Abstract
Background
The double-blind, prospective, National Surgical Adjuvant Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial (BCPT) demonstrated a 50% reduction in the risk of breast cancer (BC) for tamoxifen versus placebo, yet many women at risk of BC do not adhere to the 5-year course. This first report of the rich BCPT drug adherence data examines predictors of adherence.
Methods
13,338 women at high risk of BC were randomly assigned 6/92-9/97 to 20 mg/day tamoxifen versus placebo; we analyzed the 11,064 enrolled more than 3 years before trial unblinding. Primary endpoint was full drug adherence (100% of assigned pills per staff report, excluding protocol-required discontinuation) at 1 and 36 months; secondary was adequate adherence (76-100%). Protocol-specified multivariable logistic regression tested lifestyle factors, controlling for demographic and medical predictors.
Results
13% were current smokers. 60% were overweight/obese. 46% had moderate/heavy physical activity. 21%, 66%, 13% drank 0, 0-1, 1+ drinks/day. 91% were adequately adherent at 1 mo; 79% at 3 yrs. Alcohol use was associated with reduced full adherence at 1 mo (p=.016; odds ratio [OR]=0.79 1+ versus 0), as was age (p<.001; OR=1.4 age 60+), college education (p<.001; OR=0.78) and per-capita household annual income (p<.001; OR=1.2 per $30,000). Smoking (p=.003; OR=0.75), age (p=.024, OR=1.1), college education (p=.037; OR=1.4), tamoxifen assignment (p=.031; OR=.84), and BC risk (p<.001; OR=1.5 high v low) predicted adequate adherence at 36 months. There were no significant associations with obesity or physical activity.
Conclusions
Alcohol use and smoking might indicate a need for greater adherence support.
Keywords: Breast cancer, tamoxifen, persistence, compliance, prevention
Introduction
Oral endocrine therapies, including tamoxifen, are an important option in the prevention, as well as treatment, of breast cancer.1 However, poor utilization of self-administered, oral endocrine therapies -- that is, electing not to adopt the regimen, to adhere or conform to the regimen, or to persist or continue through the planned duration -- is a significant barrier to their efficacy, and authors have called for research to identify patients most at risk of poor utilization.2,3 Studies using varying methodologies have, for example, reported tamoxifen non-persistence rates among women with breast cancer ranging from 22% at 1 year to 35% at 3.5 years.4-6 Evidence from tamoxifen treatment trials suggests that individuals with poor persistence probably benefited less than those who took the full course.7,8
In the prevention setting, rich drug utilization data are available from the National Surgical Adjuvant Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial (BCPT) (also known as P-1), which tested whether a 5-year course of daily tamoxifen would reduce the rate of invasive breast cancer relative to a placebo in women at high risk but without a history of breast cancer. The BCPT was unblinded in 1998, following an interim analysis, and was soon approved by the Food and Drug Administration for primary reduction of breast cancer risk.9
In this first report of drug utilization in the BCPT, we focus on the lifestyle behavior factors at baseline of cigarette smoking, obesity, leisure-time physical activity, and alcohol use. We wished to examine whether these personal health behaviors, in a highly-motivated clinical trial population, would be related to medication utilization. Our interest is motivated by the strong associations among unhealthy behaviors, because together they may describe an individual who, we hypothesized, would be less likely to utilize chemoprevention as prescribed. Associations among smoking, obesity, physical activity, and alcohol use have been demonstrated in numerous studies.10-18 There is also currently a great deal of attention to the biological pathways by which these lifestyle factors are associated with cancer. The under-studied connection between lifestyle behaviors and chemoprevention utilization is another potential route by which people who engage in the behaviors may be more likely to develop cancer. In addition, the present analysis is important because few studies have addressed the associations of lifestyle behaviors and drug utilization in a healthy population. Adherence in identifying and understanding the factors that predict utilization is critical, if the long-term benefits of cancer chemopreventive therapies are to be realized.
Methods
Participants
This is a secondary analysis of the BCPT database. The BCPT, which was funded by the National Cancer Institute, was a double-blinded, placebo-controlled clinical trial that was open for accrual at several hundred clinical centers throughout North America from June 1, 1992, through September 30, 1997. During this interval, 13,338 women were randomly assigned to receive either 20 mg/day of tamoxifen or placebo for a duration of five years.9 The risk of breast cancer was estimated using the Gail model, which incorporates a woman's age at menarche, number of benign breast biopsies, histological diagnosis of atypical hyperplasia, nulliparity or age at first live birth, and number of first-degree relatives with breast cancer.19-21 Women were required to have an estimated 5-year risk of 1.66% or a history of lobular carcinoma in situ. Exclusion criteria included a history of clinical depression or addictive disorder that would preclude obtaining informed consent or interfere with protocol compliance. As in our prior analyses of QOL data from the BCPT,22,23 we used data available on the 11,064 participants recruited as of May 31, 1994 (82.6% of total accrual), as they would have been expected to have at least 36 months of follow-up data when the study was unblinded in March, 1998. We included only 36 months of follow-up. All participants provided informed consent for the BCPT, which was approved by the Institutional Review Boards of all participating institutions. The present secondary analysis was approved by the University of Pittsburgh Institutional Review Board.
