Abstract
The packing of spheres is a subject that has drawn the attention of mathematicians and philosophers for centuries, and that currently attracts the interest of the scientific community in several fields. At the nanoscale, the packing of atoms affect the chemical and structural properties of the material, and hence, its potential applications. This report describes the experimental formation of five-fold nanostructures by the packing of interpenetrated icosahedral and decahedral units. These nanowires, formed by the reaction of a mixture of metal salts (Au and Ag) in the presence of oleylamine, are obtained when the chemical composition is specifically Ag/Au=3/1. The experimental images of the icosahedral nanowires have a high likelihood with simulated electron micrographs of structures formed by two or three Boerdijk-Coxeter-Bernal helices roped on a single structure, whereas for the decahedral wires, simulations using a model of adjacent decahedra match the experimental structures. To our knowledge, this is the first report of the synthesis of nanowires formed by the packing of structures with five-fold symmetry. These icosahedral nanowire structures remind those of quasicrystals that can only be formed if at least two atomic species are present and in which icosahedral and decahedral packing has been found for bulk crystals.
Keywords: Boerdijk-Coxeter-Bernal helix, nanowires, icosahedra, decahedra, aberration corrected Electron Microscopy
A fascinating problem in material sciences is the one concerning the packing of spheres to produce a dense structure. Although this problem has been studied probably since the ancient world, the first scientific analyses came from the Kepler conjecture: how to stack cannon balls of equal size with the highest packing density. The solution is the ABC FCC stacking with a packing density of 0.7405. Packing of spheres has important applications in structure of liquids, crystals, glasses and biological systems among others, for which the reader is referred to the excellent review by Torquato and Stilinger.1
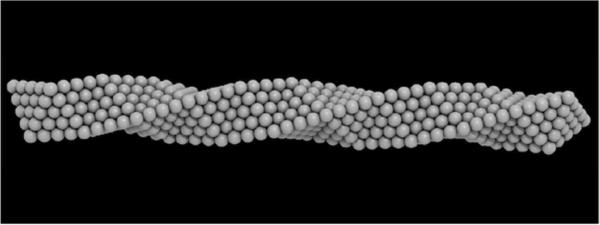
Back in 1952, Boerdijk,2 described a particular type of packing in which if spheres are forming a tetrahedra then it is possible to pack regular tetrahedra with a helical structure. If we start with a reference point in the center of the first tetrahedron, then a second tetrahedron can be stacked in such a way that one of its faces in the original position coincides with another of its faces on the second position. We can stack tetrahedra along a screw axis by rotating each tetrahedron by an angle of 131° 49' with respect to its immediate neighbor. The resulting structure is known as the Boerdijk-Coxeter helix, after the work of Coxeter who described similar structures when studying five-dimensional polytopes.3 This is the first known structure that establishes a direct relation between helices and close packing; an atomistic representation of this structure is shown in Figure 1.
Figure 1.
Boerdijk–Coxeter Bernal helix. The BCB structure is made of tetrahedral sections sharing faces and following a straight line.
Tetrahedra cannot fill Euclidean space regularly; however, in a positively curved space they make a regular polytope {335} in which each object is immersed in an icosahedral environment.4 In Euclidian space structures made with this type of helicoidal chains can be accommodated only by the introduction of topological defects. River and Sadoc4 have used the polytype {335} to describe biological helices such as the -helix and the collagen helix, and the Boerdijk-Coxeter3 spiral was used by Lord and Ranganathan,5 to describe the γ-Brass structure. This structure was popularized by Bernal in his description of the structure of liquids,6 and here we suggest that the structure should be called Boerdijk-Coxeter-Bernal helix, or BCB, in short.
Recently, Torquato and Yiao7, 8 studied the packing of platonic and Archimedean solids. A very significant result is that, this packing produces a much larger density (ϕ) which can eventually be as large as 0.95. The fact that the tetrahedron has a large aspheric and lack of central symmetry contributes to increase the packing of tetrahedral, which leads to a pentagon bipyramid or a decahedron. It is well known that tetrahedra do not fill the Euclidean space. For instance, when five tetrahedra are stacked in a cyclic way (twinning) the resulting decahedra is left with a 7°36' internal gap. Twelve interpenetrating decagonal pyramids result in an icosahedron. In a recent work, Haji-Akdari et. al.9 have shown that packing of tetrahedra will result in quasicrystalline phases; in particular they report the forming of a decagonal quasicrystal. The packing of icosahedra was discussed theoretically by Bilalbejovic,10 using Monte Carlo calculations to study the aggregation and it was found that linear, nanowire-like structures are produced. However, no mention is made of any connection with the BCB helices.
