotoxicity and the ability to fix complement on antibody bound membranes.6,45,46 The sera obtained at day 27 and day 35 gave similar results in terms of different GM3 antigens, but only the results of day 35 are shown and discussed here.
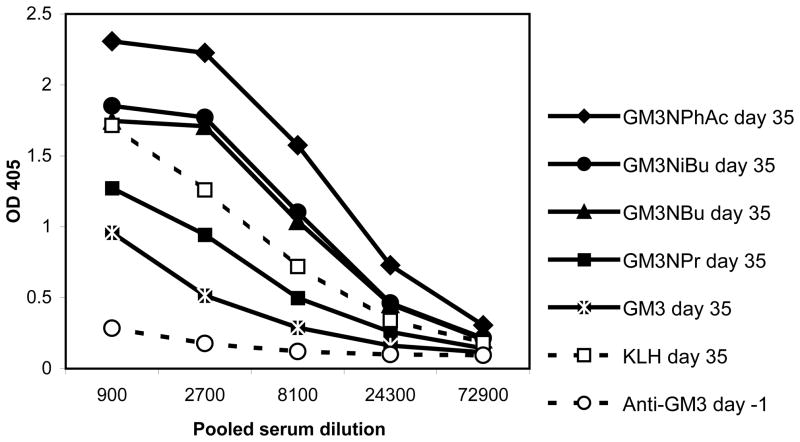
Figure 3 shows the total antibody titers with the readings of optical density (OD) plotting against pooled sera dilutions in an ELISA assay in which plate wells were coated with a derivatized GM3-HSA conjugate or KLH protein alone as the capture antigen. Higher OD values indicate higher concentrations of serum antibodies specific for the capture antigen. These wells were bound with half-log dilutions of pooled sera from day –1 and day 35 bleeds of six immunized mice per derivatized GM3-KLH conjugate. Pooling the serum of the six immunized mice gives a mean serum antibody concentration of the six immunized mice. Serum antibodies bound to the capture antigen were detected with goat anti-mouse kappa light chain specific and horseradish peroxidase (HRP) conjugated antibody. In mice, kappa light chain antibodies constitute about 95% of all antibodies, so this assay provides a good approximation of total antibody response.46 Using the GM3-HSA conjugates as capture antigens allows us to detect those antibodies which are specific for the modified GM3 component of the conjugates since the mice have never been exposed to HSA and the serum therefore will not contain antibodies to this protein. In fact, none of the pooled antisera showed binding to HSA protein (data not shown). On the other hand, the experiment using KLH as the capture antigen allows the determination of a positive control, as KLH is well established as a strong immunogen and will elicit a high titer of antibodies specific for KLH. Figure 3 indicates that while all of the tested glycoconjugates (2a–2e) were immunogenic, GM3NPhAc showed the highest level of antigen-specific antibodies. The natural GM3 antigen gave the lowest concentration of GM3-specific antibodies. There was essentially no binding of pre-immune serum to various GM3-HSA conjugates.
Figure 3.
Antigen-specific total antibody contents in sera analyzed by ELISA. Each line represents the antibody level in serum pooled from six mice. Anti-GM3 and anti-derivatized GM3 specific antibody levels were obtained from mice immunized with various GM3-KLH glycoconjugates. Anti-KLH specific antibody level, which was used as a positive control, was obtained from mice immunized with GM3NPr, and anti-GM3 specific antibody level of pre-immune serum was obtained from the same group as the negative control (equivalent results were obtained from the other groups). For ELISA assays, the corresponding GM3-HSA glycoconjugates were used as the capture antigens. Goat anti-mouse Kappa antibodies were used to detect antibodies bond to the capture antigens. Error bars are smaller than the symbol width.
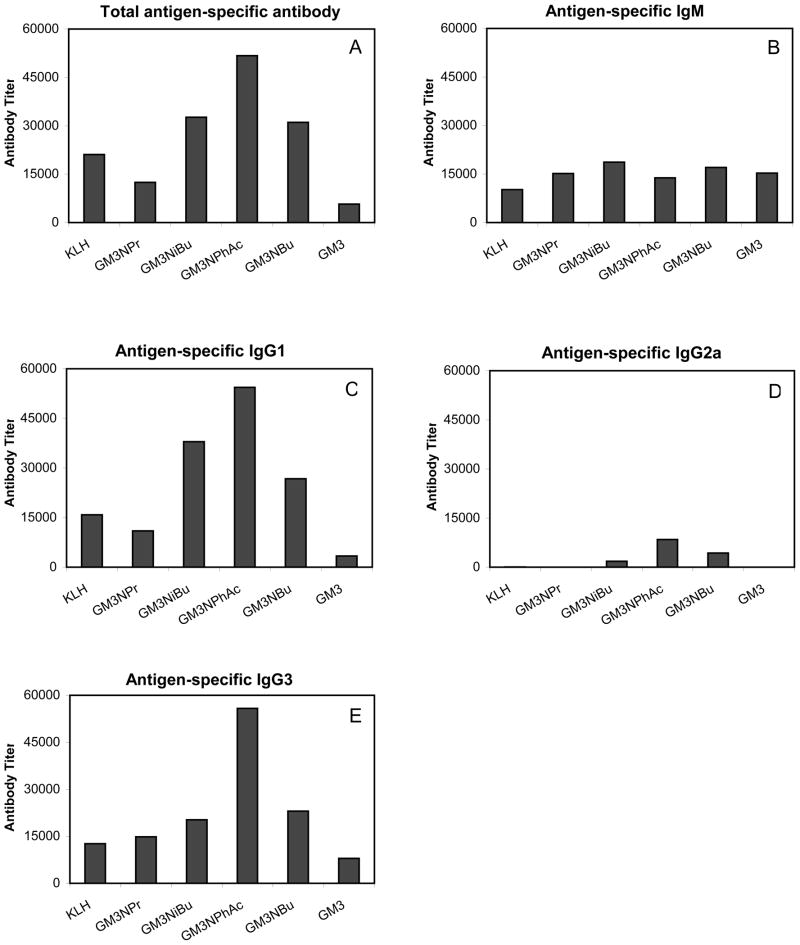
While the raw data allows us to roughly estimate the relative immunogenicities, a titer analysis was performed to normalize the data and quantify more accurately the immunogenicities of the derivatized glycoconjugates (Figure 4). Panel A of Figure 4 shows titers of total glycoconjugate-specific antibodies, and as above, the assays measured the kappa light chain. A hierarchy of immunogenicity was evident. GM3NPhAc was the most immunogenic, followed by GM3NiBu, GM3NBu and then GM3NPr. The natural GM3 antigen induced the lowest concentration of serum antibodies.