Measures
Participants' utilization of their assigned treatment (tamoxifen or placebo) was reported at 1, 3, 6, and every 6 months thereafter. The case report form included the staff assessment of the percentage of pills taken during the past 4 weeks (categorized as more than 100%, 100%, 76-99%, 51-75%, 26-50%, 1-25%, or 0%), pattern of utilization, and strategies used by the clinical staff to improve adherence. Adherence problems were recorded by the clinical staff using a checklist and an open-ended “other problem” response item. When participants went off therapy, their reasons were recorded. Until 1995, the staff also reported the number of pills remaining in the participant's medicine bottle. It was determined at that time that the qualitative pill use reporting was adequately reliable (unpublished analysis), and the forms were modified so that the physical counting of pills was no longer required. The adherence case report form was no longer required after January 19, 1996.
We use the term “adherence” for ease of expression, but non-initiation of study drug and non-persistence (early discontinuation) were also included in the measure of utilization we have examined. Participants were not included in the denominator after they formally discontinued treatment for any of the following reasons: grade 4 adverse event, cardiovascular or stroke-related event, cancer, bone fracture, non-cataract eye toxicity, pregnancy, other medical problems related to the protocol, other diagnoses or procedures potentially related to protocol, or death. Participants who withdrew consent to be followed (in the absence of a major health event) or who were lost to follow-up were considered non-adherent after that time point.
A patient-reported instrument administered at enrollment collected cigarette smoking data (at least 100 cigarettes in lifetime, age of initiation, current smoking frequency and intensity, past intensity, and age at quitting if a former smoker). The instrument also provided descriptions of physical inactivity, and light, moderate, or vigorous physical activity. Participants were asked to select one of these 4 levels to describe their activity during their leisure time in the previous 12 months. Frequency and quantity of consumed beer, wine, and alcohol were reported. Participant weight and height were recorded by clinic staff, from which body mass index (BMI) was calculated for the present study. Women with baseline BMI of at least 25 were defined as overweight or obese.
Statistical considerations
The significance of predictor variables was tested at two-sided alpha level 0.05. Pill adherence was dichotomized using cutoffs of 100% (“full adherence”) and 76% (“adequate adherence”) of pills taken during the past 4 weeks, based on the staff assessment described above under Measures. Full adherence was defined as the primary endpoint in the research proposal, but adequate adherence is an important secondary endpoint because tamoxifen is believed to retain efficacy at this level because of the long half-life of tamoxifen after chronic use.24,25 Mixed effects logistic regression analyses were performed for the adherence outcomes at 1 month and at 3 years. Predictors of interest were cigarette smoking (current versus other), unhealthy weight (overweight/obese), physical activity (inactive/light versus moderate/heavy), and alcohol use. Alcohol use was classified for the present analysis as none, up to 1, or more than 1 drink/day, distinguishing healthy or unhealthy use based on the U.S. Department of Agriculture recommendation that women drink up to 1 drink/day (12 oz. [355 ml] beer, 5 oz. [148 ml] wine or 1.5 oz. [44 ml] spirits).26 Other predictors were: assigned treatment group, age (over 60), presence of co-morbid conditions, estimated breast cancer risk (analyzed as a continuous variable), participant-reported race/ethnicity, education, marital status, family and personal history of life-threatening illness, employment status, occupation of self and spouse, per capita household income, and household inhabitants (living alone vs. with others). Pre-treatment values were used for all predictors. Because of potential confounding of demographic and medical variables with the health behavior variables of interest (e.g., smoking and income), preliminary analyses included either medical/demographic variables or health behaviors. The full model then included all the lifestyle behaviors of interest and those medical/demographic factors that reached p<.1 significance in the preliminary model. A random effect for the institution where participants were treated accounted for correlation among participants from the same institution and for an institution-specific effect on adherence. Estimated adherence for levels of the lifestyle factors was based on back-transformed least squared means.