On the experimental side most of the work has been related to model systems, pioneered by Bernal about 50 years ago.6 More recently, an experimental helical structure of gold nanowires was reported by Kondo and Takanayagi.11 These authors synthesized helical multishell gold nanowires in an ultrahigh vacuum; the thinnest wire they were able to make was 0.6 nm in diameter, corresponding to a two-shell nanowire. This kind of structures consists of a tube formed by atoms coiled around the nanowire axis. According to the theoretical models of these structures the axis has a central line of atoms (much like a coaxial cable).12, 13
On the synthesis side an important advance has been achieved in growing nanowires. Halder and N. Ravishankar14 introduced a synthesis method based on the reaction between chloroauric acid and oleylamine in a toluene medium. The problem with their method is that the growth is much uncontrolled and nanowires of all sizes are produced along with nanoparticles.
An important improvement was made by Wang and coworkers,15 who reported the growth thin gold nanowires by the reduction of HAuCl4 in oleic acid and oleylamine, which serves both as a reducing agent and a stabilizer. In this case the crystal structure corresponded to a standard fcc bulk, as confirmed by X-Ray diffraction and High Resolution Electron Microscopy.
A similar method for the growth of very thin nanowires of gold was used by Hou et al.,16 Lu et al.17 and Li et al.18 However, they added Ag to further promote linear growth by reducing a complex of AuCl and oleylamine. It has been established that because of Van deer Waals interaction between the ends of the linear oleylamine chains, which are bonded to the AuI ions, the growth of the Au metal takes place in a one-dimensional fashion. The resulting nanowires are fcc in structure and tend to be several micrometers long.
A particularly interesting case is that of the bimetallic nanowires. Krichevski et al.19 have grown Ag/Au nanowires in a proportion 1/1. These authors do not report any deep structural characterization of the nanowires. However, it must be assumed that they are fcc (this point will be discussed later). More recently Hong et al.20 reported the growth of bimetallic Au/Ag nanowires in a proportion Au/Ag = 2/1. In this case a full electron microscope characterization showed that the nanowires are fcc.
Here, we report for the first time the experimental synthesis of gold nanowires which are formed by packing of icosahedra or decahedra along the growth axis. These structures were prepared using wet chemistry synthesis following methods proposed in the literature.14–18 Specifically, we used oleylamine in mixture with AuCl and, following the method of the Xia group,18 we added silver salt to the solution and then we reduced the salt under well controlled conditions. The main differences with respect to previous work were that the reduction was made at a higher temperature, and that the amount of silver was in a mole ratio of Au/Ag was 2:1, compared to the 200:1 ratio used by Li et al.18
RESULTS
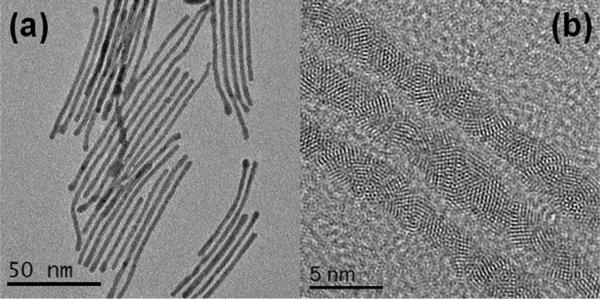
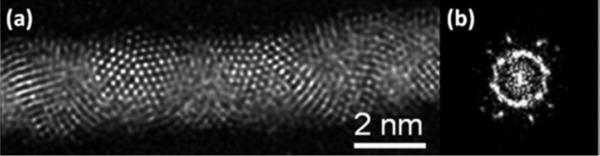
The synthesis resulted on a sample containing 85% of nanowires. The samples were examined by High Resolution Electron Microscopy (HRTEM). A typical image is shown in Figure 2a. The atomic resolution contrast clearly shows a crystal stacking which is not a simple fcc (Figure 2b). The images reveal that the nanowire is made of individual icosahedral units, which are stacked along the growth axis. The HRTEM images of icosahedral particles have been well documented on the literature and can be easily recognized (see for instance the work of Marks21 or Ascencio et al.22). Perhaps the clearest feature is that starting from the characteristic 5-fold symmetry axis and upon rotation a 3-fold and 2- fold axis can be obtained. So in order to confirm the structure we performed a tilting series of the nanowire on the HRTEM mode. The tilting sequence of images is shown in Figure 3. We also show the model of an icosahedral particle along the image as an aid to recognize the five-fold symmetry. It is clear that the nanowire contains elements of icosahedral symmetry.
Figure 2.
(a) TEM image and (b) HRTEM bright field image of the icosahedral nanowires of Ag/Au.
