Abstract
Background
AT-101 is an oral inhibitor of the anti-apoptotic Bcl proteins (Bcl-2, Bcl-XL, Bcl-W, and Mcl-1) and an inducer of the pro-apoptotic proteins noxa and puma. We studied the efficacy of AT-101 in patients with recurrent chemosensitive extensive stage – small cell lung cancer (ES-SCLC).
Methods
Patients with recurrent “sensitive” SCLC (defined as no progression during and no disease recurrence < 2 months after completion of first-line platinum-based chemotherapy) were eligible. AT-101 was administered 20 mg orally daily for 21 out of 28 days each cycle for up to 6 cycles. The primary endpoint was the objective response rate.
Results
At the time of planned interim evaluation, none of the 14 evaluable patients enrolled in the first stage had any response to therapy and the study was closed permanently for further accrual. Three patients (21 %) achieved stable disease after two cycles of therapy. Grade 3 toxicities included anorexia, fatigue, and nausea/vomiting.
Conclusions
AT-101 is not active in patients with recurrent chemosensitive SCLC. Supported by N01-CM62205.
INTRODUCTION
Small cell lung cancer (SCLC) accounts for approximately 15% of newly diagnosed lung cancer in the United States1. While initial combination chemotherapy is very effective with response rates of 60–80%, the majority of patients will eventually die from recurrent disease. Although topotecan has been approved for use in patients with relapsed SCLC2, the clinical benefit from salvage therapy is quite limited. Minimal progress has been made in the systemic therapy of SCLC over the past three decades3. Clearly, new agents need to be developed to address this problem.
AT-101 [R-(−)-gossypol acetic acid] is an orally administered Bcl-2 homology (BH3) mimetic which has been reported to inhibit the heterodimerization of Bcl-xL, Bcl-w, and Mcl-1 with pro-apoptotic Bcl-2 proteins, thus lowering the threshold for apoptosis. As the racemic mixture, AT-101 has also been administered to over 9,000 healthy volunteer men as a male contraceptive4. It has been well-tolerated with the most common adverse events being gastrointestinal toxicities and grade 1/2 fatigue. Other toxicities include cardiac effects including elevations of troponin levels and 3 possibly related cardiac rhythm abnormalities. Early studies have reported only limited single-agent antitumor activity5–8.
The majority of SCLC tumors overexpress Bcl-2 and related family members, which can increase resistance to apoptosis9. Since AT-101 is a novel small molecule which inhibits the heterodimerization to the pro-apoptotic Bcl-2 family proteins, the administration of AT-101 could potentially be of value in patients with SCLC. Preclinical activity of AT-101 in SCLC, alone and in combination with standard chemotherapy agents, has been demonstrated using in vitro and in vivo models10. We, therefore, conducted a phase II study of single agent AT-101 in patients with chemotherapy-sensitive relapsed SCLC to characterize its activity.
PATIENTS AND METHODS
Eligible patients had histologically or cytologically confirmed SCLC with measurable disease per the Response Evaluation Criteria in Solid Tumors (RECIST)11 and were previously treated with only one prior platinum-based chemotherapy regimen. They must have chemotherapy-sensitive disease defined as no progression during first-line chemotherapy and no disease recurrence sooner than two months after completion of first-line chemotherapy. Patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2 and a life expectancy of greater than 12 weeks. Required laboratory parameters included leukocytes ≥3,000/mcL, absolute neutrophil count ≥1,500/mcL, hemoglobin > 8 g/dL, platelets ≥ 100,000/mcL, total bilirubin < 1.5 mg/dL, AST(SGOT) and ALT (SGPT) ≤ 2.5 x institutional upper limit of normal (ULN), and serum creatinine ≤ 1.5 mg/dL (or creatinine clearance ≥ 60 mL/min). All patients must be able to take oral medications on a regular basis and to give written informed consent. Patients with treated brain metastases who were clinically stable were eligible. The protocol was conducted by the Mayo Phase II Consortium along with the California Cancer Consortium and approved by the Institutional Review Boards (IRB) of all participating institutions. All patients provided written informed consent before undergoing treatment.
AT-101 (NSC# 726190) was supplied by the National Cancer Institute Cancer Therapy Evaluation Program as tablets containing 10 mg of drug. AT-101 was self-administered at 20 mg orally once per day for 21 consecutive days followed by a 7 day rest. Treatment was repeated every 4 weeks for up to six cycles or until progressive disease, unacceptable toxicity, or withdrawal of consent. The dose of AT-101 was reduced to 10 mg once daily for prestudy-defined adverse event criteria. Patients who required more than one dose reduction due to toxicity were removed from study.