Figure 4.
Titer analysis of antigen-specific antibodies determined by ELISA assay (see Procedures). Each represents the titer in pooled serum obtained on day 35 after primary and booster immunizations (see Procedures) from six replicate animals.
Panels B–E of Figure 4 show the titers of different antibody isotypes. It was observed that different conjugates produced relatively equivalent concentrations of IgM antibody (panel B). IgM antibody has important complement fixing functions. However, because it is the first antibody produced by antigen-specific B cells in a T-cell independent manner and it is not subject to affinity maturation, IgM immune responses are limited by lack of memory.31 Major titer differences were observed with IgG responses to derivitized GM3 antigens. The IgG1 antibody titers seen in panel C of Figure 4 were substantially higher than that of IgM, and the glycoconjugates displayed the same hierarchy of immunogenicity seen in panel A, i.e., GM3NPhAc being the most immunogenic, followed by GM3NiBu and GM3NBu and GM3NPr. The natural GM3 induced very low levels of IgG1 antibody. Panel D of Figure 4 shows that all of the tested glycoconjugates gave very low IgG2a antibody levels with only GM3PhAc, GM3NiBu and GM3NBu having measurable IgG2a titers. IgG3 titers (Figure 4, panel E) showed the same hierarchy as seen in panels A and C with a more pronounced difference in the magnitude of the GM3NPhAc titer relative to the other gycoconjugates.
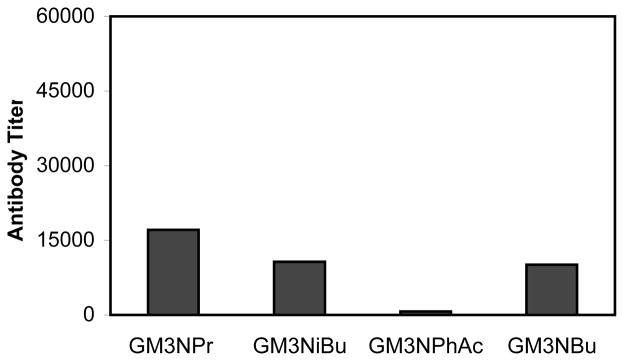
We next examined the reactivity of sera from mice immunized with derivatized GM3 conjugates to the HSA conjugate of natural GM3 (Figure 5). This allowed us to assess the potential for autoimmune reactions in response to immunization with the derivatized glycoconjugates. In general, sera from these animals showed much lower reactivity with GM3-HSA than that with the correspondingly modified GM3-HSA shown in Figure 4A. This was especially true for GM3NPhAc. In fact, the cross-reactivity of GM3NPhAc sera to the natural GM3 was almost negligible. Moreover, the hierarchy of cross-reactivity titers is the inverse of the titers of immunogenicity, with GM3NPhAc sera the least cross-reactive, followed by GM3NBu and GM3NiBu. However, the reactivity of GM3NPr sera to GM3NPr and the natural GM3 were rather similar, suggesting that the immune response induced by GM3NPr had little selectivity. The result of GM3NPhAc shown in Figure 5 can also help to conclude that the linker could not induce significant antibody responses, or a strong cross reactivity should be observed even for GM3NPhAc. These results are understandable. At the molecular level, GM3PhAc is the most dissimilar to the natural GM3. Therefore, GM3PhAc should be the most immunogenic, while antibodies against this structure should be least likely to bind to the natural form of GM3. On the other hand, GM3NPr is the very similar to the natural GM3, so it was less immunogenic, and its antibodies had the highest level of cross-reactivity with the natural GM3.
Figure 5.
Titer analysis of antibodies cross-reactive with the natural GM3 determined by ELISA (see Procedures). ELISA plates were coated with GM3-HSA conjugate. Each bar represents total anti-GM3 reactivity in pooled sera from six replicate animals immunized with the indicated derivatized GM3.
In summary, an expedient method was established to synthesize N-modified derivatives of GM3 and their protein conjugates. The properties of these glycoconjugates were studied to explore the impact of structural changes of GM3 on its immunogenicity. It was revealed that derivatized GM3 antigens are generally more immunogenic than GM3 itself. Moreover, while GM3 induces mainly IgM responses, derivatized GM3 antigens induce both IgM and IgG responses. From the viewpoint of cancer therapy, IgG responses are more interesting because of their desirable properties such as immunological memory, affinity maturation and improved antibody-dependent cell-mediated cytotoxicity.45,46
GM3NPhAc proved to be the most immunogenic among the GM3 derivatives investigated, and its anti-sera were the least cross-reactive with the natural GM3. These results indicate that GM3NPhAc is an excellent candidate as an anti-tumor vaccine. Meanwhile, our studies47 have also shown that the N-phenylacetyl derivative of mannoseamine is one of the best substrates for N-acetyl sialic acid adolase, an enzyme that may be involved in bypassing the biosynthetic bottleneck in the glycoengineering of sialic acid and sialoglycoconjugates on cells.48 In view of these results, we anticipate that the combination of a GM3PhAc-based vaccine with the glycoengineering of cancer cells by N-phenylacetylmannosamine holds great promises as an effective immunotherapy of cancer. In pursuit of this goal, we are presently focusing on the in vivo studies of the treatment of melanoma by using N-phenylacetylmannoseamine as the glycoengioneering precursor and GM3PhAc conjugates as the vaccines.
