Abstract
Given the clinical and public health significance of substance disorders and the need to identify their early risk factors, we examined the association of childhood attention-deficit/hyperactivity disorder (ADHD) with substance use (e.g., nicotine, alcohol) and abuse/dependence outcomes (nicotine, alcohol, marijuana, cocaine, other). To strengthen a potential causal inference, we meta-analyzed longitudinal studies that prospectively followed children with and without ADHD into adolescence or adulthood. Children with ADHD were significantly more likely to have ever used nicotine and other substances, but not alcohol. Children with ADHD were also more likely to develop disorders of abuse/dependence for nicotine, alcohol, marijuana, cocaine, and other substances (i.e., unspecified). Sex, age, race, publication year, sample source, and version of the Diagnostic and Statistical Manual of Mental Disorders (DSM) used to diagnose ADHD did not significantly moderate the associations with substance outcomes that yielded heterogeneous effect sizes. These findings suggest that children with ADHD are significantly more likely to develop substance use disorders than children without ADHD and that this increased risk is robust to demographic and methodological differences that varied across the studies. Finally, few studies addressed ADHD and comorbid disruptive behavior disorders (DBD), thus preventing a formal meta-analytic review. However, we qualitatively summarize the results of these studies and conclude that comorbid DBD complicates inferences about the specificity of ADHD effects on substance use outcomes.
Attention-deficit/hyperactivity disorder (ADHD) is characterized by an early onset of persistent and impairing levels of inattention-disorganization and hyperactivity-impulsivity (American Psychiatric Association, 2000). ADHD occurs in 5-10% of school-aged children (Scahill & Schwab-Stone, 2000) and represents one of the most common referrals for mental health and pediatric services in the U.S. (Barkley, 1998). ADHD is associated with comorbid mood (e.g., depression, anxiety) and disruptive behavior disorders (oppositional defiant disorder [ODD] and conduct disorder [CD]) (DBD), neuropsychological deficits (e.g., verbal working memory), family problems (e.g., negative parent-child interactions), poor academic achievement, and social dysfunction (e.g., peer rejection). These associations have been reported in boys and girls, including as young as preschool who have been followed prospectively into adolescence and young adulthood (Biederman et al., 2010; Lee, Lahey, Owens, & Hinshaw, 2008; Owens, Hinshaw, Lee, & Lahey, 2009). Thus, ADHD predicts a highly dispersed pattern of impairment across behavioral, academic, social, affective, and family domains (i.e., multifinality).
Substance use disorders (SUD) (i.e., abuse and dependence) also constitute a substantial clinical, public health, and economic concern in the United States and globally (Demyttenaere et al., 2004). In 2000, substance dependence specifically accounted for $67 billion in economic loss due to crime, social problems, foster care, and other health services (McLellan, Lewis, O'Brien, & Kleber, 2000). Among 18–59 year-old individuals participating in the National Comorbidity Survey Replication, a nationally-representative study of English-speaking adults in the United States, lifetime prevalence estimates ranged from 14.0% to 16.3% and 6.0% to 6.4% for alcohol abuse and alcohol dependence disorders, respectively (Kessler et al., 2005a). For SUD more broadly (i.e., combining abuse and dependence), the lifetime prevalence in the same age range varied from 15.3% to 18.0% (Kessler et al., 2005a). Although the 12-month prevalence of SUD in the same sample was expectedly lower (0.4% for drug dependence to 3.1% for alcohol abuse disorder), the severity of the disorders, based on functional impairment (e.g., suicide attempts, work disability, poor social relationships), was moderate to severe for most individuals (Kessler et al., 2005b). In terms of clinical significance, SUD are frequently comorbid with other disorders. Substance abuse and dependence was each uniquely associated with increased comorbidity with mood disorders across six countries, and with externalizing problems (e.g., CD, antisocial behavior [ASB]) in the U.S. and Canada specifically (Merikangas et al., 1998). Finally, in addition to comorbidity, substance problems (e.g., binge drinking) are often associated with violence, accidental injuries, risky behavior (e.g., sexually transmitted disease), and poor health outcomes (e.g., hypertension) (Courtney & Polich, 2009). Thus, SUD are highly prevalent, costly, impairing, and resistant to treatment (Goldstein et al., 2009). To facilitate the development of interventions, there is an urgent need to identify precursors of SUD, particularly early in development. Detection of individuals at risk for SUD may facilitate implementation of early, targeted interventions to prevent the onset of SUD or to minimize their negative sequelae.
There are several reasons that ADHD and substance problems may be related. First, dopamine (DA) neurotransmission is central to current models of ADHD and SUD (Bedard et al., 2009; Ray et al., 2010; Volkow, Fowler, Wang, Baler, & Telang, 2008; Volkow et al., 2009) and methylphenidate (MPH) is a highly efficacious treatment for the core symptoms of ADHD, although recent evidence suggests that therapeutic response may be time-limited (Molina et al., 2008). Positron emission tomography (PET) suggests that MPH enhances extracellular DA in the basal ganglia and anterior cingulate gyrus (Volkow, Fowler, Wang, Ding, & Gatley, 2002). MPH, by virtue of activating positive attention networks and distilling task-irrelevant stimuli, improves attention, vigilance, and motivation (Swanson, Baler, & Volkow, 2010). Second, a recent review of neuroimaging studies of humans with ADHD and SUD found replicated evidence of blunted striatal DA release and disrupted neural circuitry between the anterior cingulate cortex and striatum with prefrontal cortex (Frodl, 2010). Rodent and non-human primate models suggest the centrality of deficits in response inhibition, including dysfunctional circuitry in ventrolateral frontal, cingulate cortices, and basal ganglia regions, in both ADHD and SUD (Groman, James, & Jentsch, 2009). Third, offspring of adults with SUD are more likely to develop psychopathology, including ADHD (Clark et al., 1997; Schuckit & Smith, 1996). Elevated substance use problems have also been frequently reported in parents of children with ADHD (Chronis et al., 2003; Lahey et al., 1988; Molina, Pelham, & Lang, 1997). Finally, the prevalence of psychopathology, including SUD, is higher in first-degree relatives of ADHD probands than in healthy controls (Biederman et al., 1992). Therefore, ADHD and SUD may share common etiological influences, including similar genetic factors (Iacono, Malone, & McGue, 2008; Young et al., 2009; see Biederman et al., 2009, for an exception).
In fact, there is a sizable body of research suggesting that ADHD is associated with elevated substance use and related disorders (e.g., Boyle et al., 1993; Clure et al., 1999; Disney, Elkins, McGue, & Iacono, 1999; Katusic et al., 2005; Mannuzza, Klein, Bessler, Malloy, & LaPadula, 1993; Milberger et al., 1997a,b; Whalen, Jamner, Henker Delfino, & Lozano, 2002). In a large (n = 240) case-control study, children with ADHD were two times more likely to develop substance dependence disorders than matched controls (Biederman et al., 2006). ADHD was also robustly related (odds ratio > 9) to the likelihood of having an SUD in a study of 968 male adolescents in Brazil (Szobot et al., 2007). Thompson, Riggs, Mikulich, and Crowley (1996) assessed 171 adolescents with CD in a residential treatment program and found that ADHD was significantly associated with severe CD and substance problems. Similarly, ADHD was associated with severe substance dependence in a sample of 367 clinic-referred male and female adolescents (Whitmore et al., 1997). However, null associations between ADHD and substance problems have also been reported. In a sample of 1,302 12-16 year-old adolescents, ADHD was unrelated to substance use and related problems (Boyle & Offord, 1991). Similarly, in a prospective study of adolescents diagnosed with ADHD when they were 7-11 years old, maltreatment, but not childhood ADHD, independently predicted substance problems (De Sanctis et al., 2008). However, the inconsistent association between ADHD and SUD may also reflect methodological variability across studies including sample characteristics (e.g., sex, population-based vs. clinic-referred) and assessment methods (e.g., structured interviews, self-report, abuse/dependence vs. frequency). For example, in Biederman et al. (2010), girls were originally ascertained when they were 6-16 years old. Thus, developmentally-sensitive assessments of SUD must consider the censored nature of the age of participants at follow-up and the potential that substance patterns may reflect age-related differences in substance exposure and availability rather than diagnostic differences per se.
Overall, the predictive validity of ADHD for SUD is unknown. Although future research on ADHD and substance outcomes must improve the methodological limitations described above, it is crucial to understand what the literature currently suggests. The goal of this meta-analysis was to characterize the most informative studies, which are likely to be prospective follow-up studies of children with and without ADHD into adolescence/adulthood. Prospective longitudinal studies significantly improve the empirical basis for determining direction of effects, evaluating meditational processes, and differentiating correlates, risk factors, and causal risk factors (Kraemer, Stice, Kazin, Offord, & Kuper, 2001). Indeed, temporal ordering of predictors and outcome is one of few methodological devices available to disentangle correlated constructs (Kraemer et al., 2001). Second, meta-analysis rigorously evaluates associations from smaller studies, which is often the case for prospective longitudinal designs (Keenan & Shaw, 1997). To test the predictive validity of ADHD and SUD from methodologically diverse samples, a meta-analysis may provide superior traction relative to a single, larger study. Our aim was two-fold: (1) To meta-analyze the prospective contribution of childhood ADHD (versus control) on dichotomized measures of lifetime substance use and abuse/dependence across nicotine, alcohol, marijuana, and cocaine; and (2) To test theoretically- and methodologically-relevant moderators (i.e., age, sex, race (percent Caucasian), DSM version, sample source) of these putative associations if and when significant heterogeneity in effect size was found.
Method
Study Selection Criteria
Each study satisfied the following inclusion criteria: (a) diagnostic ascertainment of ADHD with at least one control or non-ADHD group; (b) prospective longitudinal design (i.e., ADHD diagnosis preceded the measurement of SUD); (c) binary lifetime substance use and abuse/dependence measures; (d) available data to calculate proportions of children with and without ADHD with substance use, SUD, or odds ratios provided; (e) publication between 1980 and August 2009 in English; and (f) a non-intervention design. We prioritized case-control designs to improve the generalizability of the study to other children with ADHD (i.e., diagnostic criteria + impairment) and to specifically compare the likelihood of SUD in youth with and without ADHD. We also selected substance abuse/dependence outcomes to emphasize clinical significance (Kazdin, 1999). Given that experimentation with substances is often normative in adolescence (Moffitt, 1993), we chose outcomes that included impairment (i.e., failure to fulfill obligations, failed attempts to quit). Given that ADHD also predicts functional impairment, we cannot be certain that these clinically significant outcomes are attributable to substance patterns rather than the influence of ADHD (e.g., impulsivity). However, clinically significant dichotomous outcomes also resonate with person-centered research strategies that are central to developmental psychopathology (Bergman, von Eye, & Magnusson, 2006). Dichotomization does not significantly reduce statistical power and it yields meaningful effect sizes (i.e., odds ratios) (Farrington & Loeber, 2000). Finally, the intervention selection bias is a threat to internal validity in uncontrolled studies because treatment status is often positively correlated with negative outcomes (Larzelere, Kuhn, & Johnson, 2004). That is, severity of psychopathology may account for treatment status and outcome. To avoid misrepresenting the association of early ADHD and later SUD, we did not control for treatment status. We also excluded controlled intervention studies in an effort to focus on the naturalistic course of ADHD over time.