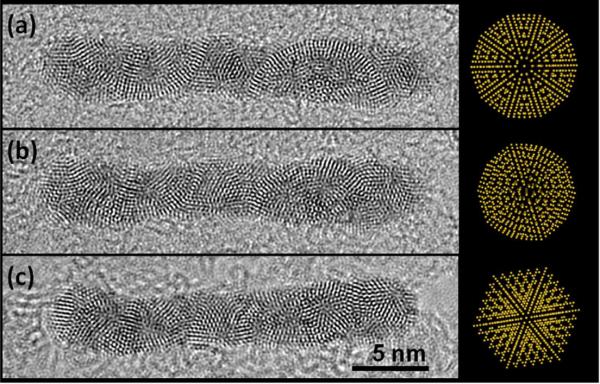
Figure 3.
A tilting sequence of HRTEM images of the Ag/Au nanowires with the icosahedral structure: (a) angle of initial reference; (b) five degree longitudinal rotation; and ten degree longitudinal rotation.
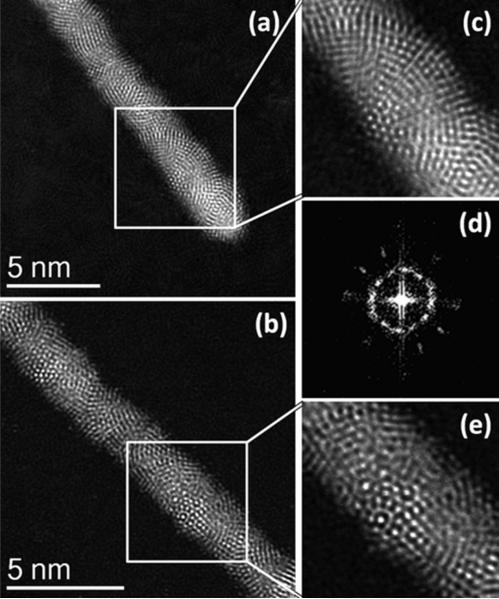
To confirm this observation, we carried out a detailed analysis of the nanowires in an aberration corrected-STEM using HAADF-STEM technique. In this technique the contrast is strongly influenced by the atomic number and mass, and the images do not change significantly with variations in defocus,23 which makes the analysis straightforward. On carrying out high resolution STEM-HAADF imaging of the nanowires we observe clearly regions in five-fold symmetry, as shown in Figure 4. In this figure we show the HAADF-STEM images of two nanowires with atomic resolution (Figure 4a and 4b). Following the structures along the growth axis in the high magnification (Figure 4c and 4e) it was not difficult to realize that the structures correspond to arrays of interpenetrated icosahedra. The two images correspond to two slightly different orientations. We also obtained the FFT along the growth axis of the nanowire of the Figure 4a; the result shown in Figure 4d confirms five-fold symmetry.
Figure 4.
(a) – (b) Aberration corrected STEM-HAADF images of the Ag/Au nanowires with the helical icosahedral structure in different orientations. The images (c) and (e) are the corresponding high magnification areas of the icosahedral packing. The image (d) shows the FFT obtained along the axis of the nanowire of figure 4(a) showing the five fold symmetry.
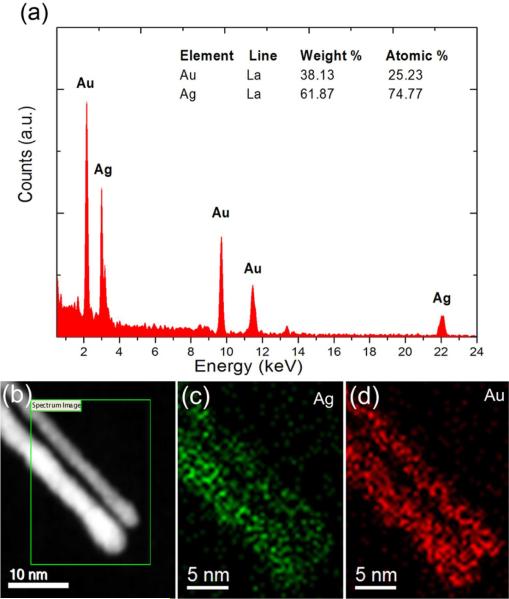
We performed X-Ray analysis in the TEM and a detailed compositional analysis of the spirals was obtained. The result is shown in Figure 5. In the figure we show the EDS spectrum and the mapping of Au and Ag. The results clearly indicate that there is an excess of silver on the spirals. In some cases we measured a majority of silver on the structure with a molar composition of Ag/Au = 3:1. It is also clear that the two elements are homogeneously distributed along the nanowire suggesting an alloy. We perform analysis of more than ten nanowires and the atomic concentration was the same. We consider this very remarkable and in contrast with the authors experience in bimetallic particles, in which the concentration of the two metals seems to vary from particle to particle despite of the initial concentration during the reaction. In the latter case we can only speak of an average concentration, being the composition of every particle different. In the present case the atomic ratio is the same in each structure, which is quite remarkable.
Figure 5.