In preparation for the primary analyses, we performed hierarchical clustering to identify profiles of women based on the health behavior variables of interest (not including adherence). Cluster analysis revealed that the two major clusters were non-smokers versus smokers, with any combination of physical activity level, obesity, and alcohol use. Using the cluster variable in place of individual behavior variables did not improve model fit according to the Akaike Information Criterion27, and we therefore used individual behavior variables in the reported analyses.
For 96 randomly selected participants who endorsed “other” problems with adherence (48 placebo, 48 tamoxifen; 6 from each of the 8 time points), an experienced data manager performed content analysis of written responses to determine whether text responses fit into categories already in the checklist or constituted distinct problems.
The statistical power for a logistic regression of full adherence was estimated in the protocol to be 80% for odds ratios in the range of 1.1-1.8 for varying assumptions about the distribution of predictor variables. Computations were performed in SAS 9.1 (Cary, NC) including PROC FASTCLUS, PROC CLUSTER, and PROC GLIMMIX.
Results
Of the 11,064 women enrolled in BCPT as of May 31, 1994, majorities were under age 60 and of white race. (Table 1) At baseline, 13% of participants were current cigarette smokers, 60% were overweight or obese, 54% were inactive or engaged in light leisure-time physical activity, and 13% consumed more than one alcoholic drink per day. Other demographic characteristics have been published elsewhere.9,22,23,28,29
Table 1.
Participant characteristics (NSABP BCPT participants randomly assigned on or before 5/31/1994).
| Participant Characteristic | Count | Percent |
|---|---|---|
| CURRENT SMOKER | 9609 | 86.8 |
| No | ||
| Yes | 1417 | 12.8 |
| Unknown | 38 | 0.3 |
| BASELINE BMI >= 25 | 6635 | 60.0 |
| LEISURE TIME PHYSICAL ACTIVITY | 5057 | 45.7 |
| Moderate - Heavy | ||
| Inactive - Light | 5968 | 53.9 |
| Unknown | 39 | 0.4 |
| ALCOHOL USE | 2268 | 20.5 |
| None | ||
| 1 drink/day | 7284 | 65.8 |
| +1 drink/day | 1471 | 13.3 |
| Unknown | 41 | 0.4 |
| AGE >= 60 | 3308 | 29.9 |
| RACE | 10572 | 95.6 |
| White | ||
| Black | 183 | 1.7 |
| Hispanic | 112 | 1.0 |
| Other/Unknown | 197 | 1.8 |
| EDUCATION | 2617 | 23.7 |
| Grade school/Some HS/HS | ||
| Associate degree/Some college/Vocational training | 4291 | 38.8 |
| College degree/Some post-college | 2466 | 22.3 |
| Graduate degree | 1651 | 14.9 |
| Unknown | 39 | 0.4 |
| TREATMENT ASSIGNMENT | 5537 | 50.0 |
| Placebo | ||
| Tamoxifen | 5527 | 50.0 |
| BREAST CANCER RISK | 2709 | 24.5 |
| <= 2% | ||
| 2-5% | 6608 | 59.7 |
| >5% | 1747 | 15.8 |
| COMORBID CONDITIONS | 8701 | 78.6 |
| LIFE-THREATENING ILLNESS | 256 | 2.3 |
| LIVING ALONE | 1717 | 15.5 |
| EMPLOYMENT STATUS | 3808 | 34.4 |
| Disabled/Homemaker/Medical leave/Retired/Unemployed | ||
| Full-time/Part-time/Student | 7216 | 65.2 |
| Unknown | 40 | 0.4 |
| OCCUPATION | 842 | 7.6 |
| Operator/Fabricator/Laborer/Other | ||
| Homemaker/Managerial/Service/Technical, sales, admin. support | 10182 | 92.0 |
| Unknown | 40 | 0.4 |
Short-term adherence to assigned treatment
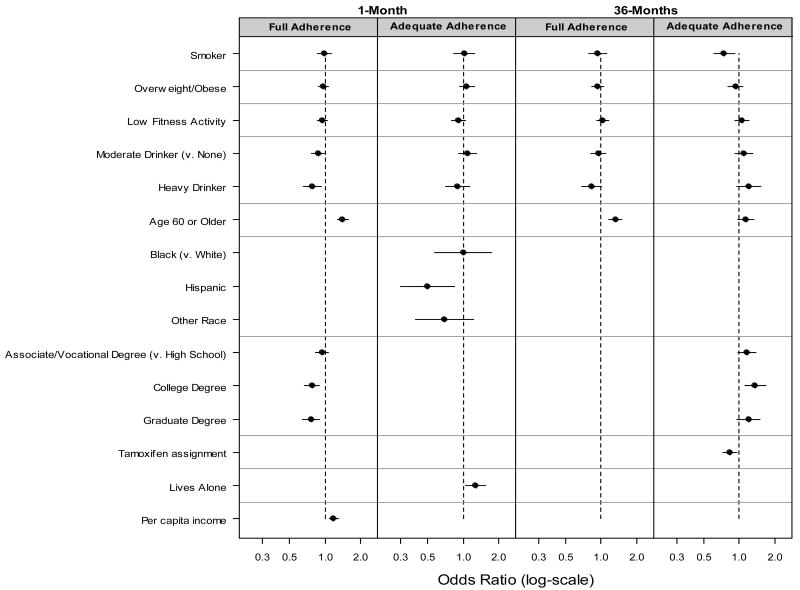