As a potential biomarker of apoptotic pathway induction in response to AT-101, peripheral blood mononuclear cells (PBMC) were collected, where feasible, within 2 weeks prior to starting AT-101, and on day 2 of therapy. PBMC were isolated by low-speed centrifugation in CPT tubes, stored in OCT medium, and frozen at -80°C for subsequent analysis. Caspase activation in PBMC was assessed using the Caspase-Glo 3/7® Assay System (Promega) according to the manufacturer's instructions. Assays were performed in triplicate.
The primary endpoint of this trial was objective response rate, determined using RECIST. All patients who completed at least one post-baseline tumor assessment were evaluable for response. A two-stage Simon design12 was utilized to test the null hypothesis (H0) that the true response probability is ≤ 0.10 versus the alternative hypothesis (H1) that the true response probability is ≥ 0.25. A total of 14 patients were to be accrued to the first stage. If two or more patients exhibited disease response, the study was to be continued to the second stage and an additional 28 patients were to be enrolled. If 7 or more patients of the 42 patients enrolled exhibited disease response, the regimen was to be considered worthy of further study in this population. This design has a probability (significance level alpha) of 0.10 to reject H0 when H0 is true, and a probability of 0.85 to reject H0 when H1 is true (power). The secondary endpoints included toxicities, time to disease progression (TTP), and overall survival (OS) as well as intratumoral Bcl-2 family member expression and possible induction of the intrinsic apoptotic pathway. All toxicities were graded according the Common Terminology Criteria for Adverse Events Version 3.0.
RESULTS
From November 2008 to January 2010, 15 patients were accrued to the study from 5 sites within the Mayo Phase 2 Consortium and the California Consortium. Patient characteristics are summarized in Table 1. The median number of cycles of AT-101 was 2 (range 1–6). One patient discontinued therapy after one cycle of treatment due to persistent grade 1 thrombocytopenia which failed to recover to retreatment level of >100,000 per protocol after three weeks and was considered non-evaluable for the primary endpoint. Fourteen patients were evaluable for the primary endpoint at the interim analysis. Two patients required a dose delay due to a non-hematologic adverse event. One patient required dose modification due to a non-hematologic adverse event.
Table 1.
Patient Characteristics
| Total (N=15) | |
|---|---|
| Age | |
| Mean | 64.5 |
| Median | 67.0 |
| Range | 46.0–76.0 |
| Gender | |
| Male | 9 (60%) |
| Female | 6 (40%) |
| ECOG Performance Score | |
| 0 | 7 (46.7%) |
| 1 | 8 (53.3%) |
| Race | |
| White | 13 (86.7%) |
| Black or African-American | 1 (6.7%) |
| Asian | 1 (6.7%) |
| Ethnicity | |
| Not Hispanic or Latino | 15 (100%) |
| Prior Radiation Therapy | |
| Yes | 12 (80%) |
| No | 3 (20%) |
All grade 3/4 toxicities (regardless of attribution) are summarized in table 2. Four patients experienced grade 3 adverse events attributable to the study treatment: nausea and vomiting (1 patient), fatigue (1 patient), and anorexia (2 patients). Two patients experienced grade 3 hematologic adverse events (leukopenia), which were determined to be unrelated to treatment. No grade 4 toxicities were observed.
Table 2.
Grade 3/4 Toxicities*
| Grade 3 | |
|---|---|
| Hematologic | |
| Febrile Neutropenia | 0 (0%) |
| Leukopenia | 2 (13.3%) |
| Thrombocytopenia | 0 (0%) |
| Non-Hematologic | |
| Anorexia | 3 (20.0%) |
| Bilirubin | 1 (6.7%) |
| Dyspnea | 1 (6.7%) |
| Fatigue | 2 (13.3%) |
| Hyperglycemia | 1 (6.7%) |
| Hypokalemia | 1 (6.7%) |
| Hypomagnesemia | 1 (6.7%) |
| Hyponatremia | 4 (26.7%) |
| Hypophosphatemia | 1 (6.7%) |
| Nausea | 2 (13.3%) |
| Small Intestine Obstruction | 1 (6.7%) |
| Vomiting | 1 (6.7%) |
No grade IV toxicities were observed.