EDS analysis of two Ag/Au nanowires showing (a) spectrum and the inset showing the concentration of Ag and Au, (b) the STEM image of two nanowires, (c) maps of the Ag L line and (d) Au L line.
In addition to the nanowires with icosahedral stacking we have also observed another type of nanowire with a five-fold symmetry as is shown in Figure 6a. In this case the five-fold symmetry corresponds to that of a decahedron as this type of contrast cannot be explained as resulting from icosahedra stacking. The FFT along a nanowire with decahedral symmetry confirm the helical structure, as shown in Figure 6b.
Figure 6.
(a) Aberration corrected STEM-HAADF image of the helical array of decahedral nanowire, (b) FFT obtained along the axis of the nanowire.
Decahedral nanowires coexist with the icosahedral ones and with fcc nanowires. In a typical sample prepared on the conditions described on this paper 70% of the nanowires will be icosahedral, 20% decahedral and 10% fcc or polycrystalline.
Structural model of the Nanowires
In order to understand the structure of the nanowires reported on the present work, it is important to make some considerations. First, the nanowires found in this work are different from the helical nanowires found experimentally by Kondo and Takanayagi11 and then studied extensively by the Ugarte group.24–26 Those helical structures are only two or three atomic layers and are only produced by strain. Sanchez-Portal et al.27 were the first ones to predict those structures theoretically using first principle simulations under tensile loading. They conclude that these structures will break and cannot be isolated. As shown by Park and Zimermann.28 The helical structure has a large amount of deformation and the atoms have a low coordination number, and because of that and the continuous stress, the atoms cannot create highly packed reoriented surface facets.
The nanowires reported in this work can have a thickness up to 20–30 atomic layers; they are also very long (as much as 50 nm). They are not the result of strain. Therefore a new structure has to be considered. A first simple model is that the growth under the conditions described on this paper induces icosahedral and decahedral particles. The nanowires would be then the result of coalescence of icosahedral and decahedral particles. A model of the nanowire formed by joining the decahedra by their (111) facets certainly induces a spiral but do not explain the observed contrast. A model is shown in the supplementary information (Figure S2). In particular the “fan” type of contrast so conspicuous in the images is not reproduced by this model.
A third model can be obtained by winding together like a rope two or more B-C-B spirals and then relaxing the structure. A model is shown in Figure 7a for the case of two spirals. The model that results is that of interpenetrating icosahedra, which corresponds to truly icosahedral growth. This model reproduces remarkable well the contrast and the FFT as shown in Figure 7b–c. As can be seen the “fan-like” 180° contrast is fully reproduced. A similar model can be obtained for the decahedral packing, which is formed by the interpenetration of decahedral spirals shown in the Figures 7d–e.
Figure 7.
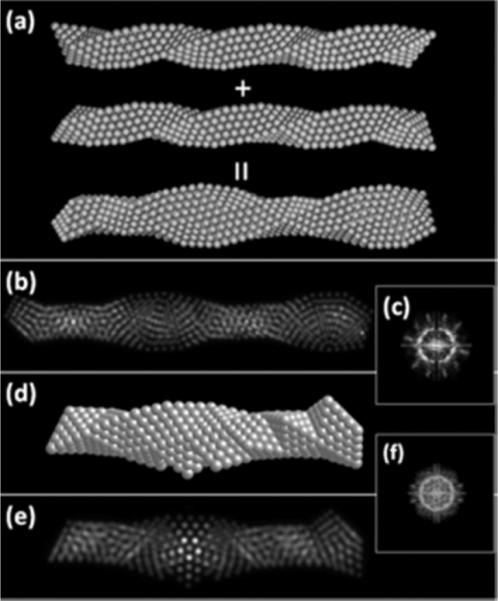
(a) Construction of a helical nanowire by the entanglement of Boerdijk-Coxeter-Bernal helices. In the figure, two BCB structures are shown before and after the entanglement. (b) Simulated STEM image of the model. (c) Simulated diffraction pattern of the structure. (d) A model of the decahedral nanowire structure. (e) Simulated image of the decahedral nanowire structure. (f) Simulated diffraction pattern of the decahedral nanowire structure
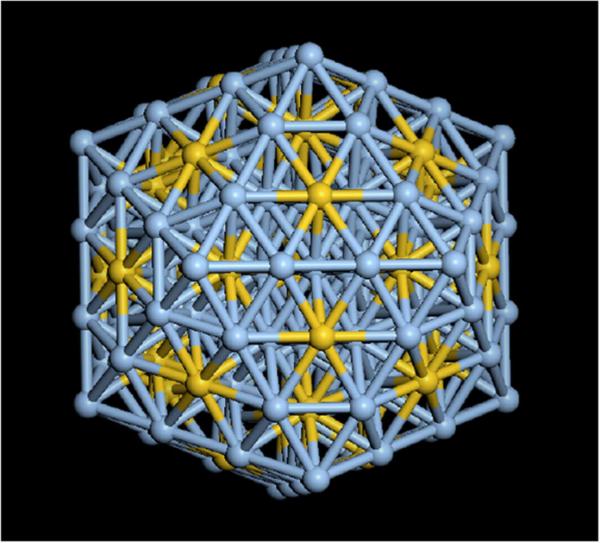
In order to understand the role of the atomic composition of silver-gold, we have performed energetic calculations on an icosahedral structure of 147 atoms with an Ag/Au ratio of 3:1. At this particular ratio of elements, it is possible to distribute both atomic species in such a way that all the tetrahedra forming the icosahedron are constituted by 1 atom of gold and 3 atoms of silver. The resulting structure is shown in Figure 8. We performed a Molecular Dynamics simulation run on this structure, using the many-body Sutton-Chen potential to describe the atomic interactions. Following the conditions of the experimental measurements the simulation was performed at 300 K, and the average configurational energy was measured and compared against the energies of two icosahedral particles of the same size and composition, but with a random elemental distribution in one particle, and an Aucore - Agshell distribution on the other. We found that the configurational energy is very similar in the three cases, around 2.8 eV per atom. These results indicate that at the particular Ag/Au ratio of 3:1, the uniformly distributed structure is energetically as possible as two of the most likely alternatives. The comparison of these results against other relative concentrations will improve the understanding of the predominance of this ratio in the experimental structures, and future work of this group this will be devoted to this issue.