Experimental Procedures
N-{O-[Methyl 4,7,8,9-tetra-O-Acetyl-3,5-dideoxy-5-trifluoroacetamido-D-glycero-α-D-galacto-non-2-ulopyranosylonate]-(2→3)-O-(2,4,6-tri-O-acetyl-β-D-galactopyranosyl)-(1→4)-2,3,6-tri-O-acetyl-β-D-glucopyranosyl} 4-Pentenamide (10)
After a mixture of 8 (500 mg, 0.85 mmol), 5 (982 mg, 1.7 mmol) and activated molecular sieves (4Å, 2.0 g) in anhydrous acetonitrile (5.0 mL) was stirred at rt for 20 h under N2, the mixture was cooled to −35 °C, and NIS (1.7 mmol) and TfOH (0.2 mmol) were added. It was kept at −35 °C for 1 h and then diluted with DCM (20 mL). The solid material was filtered off and washed with DCM (5 mL). The combined filtrates were washed with aqueous Na2S2O3 (20%) and water. The organic phase was dried over Na2SO4 and concentrated under reduced pressure. Silica gel column chromatography of the residue afforded the desired trisaccharide 9 (585 mg, 0.53 mmol, 63%) containing a small amount of impuritys. A solution of 9 (550 mg, 0.5 mmol) and 4-pentenoic anhydride (20mg) in MeOH and EtOAc (1:1, v/v, 5 mL) was stirred vigorously with Lindlar catalyst (60 mg) and H2 at rt for 3 h. The solid material was filtered off and the filtrate was condensed under reduced pressure. The residue was purified by column chromatography to afford 10 (515 mg, 90%) as a white solid: Rf 0.30 (acetone and toluene 1:1); [α]D + 2.4 (c 1.0, CHCl3); 1H NMR (CDCl3, 600 MHz): δ 7.13 (d, 1 H, J 7.8 Hz, NH), 6.25 (d, 1 H, J 9.2 Hz, NH), 5.70 (m, 1 H, J 6.0, 10.8, 16.8 Hz, CH2=CH-), 5.39 (m, 1 H, J 4.2 Hz, H-8″), 5.34 (d, 1 H, J 8.4 Hz, H-7″), 5.19 (dd, 1H, J 9.0, 10.2 Hz, H-2), 5.18 (t, 1 H, J 10.2 Hz, H-3), 4.98 (d, 1 H, J 16.8 Hz, CH2=CH-), 4.92 (d, 1 H, J 10.2 Hz, CH2=CH-), 4.90 (d, 1 H, J 9.0 Hz, H-1), 4.88 (dd, 1 H, J 5.0, 10.8 Hz, H-4″), 4.76 (dd, 1H, J 9.0, 9.6 Hz, H-2′), 4.46 (d, 1 H, J 7.8 Hz, H-1′), 4.38 (d, 1 H, J 11.4 Hz, H-9″a), 4.33 (d, 1 H, J 10.8 Hz, H-6″), 4.22 (d, 1 H, J 10.2 Hz, H-3′), 4.7 (m, 2 H), 4.13 (dd, 1 H, J 12.0, 4.2 Hz, H-6), 4.04-4.00 (m, 2 H), 3.94 (q, 1 H, J 10.2 Hz, H-5″), 3.74 (m, 1 H, J 9.0 Hz, H-4), 3.72 (s, 3 H), 3.67 (d, 1 H, J 8.4 Hz, H-5), 3.58 (t, 1 H, J 6.0 Hz, H-5′), 3.35 (bs, 1 H, H-4′), 2.82 (d, 1 H, J 3.6 Hz, OH), 2.64 (m, H-3″e), 2.26 (m, 2 H), 2.18 (m, 2 H), 2.06, 2.03, 2.02, 2.01, 2.00, 1.98, 1.97, 1.96, 1.94 (9s, 9 × 3H, OAc), 1.70 (dd, 1 H, J 12.6, 12.0 Hz, H-3″a); 19F NMR (CDCl3): δ −76.7 (s); 13C NMR (CDCl3, 50M Hz): δ 172.7, 171.2, 170.9, 170.7, 170.6, 170.6, 170.5, 169.8, 169.7, 169.4, 168.2, 158.0 (q, J 37.6 Hz), 136.4, 115.8, 115.0 (q, J 285 Hz), 100.8, 96.9, 77.9, 75.1, 74.5, 72.5, 71.7, 71.0, 69.6, 68.6, 68.5, 68.4, 66.9, 66.8, 62.7, 62.1, 61.9, 53.2, 48.7, 37.8, 35.6, 28.9, 21.4, 20.9, 20.8, 20.7, 20.7, 20.6, 20.5, 20.4, 20.3; FABMS: calcd for C47H64F3N2O28 [M + H]+ 1161.4, found 1162.0; calcd for C47H63F3N2NaO28 [M + Na]+ 1183.4, found 1184.0.
N-{O-[5-Acylamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosylonic Acid]-(2→3)-O-(β-D-galactopyranosyl)-(1→4)-β-D-glucopyranosyl} 4-Pentenamide (11a–11e)
Compound 10 (500 mg, 0.45 mmol) was dissolved in 0.5 N NaOH (30 mL) and the mixture was stirred at rt for 10 h. After neutralization and condensation under reduced pressure, the crude product was directly used for the acylation. To a solution of the resultant amine (20 mg, 0.03 mmol) in 2.5 mL MeOH and 0.5 mL of NaOH (0.5 N) was added 0.1 mL of an acyl anhydride dropwise in an ice-water bath. After the reaction finished within 6 h as indicated by TLC, the mixture was condensed under reduced pressure. The residue was purified on a Biogel P-2 column with H2O as the eluent. Fractions containing the expected product were combined and freeze-dried to afford various N-acyl GM3 derivatives 11a–11e.