Search Procedure
We employed several strategies to identify the 27 studies included in this meta-analysis. We conducted computer-based searches using the PsycInfo, PubMed, and Google Scholar databases. These inquiries entailed searching according to the following keywords (or stems when possible): alcohol, nicotine, smoking, tobacco, cigarette, marijuana, cannabis, cocaine, substance(s), drug(s), ADHD, ADD, attention-deficit, attention-deficit/hyperactivity disorder, hyperactivity, hyperactive, hyperkinetic, longitudinal, and prospective. Keywords were combined by using the Boolean operators “AND” and “OR.” Unpublished dissertations were also reviewed for potential inclusion. We also used the ancestry approach where potential studies were identified from the reference sections of existing reviews on the association of ADHD and SUD. Moreover, we thoroughly reviewed the bibliographies of identified studies for additional studies and used both forward and backward searching. To combat the file drawer problem, we also attempted to locate unpublished studies (Rosenthal, 1979). Emails describing our study and its inclusion criteria were sent to membership listservs of research organizations including the International Society for Research in Child and Adolescent Psychopathology, Division 53 (Society for Clinical Child and Adolescent Psychology) of the American Psychological Association, and the Research Society on Alcoholism. However, all of the studies included in our meta-analysis were published in peer-reviewed journals. The majority of studies was excluded for the following reasons: (a) they were qualitative reviews, (b) ADHD and substance constructs were only measured using dimensional approaches (i.e., no formal diagnosis), or (c) ADHD designations did not precede the measurement of substance use. When multiple studies with the same substance outcome were derived from the same sample, the most recent publication was used (i.e., the longest follow-up period from baseline). Coding of individual studies was conducted by two intensively trained raters. We evaluated the reliability of all moderator codes (a total of 168) and the percentage agreement was very high (95.2%). In cases where raters provided contradictory judgments, disagreements were discussed and one of the authors (KLH) made a final determination.
Moderator Variables
We tested whether demographic/methodological factors across the studies moderated the association between childhood ADHD and SUD for studies with heterogeneous effect sizes. The following demographic characteristics were coded: (a) average age of the sample at follow-up (in years); (b) gender composition (% male); and (c) racial diversity (% Caucasian). Methodological characteristics of each study were coded as follows: (a) sample source (clinic-referred vs. other), for both the ADHD and non-ADHD samples and (b) version of the Diagnostic and Statistical Manual of Mental Disorders (DSM) used to diagnose ADHD (i.e., DSM-III and DSM-III-R versus DSM-IV). The final moderator was the average number of years between the initial assessment and follow-up (less than 5 years vs. 5 to 10 years vs. greater than 10 years).
Calculation of Effect Size
We calculated the odds ratio (OR) to estimate the effect size and significance of the association between diagnostic status (ADHD vs. control) and two separate substance measures: (a) use vs. no use and (b) abuse or dependence versus non-abuse/dependence. The odds ratio was computed by the formula (a+d)/(b+c), where a represented the number of individuals with ADHD who positively reported substance use or abuse/dependence; b represented the number of youth with ADHD who denied substance use or abuse/dependence; c represented the number of individuals in the control group who positively endorsed substance use or abuse/dependence; and d represented the number of control youth without substance use or abuse/dependence. When a cell had a value of 0, we followed expert recommendations and inserted .5 to all four cells to calculate the effect size (Lipsey & Wilson, 2001). An OR of 1 indicated that membership in the dichotomous substance outcome category was equivalent in children with and without ADHD whereas an OR greater than 1 indicated that the outcome was more likely in the ADHD group. An OR of less than 1 indicated that the outcome was less likely to occur in the ADHD group. The association of ADHD and substance outcome is statistically significant when the 95% confidence interval for the OR effect size does not include 1.0. If the 95% confidence interval included 1.0, the effect indicated statistical equivalence between the ADHD and control group. For each study, the OR was separately calculated for each available substance outcome measure. Thus, in the same study, as many as eight ORs could be derived that corresponded to the three substance use outcomes (nicotine, alcohol, marijuana) and five abuse/dependence outcomes (nicotine dependence, alcohol abuse/dependence, marijuana abuse/dependence, cocaine abuse/dependence, and non-specified drug abuse/dependence). Using these procedures, a total 65 effect sizes were calculated from 27 eligible studies.
Statistical analysis
We employed a random-effects model where an OR for each substance outcome was weighted by the inverse variance of the OR. A random-effects model is more appropriate when the variability of findings is assumed to be attributed to other factors than subject-level sampling error (Lipsey & Wilson, 2001). In addition to calculating the mean effect size and its 95% confidence interval, we estimated heterogeneity of effects using the standard Cochran's Q Test and publication bias using Egger's (Egger, Smith, Schneider, & Minder, 1997) and Begg's tests (Begg & Mazumdar, 1994). We also calculated the Fail safe N to assess the potential for a file-drawer problem and evidence that any of the moderator variables predicted significant variance in the effect sizes where analyses resulted in significant heterogeneity. The meta-analysis was performed using STATA 11 with the meta, metareg, and metabias commands.
Results
To review, we evaluated the prospective contribution of childhood ADHD on measures of lifetime adolescent/adult substance use and abuse/dependence. We conducted separate meta-analyses across different substance types to examine the specificity of effects of early ADHD. We utilized random-effects models to estimate the mean effect size (and 95% confidence interval), statistical significance of the OR, and the χ2-based Q statistic for heterogeneity. The Q statistic approximates a chi-square distribution with k − 1 degrees of freedom, where k is the number of effect sizes, and it indicates the degree of consistency of findings across studies (Hedges & Olkin, 1985). Each of these parameters was estimated for the following dichotomous substance measures: (a) lifetime nicotine use, (b) nicotine dependence, (c) lifetime alcohol use, (d) alcohol abuse or dependence, (e) lifetime marijuana use, (f) marijuana abuse or dependence, (g) cocaine abuse or dependence, and (h) general illicit drug abuse or dependence (i.e., drug type not specified in the original study).
Although Table 1 summarizes the clinical, demographic, and methodological features of all 27 studies included in the meta-analysis, we note that that there were 4142 to 4175 ADHD probands and 6835 to 6880 non-ADHD controls available for analysis (depending on the specific substance outcome analyzed). Based on studies that reported relevant data, the overall sample consisted mostly of Caucasian (88.9%; 15 studies) boys (74.2%; 26 studies). The average age at follow-up for participants was 18.9 years (24 studies). The sample size for ADHD probands and non-ADHD controls, respectively, was as follows: 1648 and 2323 for lifetime nicotine use; 2459 and 2950 for nicotine dependence; 872 and 597 for lifetime alcohol use; 1337 and 1195 for alcohol abuse or dependence; 600 and 2661 for lifetime marijuana use; 943 and 1885 for marijuana abuse or dependence (excluding Gignac et al. (2005)); 652 and 481 for cocaine abuse or dependence; and 542 and 637 for non-specific substance abuse or dependence.