75% of participants were fully adherent at 1 month. In the analyses of health behaviors and adherence, without adjusting for sociodemographic and medical factors, baseline alcohol use was associated with decreased 1 month full adherence (p=.034). Smoking, weight, and physical activity were not significantly associated with full adherence. In the analysis of 1-month full adherence adjusted for other factors, alcohol use remained significant (p=.016; odds ratio [OR] .87 for moderate drinkers, OR=.79 for heavy drinkers relative to non-drinkers). Significant sociodemographic variables were age, education, and per-capita household income. (Table 2) Figure 1 provides multivariable odds ratios and 95% confidence intervals. At 1 month, 91% of participants were adequately adherent. One-month adherence was associated with race and living alone, but was not significantly associated with the lifestyle behaviors of interest.
Table 2.
Significance tests in full multivariable models of adherence in the NSABP BCPT trial at 1 and 36 months. The four lifestyle variables of interest are included. Medical and demographic variables that were significant at p<.1 in preliminary models (models not including lifestyle factors) are included.
| 1 month | 36 months | |||
|---|---|---|---|---|
| Full | Adequate | Full | Adequate | |
| Smoking | .73 | .84 | .43 | .003 |
| Overweight/Obese | .60 | .29 | .20 | .39 |
|
| ||||
| Low physical activity | .33 | .21 | .55 | .42 |
| Alcohol | .016 | .13 | .19 | .38 |
| Age (>=60) | <.001 | - | <.001 | .024 |
|
| ||||
| Race | 0.15 | .043 | - | - |
|
| ||||
| Education | <.001 | - | - | .037 |
|
| ||||
| Treatment assignment | - | - | - | .008 |
|
| ||||
| Breast cancer risk | - | - | - | <.001 |
|
| ||||
| Co-morbid conditions | - | - | - | .059 |
|
| ||||
| Single habitation | .091 | .015 | - | .074 |
|
| ||||
| Not employed | .099 | - | - | .11 |
|
| ||||
| Per capita income | <.001 | - | - | - |
Figure 1.
Odds ratios with 95% confidence intervals for full and adequate adherence, based on full multivariable models. Odds ratios are shown for variables that retained significance (p<.05) in the full models. Odds ratio for income is per $30,000 in annual per capita household income.
Long-term adherence to assigned treatment
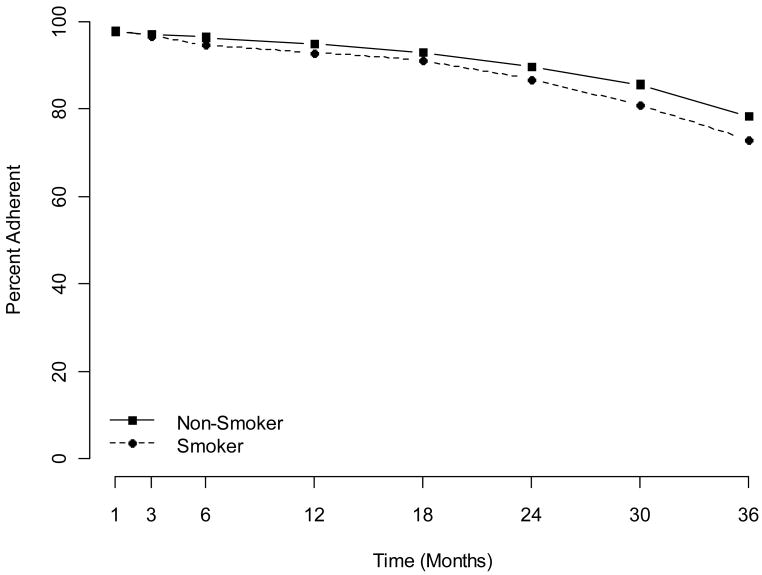
At 36 months, full adherence was 41%. In the model for 36-month adherence, including demographic and medical factors, only age was associated with full adherence. (Table 2, Figure 1) At 36 months, adequate adherence 79%. Without adjusting for other factors, 36-month adequate adherence was associated with baseline smoking (p<.001). Figure 2 reveals the gradual decline in adherence over time for women in the study, according to smoking status. In the full model, smoking retained significance (p=.003; OR=.75). From that full model, estimated adequate adherence was 80.1% for women whose weight was low/normal, 78.9% for overweight/obese; 77.9%, 79.5%, 80.9% for women whose alcohol consumption was none, moderate, or high; and 79.9%, 74.9% for non-smokers and smokers, respectively. In addition, women were less likely to adhere if they were younger than age 60, less educated, or were assigned to tamoxifen. (Table 2; Figure 1)
Figure 2.