N-{O-[5-Acetamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosylonic Acid]-(2→3)-O-(β-D-galactopyranosyl)-(1→4)-β-D-glucopyranosyl} 4-Pentenamide (11a, 20 mg, 92%)
[α]D 2.4 (c 0.7, MeOH); 1H NMR (D2O, 600 MHz): δ 5.73 (m, 1 H, J 6.6, 10.2, 17.2 Hz, CH2=CH), 4.96 (dd, 1 H, J 1.8, 17.2 Hz, CH2=CH), 4.90 (dd, 1 H, J 1.8, 10.2 Hz, CH2=CH), 4.83 (d, 1 H, J 9.0 Hz, H-1), 4.39 (d, 1 H, J 7.8 Hz, H-1′), 3.96 (dd, 1 H, J 3.0, 9.6 Hz), 3.80 (dd, 1 H, J 1.8, 12.6 Hz), 3.66-3.45 (dd, 1 H, J 1.8, 10.2 Hz), 3.44 (dd, 1 H, J 9.6, 10.2 Hz), 3.28 (dd, 1 H, J 9.0, 9.6 Hz), 2.61 (dd, 1 H, J 12.6, 4.8 Hz, H-3″e), 2.28 (m, 2 H), 2.24 (m, 2 H), 1.88 (s, 3 H, COCH3), 1.65 (t, 1 H, J 12.0 Hz, H-3″a); FABMS: calcd for C28H46N2NaO19 [M + Na]+ 737.2, found 737.3; calcd for C28H45N2Na2O19 [M − H + 2Na]+ 759.2, found 759.3, calcd for C32H58N3O21 [M + DEA + H]+ 820.3, found 820.4; HR-FABMS: calcd for C28H46N2NaO19 [M + Na]+ 737.2592, found 737.2422.
N-{O-[5-Propionylamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosylonic Acid]-(2→3)-O-(β-D-galactopyranosyl)-(1→4)-β-D-glucopyranosyl} 4-Pentenamide (11b, 20 mg, 91%)
[α]D 2.1 (c 1.0, MeOH); 1H NMR (D2O, 600MHz): δ 5.74 (m, 1 H, J 6.6, 10.2, 16.8 Hz, CH2=CH-), 4.96 (dd, 1 H, J 1.8, 17.4 Hz, CH2=CH-), 4.91 (dd, 1 H, J 1.8, 9.6 Hz, CH2=CH-), 4.83 (d, 1 H, J 9.0 Hz, H-1), 4.39 (d, 1 H, J 8.4 Hz, H-1′), 3.80 (dd, 1 H, J 2.4, 12.6 Hz), 3.42 (t, 1 H, J 10.2 Hz), 3.28 (dd, 1 H, J 9.0, 9.6 Hz), 2.62 (dd, 1 H, J 12.6, 4.8 Hz, H-3″e), 2.28 (m, 2 H), 2.24 (m, 2 H), 2.15 (q, 2 H, J 7.8 Hz, CH3CH2CO), 1.79 (t, 1 H, J 12.6 Hz, H-3″a), 0.91 (t, 3 H, J 7.8 Hz, CH3CH2CO); FABMS: calcd for C29H48N2NaO19 [M + Na]+ 751.3, found 751.4; calcd for C29H47N2Na2O19 [M − H + 2Na]+ 773.3, found 773.5; calcd for C33H60N3O21 [M + DEA + H]+ 834.4, found 834.7; HR-FABMS: calcd for C29H48N2NaO19 [M + Na]+ 751.2749, found 751.2532.
N-{O-[5-Butanoylamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosylonic Acid]-(2→3)-O-(β-D-galactopyranosyl)-(1→4)-β-D-glucopyranosyl} 4-Pentenamide (11c, 19 mg, 87%)
[α]D 3.1 (c 0.7, MeOH); 1H NMR (D2O, 600 MHz): δ 5.74 (m, 1 H, J 6.6, 10.2, 16.8 Hz, CH2=CH-), 4.96 (dd, 1 H, J 1.2, 17.4Hz, CH2=CH), 4.90 (d, 1 H, J 10.2 Hz, CH2=CH-), 4.83 (d, 1 H, J 9.6 Hz, H-1), 4.39 (d, 1 H, J 7.8 Hz, H-1′), 3.97 (dd, 1 H, J 3.0, 9.6 Hz), 3.42 (dd, 1 H, J 1.8, 9.0 Hz), 3.28 (dd, 1 H, J 9.0, 9.6 Hz), 2.62 (dd, 1 H, J 12.0, 4.2 Hz, H-3″e), 2.29 (m, 2 H), 2.24 (m, 2 H), 2.13 (t, 2 H, J 7.2 Hz, COCH2CH2CH3), 1.66 (t, 1 H, J 12.0 Hz, H-3″a), 1.47 (m, 2 H, COCH2CH2CH3), 0.77 (t, 3 H, J 7.8 Hz, COCH2CH2CH3); FABMS: calcd for C30H50N2NaO19 [M + Na]+ 765.3, found 765.4; calcd for C30H49N2Na2O19 [M − H + 2Na]+ 787.3, found 787.3; calcd for C34H62N3O21 [M + DEA + H]+ 848.4, found 848.8; HR-FABMS: calcd for C34H62N3O21 [M + DEA + H]+ 848.3876, found 848.3874.
N-{O-[5-iso-Butanoylamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosylonic Acid]-(2→3)-O-(β-D-galactopyranosyl)-(1→4)-β-D-glucopyranosyl} 4-Pentenamide (11d, 21 mg, 94%)
[α]D 2.8 (c 1.0, MeOH); 1 H NMR (D2O, 600 MHz): δ 5.74 (m, 1 H, J 6.0, 10.2, 16.8 Hz, CH2=CH-), 4.95 (d, 1 H, J 16.2 Hz, CH2=CH-), 4.90 (d, 1 H, J 10.2 Hz, CH2=CH-), 4.83 (d, 1 H, J 9.0 Hz, H-1), 4.39 (d, 1 H, J 7.2 Hz, H-1′), 3.97 (dd, 1 H, J 3.0, 9.6 Hz), 3.28 (t, 1 H, J 9.0 Hz), 2.62 (dd, 1 H, J 12.0, 4.2 Hz, H-3″e), 2.38 (m, 1 H, COCHMe2), 2.29 (m, 2 H), 2.20 (m, 2 H), 1.66 (t, 1 H, J 12.0 Hz, H-3″a), 0.96, 0.95 (2 d, 2 × 3 H, J 6.6 Hz, COCHMe2); HR-FABMS: calcd for C30H50N2NaO19 [M + Na]+ 765.2905, found 765.2936; calcd for C30H49N2Na2O19 [M − H + 2Na]+ 787.2725, found 787.2728.