Table 1.
Methodological features of studies included in meta-analysis.
| Authors | N at follow-up | Average age at follow-up | % Male | % White | Sample source | Length of follow-up | DSM version for ADHD | Outcomes |
|---|---|---|---|---|---|---|---|---|
| August et al. (2006) | ADHD: 109 Control: 91 | 18.3 | 74.58 | 89.55 | ADHD and Controls: School-based | 6-12 years | DSM-III-R | Marijuana & Alcohol Abuse & Dep.; Nicotine Use & Dep. |
| Barkley et al. (1990) | ADHD: 123 Control: 66 | 14.55 | 91.53 | Not provided | ADHD: clinical (psychological); Control: referrals from ADHD group | 8 years | Not provided | Marijuana, Alcohol, & Nicotine Use |
| Barman et al. (2004)i | ADHD: 672/705 Control: 1324/1369 | 14.04 | 50.09 | Not provided | ADHD, Control: population-based (Finnish registry) | 2-3 years | DSM-IV | Nicotine Use & Dep. |
| Biederman et al. (2006) | ADHD: 112 Control: 105 | 22 | 100 | 100 | ADHD: clinical (psychiatric, medical); Control: clinical (medical) | 10 years | DSM-III-R | Non-specific Abuse & Dep.; Alcohol Abuse & Dep.; Nicotine Dep. |
| Biederman et al. (1997) | ADHD: 128 Control: 109 | 14.77 | 100 | 100 | ADHD, Control: “psychiatric and nonpsychiatric settings” | 4 years | DSM-III-R | Marijuana & Cocaine Abuse & Dep. |
| Brook et al. (2008) | ADHD: 52 Control: 589 | 32 | 46.02 | 91 | ADHD, Control: Subset of nationally representative sample of northeastern U.S. | 19-22 years | DSM-IV | Nicotine Dep. |
| Burke et al. (2001) | ADHD: 108 Control: 96 | Not provided | 100 | 70 | ADHD, Control: clinical | 3 years | DSM-III-R | Nicotine Dep. |
| Claude &Firestone (1995) | ADHD: 52 Control: 52 | 19.7 | 100 | Not provided | ADHD: “clinical group;” Control: schools, community | 14.4 to 24.9 years for ADHD group | DSM-III-R | Non-specific Abuse & Dep.; Alcohol Abuse & Dep. |
| Elkins et al. (2007)ii | ADHD: Not provided Control: Not provided | 18.15 | 49.74 | 97.9 | ADHD, Control: population-based (Minnesota Twins) | 6-8 years | DSM-III-R, supplemented with DSM-IV | Marijuana & Alcohol Abuse & Dep.; Nicotine Dep. |
| Ernst et al. (2006) | ADHD: 50 Control: 28 | 16.0 | 69.23 | Not provided | ADHD, Control: clinical, newspapers | 3.8 years on average | DSM-IV | Marijuana, Alcohol, & Nicotine Use |
| Fergusson &Horwood (1995) | ADHD: 24 Control: 912 | 16 | Not provided | Not provided | ADHD, Control: Birth cohort | Approximately 1 year | DSM-III-R | Marijuana Use & Nicotine Dep. |
| Fischer et al. (2002) | ADHD: 147 Control: 73 | 20.5 | 91 | 94 | ADHD: clinical; Control: matched community | 13.8 years on average | Not provided | Marijuana, Cocaine, & Alcohol Abuse & Dep.; Non-specific Abuse & Dep. |
| Gignac et al. (2005) | ADHD: 28 Control: 179 | 20 | 47.83 | Not provided | ADHD, Control: psychiatric and nonpsychiatric settings | 6-17 years at baseline, 20 years on average at follow-up | DSM-III-R | Marijuana Abuse & Dep.; Marijuana Use |
| Hechtman et al. (1984) | ADHD: 75 Control: 44 | 19 | 90 | Not provided | ADHD: clinical; Control: schools | 10 years | Not provided | Marijuana, Cocaine, & Alcohol Abuse & Dep. |
| King et al. (2004)iii | ADHD: 193 Control: 163 | 14.8 | 48.75 | Not provided | ADHD, Control: community-based sample of twins | 10-12 years-old at baseline; Average age at follow-up was 14.8 years | DSM-III-R | Alcohol, Nicotine, & Marijuana Use |
| Lambert (2005) | ADHD: 217 Control: 182 | 26 | 78.05 | 76.83 | ADHD, Control: community-based | 28 years | DSM-IV | Marijuana, Cocaine, & Alcohol Abuse & Dep.; Nicotine Dep. |
| Lambert &Hartsough (1998) | ADHD: 218 Control: 182 | Not provided | 78.05 | 77 | ADHD, Control: community-based | School-age to adult | DSM-III-R | Nicotine Use |
| Mannuzza et al. (1998) | ADHD: 85 Control: 73 | 24.1 | 100 | 100 | ADHD: clinical; Control: clinical and community | 15-21 years | Not provided | Marijuana, Cocaine, & Alcohol Abuse & Dep. |
| Mannuzza et al. (1991) | ADHD: 94 Control: 78 | 18.64 | 100 | Not provided | ADHD: clinical; Control: clinical, phone-dialing | 5-16 years | Not provided | Non-specific Abuse & Dep. |
| Milberger et al. (1997a) | ADHD: 28 Control: 238 | 15 | 100 | 100 | ADHD and Control: clinical | 4 years | DSM-III-R | Nicotine Use |
| Milberger et al. (1997b) | ADHD: 34 Control: 235 | 17.34 | 49.44 | 100 | ADHD, Control: sibling referral | 4 years | DSM-III-R | Alcohol Abuse & Dep.; Non-specific Abuse & Dep. |
| Molina &Pelham (2003) | ADHD: 142 Control: 100 | 15.18 | 94.24 | 86.77 | ADHD: clinical; Control: schools, newspapers | 5.26 years on average | DSM-III-R | Marijuana & Alcohol Abuse & Dep.; Marijuana, Alcohol, & Nicotine Use; Nicotine Dep. |
| Molina et al. (2007) | ADHD: 364 Control: 240 | 17.51 | 89.24 | 82.8 | ADHD: clinical; Control: clinical, schools, newspapers | Average of 8 years | DSM-IV | Alcohol Use, Abuse, & Dep. |
| Monuteaux et al. (2008) | ADHD: 139 Control: 122 | Not provided | 0 | Not provided | ADHD and Control: clinical | 5 years | DSM-III-R, supplemented with DSM-IV | Nicotine Dep. |
| Reynolds &Kirisci (2001) | ADHD: 28 Control: 180 | 16 | 85.6 | 68.3 | Not provided | 4-6 years | Not provided | Nicotine Dep. |
| Wilens et al. (2008) | ADHD: 80 Control: 86 | 19.2 | 48.01 | Not provided | ADHD, Control: referrals from 2 previous studies | 5 years for girls and 10 years for boys | DSM-III-R | Nicotine Use & Dep. |
| Wittchen et al. (2007) | ADHD: 40 Control: 1213 | 24 | 49.94 | Not provided | ADHD, Control: government registries | 10 years | DSM-IV | Marijuana Abuse & Dep.; Marijuana Use |
Note. ADHD = attention-deficit/hyperactivity disorder (ADHD); DSM = Diagnostic and Statistical Manual; Dep. = Dependence.
Sample size differed by specific outcome measure;
Sample size not provided; odds ratio provided by authors;
Sample size provided for only one outcome (which is reported here).
Nicotine Use and Dependence
As shown in Figure 1, children with ADHD were twice as likely to have a lifetime history of ever having used nicotine (OR = 2.08, CI = 1.66, 2.60, p < .001) compared to children without ADHD. The overall homogeneity statistic for nicotine use indicated that the effect sizes of the nine studies included in this analysis were comparable (Q = 12.82, p = 0.12). A similar association was also observed for childhood ADHD and nicotine dependence. Children with ADHD were nearly three times more likely than children without ADHD to report nicotine dependence in adolescence/adulthood (OR = 2.82, CI = 2.41, 3.29, p < .001) (Figure 2). The overall homogeneity statistic for nicotine dependence indicated that the twelve studies included in this analysis were largely uniform in their estimates (Q = 5.92, p = .25).
Figure 1. Lifetime nicotine use predicted from childhood ADHD.
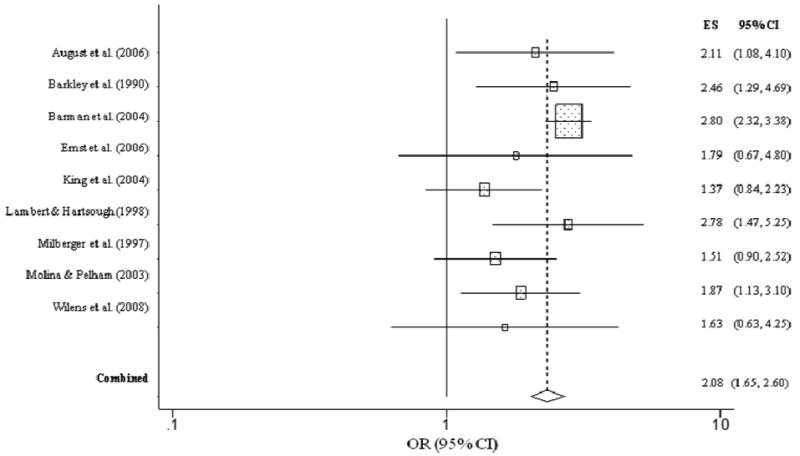
Figure 2. Nicotine dependence predicted from childhood ADHD.
Alcohol Use and Abuse/Dependence
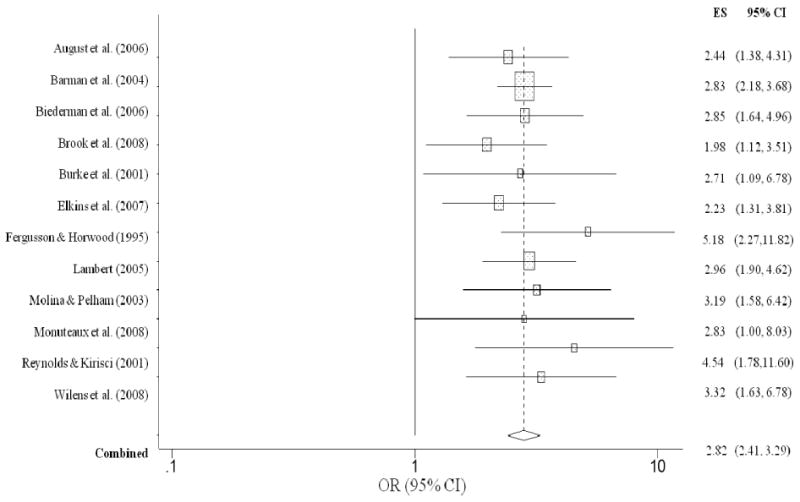
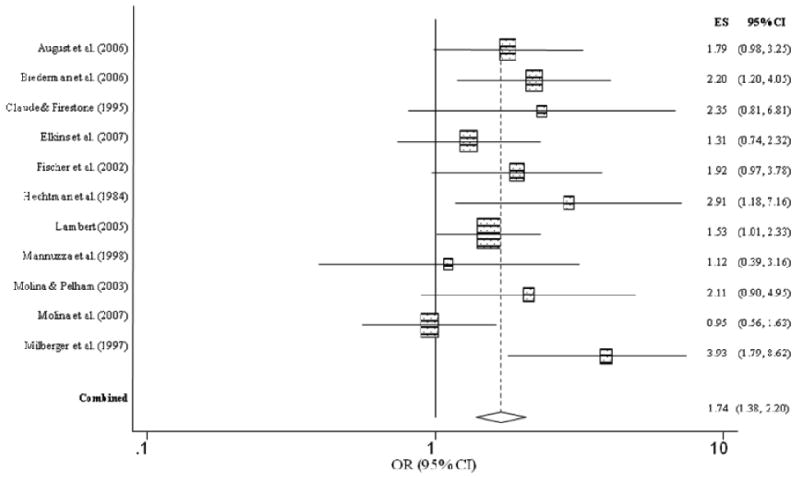
Figure 3 summarizes the individual effect sizes for each study and the aggregated estimate for lifetime history of ever having used alcohol for children with and without ADHD. Unlike nicotine use, children with ADHD were no more likely to have ever used alcohol than children without ADHD (OR = 1.27, CI = 0.85, 1.89, p = .25), as evidenced by the lower bound of the confidence interval falling below 1.0. The overall homogeneity statistic for alcohol use indicated that the five studies included in this analysis indicated a trend for effect size heterogeneity (Q = 8.26, p = .08). For alcohol abuse/dependence, childhood ADHD was significantly related to an increased risk for alcohol use disorder (OR = 1.74, CI = 1.38, 2.20, p < .001) (Figure 4). Specifically, children with ADHD were 1.7 times more likely to meet diagnostic criteria for alcohol abuse or dependence than children without ADHD. The overall homogeneity statistic for alcohol dependence indicated no significant heterogeneity across the 11 studies (Q = 13.20, p = .21).
Figure 3. Lifetime alcohol use predicted from childhood ADHD.
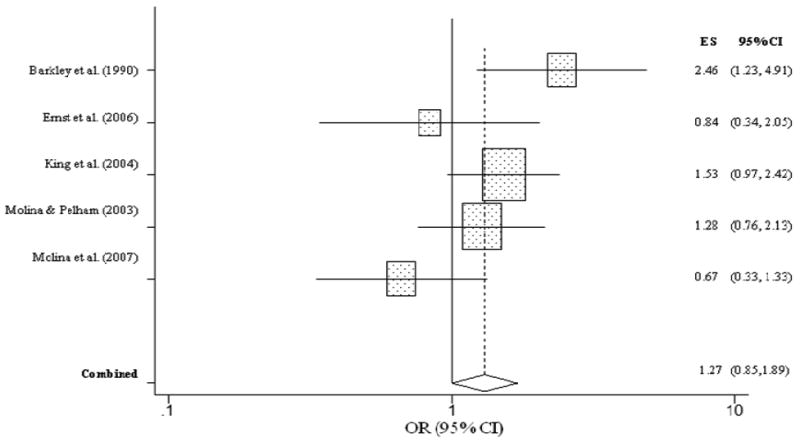
Figure 4. Alcohol abuse or dependence predicted from childhood ADHD.