Adequate adherence over time according to baseline smoking status.
Reasons for non-adherence
Adverse reactions and illness were the most frequently reported problems causing inadequate adherence at 36 months. (Table 3) Forgetting was the primary reason given for less than full adherence at 36 months (62%). The same patterns appeared at 1 month (data not shown). In the content analysis of “other problems;” 6 of 96 forms indicated cost issues and 8 indicated a loss of confidence in the study following negative publicity.
Table 3.
Problems reported for inadequate adherence at 36 months.
| Reason | % Endorsed |
|---|---|
| Adverse reaction | 22% |
| Illness | 17% |
| Testing efficacy | 12% |
| Forgot (usual schedule) | 10% |
| Forgot (disrupted schedule) | 11% |
| No medication | 3% |
| Lacked information | 1% |
| Non-acceptance | 2% |
| Emotions | 9% |
| Other priorities | 8% |
| Social criticism | 2% |
| Decision to omit dose | 2% |
| Negative thoughts | 4% |
| Not yet known | 2% |
| Other problem | 16% |
Discussion
This examination of chemoprevention adherence in the NSABP BCPT revealed that heavy alcohol users were less likely to adhere fully in the short term, and cigarette smokers were less likely to adhere adequately in the long term, whereas obesity and physical activity were not associated with adherence. A detailed pill adherence sub-study of 100 participants in the International Breast Intervention Study (IBIS), a European study of tamoxifen for prevention of breast cancer, also reported lower adherence for women who smoked.30 In the second NSABP breast cancer prevention trial, the Study of Tamoxifen and Raloxifene (STAR; also known as P-2), women with a smoking history were less likely to persist in taking their assigned drug.31 Smoking has also been associated with poor adherence to breast cancer screening.32 For those women who do smoke and yet adhere to chemoprevention, it is unclear whether this inconsistent behavior is due to differing perceptions of personal risk of breast cancer versus diseases more commonly associated with smoking, or of the relative costs and challenges of smoking cessation versus chemoprevention adherence. It has been observed that a person's perceived risk tends to reflect underestimation or optimistic bias relative to objective risk.33
The IBIS investigators did not examine the impact of alcohol, leisure-time physical activity, or obesity, and we found no other studies examining associations between these behaviors and chemoprevention adherence. Our results regarding other medical and demographic factors confirmed associations observed in IBIS including age and risk of breast cancer. STAR, as well as several studies regarding adjuvant tamoxifen therapy and other medications, found greater persistence or adherence for older patients5,6,31,34-37 although inverse or U-shaped associations have also been observed.3,38 Veronesi reported that breast cancer patients taking tamoxifen in an adjuvant therapy trial were more likely to adhere than were women in a chemoprevention trial, which is consistent with our observation that higher perceived health risk increases adherence.39 Similarly in STAR, persistence was higher among those with higher predicted breast cancer risk.31 Although having higher income was positively associated with adherence, we found inconsistent associations with education: an inverse association in the short term, positive association in the long term. Past literature in other settings has also not consistently reported a positive association with education.40,41 Our finding that being Hispanic was associated with worse short-term adherence in our study was consistent with literature, but other studies have also reported associations with Black race as well as marital status.37,38,41 Some past studies have been observational, reflecting adherence in general clinical practice rather than in the clinical trial setting, which also might account for differences.
We considered both full and adequate adherence to be of interest because this study has implications for adherence to oral therapies in general, and in other settings higher levels of adherence may be necessary to achieve efficacy. Therefore, although 75% adherence might be the most clinically important endpoint for tamoxifen, we also provide results for full adherence.