Figure 8.
147 atom icosahedron with an Ag/Au=3:1 elemental ratio. Each tetrahedron in the structure is made of 3 silver atoms and 1 gold atom.
DISCUSSION
It appears that the addition of silver in the concentration Ag/Au increases the stability of icosahedral and decahedral structures. During most solution phase synthesis an equilibrium condition is not established. Under these conditions, structures which are not the equilibrium shape (Wulff polyhedron) such as icosahedra are formed.29 In these structures, defects such as twins and steps lower the total free energy. In the case of Au, it is possible to grow very large particles with icosahedral or decahedral structure since new types of defects appear as size increases.30 The main reason is that (111) facets are preferred in gold. The case of silver (100) is also prominent and in most cases the particles tend to be pyramids or triangles, and in rare cases icosahedra or decahedra are formed. However, in a recent paper Cerbelaud et al.31 using DFT full calculations demonstrated that in Ag/Au alloy nanoparticles the icosahedral structure can be generated for ratios such as Ag/Au=5.3, Ag/Au=2.8, and Ag/Au=2.16, which is in agreement with our results. It has been reported by Huo et al.16 that during the growth of nanoparticles and nanowires using a mixing of AuCl and oleylamine, a mesostructure is formed and the gold ions are assembled within the oleylamine bilayers. This mesostructure serves as a template for the growth in one dimension, resulting in nanowires. A similar mechanism was proposed by Lu et al.17 When only Au was used, fcc nanowires are formed. However in our case when AgCl is added to the solution the mesostructure will be formed but containing Au+ and Ag+ ions. In some regions, isolated units containing Au and Ag will be formed between them, separated by the oleylamine bilayer. When the reduction takes place the atoms will tend to form icosahedra. Then when new atoms arrive the icosahedral growth will continue in one direction templated by the oleylamine bilayer. The situation is similar to the formation of quasicrystals which are produced only when at least two metals are present.32
Properties of Icosahedral nanowires
We have made some initial research on the properties of the icosahedral nanowires reported on this paper. In particular we have examined their properties as Surface Enhanced Raman Spectroscopy (SERS) substrates. Metallic substrates play a fundamental role in detecting very low concentration of molecules.33 Recently Gunawidjaja et al.34 have shown that gold nanowires mixed with silver nanoparticles are a very efficient substrate. According to those authors the Raman intensity of the best-performing silver–gold nanostructure is comparable with the dense array of silver nanowires and silver nanoplates that were prepared by means of the Langmuir–Blodgett technique. An optimized design of a single-nanostructure substrate for SERS, based on a wet-assembly technique proposed here, can serve as a compact and low-cost alternative to fabricated nanoparticle array. However, by looking at the micrographs of the samples on reference 34 it is clear that the sample might be very inhomogeneous and not very reproducible. We have found that icosahedral bimetallic nanowires are extremely efficient SERS substrates. We have performed measurements using the standard rhodamine 6G (Rh6G) by Raman spectroscopy. The peak assignment of Rh6G adsorbed on colloidal silver is clearly identified: 614 cm−1 (C- C-C) ring in plane bending; 774 cm−1 (C -H in-plane bending); 1129 cm−1 (C–H in-plane bending), and 1183, 1310, 1363, 1509 and 1572 cm−1 (aromatic C -C stretching) among others. We observe a significant amplification of the peaks using icosahedral silver –gold nanowires. The intensity increases when the direction of the polarization of the laser beam is perpendicular to the edge of the nanowires (as expected for an elongated structure). However, because of the very small size of our nanowires it is not possible to align all of them in the proper direction. In any case we estimate an amplification factor of 108–109. A typical SERS spectrum is shown in the supplementary information.