Marijuana Use and Abuse/Dependence
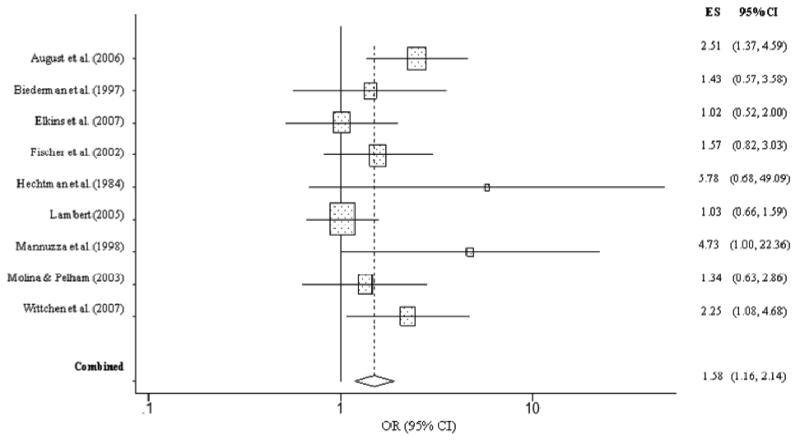
Childhood ADHD was robustly related to an increased odds of lifetime marijuana use (OR = 2.78, CI = 1.64, 4.74, p < .001). Specifically, children with ADHD were nearly three times more likely to have reported ever having used marijuana than children without ADHD (Figure 5). However, the overall homogeneity statistic for marijuana use indicated that the seven studies included in this analysis showed statistically significant variability in their effect size estimates (Q = 20.38, p < .01). This suggests that the overall effect size associating early ADHD with a higher likelihood of future adolescent/adult marijuana use should be interpreted cautiously. Results from our analysis of marijuana abuse or dependence revealed that children with ADHD were more than twice as likely to have met criteria for marijuana use disorder than children without ADHD (OR = 2.29, CI = 1.32, 3.99, p = .003). However, the overall homogeneity statistic for marijuana abuse/dependence indicated that the 10 studies included in this analysis showed significant variability in their effect sizes (Q = 45.07, p < .001). In particular, Gignac et al. (2005) reported an effect size that was more than two standard deviations above the grand mean. To assess the influence of this study, we removed it and re-analyzed the data. Without the Gignac et al. (2005) study, the homogeneity statistic was largely normalized (Q = 11.81, p = .16). The recalculated effect size (OR = 1.58, CI = 1.16, 2.14, p = .003) revealed that children with ADHD were approximately 1.5 times more likely than children without ADHD to develop marijuana abuse or dependence (Figure 6).
Figure 5. Lifetime marijuana use predicted from childhood ADHD.
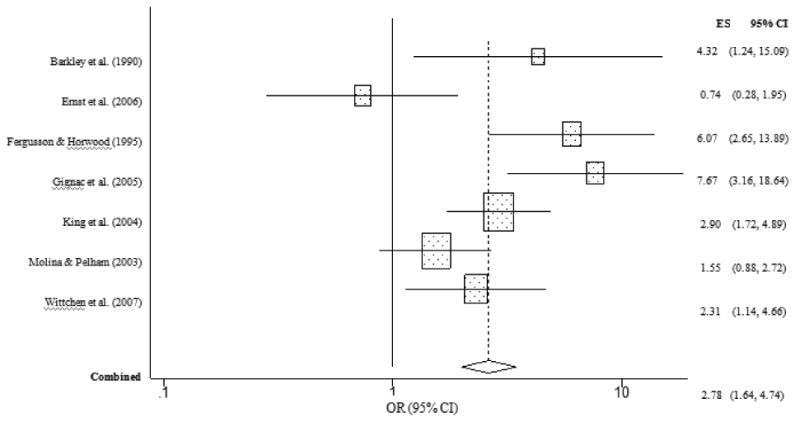
Figure 6. Marijuana abuse or dependence predicted from childhood ADHD (excluding Gignac et al. (2005).
Cocaine Abuse or Dependence
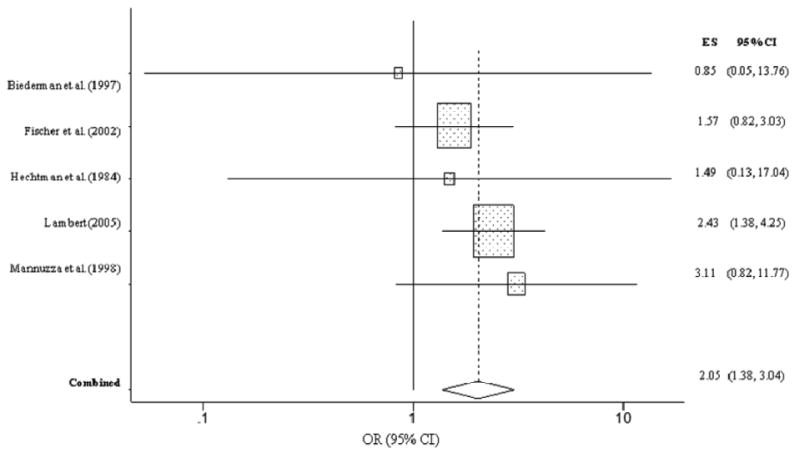
Children with ADHD were significantly more likely to develop cocaine abuse or dependence in adolescence/adulthood than children without ADHD (OR = 2.05, CI = 1.38, 3.04, p < .001) (Figure 7). Children with ADHD were twice as likely to develop cocaine abuse or dependence than children without ADHD. The Q test indicated minimal heterogeneity in the five studies (Q = 1.80, p = .77), thus strengthening our inference that each study was relatively consistent in its estimation of the association between ADHD and cocaine abuse/dependence.
Figure 7. Cocaine abuse or dependence predicted from childhood ADHD.
General Illicit Drug Abuse/Dependence
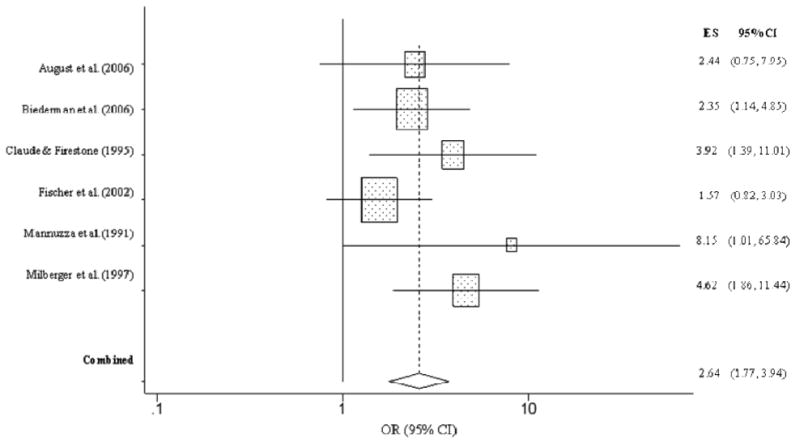
The overall effect for drug abuse/dependence, defined by studies that did not explicitly specify the substance, also revealed higher rates abuse/dependence among children with ADHD (OR = 2.64, CI = 1.77, 3.94, p < .001; Figure 8). Specifically, individuals with ADHD were more than two and half times as likely as controls to develop general illicit substance abuse/dependence. The overall homogeneity statistic indicated that the effect size of the six studies included in the analysis were not heterogeneous (Q = 5.64, p = .34).
Figure 8. Non-specific substance abuse or dependence predicted from childhood ADHD.
Publication Bias
We conducted the Egger's publication bias test and all tests were non-significant. The Begg's funnel plots also suggested no evidence of publication bias. We also used Orwin's (1983) formula for the fail-safe N to determine the number of studies that would be needed to reduce the findings to non-significance. Using an OR of 1 as the critical effect size value, for each outcome in which a significant group difference in substance use outcomes was found, we determined the number of additional studies with an effect size showing equivalence between groups (i.e., OR = 1) that would have needed to be included in our meta-analysis to alter the effect of ADHD status. The following number of studies was indicated: 9 for lifetime nicotine use, 25 for nicotine dependence, 11 for alcohol abuse/dependence, 18 for lifetime marijuana use, 12 for marijuana abuse/dependence (excluding Gignac et al., 2005), 4 for cocaine abuse/dependence, 17 for general drug abuse/dependence. These numbers suggest that it is relatively unlikely that unpublished studies would change the significant contribution of ADHD to substance problems.
Moderators
To further explore the nature of the association between ADHD and future substance patterns in those effect sizes with heterogeneity (i.e., alcohol use, marijuana use), we examined potential moderators using the metareg command for simple regressions. We separately tested the following variables as potential moderators: sex, average age at follow-up, race (i.e., percent Caucasian), sample source for both the ADHD and non-ADHD groups, version of the DSM used to ascertain ADHD, and average length of time between initial assessment and follow-up.
Comorbid Disruptive Behavior Disorders (DBD)
Given the substantial comorbidity between ADHD and disruptive behavior disorders (DBD), including ODD and CD (Barkley, 2006), as well as the robust relations between ODD/CD and substance outcomes (see Flory & Lynam, 2003 for a review), the conclusions suggested from this meta-analysis must be interpreted cautiously. We had initially intended to include comorbid ODD/CD as a moderator of ADHD predictions of substance outcomes in our meta-analysis. However, a careful review of the existing literature revealed surprisingly few studies that adequately addressed this comorbidity and that satisfied our inclusion criteria. For example, there was little consistency in the measurement of ODD/CD, particularly with respect to temporal factors (i.e., some studies measured CD during childhood, some concurrently during adolescence, and some used a combination). Therefore, this significant variability prevented our ability to formally examine ODD/CD comorbidity in the meta-analysis. Nevertheless, we strongly contend that DBD should be addressed, even if informally. Thus, we review existing findings here so that they can be interpreted concurrently with the results of the meta-analysis.
A thorough search of the literature identified 10 longitudinal studies of substance outcomes that measured ODD/CD and ADHD. For the present summary, we included only prospective longitudinal studies that utilized dichotomous measurement of all variables to enhance comparability with the studies in the meta-analysis. All 10 studies prospectively followed clinical or community samples of children with ADHD into adolescence or young adulthood where group differences (e.g., ADHD alone vs. ADHD + ODD/CD vs. controls) in substance outcomes were examined. Overall, our review suggests that comorbid ADHD + ODD/CD children may demonstrate significantly greater substance problems than children with ADHD only and controls (when included).