N-{O-[5-Phenylacetamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosylonic Acid]-(2→3)-O-(β-D-galactopyranosyl)-(1→4)-β-D-glucopyranosyl} 4-Pentenamide (11e, 20 mg, 83%)
[α]D 1.1 (c 0.7, MeOH); 1H NMR (D2O, 600 MHz): δ 7.25-7.15 (m, 5 H), 5.73 (m, 1 H, J 7.0, 10.2, 16.8 Hz, CH2=CH-), 4.96 (d, 1 H, J 1.2, 17.4 Hz, CH2=CH-), 4.89 (d, 1 H, J 10.2 Hz, CH2=CH-), 4.83 (d, 1 H, J 9.0 Hz, H-1), 4.37 (d, 1 H, J 7.8 Hz, H-1′), 3.99 (dd, 1 H, J 2.0, 9.6 Hz), 3.42 (s, 2 H, PhCH2), 3.28 (t, 1 H, J 7.2 Hz), 3.22 (dd, 1 H, J 9.0 Hz), 2.62 (dd, 1 H, J 4.0, 12.2 Hz, H-3″e), 2.29 (m, 2 H), 2.21 (m, 2 H), 1.77 (t, 1 H, J 12.2 Hz, H-3″a); HR-FABMS: calcd for C34H50N2NaO19 [M + Na]+ 813.2905, found 813.2907; calcd for C34H49N2Na2O19 [M − H + 2Na]+ 835.2725, found 835.2750.
N-{O-[5-Acylamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosylonic Acid]-(2→3)-O(β-D-galactopyranosyl)-(1→4)-β-D-glucopyranosyl} 4-Oxo-butanamide (12a–12e)
Ozone was bubbled into individual solutions of 11a–11e (0.02 mmol) in MeOH (5 ml) at −78°C until a blue color appeared and remained (ca. 30 min). The solutions were kept at −78°C for another 10 min, and then nitrogen was introduced to remove the remaining ozone. After Me2S (0.2 ml) was added at −78 °C, the resulting solution was allowed to warm to rt over a period of 1 h and stand for another 1 h before it was condensed in a vacuum. The crude product was purified on a Sephadex G10 column using distilled water as the eluent to give the aldehyde products 12a–12e as white solids, which were directly used in the conjugation reactions without further purification.
N-{O-[5-Acetamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosylonic Acid]-(2→3)-O-(β-D-galactopyranosyl)-(1→4)-β-D-glucopyranosyl} 4-Oxo-butanamide (12a)
1H NMR (D2O, 300 MHz): δ 5.05 (d, 1 H, J 9.0 Hz, H-1), 4.98 (t, 1 H, J 6.0 Hz, CHO), 4.54 (d, 1 H, J 7.7 Hz, H-1′), 3.96 (dd, 1 H, J 2.7, 9.7 Hz), 2.76 (dd, 1 H, J 12.6, 4.9 Hz, H-3″e), 2.46 (m, 1 H), 2.38 (m, 2 H), 2.02 (s, 3 H, COCH3), 1.9 (m, 1 H), 1.79 (t, 1 H, J 12.5 Hz, H-3″a); FABMS: calcd for C27H44N2NaO20 [M + Na]+ 739.3, found 739.4; calcd for C27H44N2KO20 [M + K]+ 755.2, found 755.4; HR-FABMS: calcd for C31H53N3NaO21 [M - H2O + DEA + Na]+ 826.3067, found 826.3034; calcd for C31H54N3O21 [M - H2O + DEA + H]+ 804.3247, found 804.3247.
N-{O-[5-Propionylamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosylonic Acid]-(2→3)-O-(β-D-galactopyranosyl)-(1→4)-β-D-glucopyranosyl} 4-Oxo-butanamide (12b)
1H NMR (D2O, 600 MHz): δ 4.88 (d, 1 H, J 9.0 Hz, H-1), 4.72 (t, 1 H, J 6.0 Hz, CHO), 4.40 (d, 1 H, J 7.8 Hz, H-1′), 3.98 (dd, 1 H, J 2.4, 9.7 Hz), 2.62 (dd, 1 H, J 12.6, 4.8 Hz, H-3″e), 2.50 (m, 1 H), 2.29 (m, 2 H), 2.16 (q, 2 H, J 7.8 Hz, COCH2CH3), 1.80 (m, 1 H), 1.67 (t, 1 H, J 12.0 Hz, H-3″a), 0.97 (t, 3 H, J 7.2 Hz, COCH2CH3); FABMS: calcd for C28H46N2NaO20 [M + Na]+ 753.3, found 753.4; calcd for C28H46N2KO20 [M + K]+ 769.3, found 769.4; HR-FABMS: calcd for C32H55N3NaO21 [M - H2O + DEA + Na]+ 840.3225, found 840.3286; calcd for C32H56N3O21 [M - H2O + DEA + H]+ 818.3404, found 818.3405.
N-{O-[5-Butanoylamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosylonic Acid]-(2→3)-O-(β-D-galactopyranosyl)-(1→4)-β-D-glucopyranosyl} 4-Oxo-butanamide (12c)
1H NMR (D2O, 600 MHz): δ 4.85 (d, 1 H, J 9.0 Hz, H-1), 4.70 (t, 1 H, J 4.8 Hz, CHO), 4.40 (d, 1 H, J 7.8 Hz, H-1′), 3.96 (dd, 1 H, J 2.7, 9.7 Hz), 2.60 (dd, 1 H, J 12.6, 4.8 Hz, H-3″e), 2.40 (m, 1 H), 2.28 (m, 2 H), 2.11 (t, 2 H, J 7.2 Hz, COCH2), 1.83 (m, 1 H), 1.65 (t, 1 H, J 12.0 Hz, H-3″a), 1.47 (m, 2 H, COCH2CH2), 0.75 (t, 3 H, J 7.2Hz, COCH2CH2CH3); FABMS: calcd for C29H47N2Na2O20 [M − H + 2Na]+ 789.3, found 789.5; HR-FABMS: calcd for C33H57N3NaO21 [M - H2O + DEA + Na]+ 854.3380, found 854.3334; calcd for C33H58N3O21 [M - H2O + DEA + H]+ 832.3560, found 832.3556.