Regarding electrical properties we note the work of Hong et al.20 who obtained fcc bimetallic nanowires and demonstrated that they have remarkable Coulomb blockade effects. A similar property should be expected for five-fold nanowires. We can visualize the technical feasibility of fabricating devices based on the packing of icosahedra and decahedra since the nanowires produced are in a solution and can be manipulated very easily.
CONCLUSIONS
We have shown that using an organometallic mixture it is possible to synthesize nanowires with show true icosahedral and decahedral packing. Just as in the case of bulk quasicrystals the introduction of a second atomic species was necessary to induce the five-fold packing at the nanoscale level. In the present case the composition Ag/Au 3:1 favors this type of packing. However recent theoretical work31 suggest that other concentrations should have similar effects and that other concentration of the metals should produce new structures. We can visualize the technical feasibility of fabricating devices based on the packing of icosahedra and decahedra since the nanowires produced are in a solution and can be manipulated just as in the case of bulk quasicrystals the introduction of a second atomic species was necessary to induce the five-fold packing. It is remarkable that in the case of Ag/Au the composition 3:1 appears to be remarkable stable. New combinations of metals should produce new structures.
METHODS
Synthesis
Au nanowires were synthesized as follows: 6.6 mM of HAuCl4•3H2O and 6.6 mM of AgNO3 were dissolved together in 10 mL of oleylamine (C18H37N) by vortex mixing at room temperature until the color of the solution changed from pale yellow to an intense orange color. This indicated the formation of a complex between Au3+ and OA. Then the solution was heated up to 100°C using an oil bath for 3 hours. During this process the color of the solution changed from orange to pale yellow, indicating the reduction of Au3+ to Au+. The color of the final solution that was obtained was red. This solution was dispersed in ethanol to obtain the black precipitate. This precipitate that was obtained was separated from the solution by centrifugation (3000 rpm) for 15 min and cleaned several times with ethanol and re-dispersed in 2 mL of hexane or chloroform for further analysis.
Electron Microscopy
For the electron microscopy analysis, a drop of the suspension was deposited onto a holey carbon grid. The samples were characterized using a JEOL JEM-2010F (FEG-TEM) operated at 200 kV with a 0.1 nm lattice resolution, which was employed to record high resolution (HRTEM) images and electron diffraction (ED) patterns of the materials. For the aberration (Cs) corrected characterization, the samples were analyzed using scanning transmission electron microscopy (STEM) with a JEOL ARM200F (200 kV) FEG-STEM/TEM, equipped with a CEOS Cs corrector on the illumination system. The probe size used for acquiring the HAADF as well as the BF-STEM images was 9C (23.2 pA) and the CL aperture size was 40μm. High angle annular dark field (HAADF) STEM images were acquired with a camera length of 8 cm/6cm and the collection angle of 68–280 mrad/90–270 mrad was used. The BF-STEM images were obtained using a 3mm/1mm aperture and a collection angle of 17 mrad/5.6 mrad was used (camera length in this case was 8 cm). The HAADF as well as the BF images were acquired using a DigiScan camera. In order to reduce the noise of the images and to obtain clearer images, the raw data was filtered using the 2D Wiener filter and the Richardson-Lucy/Maximum Entropy algorithm implemented by Ishizuka.35 The EDS analysis was performed using EDAX instrumentation attached to the JEOL ARM200F microscope. Spectra, line scans as well as chemical maps for the various elements were obtained using the EDAX Genesis software. For the EDS analysis the probe size used was 0.8 nm and the Condenser Lens aperture size was 40μm.
STEM and Molecular Dynamics simulations
The STEM simulations were performed using the xHREM suite, which is based on the variant of Ishizuka of the FFT multislice method.36 In accordance with the experimental setup, the simulated electron beam was of 200 kV, with a resolution close to 0.1 nm and a defocus of 100 mm. The Molecular Dynamics Simulations were made using the DL_POLY 2.14 code, by Smith and Forester.37 The canonical ensemble runs were made using a Nose-Hoover thermostat with an inertial parameter of 0.1, and a time step of 0.0015 fs. The Sutton-Chen potential was parameterized following the work of Kimura,38 with the usual rules for the definition of the alloy terms.