For instance, in a 10-year prospective study of youth with ADHD (n = 27), ADHD + ODD/CD (n = 82), and controls (n = 91), children with ADHD + ODD/CD group had significantly higher rates of regular tobacco use and alcohol and marijuana SUD than either the ADHD only or control groups (August et al., 2006). Similarly, in a longitudinal study of 364 adolescents and young adults diagnosed with ADHD in childhood and 240 matched controls, Molina, Pelham, Gnagy, Thompson, and Marshal (2007) found that adolescents with concurrent ADHD and CD were more likely to have an alcohol use disorder than controls. In this study, a direct comparison was not made between adolescents with ADHD + CD and those with ADHD alone; however, results indicated that those with ADHD + CD reported nearly five times the rate of alcohol use disorders than did youth with ADHD alone. In another prospective study of 177 clinic-referred boys, a significant bivariate relation between childhood ADHD and adolescent tobacco use was reduced to nonsignificance once childhood CD was included in the model (Burke, Loeber, & Lahey, 2001).
In an effort to further explore how the course of CD over time impacts the relation between ADHD and substance outcomes, Biederman and colleagues conducted two studies of 140 boys diagnosed with ADHD. At the 4-year follow-up, cigarette smoking, drug and alcohol dependence, and SUD in general were significantly elevated among participants with persisting symptoms of CD (N=24) versus those with desisting symptoms of CD (N=18), and those without CD (N=73) (Biederman, Mick, Faraone, & Burback, 2001). Similarly, at the 10-year follow-up of the same sample, the presence of CD along with ADHD was significantly associated with increased risk for psychoactive substance use disorders (defined as alcohol or drug abuse or dependence) compared to ADHD alone (Biederman et al., 2008). Loeber et al. (1999) also examined the effects of persistent delinquency and ADHD on substance use among a large longitudinal sample of approximately 500 boys. Results revealed that ADHD did not predict substance use once persistent delinquency and internalizing problems were controlled.
Two older studies which did not use current DSM ADHD diagnostic criteria also found results consistent with the six studies previously reviewed. August, Stewart, and Holmes (1983) followed 22 purely hyperactive (later classified as meeting DSM-III ADD criteria) and 30 hyperactive/unsocialized-aggressive boys for 4 years, examining alcohol and/or drug abuse (broadly measured) at follow-up. Results revealed that no purely hyperactive boys endorsed substance abuse, whereas a statistically significant 30% of the hyperactive/unsocialized-aggressive boys did endorse substance abuse at follow-up. Similarly, in an 8-year follow-up of 158 hyperactive children (later characterized as likely to meet DSM-III-R ADHD criteria) and 81 controls, Barkley and colleagues found that purely hyperactive children had no greater use of cigarettes and marijuana than controls whereas a comorbid hyperactive/CD group had 2-5 times the use of these substances compared to purely hyperactive or control youth (Barkley, Fischer, Edelbrock, & Smallish, 1990).
Interestingly, two studies found that ADHD was independently associated with substance outcomes. In a 28-year prospective study of 492 children with ADHD and age-matched controls, Lambert (2005) found that ADHD increased the odds of tobacco, cocaine, and amphetamine dependence whereas child conduct problems did not increase these odds after other variables were controlled. Similarly, among a very large sample of twins followed from ages 11 to 18, Elkins, McGue, and Iacono (2007) found that, at the age 14 follow-up, ADHD significantly predicted tobacco and illicit drug use initiation even when childhood CD was taken into account. However, at the age 18 follow-up, ADHD did not predict any substance outcomes independent of CD. One final study which was identified in our literature search (Ernst et al., 2006) only included one participant with ADHD + CD. Thus, their results are not generalizable.
Discussion
Childhood ADHD is a reliable predictor of negative outcomes across academic, social, neuropsychological, and affective domains. Hence, multifinality, where multiple negative outcomes share a common developmental origin, is a defining feature of ADHD (Cicchetti, 2006). However, far less is known about the prospective contribution of childhood ADHD to subsequent substance use and related disorders (abuse/dependence) than these other domains. To quantitatively characterize the association of ADHD on SUD and to strengthen a potential causal inference by establishing temporal ordering, we focused on prospective longitudinal studies (Kraemer et al., 2001). Our meta-analysis provides persuasive evidence of three key findings: (a) childhood ADHD conferred a significant increase in the odds of ever having used nicotine or illicit drugs, but not for alcohol; (b) childhood ADHD prospectively predicted the likelihood of developing adolescent/adult nicotine, alcohol, marijuana, and cocaine use disorders (i.e., abuse or dependence), as well as unspecified drug abuse/dependence; and (c) empirical tests of potential moderators for outcomes with heterogeneity in effect size estimates, consisting of demographic or methodological features that varied across studies, were not significant. That is, the reported effect sizes for ADHD and substance problems did not differ significantly by average age at follow-up, gender, race, sample source (clinic-referred vs. school-/population-based), or DSM version used to determine ADHD. In addition to the statistical significance of the association between ADHD and substance problems, we emphasize the size of the effects: children with ADHD were at least 1.5 times more likely to develop SUD across diverse forms of substances, including nearly 3 times higher for nicotine dependence. Considered together, our results suggest that early ADHD strongly predicts future substance abuse/dependence in adolescence/adulthood and that this association is largely impervious to demographic and methodological factors that varied across each study.
Overall, findings from previous studies of ADHD and comorbid ODD/CD predicting substance use outcomes are somewhat consistent, and they suggest that the relation between ADHD and substance outcomes in the literature (and potentially in our meta-analysis) may be partially or fully accounted for by the comorbidity between ADHD and ODD/CD, which is robustly related to substance outcomes. However, it is crucial to note that this characterization is based on only ten published studies and a literature with highly variable methods, including sample characteristics. For example, the study of Burke et al. (2001) was based exclusively on clinic-referred boys whereas the Loeber et al. (1999) study was based on a high-risk epidemiological sample. In fact, we were surprised at how infrequent comorbid ODD/CD was accounted for in the literature (either statistically controlled or explicit interactive effects between ADHD and ODD/CD). Thus, we echo the prescient review of Lilienfeld and Waldman (1990) who observed that prospective studies of ADHD and antisocial behavior (ASB) outcomes were likely contaminated by high rates of ODD/CD. That is, the association between ADHD and substance problems cannot be adequately discerned until its frequent comorbidity with ODD/CD is cogently addressed. Additionally, researchers must adopt greater consistency in how ODD/CD is measured and conceptualized. For instance, studies varied in their ascertainment of ODD/CD, ranging from adult retrospective recall of childhood CD, to concurrent assessment of adolescent CD, to measures of childhood ADHD and CD gathered concurrently in a prospective study, to some combination of these strategies. This variability may influence how or whether the relation between ADHD and substance outcomes is accounted for by comorbid ODD/CD. Moreover, equifinality suggests that there are multiple pathways to SUD that may involve ADHD and ODD/CD to varying degrees (Cicchetti, 2006).
Limitations and Guidelines for Future Research
Although dimensional perspectives on psychopathology, including ADHD and SUD, have considerable empirical support (Barkley, 2003; Helzer et al., 2006; Neuman et al., 1999), our meta-analysis was limited to dichotomous designations of ADHD and substance use and abuse/dependence disorders. Thus, within each diagnostic group, there is likely to be significant variability. For example, in two separate studies, children with ADHD varied dramatically with respect to the precise combination of functional impairments demonstrated in adolescence across affective, social, and behavioral domains, although ADHD probands were reliably more impaired than youth without ADHD (Lee et al,. 2008; Owens et al., 2009). Similarly, the family context of ADHD is likely to contribute to the developmental course of ADHD (i.e., persistent vs. less severe) based on factors such as parenting and parent psychopathology (Chronis et al., 2007). An important question not addressed by this meta-analysis is if ADHD is associated with the timing of substance use disorders (e.g., earlier onset). Other studies (Odgers et al., 2008) suggest that age of onset of substance use is inversely related to negative outcomes and that this association is not exclusively explained by more conduct problems in early-onset substance users. If ADHD is related to an earlier onset of substance use/problems, this would suggest that preventive interventions must be implemented earlier in development for children with ADHD.
This meta-analysis also did not clarify potential differences among ADHD subtypes, an important consideration given that Combined-type ADHD is more strongly associated with externalizing disorders than other subtypes (Lahey et al., 1994; 2004). Moreover, even if all ADHD subtypes show significant associations with future SUD, one cannot assume that the mechanisms underlying those associations are identical (Hinshaw & Lee, 2003). For example, the association between Combined-type ADHD and SUD may be driven by comorbidity with ODD/CD whereas the association between Inattentive-type ADHD and SUD may be driven by the need to temporarily ameliorate neurocognitive deficits, including sluggish cognitive tempo (Milich, Balentine, & Lynam, 2001). Similarly, because of our dichotomous approach to ADHD, we were unable to examine the differential contribution of inattention-disorganization versus hyperactivity-impulsivity symptoms to SUD. Previous evidence suggests that inattention and hyperactivity may be differentially related to substance outcomes, including some studies implicating a stronger contribution from inattention (Molina & Pelham, 2003) and others from hyperactivity (Lee & Hinshaw, 2006). Finally, the intervention selection bias (Larzelere, Kuhn, & Johnson, 2004) threatens the internal validity of uncontrolled studies because intervention status typically correlates positively with psychopathology and its severity. Although our meta-analysis excluded controlled intervention studies, the precise contribution of treatment status to variability in substance outcomes was not definitively addressed.
Our preceding review of the potential importance of comorbid ODD/CD in studies of ADHD and substance outcomes necessitates a careful discussion of comorbidity more broadly, including ADHD and its frequent overlap with mood and anxiety disorders (Angold, Costello, & Erkanli, 1999). Unfortunately, our meta-analysis was unable to accommodate moderator analyses for disorders commonly comorbid with ADHD, including ODD/CD, depression, and anxiety. Although there may be differential patterns of prediction of SUD based on ADHD and its comorbidity (e.g., ADHD + ODD/CD), these groups may also represent a latent continuums characterized by heterotypic continuity rather than discrete disorders with separate etiologies (Patterson, DeGarmo, & Knutson, 2000; Shaw & Winslow, 1997). In fact, common influences across these phenotypes (e.g., disruptive parenting practices, deviant peer affiliation) may work synergistically with neurodevelopmental influences that are particularly salient in adolescence, including striatal dopamine and its influence on reward sensitivity and risk-taking (Galvan, 2010). Finally, some dimensions of anxiety may actually protect individuals with ADHD from risk-taking, externalizing behavior, or SUD (Levy, 2004). Thus, future research must consider comorbidity and the potential for interactive effects with ADHD, particularly across time.