N-{O-[5-iso-Butanoylamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosylonic Acid]-(2→3)-O-(β-D-galactopyranosyl)-(1→4)-β-D-glucopyranosyl} 4-Oxo-butanamide (12d)
1H NMR (D2O, 600 MHz): 4.90 (d, 1 H, J 9.0 Hz, H-1), 4.82 (t, 1 H, J 6.0 Hz, CHO), 4.40 (d, 1 H, J 7.8 Hz, H-1′), 3.85 (t, 1 H, J 9.6Hz), 3.44 (dd, 1 H, J 8.0, 10.0 Hz), 3.40 (dd, 1 H, J 1.8, 9.6 Hz), 2.62 (dd, 1 H, J 12.6, 4.8 Hz, H-3″e), 2.57 (m, 1 H), 2.38 (m, 1 H, COCH), 2.32 (m, 1 H), 2.25 (m, 1 H), 1.82 (m, 1 H), 1.66 (t, 1 H, J 12.0 Hz, H-3″a), 0.96, 0.95 (2d, 6 H, J 6.6 Hz, CHMe2); FABMS: calcd for C29H48N2NaO20 [M + Na]+ 767.3, found 767.3; calcd for C29H48N2KO20 [M + K]+ 783.3, found 783.3; HR-FABMS: calcd for C33H57N3NaO21 [M - H2O + DEA + Na]+ 854.3380, found 854.3367; calcd for C33H58N3O21 [M - H2O + DEA + H]+ 832.3560, found 832.3576.
N-{O-[5-Phenylacetamido-3,5-dideoxy-D-glycero-α-D-galacto-non-2-ulopyranosylonic Acid]-(2→3)-O-(β-D-galactopyranosyl)-(1→4)-β-D-glucopyranosyl} 4-Oxo-butanamide (12e)
1H NMR (D2O, 600 MHz): δ 7.25-7.18 (m, 5 H), 4.90 (d, 1 H, J 9.6 Hz, H-1), 4.41 (t, 1 H, J 7.2 Hz, CHO), 4.39 (d, 1 H, J 7.8 Hz, H-1′), 3.95 (dd, 1 H, J 3.0, 9.6 Hz), 3.84 (t, 1 H, J 9.6 Hz), 3.48 (s, 2 H, PhCH2), 3.43 (dd, 1 H, J 9.6, 7.8 Hz), 3.22 (d, 1 H, J 9.0 Hz), 2.62 (dd, 1 H, J 4.8, 12.6 Hz, H-3″e), 2.55 (m, 1 H), 2.23–2.33 (m, 2 H), 1.82 (m, 1 H), 1.65 (t, 1 H, J 12.0 Hz, H-3″a); FABMS: calcd for C33H48N2NaO20 [M + Na]+ 815.2698, found 815.3; calcd for C33H48N2KO20 [M + K]+ 831.3, found 831.3. HR-FABMS: calcd for C37H57N3NaO21 [M - H2O + DEA + Na]+ 902.3380, found 902.3360; calcd for C37H58N3O21 [M - H2O + DEA + H]+ 880.3560, found 880.3510.
General procedure for the coupling between 12a-12e and KLH or HSA
A solution of 12a-12e (6 mg each), KLH or HSA (5 mg) and NaBH3CN (5 mg) in 0.1M NaHCO3 (0.4 mL, pH 7.5–8.0) was allowed to stand at rt in the dark for 3 days with occasional shaking. The reaction mixture was then loaded onto a Biogel A0.5 column (1 cm × 15 cm) and eluted with 0.1 M PBS buffer (I = 0.1, pH = 7.8). The fractions containing the glycoconjugate, characterized by BCA assay for proteins and by the Svennerholm 43 method for sialic acid, were combined and dialyzed against distilled water for 2 days. It was then lyophilized to give a white powder of the expected glycoconjugate.
Analysis of the carbohydrate loading levels of the glycoconjugates
After the solution of an exactly weighed sample of the glycoconjugate (0.5 mg) in distilled water (1.0 mL) was mixed well with the resorcinol reagent (2.0 mL), the mixture was heated in a boiling water bath for 30 min. It was then cooled to rt, and to the mixture was added an extraction solution (1-butanol acetate and 1-butanol, 85:15 v/v, 3.0 mL). The mixture was shaken vigorously before it was allowed to stand still for ca. 10 min letting the organic layer well separated from the inorganic layer. The organic layer was transferred to a 1.0 cm cuvette, and its absorbance at 580 nm was determined by an UV/visible spectrometer, using a blank organic solution as the control. The sialic acid content of the glycoconjugate is determined against a calibration curve created with the standard NeuNAcyl (Acyl = Ac, Pr, Bu, iBu, PhAc) solutions analyzed under the same conditions.43 The carbohydrate loading of each glycoconjugate was calculated according to the equation shown below.
Immunization of mice
Six female C57BL/6 mice at the age of eight weeks (Jackson Laboratories, Bar Harbor, ME) were immunized for each GM3-KLH glycoconjugate. Immunizations were intraperitoneal with glycoconjugate containing 2 μg of carbohydrate in 200 μl of saline mixed with 200 μl of MPL/TDM Ribi adjuvant (Sigma Chemical, St. Louis, MO) following the manufacturer’s protocol. The mice were boosted with identical immunizations on days 14, 21 and 28 following the initial immunization. The mice were bled by tail vein prior to the initial immunization on day 0 and after immunization on day 27 and day 35. Bleed was clotted to obtain sera, which were stored at −80 °C.
Protocols for ELISA analysis
ELISA plates were coated with KLH or GM3 and GM3 derivatives conjugated to HSA. These capture reagents allowed detection of antibodies specific for KLH or the GM3 and derivatized GM3 components of the glycoconjugates. Maxisorp ELISA plates (NuncNalgene, Rochester, N.Y.) were coated overnight at 4 °C with 100 μl of HSA glycoconjugates or KHL (1 μg/ml 0.1 M bicarbonate buffer), and then washed with PBS. Sera from the six mice per group were pooled, diluted 1:300 to 1:72900 in serial half-log dilutions in PBS with 0.02% azide, and incubated overnight in the coated ELISA plates (100 μl/well). The plates were then washed and incubated with 1:1000 dilution of alkaline phosphatase linked anti-kappa, anti-IgM or anti-IgG2a antibodies, or with 1:2000 dilution of anti-IgG1 or anti-IgG3 antibodies (Southern Biotechnology, Buckingham, AL) for 1 h at rt. Plates were washed and developed with PNPP substrate for colorimetric readout using a BioRad 550 plate reader (BioRad, Hercules, CA) at 405 nM wavelength.