Supplementary Material
Acknowledgement
The authors would like to acknowledge The Welch Foundation Project # AX-1615. “Controlling the Shape and Particles Using Wet Chemistry Methods and Its Application to Synthesis of Hollow Bimetallic Nanostructures”. The authors would also like to acknowledge the NSF PREM Grant # DMR 0934218, Title: Oxide and Metal Nanoparticles – The Interface between life sciences and physical sciences. The authors would also like to acknowledge RCMI Center for Interdisciplinary Health Research CIHR. “The project described was supported by Award Number 2G12RR013646-11 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources of the National Institutes of Health.”
Footnotes
Supporting Information Available: This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES AND NOTES
- 1.Torquato S, Stilinger FH. Jammed Hard-Particle Packings: From Kepler to Bernal and Beyond. Rev. Mod. Phys. 2010;82:2633–2672. [Google Scholar]
- 2.Boerdijk AH. Some Remarks Concerning Close Packing of Equal Spheres. Philips Res. Rep. 1952;7:303–313. [Google Scholar]
- 3.Coxeter HSM. Regular Complex Polytopes. 1st ed. Cambridge University Press; 1974. 2nd ed. 1991. [Google Scholar]
- 4.Sedoc JF, Rivier N. Boerdijk–Coxeter Helix and Biological Helices as Quasicrystals. Mat. Sci. Eng. 2000;294–296:397–400. [Google Scholar]
- 5.Lord EA, Ranganatan S. The γ-Brass Structure and the Boerdijk–Coxeter Helix. J. of Non-Crystalline Solids. 2004;334–335:121–125. [Google Scholar]
- 6.Bernal JD. Geometry and the Structure of Monatomic Liquids. Nature (London) 1960;185:68–70. [Google Scholar]
- 7.Torquato S, Jiao Y. Exact Constructions of a Family of Dense Periodic Packings of Tetrahedra. Phys. Rev E. 2010;81:041310. doi: 10.1103/PhysRevE.81.041310. [DOI] [PubMed] [Google Scholar]
- 8.Torquato S, Jiao Y. Dense Packings of the Platonic and Archimedean Solids. Nature. 2009;460:876–881. doi: 10.1038/nature08239. [DOI] [PubMed] [Google Scholar]
- 9.Haji Akbari A, Engel M, Keys A, Zheng X, Petschek R, Palffy-Muhoray P, Glotzer SC. Disordered, Quasicrystalline, and Crystalline Phases of Densely Packed Tetrahedra. Nature. 2009;462:773–777. doi: 10.1038/nature08641. [DOI] [PubMed] [Google Scholar]
- 10.Bilalbegovic G. Assemblies of Gold Icosahedra. Comput. Mat. Sci. 2004;31:181–186. [Google Scholar]
- 11.Kondo Y, Takanayagi K. Synthesis and Characterization of Helical Multi-Shell Gold Nanowires. Science. 2000;289:606–608. doi: 10.1126/science.289.5479.606. [DOI] [PubMed] [Google Scholar]
- 12.Bilalbegovic G. Structure and Stability of Finite Gold Nanowires. Phys. Rev. B. 1998;58:15412–15415. [Google Scholar]
- 13.Wang B, Yin S, Wang G, Buldum A, Zhao J. Novel Structures and Properties of Gold Nanowires. Phys. Rev. Lett. 86:2046–2049. doi: 10.1103/PhysRevLett.86.2046. [DOI] [PubMed] [Google Scholar]
- 14.Halder A, Ravishankar N. Ultrafine Single-Crystalline Gold Nanowire Arrays by Oriented Attachment. Advanced Materials. 2007;19:1854–1858. [Google Scholar]
- 15.Wang C, Hu Y, Lieber CM, Sun S. Ultrathin Au Nanowires and Their Transport Properties. J. Am. Chem. Soc. 2008;130:892002–8903. doi: 10.1021/ja803408f. [DOI] [PubMed] [Google Scholar]
- 16.Huo Z, Tsung C, Huang W, Zhang X, Yang P. Sub-Two Nanometer Single Crystal Au Nanowires. Nano. Lett. 2008;8:2041–2044. doi: 10.1021/nl8013549. [DOI] [PubMed] [Google Scholar]
- 17.Lu X, Yavuz MS, Tuan H, Korgel B, Xia Y. Ultrathin Gold Nanowires Can Be Obtained by Reducing Polymeric Strands of Oleylamine-AuCl Complexes Formed via Aurophilic Interaction. J. Am. Chem. Soc. 2008;130:8900. doi: 10.1021/ja803343m. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Tao J, Lu X, Zhu Y, Xia Y. Facile Synthesis of Ultrathin Au Nanorods by Aging the AuCl (oleylamine) Complex with Amorphous Fe Nanoparticles in Chloroform. Nano. Lett. 2008;8:3052–3055. doi: 10.1021/nl8017127. [DOI] [PubMed] [Google Scholar]