Our meta-analysis provides further evidence that childhood ADHD is prospectively associated with a distressing array of negative outcomes, adding to previous studies that implicated ADHD with elevated rates of comorbid mood, anxiety, and externalizing problems (Biederman et al., 2010; Hinshaw, Owens, Sami, & Fargeon, 2006). In addition to conducting prospective longitudinal studies, future research must obtain greater traction on identifying the complex and potentially diverse mechanisms and pathways that lead from early ADHD to later SUD. There is evidence that academic problems and peer difficulties mediate the association between early behavior problems, including ADHD, and subsequent depression (i.e., dual failure model; Herman, Lambert, Ialongo, & Ostrander, 2007; Burke, Loeber, Lahey, & Rathouz, 2005; Capaldi & Stoolmiller, 1999). Previously identified mediators of ADHD and SUD also include deviant peer affiliation, coping, and parental support (Molina, Marshal, Pelham, & Wirth, 2005; Marshal, Molina, & Pelham, 2003). We propose that neuropsychological correlates of ADHD, including executive function (EF) deficits (e.g., response inhibition, planning, cognitive flexibility), should be tested as mediators given that EF is strongly associated with ADHD and independently predicts externalizing behavior and substance problems (Nigg et al., 2006). Given that mediators are targets for intervention, they offer the possibility to interrupt the cascade of negative sequelae associated with a predictor of negative outcomes. Thus, meditational analyses of ADHD-substance linkages must be prioritized in future studies. We also advocate that careful attention be paid to design features of existing studies and their potential influence on results. Wide age ranges in prospective studies of children with ADHD require extended follow-up to ensure that participants have equally entered periods of adolescent risk when substance experimentation often emerges (Moffitt, 1993).
Although meta-analyses support the efficacy of MPH in the treatment of ADHD (Faraone, Spencer, Aleardi, Pagano, & Biederman, 2004; Schachter, Pham, King, Langford, & Moher, 2001), there are unresolved questions about the development of future substance problems in children treated with stimulant medication (Kollins, 2008; Volkow, 2008). For example, Wilens, Faraone, Biederman, & Gunawardene (2003) reported that stimulant medication substantially reduced the risk for future substance problems, but two recent studies failed to replicate the protective effects of MPH (Biederman et al., 2008; Mannuzza et al., 2008). In one study, exposure to stimulant medication was positively associated with future substance use (Lambert & Hartsough, 1998). Given that MPH and amphetamines have similar neural reward properties and that repeated exposure to stimulants alters the sensitivity of dopamine receptors in non-human animals, concerns have been raised about the relative safety of MPH and its potential to increase neural sensitivity to the reinforcing properties of stimulants (and to substances more broadly) (Vitiello, 2001; Kollins, 2003). Despite neurodevelopmental evidence that the timing of exposure to stimulants in non-human animals adversely affects outcomes (i.e., earlier exposure was associated with worse outcomes), a recent study found that children who received treatment with stimulants early in development (< 8 years old) were less likely to develop substance problems than those treated with stimulants later in life (8-12 years). Specifically, children in the latter group were more likely to develop non-alcohol substance problems, and the association between age of stimulant treatment and substance problems was mediated by antisocial personality disorder (Mannuzza et al., 2008). Collectively, these studies underscore that treatment of ADHD with stimulant medication and its prospective contribution to substance use problems must be more precisely characterized in future studies.
Although the cost-effectiveness of interventions designed to reduce substance problems primarily through the prevention of mental disorders may be prohibitive, there is considerable interest in the potential contribution of childhood ADHD to the development of future SUD (Armstrong & Costello, 2002; Flory & Lynam, 2003; Molina et al., 2007; Molina & Pelham, 2003). If ADHD is causally related to SUD, then tailored prevention programs could be delivered to youth with ADHD prior to the developmental periods of greatest risk for substance initiation and progression to problem use. For example, parent- and family-based interventions for ADHD youth should emphasize the potential importance of parental monitoring (i.e., knowing the child's peers, recreational activities, and whereabouts after school), given its centrality to the onset of adolescent substance use and subsequent prevention of deviant peer affiliation (Steinberg, Fletcher, & Darling, 1994). In addition, interventions that reduce ADHD may also effectively reduce the risk of SUD and/or make substance problems more responsive to interventions. This is a crucial consideration given that psychopathology (e.g., ADHD, depression) and comorbid SUD are particularly resistant to intervention (Goldstein et al., 2009; Wilens, 2003) and that patients often delay treatment many years after the onset of the disorder (Kessler et al., 2001). We also emphasize that SUD must be thoughtfully assessed among adolescents and adults with a history of early ADHD. In particular, clinical ascertainment must differentiate experimentation from problematic substance use (e.g., functional impairment), given that children with ADHD were no more likely to have used alcohol than non-ADHD controls in our analysis. In other words, over-simplified designations of substance use are likely to betray important differences in underlying risk factors. For example, clinicians should separately consider positive versus negative reinforcement processes underlying substance problems given their differential contribution to risk-taking behavior in adolescents, particularly as a function of individual differences in distress tolerance (MacPherson et al., 2010).
In sum, childhood ADHD is associated with a substantially higher risk of a lifetime history of nicotine and illicit substance use, in addition to nicotine dependence, alcohol, marijuana, cocaine, and illicit drug abuse/dependence. Although the mechanisms governing these associations are not yet fully understood, the findings from this meta-analysis underscore the clinical and public health significance of ADHD and its persistent effects into adolescence and adulthood (i.e., multifinality). To more rigorously evaluate a potential causal role of ADHD in the development of SUD, ongoing intervention studies of ADHD must concurrently assess substance problems and whether they respond favorably to standard ADHD intervention methods (e.g., methylphenidate, behavior management). Furthermore, more intensive neural (e.g., striatal, anterior cingulate cortex) and genetic (e.g., dopamine neurotransmission) assays of potential common etiological influences on ADHD and SUD should refine our understanding of their comorbidity and may signal logical targets for pharmacological interventions.
Acknowledgments
This work was supported by the Consortium of Neuropsychiatric Phenomics (CNP) (NIH Roadmap for Medical Research grant UL1-DE019580, RL1DA024853) and NIH Grant 1R03AA020186-01 to Steve S. Lee.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text rev. Washington, DC: Author; 2000. [Google Scholar]
- Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of Child Psychology and Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. Journal of Consulting and Clinical Psychology. 2002;70:1224–1239. doi: 10.1037//0022-006x.70.6.1224. [DOI] [PubMed] [Google Scholar]
- August GJ, Stewart MA, Holmes CS. A four-year follow-up of hyperactive boys with and without conduct disorder. The British Journal of Psychiatry: The Journal of Mental Science. 1983;143:192–198. doi: 10.1192/bjp.143.2.192. [DOI] [PubMed] [Google Scholar]
- *.August GJ, Winters KC, Realmuto GM, Fahnhorst T, Botzet A, Lee S. Prospective study of adolescent drug use among community samples of ADHD and non-ADHD participants. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:824–832. doi: 10.1097/01.chi.0000219831.16226.f8. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment. 2nd. New York, NY, US: Guilford Press; 1998. [Google Scholar]
- Barkley RA. Attention-deficit/hyperactivity disorder. In: Mash EJ, Barkley RA, editors. Child psychopathology. 2nd. New York, NY, US: Guilford Press; 2003. pp. 75–143. [Google Scholar]
- Barkley RA. The relevance of the still lectures to attention-deficit/hyperactivity disorder: a commentary. Journal of Attention Disorders. 2006;10:137–140. doi: 10.1177/1087054706288111. [DOI] [PubMed] [Google Scholar]
- *.Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. Journal of the American Academy of Child & Adolescent Psychiatry. 1990;29:546–557. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- *.Barman SK, Pulkkinen L, Kaprio J, Rose RJ. Inattentiveness, parental smoking and adolescent smoking initiation. Addiction. 2004;99:1049–1061. doi: 10.1111/j.1360-0443.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- Bédard A, Schulz KP, Cook EH, Fan J, Clerkin SM, Ivanov I, Halperin JM, Newcorn JH. Dopamine transporter gene variation modulates activation of striatum in youth with ADHD. NeuroImage. 2009 doi: 10.1016/j.neuroimage.2009.12.041. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- Bergman LR, von Eye A, Magnusson D. Person-oriented research strategies in developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology, Volume 1: Theory and method. 2nd. New York: Wiley; 2006. pp. 850–888. [Google Scholar]
- Biederman J, Faraone SV, Keenan K, Benjamin J. Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder: Patterns of comorbidity in probands and relatives in psychiatrically and pediatrically referred samples. Archives of General Psychiatry. 1992;49:728–738. doi: 10.1001/archpsyc.1992.01820090056010. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV, Burback M. Patterns of remission and symptom decline in conduct disorder: A four-year prospective study of an ADHD sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:290–98. doi: 10.1097/00004583-200103000-00008. [DOI] [PubMed] [Google Scholar]
- *.Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, et al. Young adult outcome of attention deficit hyperactivity disorder: A controlled 10-year follow-up study. Psychological Medicine. 2006;36:167–179. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Dolan C, Hughes S, Mick E, Monuteaux MC, et al. The long-term longitudinal course of oppositional defiant disorder and conduct disorder in ADHD boys: findings from a controlled 10-year prospective longitudinal follow-up study. Psychological Medicine. 2008;38:1027–1036. doi: 10.1017/S0033291707002668. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Monuteaux MC, Fried R, Byrne D, Mirto T, et al. Faraone SV. Adult psychiatric outcomes of girls with attention deficit hyperactivity disorder: 11-year follow-up in a longitudinal case-control study. American Journal of Psychiatry. 2010 doi: 10.1176/appi.ajp.2009.09050736. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Monuteaux MC, Mick E, Clarke A, Haagen KT, Faraone SV. Familial risk analysis of the association between attention-deficit/hyperactivity disorder and psychoactive substance use disorder in female adolescents: A controlled study. Journal of Child Psychology and Psychiatry. 2009;50:352–358. doi: 10.1111/j.1469-7610.2008.02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Biederman J, Wilens T, Mick E, Faraone SV, Weber W, Curtis S, et al. Soriano J. Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:21–29. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- Boyle MH, Offord DR. Psychiatric disorder and substance use in adolescence. Canadian Journal of Psychiatry. 1991;36:699–705. [PubMed] [Google Scholar]