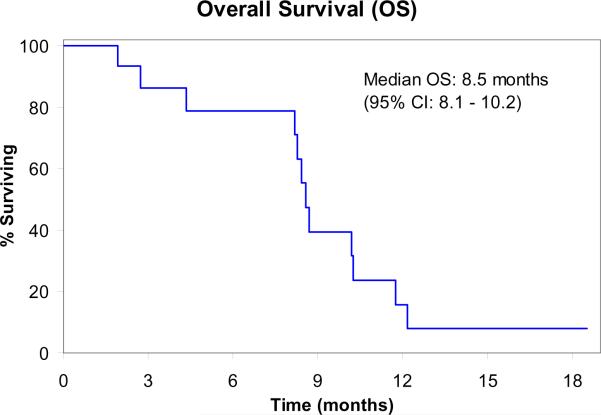
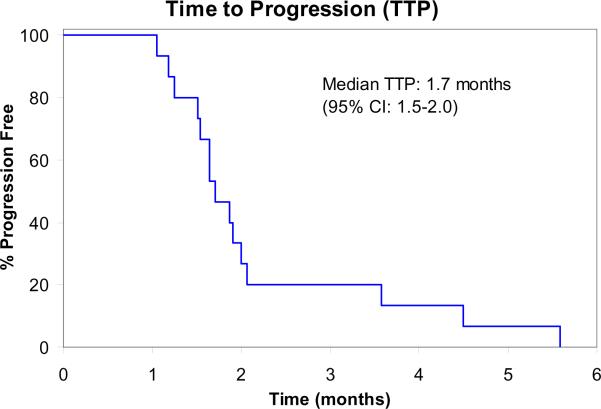
At the time of planned interim evaluation, among the 14 evaluable patients, no objective responses were observed. Therefore, accrual to the trial was terminated due to failure to pass the pre-specified interim analysis per study design. Three patients (21%) achieved stable disease after two cycles but subsequently progressed on treatment. Median time to progression was 1.7 months (95% confidence intervals: 1.5–2.0 months). Median overall survival was 8.5 months (95% confidence intervals: 8.1–10.2 months).
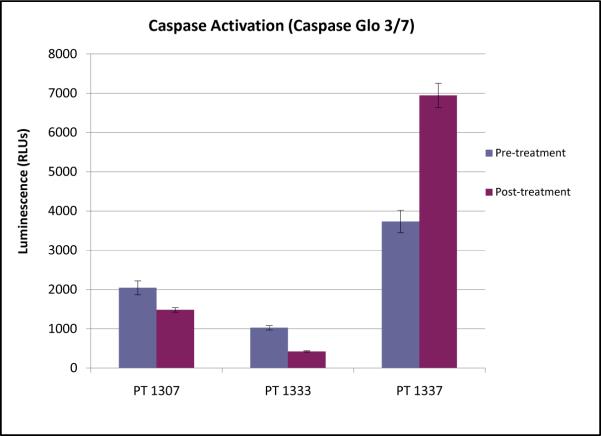
Caspase assays were performed on PBMC samples as a measure of the extent of activation of the apoptotic signaling cascade induced by AT-101 in vivo. Only three subjects had pre- and post-treatment samples available for analysis. There was marked inter-patient variability in caspase activation, and no consistent evidence of apoptotic induction by AT-101 (Figure 3). Only one of the three patients tested demonstrated evidence of increased caspase activity following AT-101, with two patients paradoxically showing lower caspase activity post-treatment. Each of the three patients had progressive disease as their best response.
Figure 3.
Relative caspase activity in peripheral blood mononuclear cells
DISCUSSION
Unfortunately, AT-101 joins the long list of agents that have shown disappointing results in patients with small cell lung cancer. As a single agent in patients with chemosensitive recurrent SCLC in this trial, AT-101 did achieve stable disease in 3 patients after 2 cycles of treatment but those patients progressed while still on therapy. A phase I/II study of AT-101 in combination with topotecan in recurrent SCLC was recently published13. The trial looked at two cohorts: chemorefractory and chemosensitive. The study did not meet its primary endpoints at the planned interim analysis and was stopped early.