- 19.Krichevski O, Tirosh E, Markovich G. Formation of Gold-Silver Nanowires in Thin Surfactant Solution Films. Langmuir. 2006;22:867–870. doi: 10.1021/la051618v. [DOI] [PubMed] [Google Scholar]
- 20.Hong X, Wang D, Yu R, Yan H, Sun Y, He L, Niu Z, Peng Q, Li Y. Ultrathin Au–Ag Bimetallic Nanowires with Coulomb Blockade Effects. Chem. Commun. 2011;47:5160–5162. doi: 10.1039/c1cc10903k. [DOI] [PubMed] [Google Scholar]
- 21.Marks LD. Particle size effects on Wulff constructions Surface Science. 1985 February 2;150(2):358–366. [Google Scholar]
- 22.Ascencio JA, Gutiérrez-Wing C, Espinosa ME, Marín M, Tehuacanero S, Zorrilla C, José Yacamán M. Structure Determination of Small Particles by HREM Imaging: Theory and Experiment. Surface Science. 1998;396:349–368. [Google Scholar]
- 23.Varela M, Lupini AR, Van Benthem K, Borisevich AY, Chisholm MF, Shibata N, Abe E, Pennycook SJ. Materials Characterization in the Aberration-Corrected Scanning Transmission Electron Microscope. Ann. Rev. Mat. Res. 2005;35:539–569. [Google Scholar]
- 24.Legoas SB, Galvão DS, Rodrigues V, Ugarte D. Origin of Anomalously Long Interatomic Distances in Suspended Gold Chains. Phys. Rev. Lett. 2002;88:076105. doi: 10.1103/PhysRevLett.88.076105. [DOI] [PubMed] [Google Scholar]
- 25.Coura PZ, Legoas SB, Moreira AS, Sato F, Rodrigues V, Dantas SO, Ugarte D, Galvão DS. On the Structural and Stability Features of Linear Atomic Suspended Chains Formed from Gold Nanowires Stretching. Nano Letters. 2004;4:1187–1191. [Google Scholar]
- 26.González JC, Rodrigues V, Bettini J, Rego LGC, Rocha AR, Coura PZ, Dantas SO, Sato F, Galvão DS, Ugarte D. Indication of Unusual Pentagonal Structures in Atomic Size Cu Nanowires. Phys. Rev. Lett. 2004;93(126103):1–4. doi: 10.1103/PhysRevLett.93.126103. [DOI] [PubMed] [Google Scholar]
- 27.Sánchez Portal D, Artacho E, Junquera J, Ordejón P, Garcıa A, Soler JM. Stiff Monatomic Gold Wires with a Spinning Zigzag. Geometry. 1999;83:3884–3887. [Google Scholar]
- 28.Park HS, Zimmerman JA. Modeling inelasticity and failure in gold nanowires. Phys. Rev. B. 2005;72:054106. [Google Scholar]
- 29.Marks LD. Modified Wulff constructions for twinned particles. Journal of Crystal Growth. 1983;61:556–566. [Google Scholar]
- 30.Kroner E, Anthony KH. Dislocations and Disclinations in Material Structures: The Basic Topological Concepts. Annu. Rev. Mater. Sci. 1975;5:43–72. [Google Scholar]
- 31.Cerbelaud M, Ferrando R, Barcaro G, Fortunelli A. Optimization of chemical ordering in AgAu nanoalloys. Phys. Chem. Chem. Phys. 2011:1–9. doi: 10.1039/c0cp02845b. [DOI] [PubMed] [Google Scholar]
- 32.Louzguine-Luzgin DV, Inoue A. Formation and Properties of Quasicrystals. Ann Rev. Mater. Res. 2008;38:403–423. [Google Scholar]
- 33.Fan M, Andrade G, Brolo AG. A Review on the Fabrication Of substrates For Surface Enhanced Raman Spectroscopy and Their applications In Analytical Chemistry. Analytica Chimica Acta. 2011;693:7–25. doi: 10.1016/j.aca.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Gunawidjaja Ray., Kharlampieva E, Choi I, Tsukruk VV. Bimetallic Nanostructures as Active Raman Markers: Gold-Nanoparticle Assembly on 1D and 2D Silver Nanostructure Surfaces. Small. 2009;5:2460–2466. doi: 10.1002/smll.200900688. [DOI] [PubMed] [Google Scholar]
- 35.Ishizuka K. A Practical Approach For STEM Image Simulation Based on the FFT Multislice Method. Ultramicroscopy. 2001;90:71–83. doi: 10.1016/s0304-3991(01)00145-0. [DOI] [PubMed] [Google Scholar]
- 36.HREM Research Inc. http://www.hremresearch.com.
- 37.Forester TR, Smith W. DL_POLY Molecular Dynamics Code. CCP5. 1995. [Google Scholar]
- 38.Kimura Y. Technical report 3, Caltech ASCI. 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.