- Boyle MH, Offord DR, Racine YA, Fleming JE, Szatmari P, Links PS. Predicting substance use in early adolescence based on parent and teacher assessments of childhood psychiatric disorder: Results from the Ontario Child Health Study follow-up. Journal of Child Psychology and Psychiatry. 1993;34:535–544. doi: 10.1111/j.1469-7610.1993.tb01034.x. [DOI] [PubMed] [Google Scholar]
- *.Brook JS, Duan T, Zhang C, Cohen PR, Brook DW. The association between attention deficit hyperactivity disorder in adolescence and smoking in adulthood. American Journal on Addictions. 2008;17:54–59. doi: 10.1080/10550490701756039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Burke JD, Loeber R, Lahey BB. Which aspects of ADHD are associated with tobacco use in early adolescence? Journal of Child Psychology and Psychiatry. 2001;42:493–502. [PubMed] [Google Scholar]
- Burke JD, Loeber R, Lahey BB, Rathouz PJ. Developmental transitions among affective and behavioral disorders in adolescent boys. Journal of Child Psychology and Psychiatry. 2005;46:1200–1210. doi: 10.1111/j.1469-7610.2005.00422.x. [DOI] [PubMed] [Google Scholar]
- Capaldi DM, Stoolmiller M. Co-occurrence of conduct problems and depressive symptoms in early adolescent boys: III. prediction to young-adult adjustment. Development and Psychopathology. 1999;11:59–84. doi: 10.1017/s0954579499001959. [DOI] [PubMed] [Google Scholar]
- Chronis AM, Lahey BB, Pelham WE, Kipp HL, Baumann BL, Lee SS. Psychopathology and substance abuse in parents of young children with attention-Deficit/Hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:1424–1432. doi: 10.1097/00004583-200312000-00009. [DOI] [PubMed] [Google Scholar]
- Chronis AM, Lahey BB, Pelham WE, Williams SH, Baumann BL, Kipp H, et al. Rathouz P. Maternal depression and early positive parenting predict future conduct problems in young children with attention-deficit/hyperactivity disorder. Developmental Psychology. 2007;43:70–82. doi: 10.1037/0012-1649.43.1.70. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. Development and psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, vol 1: Theory and method. 2nd. Hoboken, NJ, US: John Wiley & Sons Inc; 2006. pp. 1–23. [Google Scholar]
- Clark DB, Moss HB, Kirisci L, Mezzich AC, Miles R, Ott P. Psychopathology in preadolescent sons of fathers with substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:495–502. doi: 10.1097/00004583-199704000-00012. [DOI] [PubMed] [Google Scholar]
- *.Claude D, Firestone P. The development of ADHD boys: A 12-year follow-up. Canadian Journal of Behavioural Science. 1995;27:226–249. [Google Scholar]
- Clure C, Brady KT, Saladin ME, Johnson D, Waid R, Rittenbury M. Attention deficit/hyperactivity disorder and substance use: Symptoms pattern and drug choice. American Journal of Drug and Alcohol Abuse. 1999;25:441–448. doi: 10.1081/ada-100101871. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychological Bulletin. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sanctis VA, Trampush JW, Harty SC, Marks DJ, Newcorn JH, Miller CJ, Halperin JM. Childhood maltreatment and conduct disorder: Independent predictors of adolescent substance use disorders in youth with attention deficit/hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology. 2008;37:785–793. doi: 10.1080/15374410802359650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney ER, Elkins IJ, McGue M, Iacono WG. Effects of ADHD, conduct disorder, and gender of substance use and abuse in adolescence. American Journal of Psychiatry. 1999;156:1515–1521. doi: 10.1176/ajp.156.10.1515. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, Angermeyer MC, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA: The Journal of the American Medical Association. 2004;291:2581–2590. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- *.Ernst M, Luckenbaugh DA, Moolchan ET, Leff MK, Allen R, Eshel N, et al. Kimes A. Behavioral predictors of substance-use initiation in adolescents with and without attention-deficit/hyperactivity disorder. Pediatrics. 2006;117:2030–2039. doi: 10.1542/peds.2005-0704. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Spencer T, Aleardi M, Pagano C, Beiderman J. Meta-analysis of the efficacy of methylphenidate for treating adult attention-Deficit/Hyperactivity disorder. Journal of Clinical Psychopharmacology. 2004;24:24–29. doi: 10.1097/01.jcp.0000108984.11879.95. [DOI] [PubMed] [Google Scholar]
- Farrington DP, Loeber R. Some benefits of dichotomization in psychiatric and criminological research. Criminal Behaviour and Mental Health. 2000;10:100–122. [Google Scholar]
- *.Fergusson DM, Horwood LJ. Predictive validity of categorically and dimensionally scored measures of disruptive childhood behaviors. Journal of the American Academy of Child & Adolescent Psychiatry. 1995;34:477–485. [PubMed] [Google Scholar]
- *.Fischer M, Barkley RA, Smallish L, Fletcher K. Young adult follow-up of hyperactive children: Self-reported psychiatric disorders, comorbidity and the role of childhood conduct problems and teen CD. Journal of Abnormal Child Psychology. 2002;30:463–475. doi: 10.1023/a:1019864813776. [DOI] [PubMed] [Google Scholar]
- Flory K, Lynam DR. The relation between attention deficit hyperactivity disorder and substance abuse: What role does conduct disorder play? Clinical Child and Family Psychology Review. 2003;6:1–16. doi: 10.1023/a:1022260221570. [DOI] [PubMed] [Google Scholar]
- Frodl T. Comorbidity of ADHD and substance use disorder (SUD): A neuroimaging perspective. Journal of Attention Disorders. 2010;14:109–120. doi: 10.1177/1087054710365054. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:1–9. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Gignac M, Wilens TE, Biederman J, Kwon A, Mick E, Swezey A. Assessing cannabis use in adolescents and young adults: What do urine screen and parental report tell you? Journal of Child and Adolescent Psychopharmacology. 2005;15:742–750. doi: 10.1089/cap.2005.15.742. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Shamseddeen W, Spirito A, Emslie G, Clarke G, Wagner KD, et al. Brent DA. Substance use and the treatment of resistant depression in adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 2009 doi: 10.1097/CHI.0b013e3181bef6e8. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, James AS, Jentsch JD. Poor response inhibition: At the nexus between substance abuse and attention deficit/hyperactivity disorder. Neuroscience and Biobehavioral Reviews. 2009;33:690–698. doi: 10.1016/j.neubiorev.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hechtman L, Weiss G, Perlman T. Hyperactives as young adults: Past and current substance abuse and antisocial behavior. American Journal of Orthopsychiatry. 1984;54:415–425. doi: 10.1111/j.1939-0025.1984.tb01507.x. [DOI] [PubMed] [Google Scholar]
- Hedges L, Olkin I. Statistical Methods for Meta-analysis. San Diego,CA: Academic Press; 1985. [Google Scholar]
- Helzer JE, Bucholz KK, Bierut LJ, Regier DA, Schuckit MA, Guth SE. Should DSM-V include dimensional diagnostic criteria for alcohol use disorders? Alcoholism: Clinical and Experimental Research. 2006;30:303–310. doi: 10.1111/j.1530-0277.2006.00028.x. [DOI] [PubMed] [Google Scholar]
- Herman KC, Lambert SF, Ialongo NS, Ostrander R. Academic pathways between attention problems and depressive symptoms among urban african american children. Journal of Abnormal Child Psychology. 2007;35:265–274. doi: 10.1007/s10802-006-9083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw SP, Lee SS. Conduct and oppositional defiant disorders. In: Mash EJ, Barkley RA, editors. Child psychopathology. 2nd. New York, NY, US: Guilford Press; 2003. pp. 144–198. [Google Scholar]
- Hinshaw SP, Owens EB, Sami N, Fargeon S. Prospective follow-up of girls with attention-deficit/hyperactivity disorder into adolescence: Evidence for continuing cross-domain impairment. Journal of Consulting and Clinical Psychology. 2006;74:489–499. doi: 10.1037/0022-006X.74.3.489. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Katusic SK, Barbaresi WJ, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Psychostimulant treatment and risk for substance abuse among young adults with a history of attention-deficit/hyperactivity disorder: A population-based, birth cohort study. Journal of Child and Adolescent Psychopharmacology. 2005;15:764–776. doi: 10.1089/cap.2005.15.764. [DOI] [PubMed] [Google Scholar]
- Kazdin AE. The meanings and measurement of clinical significance. Journal of Consulting and Clinical Psychology. 1999;67:332–339. doi: 10.1037//0022-006x.67.3.332. [DOI] [PubMed] [Google Scholar]
- Keenan K, Shaw D. Developmental and social influences on young girls' early problem behavior. Psychological Bulletin. 1997;121:95–113. doi: 10.1037/0033-2909.121.1.95. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Berglund PA, Caraveo-Anduaga JJ, DeWit DJ, Greenfield SF, Kolody B, Olfson M, Vega WA. Patterns and predictors of treatment seeking after onset of a substance use disorder. Archives of General Psychiatry. 2001;58:1065–1071. doi: 10.1001/archpsyc.58.11.1065. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005a;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005b;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.King SM, Iacono WG, McGue M. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction. 2004;99:1548–1559. doi: 10.1111/j.1360-0443.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- Kollins SH. Comparing the abuse potential of methylphenidate versus other stimulants: A review of available evidence and relevance to the ADHD patient. Journal of Clinical Psychiatry. 2003;64:14–18. [PubMed] [Google Scholar]
- Kollins SH. ADHD, substance use disorders, and psychostimulant treatment: Current literature and treatment guidelines. Journal of Attention Disorders. 2008;12:115–125. doi: 10.1177/1087054707311654. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American Journal of Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, McBurnett K, Biederman J. DMS-IV field trials for attention deficit hyperactivity disorder in children and adolescents. American Journal of Psychiatry. 1994;151:1673–1685. doi: 10.1176/ajp.151.11.1673. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Kipp H, Ehrhardt A, Lee SS, Willcutt EG, Hartung CM, Chronis A, Massetti G. Three-year predictive validity of DSM-IV attention deficit hyperactivity disorder in children diagnosed at 4-6 years of age. American Journal of Psychiatry. 2004;161:2014–2020. doi: 10.1176/appi.ajp.161.11.2014. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Piacentini JC, McBurnett K, Stone P. Psychopathology in the parents of children with conduct disorder and hyperactivity. Journal of the American Academy of Child & Adolescent Psychiatry. 1988;27:163–170. doi: 10.1097/00004583-198803000-00005. [DOI] [PubMed] [Google Scholar]
- *.Lambert N. The contribution of childhood ADHD, conduct problems, and stimulant treatment to adolescent and adult tobacco and psychoactive substance abuse. Ethical Human Psychology and Psychiatry. 2005;7:197–221. [Google Scholar]