The lack of strong associations with obesity and physical activity suggest that poor adherence is not simply based on a pattern of unhealthy behavior in general, but could be related to common sociological, psychological, biological, or genetic mechanisms that impact both substance use and medication adherence. The present report was limited to baseline factors – those factors that would enable a care provider to predict poor adherence at the start of treatment. Separately, we will report associations of adherence with the participant's experience while on treatment, including quality of life and symptoms. In that report, we will examine whether smoking and alcohol use were associated with worse symptoms and quality of life, mediating their effects on adherence. Past studies have examined how emotional factors influence decision making about tamoxifen.42,43 Differences in emotional factors and symptoms might also help explain the higher adherence among older women. Older women had lower levels of emotional distress and fewer vasomotor symptoms.28
The effectiveness of breast cancer chemoprevention for high-risk women has been demonstrated,9,44 but that benefit will only be translated to the general population if women adopt and adhere to a chemopreventive agent. While we are ultimately concerned with adherence to the 5-year regimen in clinical practice, our study examined adherence in the context of a placebo-controlled clinical trial. In clinical practice, adherence might be supported because all patients are receiving a proven therapy. However, given the active adherence promotion by BCPT headquarters and institutional clinical staff, and other factors noted in the literature, it is likely to have been higher in the BCPT.45 Our report does not address strategies to improve adherence in clinical practice, and does not provide data beyond three years, after which adherence might continue to decline. It is worth noting that in our context, even women who were of healthy weight, non-smoking, moderate alcohol users, white, older than 60, and college educated had some problems with adherence. However, the need to better define the patient groups at risk for non-adherence has been noted in the literature.40,46 Additional adherence interventions targeting women who smoke cigarettes and drink alcohol and addressing the underlying factors that lead to the unhealthy behaviors may be necessary to maximize the benefits of chemoprevention treatment.
Acknowledgments
The authors gratefully acknowledge the courageous participants, without whom this trial could not have been accomplished; Lynne Anderson, Data Manager; Barbara C. Good, PhD, Director of Scientific Publications for the NSABP, and Wendy L. Rea, Editorial Assistant for the NSABP. None of the acknowledged people was compensated beyond normal salary for this work.
This work was supported by Public Health Service Grants U10-CA-37377, U10-CA-69974, and R03CA134199-02 from the National Cancer Institute, Department of Health and Human Services.
Authors' research support: Public Health Service Grants U10-CA-37377 (SRL, WC, DLW, JPC, NC, PAG) U10-CA-69974 (SRL, WC, DLW, JPC, NC, PAG), and R03CA134199-02 (SRL, WC, NC, PAG) from the National Cancer Institute, Department of Health and Human Services.
Footnotes
Conflicts of interest: SR Land: None. WM Cronin: None. DL Wickerham: Astra Zeneca Speakers' Bureau and Eli Lilly Consultant/Advisory Board. JP Costantino: None. NJ Christian: None. Wm MP Klein: None. PA Ganz: None.
The authors retain the right to provide a copy of the final manuscript to the NIH upon acceptance for journal publication, for public archiving in PubMed Central as soon as possible but no later than 12 months after publication by the journal.
Confidential: Not to be distributed or submitted without explicit permission of the NSABP Operations Office.
References
- 1.Visvanathan K, Chlebowski RT, Hurley P, Col NF, Ropka M, Collyar D, et al. American society of clinical oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235–58. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–62. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 3.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–8. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer. 2007;109:832–9. doi: 10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]
- 5.Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99:215–20. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 6.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19:322–8. doi: 10.1200/JCO.2001.19.2.322. [DOI] [PubMed] [Google Scholar]