A titer analysis was performed to normalize data and calculate relative immunogenicities of derivatized glycoconjugates.30 Optical density (OD) values were plotted against dilution values, and a best-fit line was obtained. The equation of this line was used to calculate the dilution value at which an OD of 0.5 was achieved, and antibody titer was calculated at the inverse of this dilution value.
Supplementary Material
Acknowledgments
This work was supported by a research grant from NIH/NCI (1R01 CA95142).
Footnotes
Supporting Information Available. Experimental procedures for 5 and 8, and the 1H NMR and MS spectra of compounds 10 and 11a–11e. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hakomori S. Possible functions of tumor-associated carbohydrate antigens. Curr Opin Immunol. 1991;3:646–653. doi: 10.1016/0952-7915(91)90091-e. [DOI] [PubMed] [Google Scholar]
- 2.Hakomori S, Zhang Y. Glycosphingolipid antigens and cancer therapy. Chem Biol. 1997;3:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 3.Reisfeld RA, Cheresh DAH. Human tumor antigens. Adv Immunol. 1987;40:323–377. doi: 10.1016/s0065-2776(08)60242-4. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Efraim S. One hundred years of cancer immunotherapy: a critical appraisal. Tumor Biol. 1999;20:1–24. doi: 10.1159/000056517. [DOI] [PubMed] [Google Scholar]
- 5.Ragupathi G. Carbohydrate antigens as targets for active specific immunotherapy. Cancer Immunol Immunother. 1998;46:82–87. doi: 10.1007/s002620050316. [DOI] [PubMed] [Google Scholar]
- 6.Danishefsky SJ, Allen JR. From the laboratory to the clinic: A retrospective on fully synthetic carbohydrate-based anticancer vaccines. Angew Chem Int Ed. 2000;39:837–863. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Hellstrom KE, Gladstone P, Hellstrom I. Cancer vaccines: Challenges and potential solutions. Mol Med Today. 1997:286–290. doi: 10.1016/s1357-4310(97)01048-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, Wu TC. Experimental vaccine strategies for cancer immunotherapy. J Biomed Sci. 1998;5:231–252. doi: 10.1007/BF02255855. [DOI] [PubMed] [Google Scholar]
- 9.Livingston PO. Construction of cancer vaccines with carbohydrate and protein tumor antigens. Curr Opin Immunol. 1992;4:624–629. doi: 10.1016/0952-7915(92)90038-g. [DOI] [PubMed] [Google Scholar]
- 10.Toyokuni T, Singhal AK. Synthetic carbohydrate vaccines based on tumor-associated antigens. Chem Soc Rev. 1995:231–242. [Google Scholar]
- 11.Stipp D. Closing in on the cancer vaccines. Fortune. 1998;138:168–176. [Google Scholar]
- 12.Liu T, Guo Z, Yang Q, Sad S, Jennings HJ. Biochemical engineering of surface α(2–8)polysialic acid for immunotargeting cancer cells. J Biol Chem. 2000;275:32832–32836. doi: 10.1074/jbc.C000573200. [DOI] [PubMed] [Google Scholar]
- 13.Bertozzi CR, Kiessling LL. Chemical glycobiology. Science. 2001;291:2357–2364. doi: 10.1126/science.1059820. [DOI] [PubMed] [Google Scholar]
- 14.Saxon E, Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 15.Mahal LK, Bertozzi CR. Engineering cell surfaces: fertile ground for molecular landscaping. Chem Biol. 1997;4:415–422. doi: 10.1016/s1074-5521(97)90193-9. [DOI] [PubMed] [Google Scholar]
- 16.Yarema KJ, Mahal LK, Bruehl RE, Rodriguez EC, Bertozzi CR. Metabolic delivery of ketone groups to sialic acid residues. Application to cell surface glycoform engineering. J Biol Chem. 1998;273:31168–31179. doi: 10.1074/jbc.273.47.31168. [DOI] [PubMed] [Google Scholar]
- 17.Mahal KL, Yarema KJ, Bertozzi CR. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science. 1997;276:1125–1128. doi: 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- 18.Kayser H, Geile CC, Paul C, Zeitler R, Reutter W. Incorporation of N-acyl-2-amino-2-deoxy-hexoses into glycosphingolipids of pheochromocytoma cell line PC12. FEBS. 1992;301:137–140. doi: 10.1016/0014-5793(92)81233-c. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt C, Stehling P, Schnitzer J, Reutter W, Horskorte R. Biological engineering of neural cell surfaces by the synthetic N-propanoyl-substituted neuraminic acid precursor. J Biol Chem. 1998;273:19146–19152. doi: 10.1074/jbc.273.30.19146. [DOI] [PubMed] [Google Scholar]
- 20.Takano R, Muchmore E, Dennis JW. Sialylation and malignant potential in tumor cell glycosylation mutants. Glycobiology. 1994;4:665–674. doi: 10.1093/glycob/4.5.665. [DOI] [PubMed] [Google Scholar]
- 21.Zou W, Borrelli S, Gilbert M, Liu T, Pon RA, Jennings HJ. Bioengineering of surface GD3 ganglioside for immunotargeting human melanoma cells. J Biol Chem. 2004;279:25390–25399. doi: 10.1074/jbc.M402787200. [DOI] [PubMed] [Google Scholar]
- 22.Bitton RJ, Guthmann MD, Babri MR, Carnero AJL, Alonso DF, Fainboim L, Gomez DE. Cancer vaccines: An update with special focus on ganglioside antigens (Review) Oncol Rep. 2002;9:267–276. [PubMed] [Google Scholar]
- 23.Livingston PO. Approaches to augmenting the immunogenicity of melanoma gangliosides: from the whole melanoma cells to ganglioside-KLH conjugate vaccines. Immunol Rev. 1995;145:147–156. doi: 10.1111/j.1600-065x.1995.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 24.Estevez F, Carr A, Solorzano L, Valiente O, Mesa C, Barroso O, Sierra GV, Fernandez LE. Enhancement of the immune response to poorly immunogenic gangliosides after incorporation into very small size proteoliposomes (VSSP) Vaccine. 1999;18:190–197. doi: 10.1016/s0264-410x(99)00219-4. [DOI] [PubMed] [Google Scholar]