- *.Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. Journal of Learning Disabilities. 1998;31:533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Larzelere RE, Kuhn BR, Johnson B. The intervention selection bias: An under-recognized confound in intervention studies. Psychological Bulletin. 2004;130:289–303. doi: 10.1037/0033-2909.130.2.289. [DOI] [PubMed] [Google Scholar]
- Lee SS, Hinshaw SP. Predictors of adolescent functioning in girls with attention deficit hyperactivity disorder (ADHD): The role of childhood ADHD, conduct problems, and peer status. Journal of Clinical Child and Adolescent Psychology. 2006;35:356–368. doi: 10.1207/s15374424jccp3503_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Lahey BB, Owens EB, Hinshaw SP. Few preschool boys and girls with ADHD are well-adjusted during adolescence. Journal of Abnormal Child Psychology. 2008;36:373–383. doi: 10.1007/s10802-007-9184-6. [DOI] [PubMed] [Google Scholar]
- Levy F. Synaptic gating and ADHD: A biological theory of comorbidity of ADHD and anxiety. Neuropsychopharmacology. 2004;29:1589–1596. doi: 10.1038/sj.npp.1300469. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, Waldman ID. The relation between childhood attention-deficit hyperactivity disorder and adult antisocial behavior reexamined: The problem of heterogeneity. Clinical Psychology Review. 1990;10:699–725. [Google Scholar]
- Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, CA: SAGE; 2001. [Google Scholar]
- Loeber R, Stouthamer-Loeber M, White HR. Developmental Aspects of Delinquency and Internalizing Problems and Their Association With Persistent Juvenile Substance Use Between Ages 7 and 18. Journal of Clinical Child Psychology. 1999;28:322. doi: 10.1207/S15374424jccp280304. [DOI] [PubMed] [Google Scholar]
- MacPherson L, Reynolds EK, Daughters SB, Wang F, Cassidy J, Mayes LC, et al. Positive and negative reinforcement underlying risk behavior in early adolescents. Prevention Science. 2010;11:331–342. doi: 10.1007/s11121-010-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult psychiatric status of hyperactive boys grown up. American Journal of Psychiatry. 1998;155:493–498. doi: 10.1176/ajp.155.4.493. [DOI] [PubMed] [Google Scholar]
- *.Mannuzza S, Klein RG, Bonagura N, Malloy P, Giampino TL, Addalli KA. Hyperactive boys almost grown up. V. Replication of psychiatric status. Archives of General Psychiatry. 1991;48:77–83. doi: 10.1001/archpsyc.1991.01810250079012. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Truong NL, Moulton JL, Roizen ER, Howell KH, Castellanos FX. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: Prospective follow-up into adulthood. American Journal of Psychiatry. 2008;165:604–609. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshal MP, Molina BSG, Pelham WE., Jr Childhood ADHD and adolescent substance use: An examination of deviant peer group affiliation as a risk factor. Psychology of Addictive Behaviors. 2003;17:293–302. doi: 10.1037/0893-164X.17.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. Journal of the American Medical Association. 2000;284:1689–95. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Mehta RL, Molnar BE, Walters EE, Swendsen JD, Auilar-Gaziola S, Bijl R, Borges G, Caraveo-Anduaga JJ, Dewit DJ, Kolody B, Vega W, Wittchen H, Kessler RC. Comorbidity of substance use disorders with mood and anxiety disorders: Results of the international consortium in psychiatric epidemiology. Addictive Behaviors. 1998;23:893–908. doi: 10.1016/s0306-4603(98)00076-8. [DOI] [PubMed] [Google Scholar]
- *.Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Further evidence of an association between attention-deficit/hyperactivity disorder and cigarette smoking: Findings from a high-risk sample of siblings. American Journal on Addiction. 1997;6:205–217. [PubMed] [Google Scholar]
- *.Milberger S, Biederman J, Faraone SV, Wilens T, Chu MP. Associations between ADHD and psychoactive substance use disorders: Findings from a longitudinal study of high risk siblings of ADHD children. American Journal on Addictions. 1997;6:318–329. [PubMed] [Google Scholar]
- Milich R, Balentine AC, Lynam DR. ADHD combined type and ADHD predominantly inattentive type are distinct and unrelated disorders. Clinical Psychology: Science and Practice. 2001;8:463–488. [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100(4):674–701. [PubMed] [Google Scholar]
- Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Marshal MP, Pelham WE, Jr, Wirth RJ. Coping skills and parent support mediate the association between childhood attention-deficit/hyperactivity disorder and adolescent cigarette use. Journal of Pediatric Psychology. 2005;30:345–357. doi: 10.1093/jpepsy/jsi029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Molina BSG, Pelham WE. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal of Abnormal Psychology. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE, Lang AR. Alcohol expectancies and drinking characteristics in parents of children with attention deficit hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 1997;21:557–566. doi: 10.1111/j.1530-0277.1997.tb03802.x. [DOI] [PubMed] [Google Scholar]
- *.Molina BSG, Pelham WE, Gnagy EM, Thompson AL, Marshal MP. Attention-deficit/hyperactivity disorder risk for heavy drinking and alcohol use disorder is age specific. Alcoholism: Clinical and Experimental Research. 2007;31:643–654. doi: 10.1111/j.1530-0277.2007.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Monuteaux MC, Faraone SV, Hammerness P, Wilens TE, Fraire M, Biederman J. The familial association between cigarette smoking and ADHD: A study of clinically referred girls with and without ADHD, and their families. Nicotine & Tobacco Research. 2008;10:1549–1558. doi: 10.1080/14622200802326137. [DOI] [PubMed] [Google Scholar]
- Neuman RJ, Todd RD, Heath AC, Reich W, Hudziak JJ, Bucholz KK, Madden PAF, Begleiter H, Porjesz B, Kuperman S, Hesselbrock V, Reich T. Evaluation of ADHD typology in three contrasting samples: A latent class approach. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:25–33. doi: 10.1097/00004583-199901000-00016. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Odgers CL, Caspi A, Nagin DS, Piquero AR, Slutske WS, Milne BJ, Dickson N, et al. Is it important to prevent early exposure to drugs and alcohol among adolescents? Psychological Science: A Journal of the American Psychological Society / APS. 2008;19:1037–1044. doi: 10.1111/j.1467-9280.2008.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwin RG. A Fail-Safe N for Effect Size in Meta-Analysis. Journal of Educational Statistics. 1983;8:157–159. [Google Scholar]
- Owens EB, Hinshaw SP, Lee SS, Lahey BB. Few girls with childhood attention-deficit/hyperactivity disorder show positive adjustment during adolescence. Journal of Clinical Child and Adolescent Psychology. 2009;38:132–143. doi: 10.1080/15374410802575313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GR, DeGarmo DS, Knutson N. Hyperactive and antisocial behaviors: Comorbid or two points in the same process? Development and Psychopathology. 2000;12:91–106. doi: 10.1017/s0954579400001061. [DOI] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Jr, Tidey JW, McGeary JE, MacKillop J, Gwaltney CJ, Rohsenow DJ, Swift RM, Monti PM. Polymorphisms of the μ-opioid receptor and dopamine D4 receptor genes and subjective responses to alcohol in the natural environment. Journal of Abnormal Psychology. 2010;119:115–125. doi: 10.1037/a0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Reynolds M, Kirisci L. The relationship between behavioral dysregulation in late childhood and cigarette smoking at age 16. Journal of Child & Adolescent Substance Abuse. 2001;10:91–99. [Google Scholar]
- Rosenthal R. The file drawer problem and tolerance for null effects. Psychological Bulletin. 1979;86:638–641. [Google Scholar]
- Scahill L, Schwab-Stone M. Epidemiology of ADHD in school-age children. Child and Adolescent Psychiatric Clinics of North America. 2000;9:541–555. [PubMed] [Google Scholar]
- Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. Canadian Medical Association Journal. 2001;165:1475–1488. [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Archives of General Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Winslow EB. Precursors and correlates of antisocial behavior from infancy to preschool. In: Stoff DM, Breiling J, Maser JD, editors. Handbook of antisocial behavior. Hoboken, NJ, US: John Wiley & Sons Inc; 1997. pp. 148–158. [Google Scholar]
- Steinberg L, Fletcher A, Darling N. Parental monitoring and peer influences on adolescent substance use. Pediatrics. 1994;93:1060–1064. [PubMed] [Google Scholar]
- Swanson J, Baler RD, Volkow ND. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: A decade of progress. Neuropsychopharmacology. 2010:1–20. doi: 10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szobot CM, Rohde LA, Bukstein O, Molina BSG, Martins C, Ruaro P, Pechansky F. Is attention-deficit/hyperactivity disorder associated with illicit substance use disorders in male adolescents? A community-based case-control study. Addiction. 2007;102:1122–1130. doi: 10.1111/j.1360-0443.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- Thompson LL, Riggs PD, Mikulich SK, Crowley TJ. Contribution of ADHD symptoms to substance problems and delinquency in conduct-disordered adolescents. Journal of Abnormal Child Psychology. 1996;24:325–347. doi: 10.1007/BF01441634. [DOI] [PubMed] [Google Scholar]
- Vitiello B. Long-term effects of stimulant medications on the brain: Possible relevance to the treatment of attention deficit hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology. 2001;11:25–34. doi: 10.1089/104454601750143384. [DOI] [PubMed] [Google Scholar]
- Volkow ND. Addiction reviews Introduction. Annals of the New York Academy of Sciences. 2008;1141:xi–xii. doi: 10.1196/annals.1441.034. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56:3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Ding Y, Gatley SJ. Mechanism of action of methylphenidate: insights from PET imaging studies. Journal of Attention Disorders. 2002;6:S31–43. doi: 10.1177/070674370200601s05. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM. Evaluating dopamine reward pathway in ADHD: Clinical implications. Journal of the American Medical Association. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen CK, Jamner LD, Henker B, Delfino RJ, Lozano JM. The ADHD spectrum and everyday life: experience sampling of adolescent moods, activities, smoking, and drinking. Child Development. 2002;73(1):209–227. doi: 10.1111/1467-8624.00401. [DOI] [PubMed] [Google Scholar]
- Whitmore EA, Mikulich SK, Thompson LL, Riggs PD, Aarons GA, Crowley TJ. Influences on adolescent substance dependence: Conduct disorder, depression, attention deficit hyperactivity disorder, and gender. Drug and Alcohol Dependence. 1997;4:87–97. doi: 10.1016/s0376-8716(97)00074-4. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Does the medicating ADHD increase or decrease the risk for later substance abuse? Revista Brasileira De Psiquiatria. 2003;25:127–128. doi: 10.1590/s1516-44462003000300001. [DOI] [PubMed] [Google Scholar]
- *.Wilens TE, Vitulano M, Upadhyaya H, Adamson J, Sawtelle R, Utzinger L, Biederman J. Cigarette smoking associated with attention deficit hyperactivity disorder. Journal of Pediatrics. 2008;153:414–419. doi: 10.1016/j.jpeds.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- *.Wittchen H, Fröhlicha C, Behrendta S, Günthera A, Rehma J, Zimmermanna P, et al. Cannabis use and cannabis use disorders and their relationship to mental disorders: A 10-year prospective-longitudinal community study in adolescents. Drug and Alcohol Dependence. 2007;88:S60–S70. doi: 10.1016/j.drugalcdep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]