- 7.Bryant J, Fisher B, Dignam J. Duration of adjuvant tamoxifen therapy. J Natl Cancer Inst Monogr. 2001:56–61. doi: 10.1093/oxfordjournals.jncimonographs.a003462. [DOI] [PubMed] [Google Scholar]
- 8.Thompson AM, Dewar J, Fahey T, McCowan C. Association of poor adherence to prescribed tamoxifen with risk of death from breast cancer. American Society of Clinical Oncology Breast Cancer Symposium; 2007. [Google Scholar]
- 9.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 10.Clark MM, Niaura R, King TK, Pera V. Depression, smoking, activity level, and health status: pretreatment predictors of attrition in obesity treatment. Addict Behav. 1996;21:509–13. doi: 10.1016/0306-4603(95)00081-x. [DOI] [PubMed] [Google Scholar]
- 11.Coulson NS, Eiser C, Eiser JR. Diet, smoking and exercise: interrelationships between adolescent health behaviours. Child Care Health Dev. 1997;23:207–16. doi: 10.1111/j.1365-2214.1997.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 12.Lahti-Koski M, Pietinen P, Heliovaara M, Vartiainen E. Associations of body mass index and obesity with physical activity, food choices, alcohol intake, and smoking in the 1982-1997 FINRISK Studies. Am J Clin Nutr. 2002;75:809–17. doi: 10.1093/ajcn/75.5.809. [DOI] [PubMed] [Google Scholar]
- 13.Streater JA, Jr, Sargent RG, Ward DS. A study of factors associated with weight change in women who attempt smoking cessation. Addict Behav. 1989;14:523–30. doi: 10.1016/0306-4603(89)90072-5. [DOI] [PubMed] [Google Scholar]
- 14.French SA, Hennrikus DJ, Jeffery RW. Smoking status, dietary intake, and physical activity in a sample of working adults. Health Psychol. 1996;15:448–54. doi: 10.1037//0278-6133.15.6.448. [DOI] [PubMed] [Google Scholar]
- 15.Blair SN, Goodyear NN, Wynne KL, Saunders RP. Comparison of dietary and smoking habit changes in physical fitness improvers and nonimprovers. Prev Med. 1984;13:411–20. doi: 10.1016/0091-7435(84)90032-x. [DOI] [PubMed] [Google Scholar]
- 16.Lazarus NB, Kaplan GA, Cohen RD, Leu DJ. Smoking and body mass in the natural history of physical activity: prospective evidence from the Alameda County Study, 1965-1974. Am J Prev Med. 1989;5:127–35. [PubMed] [Google Scholar]
- 17.Klesges RC, Eck LH, Isbell TR, Fulliton W, Hanson CL. Smoking status: effects on the dietary intake, physical activity, and body fat of adult men. Am J Clin Nutr. 1990;51:784–9. doi: 10.1093/ajcn/51.5.784. [DOI] [PubMed] [Google Scholar]
- 18.Perkins KA, Rohay J, Meilahn EN, Wing RR, Matthews KA, Kuller LH. Diet, alcohol, and physical activity as a function of smoking status in middle-aged women. Health Psychol. 1993;12:410–5. doi: 10.1037//0278-6133.12.5.410. [DOI] [PubMed] [Google Scholar]
- 19.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 20.Costantino JP, Gail MH, Pee D, Anderson S, Redmond CK, Benichou J, et al. Validation studies for models projecting the risk of invasive and total breast cancer incidence. J Natl Cancer Inst. 1999;91:1541–8. doi: 10.1093/jnci/91.18.1541. [DOI] [PubMed] [Google Scholar]
- 21.Gail MH, Costantino JP, Bryant J, Croyle R, Freedman L, Helzlsouer K, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91:1829–46. doi: 10.1093/jnci/91.21.1829. [DOI] [PubMed] [Google Scholar]
- 22.Land S, Wieand HS, Day R, Ten Have T, Costantino JP, Lang W, et al. Methodological issues in the analysis of quality of life data in clinical trials: Illustrations from the National Surgical Adjuvant Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial. In: Mesbah M, Cole B, Lee M, editors. Statistical Design, Measurement and Analysis of Health Related Quality of Life. Kluwer Academic Publishers; 2002. pp. 71–85. [Google Scholar]
- 23.Land S, Ganz PA. Quality of life issues with endocrine chemoprevention. In: Morrow M, Jordan VC, editors. Managing Breast Cancer Risk. Hamilton, Ontario, Canada: BC Decker, Inc; 2003. [Google Scholar]
- 24.Fabian C, Sternson L, El-Serafi M, Cain L, Hearne E. Clinical pharmacology of tamoxifen in patients with breast cancer: correlation with clinical data. Cancer. 1981;48:876–82. doi: 10.1002/1097-0142(19810815)48:4<876::aid-cncr2820480403>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 25.Guerrieri-Gonzaga A, Baglietto L, Johansson H, Bonanni B, Robertson C, Sandri MT, et al. Correlation between tamoxifen elimination and biomarker recovery in a primary prevention trial. Cancer Epidemiol Biomarkers Prev. 2001;10:967–70. [PubMed] [Google Scholar]
- 26.Dietary Guidelines for Americans 2005. U.S. Department of Health and Human Services and U.S. Department of Agriculture; 2005. [Google Scholar]