- 25.Jennings H. N-propionylated group B meningococcal polysaccharide glycoconjugate vaccine against group B meningococcal meningitis. Int J Infect Dis. 1997;1:158–164. [Google Scholar]
- 26.Jennings HJ, Sood RK. In: Neoglycoconjugates: Preparation and Applications. Lee YC, Lee RT, editors. Academic Press; San Diego: 1994. pp. 325–371. [Google Scholar]
- 27.Ritter G, Boosfeld E, Calves MJ, Oettgen HF, Old LJ, Livingston PO. Antibody response after immunization with ganglioside GD3, GD3 lactones, GD3 amide and GD3 gangliosidol in the mouse. GD3 lactone I induces antibodies reactive with human melanoma. Immunobiology. 1990;182:32. doi: 10.1016/S0171-2985(11)80581-4. [DOI] [PubMed] [Google Scholar]
- 28.Ritter G, Boosfeld E, Asluri S, Calves MJ, Oettgen HF, Old LJ, Livingston PO. Antibody response after immunization with ganglioside GD3, GD3 congeners (lactones, amide and gangliosidol) in patients with malignant melanoma. Int J Cancer. 1991;48:379–385. doi: 10.1002/ijc.2910480312. [DOI] [PubMed] [Google Scholar]
- 29.Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad USA. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chefalo P, Pan YB, Nagy N, Harding CV, Guo Z. Preparation and immunological studies of protein conjugates of N-acylneuraminic Acids. Glycoconjugate J. 2004;20:407–414. doi: 10.1023/B:GLYC.0000033997.01760.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 32.Xue J, Pan Y, Guo Z. Neoglycoprotein cancer vaccines: Synthesis of an azido derivative of GM3 and its efficient coupling to proteins through a new linker. Tetrahedron Lett. 2002;43:1599–1602. [Google Scholar]
- 33.Earle MA, Manku S, Hultin PG, Li H, Palcic MM. Chemoenzymatic synthesis of a trimeric ganglioside GM3 analogue. Carbohydr Res. 1997;301:1–4. doi: 10.1016/s0008-6215(97)00085-2. [DOI] [PubMed] [Google Scholar]
- 34.Nagao Y, Nekado T, Ikeda K, Achiwa K. Synthesis of ganglioside GM3 and GM4 analogs having mimics of ceramide moieties and their binding activities with inluenza virus A. Chem Pharm Bull. 1995;43:1536–1642. doi: 10.1248/cpb.43.1536. [DOI] [PubMed] [Google Scholar]
- 35.Eisele T, Schmidt RR. Synthesis of the thio-linked ganglioside GM3 epitope. Liebigs Ann. 1997:865–872. [Google Scholar]
- 36.Lonn H, Stenvall K. Exceptionally high yield in glycosylation with sialic acid: Synthesis of a GM3 glycoside. Tetrahedron Lett. 1992;33:115–116. [Google Scholar]
- 37.Fujita S, Numata M, Sugimot M, Tomita K, Ogawa T. Total synthesis of the modified ganglioside de-N-acetyl-GM3 and some analogs. Carbohydr Res. 1992;228:347–370. doi: 10.1016/0008-6215(92)84130-k. [DOI] [PubMed] [Google Scholar]
- 38.Murase T, Ishida H, Kiso M, Hasegawa A. A facile regio- and stereoselective synthesis of ganglioside GM3. Carbohydr Res. 1989;188:71–80. doi: 10.1016/0008-6215(89)84060-1. [DOI] [PubMed] [Google Scholar]
- 39.Yan F, Mehta S, Eichler E, Wakarchuk WW, Gilbert M, Schur MJ, Whitfield DM. Simplifying oligosaccharide synthesis: Efficient synthesis of lactosamine and siaylated lactosamine oligosaccharide donors. J Org Chem. 2003;68:2426–2431. doi: 10.1021/jo026569v. [DOI] [PubMed] [Google Scholar]
- 40.De Meo C, Demchenko AV, Boons G-J. A stereoselective approach for the synthesis of α-sialosides. J Org Chem. 2001:5490–5497. doi: 10.1021/jo010345f. [DOI] [PubMed] [Google Scholar]
- 41.De Meo C, Demchenko A, Boons GJ. Triflouroacetamido substituted sialyl donors for the preparation of sialyl galactosides. Aus J Chem. 2002;55:131–134. [Google Scholar]
- 42.Hasegawa A, Murase T, Ogawa M, Ishida H, Kiso M. Synthetic studies on sialoglycoconjugates 17: Synthesis of 4-O-, 9-O-, and 4,9-di-O-acetyl-N-acetylneuraminic acids and their derivatives. J Carbohydr Chem. 1990;9:415–428. [Google Scholar]
- 43.Svennerholm L. Estimation of sialic acids. II. Colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957;24:604–11. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- 44.Evans JT, Cluff CW, Johnson DA, Lacy MJ, Persing DH, Baldridge JR. Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi.529. Expert Rev Vaccines. 2003;2:219–229. doi: 10.1586/14760584.2.2.219. [DOI] [PubMed] [Google Scholar]
- 45.Casadevall A, Pirofski LA. Antibody-mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 2003;24:474–478. doi: 10.1016/s1471-4906(03)00228-x. [DOI] [PubMed] [Google Scholar]
- 46.Goldsby RA, Kindt TJ, Osborne BA, Kuby J. Fundamental Immunology. W.H. Freeman and Company; New York, N.Y: 2003. pp. 76–105. [Google Scholar]
- 47.Pan Y, Ayani T, Nadas J, Guo Z. Accessibility of N-acyl derivatives of D-mannosamine to N-acetylneuraminic acid aldolase. Carbohydr Res. 2004;339:2091–2100. doi: 10.1016/j.carres.2004.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobs CL, Goon S, Yarema KJ, Hinderlich S, Hang HC, Chai DH, Bertozzi CR. Substrate specificity of the sialic acid biosynthetic pathway. Biochemistry. 2001;40:12864–12874. doi: 10.1021/bi010862s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.