Several hypotheses have been proposed as to the disappointing activity of Bcl-2 inhibitors in SCLC. To date, the promising pre-clinical findings with Bcl-2 inhibitors have failed to materialize in the clinic. A possible explanation is that the agents do not reach sufficient concentration in the tumor cells to suppress the Bcl-2 pathway in vivo. Another contributor to drug resistance could be the long half-life of Bcl-2 protein. A third explanation is the possibility of off-target cytotoxic mechanisms, and a failure to inhibit apoptotic induction specifically. In a preclinical study of six Bcl-2 inhibitors, both obatoclax and AT-101 treatment caused cell death in a caspase-independent manner instead of inducing classic apoptosis 14. Only ABT-737 was noted to induce classic apoptosis. This failure to induce caspase-dependent cell death is in keeping with our limited correlative analyses, which demonstrated no consistent induction on caspases following AT-101. One recent study identified some gene expression signatures that could predict sensitivity to ABT-263 in both SCLC and leukemia/lymphoma lines15. Perhaps the combination of the current Bcl-2 inhibitors with agents that target other areas of the apoptotic pathway may cause adequate suppression. Another avenue would be to further explore gene expression signatures as predictors of tumor sensitivity to target subsets of the patient population that would be sensitive to Bcl-2 inhibition.
Figure 1.
Overall Survival (N=15)
CI: Confidence Intervals
Figure 2.
Time to Progression (N=15)
CI: Confidence Intervals
Acknowledgements
Supported by N01-CM62205. Supported in part by Cancer Center Support Grant P30 CA91842 and the Clinical Trials Core of the Siteman Cancer Center at Washington University and Barnes-Jewish Hospital in St. Louis, Missouri.
Footnotes
Financial disclosures: Informed consent was obtained from all of the patients on study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–44. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 2.von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–67. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez E, Lilenbaum RC. Small cell lung cancer: past, present, and future. Curr Oncol Rep. 12:327–34. doi: 10.1007/s11912-010-0120-5. [DOI] [PubMed] [Google Scholar]
- 4.Coutinho EM. Gossypol: a contraceptive for men. Contraception. 2002;65:259–63. doi: 10.1016/s0010-7824(02)00294-9. [DOI] [PubMed] [Google Scholar]
- 5.Stein RC, Joseph AE, Matlin SA, et al. A preliminary clinical study of gossypol in advanced human cancer. Cancer Chemother Pharmacol. 1992;30:480–2. doi: 10.1007/BF00685601. [DOI] [PubMed] [Google Scholar]
- 6.Flack MR, Pyle RG, Mullen NM, et al. Oral gossypol in the treatment of metastatic adrenal cancer. J Clin Endocrinol Metab. 1993;76:1019–24. doi: 10.1210/jcem.76.4.8473376. [DOI] [PubMed] [Google Scholar]
- 7.Bushunow P, Reidenberg MM, Wasenko J, et al. Gossypol treatment of recurrent adult malignant gliomas. J Neurooncol. 1999;43:79–86. doi: 10.1023/a:1006267902186. [DOI] [PubMed] [Google Scholar]
- 8.Van Poznak C, Seidman AD, Reidenberg MM, et al. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial. Breast Cancer Res Treat. 2001;66:239–48. doi: 10.1023/a:1010686204736. [DOI] [PubMed] [Google Scholar]
- 9.Choi J, Choi K, Benveniste EN, et al. Bcl-2 promotes invasion and lung metastasis by inducing matrix metalloproteinase-2. Cancer Res. 2005;65:5554–60. doi: 10.1158/0008-5472.CAN-04-4570. [DOI] [PubMed] [Google Scholar]
- 10.Ascenta Therapeutics. Version 7 2009. Investigator's Brochure. [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 13.Heist RS, Fain J, Chinnasami B, et al. Phase I/II study of AT-101 with topotecan in relapsed and refractory small cell lung cancer. J Thorac Oncol. 5:1637–43. doi: 10.1097/JTO.0b013e3181e8f4dc. [DOI] [PubMed] [Google Scholar]
- 14.Paik PK, Rudin CM, Brown A, et al. A phase I study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in solid tumor malignancies. Cancer Chemother Pharmacol. 2010 doi: 10.1007/s00280-010-1265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tahir SK, Wass J, Joseph MK, et al. Identification of expression signatures predictive of sensitivity to the Bcl-2 family member inhibitor ABT-263 in small cell lung carcinoma and leukemia/lymphoma cell lines. Mol Cancer Ther. 9:545–57. doi: 10.1158/1535-7163.MCT-09-0651. [DOI] [PubMed] [Google Scholar]