- 27.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
- 28.Day R, Ganz PA, Costantino JP, Cronin WM, Wickerham DL, Fisher B. Health-related quality of life and tamoxifen in breast cancer prevention: a report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17:2659–69. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 29.Cella D, Land SR, Chang CH, Day R, Costantino JP, Wolmark N, et al. Symptom measurement in the Breast Cancer Prevention Trial (BCPT) (P-1): psychometric properties of a new measure of symptoms for midlife women. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-007-9682-9. [DOI] [PubMed] [Google Scholar]
- 30.Maurice A, Howell A, Evans DG, O'Neil AC, Scobie S. Predicting compliance in a breast cancer prevention trial. Breast J. 2006;12:446–50. doi: 10.1111/j.1075-122X.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 31.Cronin WM, Cecchini RS, Wickerham DL, Vogel VG, Costantino JP, Wolmark N. Factors associated with participant adherence in the NSABP Study of Tamoxifen and Raloxifene (STAR). American Society of Clinical Oncology (ASCO) Annual Meeting; 2008. [Google Scholar]
- 32.Caleffi M, Ribeiro RA, Bedin AJ, Jr, Viegas-Butzke JM, Baldisserotto FD, Skonieski GP, et al. Adherence to a breast cancer screening program and its predictors in underserved women in southern Brazil. Cancer Epidemiol Biomarkers Prev. 2010;19:2673–9. doi: 10.1158/1055-9965.EPI-10-0338. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein ND, Klein WM. Unrealistic optimism: Present and future. Journal of Social and Clinical Psychology. 1996;15:1–8. [Google Scholar]
- 34.Doro P, Benko R, Kosik E, Matuz M, Toth K, Soos G. Utilization of oral antihyperglycemic drugs over a 7-year period (1998-2004) in a Hungarian population and adherence to drug therapy. Eur J Clin Pharmacol. 2005;61:893–7. doi: 10.1007/s00228-005-0031-9. [DOI] [PubMed] [Google Scholar]
- 35.Sirey JA, Bruce ML, Alexopoulos GS, Perlick DA, Friedman SJ, Meyers BS. Stigma as a barrier to recovery: Perceived stigma and patient-rated severity of illness as predictors of antidepressant drug adherence. Psychiatr Serv. 2001;52:1615–20. doi: 10.1176/appi.ps.52.12.1615. [DOI] [PubMed] [Google Scholar]
- 36.Bardel A, Wallander MA, Svardsudd K. Factors associated with adherence to drug therapy: a population-based study. Eur J Clin Pharmacol. 2007;63:307–14. doi: 10.1007/s00228-006-0246-4. [DOI] [PubMed] [Google Scholar]
- 37.Morris AB, Li J, Kroenke K, Bruner-England TE, Young JM, Murray MD. Factors associated with drug adherence and blood pressure control in patients with hypertension. Pharmacotherapy. 2006;26:483–92. doi: 10.1592/phco.26.4.483. [DOI] [PubMed] [Google Scholar]
- 38.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–6. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 39.Veronesi A, Pizzichetta MA, Ferlante MA, Zottar M, Magri MD, Crivellari D, et al. Tamoxifen as adjuvant after surgery for breast cancer and tamoxifen or placebo as chemoprevention in healthy women: different compliance with treatment. Tumori. 1998;84:372–5. doi: 10.1177/030089169808400312. [DOI] [PubMed] [Google Scholar]
- 40.Ulfvarson J, Bardage C, Wredling RA, von Bahr C, Adami J. Adherence to drug treatment in association with how the patient perceives care and information on drugs. J Clin Nurs. 2007;16:141–8. doi: 10.1111/j.1365-2702.2005.01477.x. [DOI] [PubMed] [Google Scholar]
- 41.Kulkarni SP, Alexander KP, Lytle B, Heiss G, Peterson ED. Long-term adherence with cardiovascular drug regimens. Am Heart J. 2006;151:185–91. doi: 10.1016/j.ahj.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 42.Bober SL, Hoke LA, Duda RB, Regan MM, Tung NM. Decision-making about tamoxifen in women at high risk for breast cancer: clinical and psychological factors. J Clin Oncol. 2004;22:4951–7. doi: 10.1200/JCO.2004.05.192. [DOI] [PubMed] [Google Scholar]
- 43.Rondanina G, Puntoni M, Severi G, Varricchio C, Zunino A, Feroce I, et al. Psychological and clinical factors implicated in decision making about a trial of low-dose tamoxifen in hormone replacement therapy users. J Clin Oncol. 2008;26:1537–43. doi: 10.1200/JCO.2007.13.6739. [DOI] [PubMed] [Google Scholar]
- 44.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. Jama. 2006;295:2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 45.Horwitz RI, Horwitz SM. Adherence to treatment and health outcomes. Arch Intern Med. 1993;153:1863–8. [PubMed] [Google Scholar]
- 46.Murthy V, Bharia G, Sarin R. Tamoxifen non-compliance: does it matter? Lancet Oncol. 2002;3:654. doi: 10.1016/s1470-2045(02)00895-1. [DOI] [PubMed] [Google Scholar]